Abstract
The growing incidence of antibiotic-resistant Gram-negative bacterial infections poses a serious threat to public health. Molecules that have yet to be exploited as antibiotics are potent protein toxins called bacteriocins that are produced by Gram-negative bacteria during competition for ecological niches. This review discusses the state of the art regarding the use for therapeutic purposes of two types of Gram-negative bacteriocins: colicin-like bacteriocins (CLBs) and tailocins. In addition to in vitro data, the potency of eight identified CLBs or tailocins has been demonstrated in diverse animal models of infection with no adverse effects for the host. Although the characteristics of bacteriocins will need further study, results obtained thus far regarding their in vivo potency, immunogenicity and low levels of resistance are encouraging. This leads the way for the development of novel treatments using bacteriocins as protein antibiotics.
Keywords: antibiotics, bacteriocins, colicin, infection, pyocin
Introduction
Antibiotic resistance has increased at an alarming rate worldwide, yet at the same time the discovery of new antibiotics is stalling. Of particular concern are infections caused by Gram-negative bacteria, which possess a highly impermeable outer membrane that limits the entry of multiple classes of antibiotics, leaving limited therapeutic options that are increasingly less efficacious as resistance spreads [1–4]. Foremost among the Gram-negative pathogens are Pseudomonas aeruginosa, Escherichia coli, Klebsiella pneumoniae and Acinetobacter baumannii, all of which pose serious threats to global healthcare and patient safety; in 2008, US intensive care units reported 17, 13, 13 and 74%, respectively, of clinical isolates of these species as multidrug-resistant [5]. Among these pathogens, E. coli and K. pneumoniae are well-known producers of extended-spectrum β-lactamases and carbapenemases that render producing bacteria resistant to β-lactams including cephalosporins and carbapenems, which are considered to be antibiotics of last resort [2,6,7]. In addition, chronic biofilm-mediated infections, such as P. aeruginosa lung infections in cystic fibrosis patients and E. coli urinary tract infections, are relatively poorly treated with existing antibiotics [8–10]. Consequently, treatment options for infections caused by Gram-negative bacteria are limited and patient outcome is increasingly poor [8–10].
Bacteriocins offer a potential alternative therapeutic strategy to treat both multidrug-resistant and chronic bacterial infections. These antimicrobial peptides or proteins are diverse and widespread among Gram-positive and Gram-negative bacteria [11]. It is becoming evident that they are linked to pathogenesis as they play a role in displacing bacterial flora, enhancing colonization and therefore infection ([11–13] and Sharp et al. unpublished data). In fact, protein bacteriocin-encoding genes can be found in the genomes of most Gram-negative pathogens, including P. aeruginosa, E. coli and K. pneumoniae ([14] and Sharp et al. unpublished data). Importantly, they are highly specific antibacterials that kill only bacteria closely related to the producer and are deployed during the fight for resources with competitor strains [14–16]. This specificity makes them attractive as therapeutics as they offer a more targeted approach. Indeed, one major issue with conventional antibiotics is the dysbiosis induced by broad-range killing of bacteria [17]. While the narrow killing spectrum of bacteriocins means that the bacteria responsible for the infection have to be identified prior to treatment, it gives the advantage of being able to specifically target one species, or even one strain of bacteria, leaving the normal healthy microflora intact [17]. The narrow killing spectrum also reduces the selective pressure for resistance on bystander microorganisms [18].
Gram-negative bacteriocins can be classified into three groups: peptide bacteriocins, which have been reviewed elsewhere [19,20], colicin-like bacteriocins (CLBs) and tailocins. The potential of the latter two, CLBs and tailocins, to treat bacterial infections is reviewed here. Previous reviews have discussed Gram-positive bacteriocins as antibiotics [11,21,22] and the in vitro potential of bacteriocins against Gram-negative bacteria [11,23–25]. Our aim is to focus on the potential ability of CLBs and tailocins to treat Gram-negative infections and give an exhaustive report of the results available from in vivo models of infection to date. We will not discuss data from probiotic applications of bacteriocins as the role of bacteriocins in probiotics is complex and only beginning to be understood [26], and although recent progress has been made in understanding the role of peptide bacteriocins in E. coli, equivalent investigations for CLBs and tailocins are still lacking [27].
In vitro studies have demonstrated the activity of bacteriocins against both planktonic and biofilm-embedded bacteria [28–31] and, interestingly, Rendueles et al. demonstrated that colicin R preferentially targets bacteria growing in the biofilm state. As a result, there is renewed interest in investigating the efficacy of bacteriocins in animal models. The use of animal models of infection to test antibacterial compounds is a long-established practice and acknowledged as a useful tool in predicting if translation into the treatment of human infections is likely to be successful. While there is no such thing as a standard animal model, new models have been developed to more closely resemble specific human infections (acute or chronic infections, sepsis and meningitis), giving improved insights into host–pathogen interactions during antibiotic treatment (reviewed in ref. [32] for P. aeruginosa). Since their discovery, bacteriocins have been tested in a variety of animal models, with a range of modes of administration and dosing regimens. Here, we summarize the results of these investigations and discuss factors that will ultimately determine the success of bacteriocins as therapeutic agents. Although further research is required, bacteriocins have key properties such as high potency in vivo and exquisite specificity that suggest they may be excellent candidates for further therapeutic development.
In vivo activity of bacteriocins
Colicin-like bacteriocins
Colicins are 40–70 kDa toxins that specifically target E. coli. Given the fact that these molecules were the first to be discovered and have been the best studied to date [33], we refer to similar molecules produced by other Gram-negative bacteria as CLBs. CLBs target strains closely related to the producing strain, typically of the same species, and have species-specific names: colicins from E. coli, klebicins from Klebsiella and pesticins from Yersinia pestis. Pseudomonas CLBs are named S-type pyocins (named after P. pyocyanea) [34] and are produced by >70% of strains [35]. CLBs are protease-susceptible, modular proteins with domains involved in receptor binding, translocation and cytotoxicity.
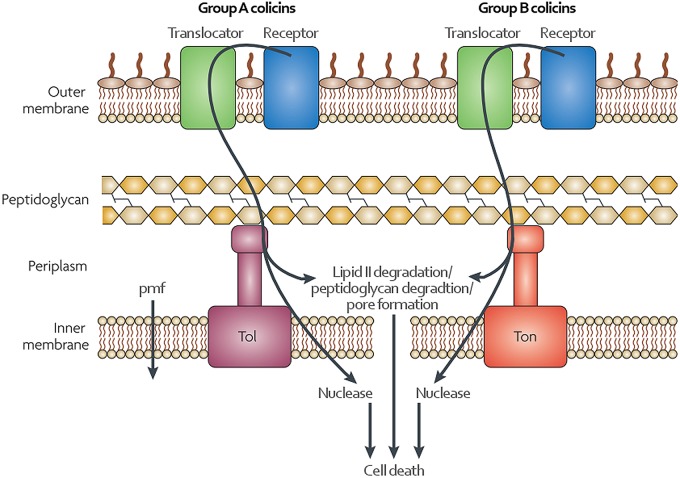
Using their receptor-binding domain, CLBs bind to bacterial outer membrane proteins before translocation through either the Ton or Tol systems of Gram-negative bacteria [36]. Many CLBs exploit TonB-dependent transporters to enter the cell, the physiological role of which is to import siderophores or vitamins. Although less well understood, the Tol system is also required for the entry of some CLBs. Several CLBs, such as colicin N and pyocin L1, have also been shown to interact with lipopolysaccharides (LPSs) on the cell surface [37,38]. The cytotoxic domains of CLBs are commonly pore-forming ionophores or nucleases, but some also have lipid II- or peptidoglycan-degrading activity (Figure 1) [39]. For the producing organism to be protected from its own bacteriocin, it produces an immunity protein that, at least in the case of the nuclease CLBs, binds the cytotoxic domain and neutralizes its activity [15].
Figure 1. Import of colicin-like bacteriocins.
Specificity of CLBs is mediated by binding a receptor in the outer membrane of a target cell. An adjacent translocator will then mediate transport into the periplasm. Energy for this transport is provided by either the Tol system for group A CLBs or by the Ton system for group B CLBs. Pore-forming colicin-like bacteriocins then insert into the inner membrane to disrupt the proton motive force (pmf) while nucleases translocate into the cytoplasm. Adapted from ref. [36].
Although data are currently limited, the efficacy of both the colicins and S-type pyocins has been tested in vivo. Indeed, a previous study showed that colicin E1 in pig feed can prevent diarrhoea and increase growth and feed efficiency; however, the dose was not sufficient to completely eradicate the targeted pathogenic strain of E. coli [40]. Pyocin S2 was shown to protect against a lethal P. aeruginosa infection in the greater wax moth Galleria mellonella larvae, a model of P. aeruginosa infections [41], and in murine lung infections when given 1 h post-infection [42]. The latter effect was also observed with pyocins AP41, S5 and L1, with pyocin S5 being particularly potent. Indeed, pyocin S5 was at least two orders of magnitude more potent than tobramycin in this model (Table 1) [42]. Similarly, all four pyocins were protective when given 6 h before infection. Interestingly, none of the bacteria isolated after pyocin treatment were resistant to the pyocins used, one isolate displaying increased tolerance to pyocin AP41. However, infection with this pyocin AP41-tolerant strain could still be controlled with this pyocin in vivo. Combinations of pyocins were also tested in vivo and were found to reduce colony forming units counts further, although not significantly [42].
Table 1. Effectiveness of protein antibiotics in in vivo challenge models.
| Model host | Protein | Treatment route | Challenge organism | Challenge route | Prophylactic (P) or therapeutic (T)?* | Effective? | |
|---|---|---|---|---|---|---|---|
| Colicin-like bacteriocins | |||||||
| Wax moth G. mellonella larvae | Pyocin S2 | Injection | P. aeruguinosa | Injection | T | Yes | [29] |
| Mouse | Pyocin S2 | IN | P. aeruguinosa | IN | P | Yes | [42] |
| Pyocin AP41 | Yes | ||||||
| Pyocin S5 | Yes | ||||||
| Pyocin L1 | Yes | ||||||
| Pyocin S2 | T | Yes | [42] | ||||
| Pyocin AP41 | Yes | ||||||
| Pyocin S5 | Yes | ||||||
| Pyocin L1 | Yes | ||||||
| Pig | Colicin E1 | Oral | E. coli | Oral | P | Yes | [40] |
| Tailocins | |||||||
| Mouse | Pyocin R2 | IP | P. aeruginosa | IP | T | Yes | [43] |
| IV | T | Yes | |||||
| Mouse | Enterocoliticin | Oral | Yersinia enterocolitica | Oral | T | Yes | [49] |
| Engineered tailocins | |||||||
| Rabbit | AvR2-V10.3 | Orogastrically | E. coli | Orogastrically | T | Yes | [63] |
| Unknown and unidentified bacteriocins | |||||||
| Mouse | Unknown pyocin(s) P10 | IP | P. aeruginosa | IP | P | Yes | [52] |
| IP | IP | T | No | ||||
| Chick embryo | Unknown pyocin | Allantoic cavity | P. aeruginosa | Allantoic cavity | P | Yes | [50] |
| Mouse | Pyocin H108 (S-type) | IP | P. aeruginosa | IP | P | No | [54] |
| T | No | ||||||
| Mouse | Colicin ‘wash’ | ‘Bladder wash’ | ? | ‘Established UTI’ | T | Yes | [53] |
| Mouse | Unknown pyocin 78-C2 | IV | P. aeruginosa | IV | P | Yes | [51] |
| IP | No | ||||||
| Mouse | Pyocin 1577 (R-type) | IP | P. aeruginosa | IP | P | Yes | [54] |
| T | No | ||||||
| Pyocin 5882 (F-type) | P | Yes | |||||
| T | No | ||||||
| Pyocin 1577 (R-type) | Topical | Topical on burn | T | No | |||
| IV | T | No | |||||
| Pyocin 5882 (F-type) | Topical | Topical on burn | T | Minimal | |||
Detailed description of studies with known and identified bacteriocins can be found in the text. Abbreviations: IV, intravenous; IP, intraperitoneal; IN, intranasal; UTI, urinary tract infection. *Bacteriocin given at the same time as the challenge is considered prophylactic.
Tailocins
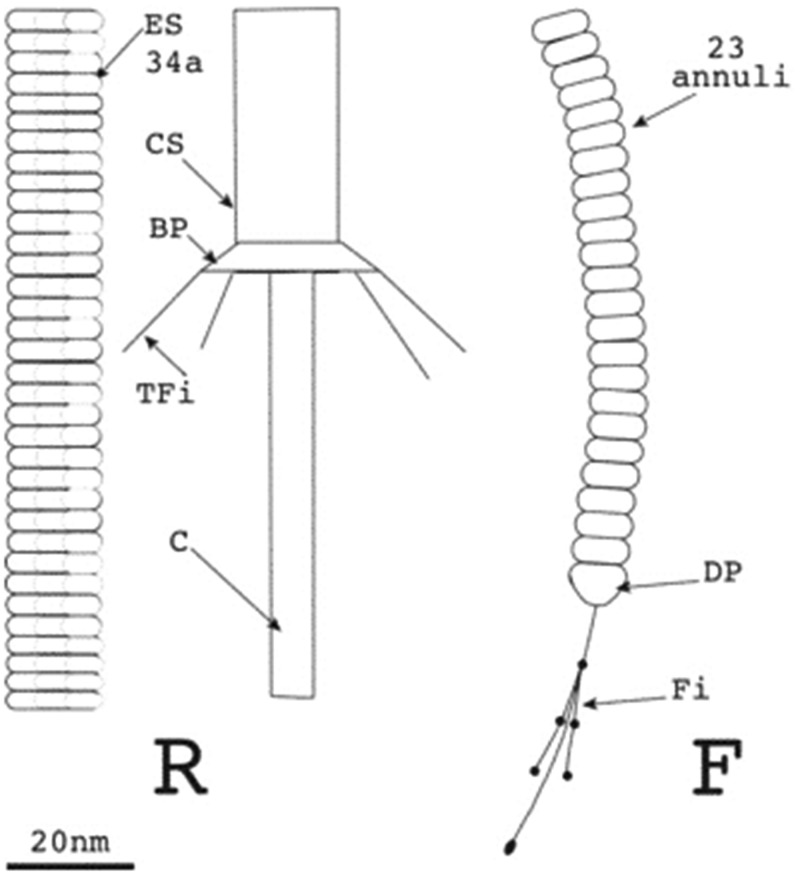
Tailocins, also called high molecular mass bacteriocins, resemble the tail structures of bacteriophages from the Myoviridae and Siphoviridae families [39]. Although they are thought to be derived from phages, they are not simply defective phages, but are adapted specifically to their function as bacteriocins [43]. Tailocin gene clusters are found in the genomes of many Gram-negative and Gram-positive bacteria [44,45]. The best studied tailocins are the R- and F-type pyocins of P. aeruginosa. R-type pyocins are morphologically and genetically similar to the contractile tail of P2-like temperate enterophages [46]. They consist of a core and sheath, and are contractile (Figure 2). F-type pyocins resemble λ phage, have no sheath and are flexible (Figure 2) [39]. Tailocin specificity is modulated by tail fibres, which have been demonstrated to bind to LPSs, with different residues involved depending on the tailocin [47,48]. Upon contact with the receptor, the tailocin undergoes conformational changes that result in lethal membrane damage, detected as the depletion of the proton motive force. As opposed to CLBs, tailocins are not protease-susceptible and no immunity proteins have been described [39]. Very little is known about the mechanisms of resistance to tailocins; however, it is suggested to be mediated by an alteration of cell surface receptors recognized by the tailocin [47].
Figure 2. R-type tailocins consist of an inner core (C) and sheath, which can be contracted (CS) or extended (ES).
Upon binding a target cell with tail fibres (TFi), attached to the sheath via the base plate (BP), the sheath contracts and inserts the core into the inner membrane of a target cell, consequently depleting the proton motive force. F-type tailocins consist of a flexible rod, made from 23 annuli, to which fibres (Fi) with some globular structures are attached via a distal part (DP). Taken from ref. [46].
Studies of tailocins in animal models have shown that, like CLBs, they show strong efficacy against Gram-negative infections. Scholl and Martin [43] conducted a thorough study of pyocin R2 efficacy in a murine peritonitis model. They determined the time window after infection in which treatment is possible and the dose necessary for adequate treatment. Protection via immune system stimulation was ruled out and pyocin R2 was found to be an effective therapeutic up to 4 h after infection in an aggressive infection model. Their results suggest that higher or repeated doses would allow for later treatment. The treatment of a second infection was less effective due to protective antibodies that had formed. They also found that the effective dose of pyocin R2 to treat the intraperitoneal (IP) challenge was lower when delivered through IP injection than through intravenous (IV) injection, suggesting that site-specific delivery of this pyocin can improve its efficacy (Table 1) [43].
Enterocoliticin, an R-type tailocin from Yersinia enterocolitica, was found to be effective for the treatment of mice orally infected with Y. enterocolitica when given orally [49]. The tailocin was only found to be active in vivo for 5 h; however, doses were given up to 24 h apart. Taking this into consideration, only those mice that received enterocoliticin 1 h after infection and were analyzed 5 h later can inform on the effect of the tailocin, and these mice did indeed show significantly reduced bacterial numbers (Table 1) [49].
Unfortunately, many CLBs and tailocins that were shown to be active in vivo [50–55] cannot be identified today, due to incoherent naming and lack of identification of the active molecules (Table 1).
Engineered bacteriocins
An important aspect of bacteriocin structure and function is their organization into domains that can be engineered to generate chimeric bacteriocins. Indeed, domains from different CLBs can be combined to customize their features. These include the target specificity via change of the receptor recognition domain, the immunity profile via change of immunogenic domains, mode of action via change of the cytotoxic domain and stability via change of structure, length or amino acid composition.
A combination of receptor binding and translocation domains of the CLB pesticin and bacteriophage T4 lysozyme was shown to be active against Y. pestis in mice [56]. The cytotoxic domain of CLBs can be combined with other pathogen-targeting proteins or domains, such as phage proteins [57], pheromones [58,59], antibodies or parts thereof, or ligands [60]. Combinations of the colicin Ia killing domain with pheromones, PMC-SA [58] against Gram-positive Staphylococcus aureus and PMC-EF [61] against Gram-positive Enterococcus faecalis, have been tested in vivo and were found to be effective.
Tailocin chimeras can be created by combining tailocins with parts of other tailocins or phages [62,63]. As the specificity of the tailocin is mediated by tail fibres, replacing them can enable the complex to recognize new targets [46]. AvR2-V10.3, an R-type pyocin with tail fibres engineered to target E. coli O157:H7, was shown to be effective in a diarrhoea model in rabbits, even when given after the onset of diarrhoea [63].
Bacteriocins as protein antibiotics
While data on the use of bacteriocins as protein antibiotics obtained in the last few years from in vivo experiments are encouraging, there are key questions related to their suitability as therapeutics that remain to be addressed. First, only preliminary data on the effect of bacteriocins on the host in terms of toxicity or immune response are available. However, the limited data that are available in the majority of cases report no adverse or toxic effects against the host organism (Table 2). There is one exception to this: Bird and Grieble [50] observed 11% mortality in pyocin-treated chick embryos, while in the control group 6% mortality occurred from injection alone, although it is not clear whether this pyocin preparation was free of endotoxin. In addition, more research is required on dosing regimens and in particular the timing of bacteriocin administration pre- or post-infection. To address this, testing in well-defined infection models and detailed pharmacokinetic studies are required.
Table 2. Adverse effects of protein antibiotics in vivo.
| Model host | Protein | Treatment route | Adverse effect | |
|---|---|---|---|---|
| Colicin-like bacteriocins | ||||
| Pig | Colicin E1 | Oral | None | [40] |
| G. mellonella caterpillar | Pyocin S2 | Injection | None | [41] |
| Mouse | Pyocin S2 | IN | None | [42] |
| Pyocin AP41 | None | |||
| Pyocin S5 | None | |||
| Pyocin L1 | None | |||
| Tailocins | ||||
| – | – | – | – | – |
| Unknown and unidentified bacteriocins | ||||
| Mouse | Unknown pyocin 78-C2 | IV | None | [51] |
| IP | None | |||
| Mouse | Unknown pyocin(s) P10 | IP | None | [52] |
| Chick embryo | Unknown pyocin | Allantoic cavity | 11% mortality after pyocin treatment and 6% mortality in controls | [50] |
| Mouse | Pyocin H108 (S-type) | IP | None | [54] |
| Mouse | Pyocin 1577 (R-type) | IP | None | [54] |
| Pyocin 5882 (F-type) | None | |||
| Pyocin 1577 (R-type) | Topical | None | ||
| Pyocin 5882 (F-type) | None | |||
| Mouse | Unknown pyocin (tailocin) | IP | None | [55] |
| Rabbit | SC | None | ||
Abbreviations: IV, intravenous; IP, intraperitoneal; IN, intranasal; SC, subcutaneous.
What about immunogenicity? One might assume that bacteriocins and phage proteins are not immunogenic as they have been employed in bacterial and viral warfare within our bodies for millennia. Nonetheless, some groups have found low levels of antibodies against these proteins [42,43]. Repeated exposure to high doses of pyocin S5 via the IP route in mice did not lead to the development of pyocin S5-specific IgA or IgG, whereas exposure via the intranasal route led to low levels of S5-specific IgG, which did not interfere with pyocin S5 treatment of subsequent infection [42]. Conversely, another study demonstrated that a single IV exposure of pyocin R2 to mice induced the production of neutralizing antibodies, consequently making a second treatment ineffective in 90% of mice. Four of five mice that died in the second treatment round had formed neutralizing antibodies [43]. Overall, immunogenicity has barely been investigated and more research is required.
Comparison of the potency of bacteriocins with that of conventional antibiotics has elicited encouraging results. Indeed, pyocin S5 is at least 100-fold more potent than tobramycin in a murine lung infection model, making pyocin S5 a promising replacement for traditional antibiotics to target P. aeruginosa [42]. The same study demonstrated that pyocins are stable and active in vivo for at least 24 h [42]. However, most protein antibiotic candidates are not as stable as traditional antibiotics and may need to be applied in higher doses and with shorter dosing intervals between repeated doses. While this may be perceived as a disadvantage, low stability can also reduce whole-body and environmental exposure to the antibiotic, which may minimize the selective pressure for the development of resistance [64].
Finally, at the origin of the antibiotic crisis, we are currently facing is the emergence of resistance to conventional antibiotics. While emergence of resistance to conventional antibiotics is widely documented, little is known about how resistance to bacteriocins might arise and evolve in vivo. Several studies have shown resistance to bacteriocins in environmental settings and that resistance to bacteriocins can occur through modification of cell surface receptors in vitro. Recent results suggest that mechanisms of resistance are dependent on environmental factors and that resistance may be reduced in vivo [17,65]. It is interesting to note that while resistance is possible, many bacteria remain sensitive to a certain level to the bacteriocin, suggesting that complete resistance is hard to evolve or comes at a great cost for the bacteria [66].
Immunity, as opposed to resistance, is mediated by immunity proteins that specifically bind CLBs. The natural immunity of bacteriocin-producing strains could prove to be a drawback to the use of bacteriocins as protein antibiotics. However, it has recently been shown that immunity, too, comes at a great cost, as immune strains are outcompeted by non-immune strains under non-selective conditions [67]. Moreover, while some bacteriocins share killing mechanisms or have identical cytotoxic domains that could lead to cross-immunity between bacterial strains, it is to be noted that many different killing mechanisms exist. These varied mechanisms limit the emergence of cross-resistance and immunity among protein antibiotics. It is also interesting to note that most bacterial strains do not express CLBs and among those that do, the majority produce only one CLB (Sharp et al. unpublished data). This suggests that most strains will either be susceptible to bacteriocins or be immune to a limited number of them. Therefore, it is expected that the use of a cocktail of bacteriocins will be able to circumvent individual immunity. Interestingly, in rare cases, in vitro susceptibility of strains to protein antibiotics did not correspond to in vivo susceptibility [51,68].
Conclusions
Commercial interest in bacteriocins comes from many sides. Farming industries, such as the poultry industry [21,69] and aquaculture industry [70], look for more efficient ways to treat their stock. Interest in human applications comes from scientists and companies that realize the global health and economic potential of bacteriocin therapeutics. As early as 1986, a potential application of colicin E1 was patented [71]. Many researchers have since patented applications of CLBs to treat infections [41,60,68,72,73]. Two patents focus on the delivery of CLBs to specific organs [68,74]. Scientists at Avid-Biotics Corporation have been engineering tailocin tail fibres for over a decade, resulting in five patents [75–79] with either diagnostic or therapeutic aims.
Eventually, application of probiotic bacteria-producing bacteriocins rather than application of purified bacteriocins might be more cost-effective. Recently, production of bacteriocins in plants has been achieved at costs that may encourage commercialization [80]. However, interactions between pathogenic and probiotic species in the setting of an in vivo microbiome are not yet understood well enough to predict their success [27].
This review provides an overview of in vivo studies undertaken to date. However, the lack of systematic identification and naming of bacteriocins have rendered the results of in vivo studies, e.g. that of Rosamund Williams [54], useless for posterity, highlighting the need for a uniform nomenclature accessible and used by all. While databases on bacteriocins exist (BACTIBASE, BAGEL), they mostly focus on Gram-positive peptide-type bacteriocins, leaving a big gap to be filled for Gram-negative bacteria, which also use protein bacteriocins.
Recently, over 2000 protein bacteriocins were identified across a range of Gram-negative bacteria including the important pathogens P. aeruginosa, E. coli and K. pneumoniae (Sharp et al. unpublished data). Less than 100 of these have been studied in vitro [15] and only eight of them have been studied in vivo. More preclinical studies are required to better characterize bacteriocins, especially their immunogenicity and the emergence of resistance in vivo. However, the results of the reviewed in vivo experiments, as well as the use of bacteriocins against Gram-positive bacteria such as nisin as a food preservative and topical antibiotic in humans [81], are encouraging. They showcase the potential that might be hidden among the thousands of unstudied bacteriocins in terms of therapeutics and indicate promise for the use of bacteriocins as protein antibiotics.
Summary
Bacteriocins have been shown to be effective in a range of acute infection models and offer the potential for highly targeted antibiotic therapy.
There is a lack of key data on issues such as the ability of bacteria to develop resistance to bacteriocins in vivo and the toxicity and immunogenicity of bacteriocins on repeat administration.
High quality preclinical data that address these key questions are required to support the development of bacteriocins as therapeutic protein antibiotics.
Abbreviations
- CLBs
colicin-like bacteriocins
- IP
intraperitoneal
- IV
intravenous
- LPSs
lipopolysaccharides
- UTI
urinary tract infection
Competing Interests
The University of Glasgow has filed patents on the use of the protein antibiotics (colicins and pyocins) as therapeutics with DW listed as an inventor. The University of Oxford has filed a patent on methods for the discovery and engineering of bacteriocins with CK listed as an inventor. The authors have no other conflicts of interest.
References
- 1.Fair R.J. and Tor Y. (2014) Antibiotics and bacterial resistance in the 21st century. Perspect. Med. Chem., 6, 25–64 doi: 10.4137/PMC.S14459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cadman H. and Marinez L. (eds) (2014) Antimicrobial Resistance Global Report on Surveillance, World Health Organization, pp. 1–42 [Google Scholar]
- 3.Tommasi R., Brown D.G., Walkup G.K., Manchester J.I. and Miller A.A. (2015) ESKAPEing the labyrinth of antibacterial discovery. Nat. Rev. Drug Discov. 14, 529–542 doi: 10.1038/nrd4572 [DOI] [PubMed] [Google Scholar]
- 4.European Centre for Disease Prevention and Control (ECDC) (2015) Antimicrobial resistance surveillance in Europe 2014. Annual Report of the European Antimicrobial Resistance Surveillance Network (EARS-Net), ECDC, Stockholm, pp. 1–118 [Google Scholar]
- 5.Center for Disease Control and Prevention (2011) Gram-negative bacteria infections in healthcare settings [Internet]. http://www.cdc.gov/hai/organisms/gram-negative-bacteria.html
- 6.Pitout J.D.D., Nordmann P. and Poirel L. (2015) Carbapenemase-producing Klebsiella pneumoniae, a key pathogen set for global nosocomial dominance. Antimicrob. Agents Chemother. 59, 5873–5884 doi: 10.1128/AAC.01019-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Willyard C. (2017) The drug-resistant bacteria that pose the greatest health threats. Nature 543, 15 doi: 10.1038/nature.2017.21550 [DOI] [PubMed] [Google Scholar]
- 8.Soto S.M. (2014) Importance of biofilms in urinary tract infections: new therapeutic approaches. Adv. Biol. 2014, 1–13 doi: 10.1155/2014/543974 [DOI] [Google Scholar]
- 9.Costerton J.W., Stewart P.S. and Greenberg E.P. (1999) Bacterial biofilms: a common cause of persistent infections. Science 284, 1318–1322 doi: 10.1126/science.284.5418.1318 [DOI] [PubMed] [Google Scholar]
- 10.Lyczak J.B., Cannon C.L. and Pier G.B. (2002) Lung infections associated with cystic fibrosis. Clin. Microbiol. Rev. 15, 194–222 doi: 10.1128/CMR.15.2.194-222.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hammami R., Fernandez B., Lacroix C. and Fliss I. (2013) Anti-infective properties of bacteriocins: an update. Cell Mol. Life Sci. 70, 2947–2967 doi: 10.1007/s00018-012-1202-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Micenková L., Štaudová B., Bosák J., Mikalová L., Littnerová S., Vrba M. et al. (2014) Bacteriocin-encoding genes and ExPEC virulence determinants are associated in human fecal Escherichia coli strains. BMC Microbiol. 14, 109 doi: 10.1186/1471-2180-14-109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Petkovšek Ž., Žgur-Bertok D. and Erjavec M.S. (2012) Colicin insensitivity correlates with a higher prevalence of extraintestinal virulence factors among Escherichia coli isolates from skin and soft-tissue infections. J. Med. Microbiol. 61(Pt_6), 762–765 doi: 10.1099/jmm.0.037234-0 [DOI] [PubMed] [Google Scholar]
- 14.Holt K.E., Thieu Nga T.V., Thanh D.P., Vinh H., Kim D.W., Vu Tra M.P. et al. (2013) Tracking the establishment of local endemic populations of an emergent enteric pathogen. Proc. Natl Acad. Sci. U.S.A. 110, 17522–17527 doi: 10.1073/pnas.1308632110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Majeed H., Gillor O., Kerr B. and Riley M.A. (2011) Competitive interactions in Escherichia coli populations: the role of bacteriocins. ISME J. 5, 71–81 doi: 10.1038/ismej.2010.90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nedialkova L.P., Denzler R., Koeppel M.B., Diehl M., Ring D., Wille T. et al. (2014) Inflammation fuels colicin Ib-dependent competition of Salmonella serovar typhimurium and E. coli in Enterobacterial blooms. PLoS Pathog. 10, e1003844 doi: 10.1371/journal.ppat.1003844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Francino M.P. (2016) Antibiotics and the human gut microbiome: dysbioses and accumulation of resistances. Front. Microbiol. 6, 1543 doi: 10.3389/fmicb.2015.01543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Isaacs D. (2006) Unnatural selection: reducing antibiotic resistance in neonatal units. Arch. Dis. Child. Fetal Neonatal Ed. 91, F72–F74 doi: 10.1136/adc.2005.074963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cotter P.D., Ross R.P. and Hill C. (2013) Bacteriocins — a viable alternative to antibiotics? Nat. Rev. Microbiol. 11, 95–105 doi: 10.1038/nrmicro2937 [DOI] [PubMed] [Google Scholar]
- 20.Joerger R.D. (2003) Alternatives to antibiotics: bacteriocins, antimicrobial peptides and bacteriophages. Poult. Sci. 82, 640–647 doi: 10.1093/ps/82.4.640 [DOI] [PubMed] [Google Scholar]
- 21.Fields F.R., Lee S.W. and McConnell M.J. (2016) Using bacterial genomes and essential genes for the development of new antibiotics. Biochem. Pharmacol. 1–13 doi: 10.1016/j.bcp.2016.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rios A.C., Moutinho C.G., Pinto F.C., Del Fiol F.S., Jozala A., Chaud M.V. et al. (2016) Alternatives to overcoming bacterial resistances: state-of-the-art. Microbiol. Res. 191, 51–80 doi: 10.1016/j.micres.2016.04.008 [DOI] [PubMed] [Google Scholar]
- 23.Yang S.-C., Lin C.-H., Sung C.-T. and Fang J.-Y. (2014) Antibacterial activities of bacteriocins: application in foods and pharmaceuticals. Front. Microbiol. 5, 241 doi: 10.3389/fmicb.2014.00241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cavera V.L., Arthur T.D., Kashtanov D. and Chikindas M.L. (2015) Bacteriocins and their position in the next wave of conventional antibiotics. Int. J. Antimicrob. Agents 46, 494–501 doi: 10.1016/j.ijantimicag.2015.07.011 [DOI] [PubMed] [Google Scholar]
- 25.Brown C.L., Smith K., McCaughey L. and Walker D. (2012) Colicin-like bacteriocins as novel therapeutic agents for the treatment of chronic biofilm-mediated infection. Biochem. Soc. Trans. 40, 1549–1552 doi: 10.1042/BST20120241 [DOI] [PubMed] [Google Scholar]
- 26.Kirkup B.C. (2006) Bacteriocins as oral and gastrointestinal antibiotics: theoretical considerations, applied research, and practical applications. Curr. Med. Chem. 13, 3335–3350 doi: 10.2174/092986706778773068 [DOI] [PubMed] [Google Scholar]
- 27.Sassone-Corsi M., Nuccio S.-P., Liu H., Hernandez D., Vu C.T., Takahashi A.A. et al. (2016) Microcins mediate competition among Enterobacteriaceae in the inflamed gut. Nature 540, 280–283 doi: 10.1038/nature20557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith K., Martin L., Rinaldi A., Rajendran R., Ramage G. and Walker D. (2012) Activity of pyocin S2 against Pseudomonas aeruginosa biofilms. Antimicrob. Agents Chemother. 56, 1599–1601 doi: 10.1128/AAC.05714-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rendueles O., Beloin C., Latour-Lambert P. and Ghigo J.-M. (2014) A new biofilm-associated colicin with increased efficiency against biofilm bacteria. ISME J. 8, 1275–1288 doi: 10.1038/ismej.2013.238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brown C.L., Smith K., Wall D.M. and Walker D. (2015) Activity of species-specific antibiotics against Crohn's disease-associated adherent-invasive Escherichia coli. Inflamm. Bowel Dis. 21, 2372–2382 doi: 10.1097/MIB.0000000000000488 [DOI] [PubMed] [Google Scholar]
- 31.Trautner B.W., Hull R.A. and Darouiche R.O. (2005) Colicins prevent colonization of urinary catheters. J. Antimicrob. Chemother. 56, 413–415 doi: 10.1093/jac/dki228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lorenz A., Pawar V., Häussler S. and Weiss S. (2016) Insights into host-pathogen interactions from state-of-the-art animal models of respiratory Pseudomonas aeruginosa infections. FEBS Lett. 590, 3941–3959 doi: 10.1002/1873-3468.12454 [DOI] [PubMed] [Google Scholar]
- 33.Cascales E., Buchanan S.K., Duché D., Kleanthous C., Lloubès R., Postle K. et al. (2007) Colicin biology. Microbiol. Mol. Biol. Rev. 71, 158–229 doi: 10.1128/MMBR.00036-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jacob F. (1954) Biosynthèse induite et mode d'action d'une pyocine, antibiotique de Pseudomonas pyocyanea. Ann. Inst. Pasteur. 86, 149–160 PMID: [PubMed] [Google Scholar]
- 35.Fyfe J.A., Harris G. and Govan J.R. (1984) Revised pyocin typing method for Pseudomonas aeruginosa. J. Clin. Microbiol. 20, 47–50 PMCID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kleanthous C. (2010) Swimming against the tide: progress and challenges in our understanding of colicin translocation. Nat. Rev. Microbiol. 8, 843–848 doi: 10.1038/nrmicro2454 [DOI] [PubMed] [Google Scholar]
- 37.McCaughey L.C., Grinter R., Josts I., Roszak A.W., Waløen K.I., Cogdell R.J. et al. (2014) Lectin-like bacteriocins from Pseudomonas spp. utilise d-rhamnose containing lipopolysaccharide as a cellular receptor. PLoS Pathog. 10, e1003898 doi: 10.1371/journal.ppat.1003898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Johnson C.L., Ridley H., Marchetti R., Silipo A., Griffin D.C., Crawford L. et al. (2014) The antibacterial toxin colicin N binds to the inner core of lipopolysaccharide and close to its translocator protein. Mol. Microbiol. 92, 440–452 doi: 10.1111/mmi.12568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ghequire M.G.K. and De Mot R. (2014) Ribosomally encoded antibacterial proteins and peptides from Pseudomonas. FEMS Microbiol. Rev. 38, 523–568 doi: 10.1111/1574-6976.12079 [DOI] [PubMed] [Google Scholar]
- 40.Cutler S.A., Lonergan S.M., Cornick N., Johnson A.K. and Stahl C.H. (2007) Dietary inclusion of colicin E1 is effective in preventing postweaning diarrhea caused by F18-positive Escherichia coli in pigs. Antimicrob. Agents Chemother. 51, 3830–3835 doi: 10.1128/AAC.00360-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Walker D. and Smith K. (2015) Colicins for treating bacterial infections. United States; US20150164984A1
- 42.McCaughey L.C., Ritchie N.D., Douce G.R., Evans T.J. and Walker D. (2016) Efficacy of species-specific protein antibiotics in a murine model of acute Pseudomonas aeruginosa lung infection. Sci. Rep. 6, 1–8 doi: 10.1038/srep30201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Scholl D. and Martin D.W. (2008) Antibacterial efficacy of R-type pyocins towards Pseudomonas aeruginosa in a murine peritonitis model. Antimicrob. Agents Chemother. 52, 1647–1652 doi: 10.1128/AAC.01479-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Coetzee H.L., De Klerk H.C., Coetzee J.N. and Smit J.A. (1968) Bacteriophage-tail-like particles associated with intra-species killing of Proteus vulgaris. J. Gen. Virol. 2, 29–36 doi: 10.1099/0022-1317-2-1-29 [DOI] [PubMed] [Google Scholar]
- 45.Zink R., Loessner M.J. and Scherer S. (1995) Charaterization of cryptic prophages (monocins) in Listeria and sequence analysis of a holin/endolysin gene. Microbiology 141, 2577–2584 doi: 10.1099/13500872-141-10-2577 [DOI] [PubMed] [Google Scholar]
- 46.Michel-Briand Y. and Baysse C. (2002) The pyocins of Pseudomonas aeruginosa. Biochimie 84, 499–510 doi: 10.1016/S0300-9084(02)01422-0 [DOI] [PubMed] [Google Scholar]
- 47.Köhler T., Donner V. and van Delden C. (2010) Lipopolysaccharide as shield and receptor for R-pyocin-mediated killing in Pseudomonas aeruginosa. J. Bacteriol. 192, 1921–1928 doi: 10.1128/JB.01459-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kocíncová D. and Lam J.S. (2013) A deletion in the wapB promoter in many serotypes of Pseudomonas aeruginosa accounts for the lack of a terminal glucose residue in the core oligosaccharide and resistance to killing by R3-pyocin. Mol. Microbiol. 89, 464–478 doi: 10.1111/mmi.12289 [DOI] [PubMed] [Google Scholar]
- 49.Damasko C., Konietzny A., Kaspar H., Appel B., Dersch P. and Strauch E. (2005) Studies of the efficacy of enterocoliticin, a phage-tail like bacteriocin, as antimicrobial agent against Yersinia enterocolitica serotype O3 in a cell culture system and in mice. J. Vet. Med. Ser. B 52, 171–179 doi: 10.1111/j.1439-0450.2005.00841.x [DOI] [PubMed] [Google Scholar]
- 50.Bird T.J. and Grieble H.G. (1969) Pyocin antibiosis in chick embryos. Antimicrob. Agents Chemother. 9, 495–498 PMID: [PubMed] [Google Scholar]
- 51.Merrikin D.J. and Terry C.S. (1972) Use of pyocin 78-C2 in the treatment of Pseudomonas aeruginosa infection in mice. Appl. Microbiol. 23, 164–165 PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Haas H., Sacks T. and Saltz N. (1974) Protective effect of pyocin against lethal Pseudomonas aeruginosa infections in mice. J. Infect. Dis. 129, 470–472 doi: 10.1093/infdis/129.4.470 [DOI] [PubMed] [Google Scholar]
- 53.Riley M.A., Robinson S.M., Roy C.M., Dennis M., Liu V. and Dorit R.L. (2012) Resistance is futile: the bacteriocin model for addressing the antibiotic resistance challenge. Biochem. Soc. Trans. 40, 1438–1442 doi: 10.1042/BST20120179 [DOI] [PubMed] [Google Scholar]
- 54.Williams R.J. (1976) Treatment of Pseudomonas aeruginosa infections with pyocins. J. Med. Microbiol. 9, 153–161 doi: 10.1099/00222615-9-2-153 [DOI] [PubMed] [Google Scholar]
- 55.Higerd T.B., Baechler C.A. and Berk R.S. (1967) In vitro and in vivo characterization of pyocin. J. Bacteriol. 93, 1976–1986 PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lukacik P., Barnard T.J. and Buchanan S.K. (2012) Using a bacteriocin structure to engineer a phage lysin that targets Yersinia pestis. Biochem. Soc. Trans. 40, 1503–1506 doi: 10.1042/BST20120209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jakes K.S., Davis N.G. and Zinder N.D. (1988) A hybrid toxin from bacteriophage f1 attachment protein and colicin E3 has altered cell receptor specificity. J. Bacteriol. 170, 4231–4238 doi: 10.1128/jb.170.9.4231-4238.1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Qiu X.-Q., Wang H., Lu X.-F., Zhang J., Li S.-F., Cheng G. et al. (2003) An engineered multidomain bactericidal peptide as a model for targeted antibiotics against specific bacteria. Nat. Biotechnol. 21, 1480–1485 doi: 10.1038/nbt913 [DOI] [PubMed] [Google Scholar]
- 59.Dorit R., Roy S.M. and Riley M.A. (eds) (2016) The Bacteriocins, 1st edn Caister Academic Press, Norfolk, UK [Google Scholar]
- 60.Qiu X.-Q. (2006) Methods and compositions for the treatment of infection, vol. 1 United States; US2006/0156009A1 [Google Scholar]
- 61.Qiu X.-Q., Zhang J., Wang H. and Wu G.Y. (2005) A novel engineered peptide, a narrow-spectrum antibiotic, is effective against vancomycin-resistant Enterococcus faecalis. Antimicrob. Agents Chemother. 49, 1184–1189 doi: 10.1128/AAC.49.3.1184-1189.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Williams S.R., Gebhart D., Martin D.W. and Scholl D. (2008) Retargeting R-type pyocins to generate novel bactericidal protein complexes. Appl. Environ. Microbiol. 74, 3868–3876 doi: 10.1128/AEM.00141-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ritchie J.M., Greenwich J.L., Davis B.M., Bronson R.T., Gebhart D., Williams S.R. et al. (2011) An Escherichia coli O157-specific engineered pyocin prevents and ameliorates infection by E. coli O157:H7 in an animal model of diarrheal disease. Antimicrob. Agents Chemother. 55, 5469–5474 doi: 10.1128/AAC.05031-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Olofsson S.K. and Cars O. (2007) Optimizing drug exposure to minimize selection of antibiotic resistance. Clin. Infect. Dis. 45(Suppl 2), S129–S136 doi: 10.1086/519256 [DOI] [PubMed] [Google Scholar]
- 65.Inglis R.F., Scanlan P. and Buckling A. (2016) Iron availability shapes the evolution of bacteriocin resistance in Pseudomonas aeruginosa. ISME J. 10, 2060–2066 doi: 10.1038/ismej.2016.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hawlena H., Bashey F. and Lively C.M. (2010) The evolution of spite: population structure and bacteriocin-mediated antagonism in two natural populations of xenorhabdus bacteria. Evolution 64, 3198–3204 doi: 10.1111/j.1558-5646.2010.01070.x [DOI] [PubMed] [Google Scholar]
- 67.Gerardin Y., Springer M. and Kishony R. (2016) A competitive trade-off limits the selective advantage of increased antibiotic production. Nat. Microbiol. 1, 1–7 doi: 10.1038/nmicrobiol.2016.175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Walker D. and McCaughey L. (2016) Pulmonary Administration of Pyocins for Treating Bacterial Respiratory Infections, World Property Organization, WO2016046218A1 [Google Scholar]
- 69.Svetoch E.A. and Stern N.J. (2010) Bacteriocins to control Campylobacter spp. in poultry — a review. Poult. Sci. 89, 1763–1768 doi: 10.3382/ps.2010-00659 [DOI] [PubMed] [Google Scholar]
- 70.Desriac F., Defer D., Bourgougnon N., Brillet B., Le Chevalier P. and Fleury Y. (2010) Bacteriocin as weapons in the marine animal-associated bacteria warfare: inventory and potential applications as an aquaculture probiotic. Mar. Drugs 8, 1153–1177 doi: 10.3390/md8041153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bycroft N.L., Byng G.S. and Good S.R. (1986) Synergistic antimicrobial compositions, vol. 104 US pat. 5,043,176. p. 54–55 [Google Scholar]
- 72.Shanks R.M.Q. and Kadouri D.E. (2015) Citrobacter freundii antibacterial agents and their use. United States; US9139622B2 [DOI] [PMC free article] [PubMed]
- 73.Dorit R. and Riley M.A. (2006) Engineered bacteriocins and bacteriocin combinations and methods for treating bacterial based infections. United States; US20060229244A1
- 74.Lichter J., Lebel C., Piu F. and Harris J.P. (2011) Compositions and methods for the treatment of sinonasal disorders. World Intellectual Property Organization; WO2011050206A2 [Google Scholar]
- 75.Scholl D.M., Gebhart D., Williams S.R., Govoni G.R. and Martin J.R (2011) Diffocin and methods of use thereof. United States; US20110293566A1
- 76.Scholl D.M. and Williams S.R. (2014) Recombinant bacteriophage and methods for their use. United States; US8673553B2
- 77.Scholl D. and Williams S.R. (2012) Recombinant P4 bacteriophage and methods for their use. World Intellectual Property Organization; WO2012064660A1 [Google Scholar]
- 78.Gebhart D. and Scholl D.M. (2008) Inhibition of Yersinia pestis. United States; US20080286236A1
- 79.Scholl D.M. and Williams S.R. (2010) Modified bacteriocins and methods for their use. United States; US20100261258A1
- 80.Schulz S., Stephan A., Hahn S., Bortesi L., Jarczowski F., Bettmann U. et al. (2015) Broad and efficient control of major foodborne pathogenic strains of Escherichia coli by mixtures of plant-produced colicins. Proc. Natl Acad. Sci. U.S.A. 112, E5454–E5460 doi: 10.1073/pnas.1513311112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fernández L., Delgado S., Herrero H., Maldonado A. and Rodríguez J.M. (2008) The bacteriocin nisin, an effective agent for the treatment of staphylococcal mastitis during lactation. J. Hum. Lact. 24, 311–316 doi: 10.1177/0890334408317435 [DOI] [PubMed] [Google Scholar]