Abstract
Npr2 (Natriuretic peptide receptor 2) affects bifurcation of neural crest or placode derived afferents upon entering the brainstem/spinal cord, leading to lack of either rostral or caudal branches. Previous work has shown that early embryonic growth of cochlear and vestibular afferents are equally affected in this mutant but later work on postnatal Npr2 point mutations suggested some additional effects on the topology of afferent projections and mild functional defects. Using multicolor lipophilic dye tracing we show that absence of Npr2 has little to no effect on the initial patterning of inner ear afferents with respect to their dorso-ventral cochleotopic specific projections. However, in contrast to control animals we found a variable degree of embryonic extension of auditory afferents beyond the boundaries of the anterior cochlear nucleus into the cerebellum that emanates only from apical spiral ganglion neurons. Such expansion has previously only been reported for Hox gene mutants and implies an unclear interaction of Hox codes with Npr2-mediated afferent projection patterning to define boundaries. Some vestibular ganglion neurons expand their projections to reach the cochlear apex and the cochlear nuclei, comparable to previous findings in Neurod1 mutant mice. Before birth such expansions are reduced or lost leading to truncated projections to the antero-ventral cochlear nucleus and an expansion of low frequency fibers of the apex to the postero-ventral cochlear nucleus.
Introduction:
Previous work has identified the receptor guanylyl cyclase Natriuretic peptide receptor 2 (Npr2, also designated guanylate cyclase-B), its ligand C-type natriuretic peptide (CNP) and the cGMP-dependent kinase I (cGKI) as leading factors that regulate bifurcation of central axons of dorsal root ganglion (DRG) neurons along the spinal cord (Schmidt, et al., 2009, Schmidt, et al., 2007, Schmidt, et al., 2002). More recent work has expanded these initial findings to neural crest and placode derived fibers associated with the V, VII, IX and X cranial nerves as well as to the placode derived inner ear ganglia (Ter-Avetisyan, et al., 2014) and most recently the midbrain sensory neurons innervating muscles of mastication, the mesencephalic trigeminal (MesV) neurons (Ter-Avetisyan, et al., 2018). Without CNP, Npr2, or cGKI, DRG or cranial sensory ganglion (CSG) axons do not bifurcate when entering the spinal cord or the hindbrain, respectively. Instead, they either turn in an ascending or descending direction. Collateral formation from these stem axons is not impaired (Dumoulin, et al., 2018, Schmidt, et al., 2007, Tröster, et al., 2018). Expression of receptor or ligand suggest a progressive alteration over time from a restricted initial expression in sensory neurons (Npr2, cGKI) and rhombomeres (CNP) that have cranial nerve entry points (Ter-Avetisyan, et al., 2018).
Detailed growth of fibers either rostral or caudal in the hindbrain have previously been reported for the vestibular system and sparse labeling suggests immediate bifurcation of inner ear afferents comparable to other sensory neurons (Ter-Avetisyan, et al., 2014). However, judging from mass labeling of all vestibular afferents many appear to behave differently from other sensory systems by developing for a given ganglion neuron either a rostral or caudal directed axon that only later is supplemented by a secondary branch in the opposite direction (Maklad, et al., 2010). This development appears to help sort sensory organ specific polarity information flow for the gravistatic vestibular organs to the hindbrain (Fritzsch and López-Schier, 2014). In contrast, other sensory organs of the ear such as canal cristae and the organ of Corti of the cochlea have hair cells of only one polarity and organize their afferents according to different principles reflecting angular acceleration in three dimensions (Chagnaud, et al., 2017) or project the tonotopic organization of the organ of Corti as regular cochleotopic projection onto the cochlear nuclei (Fritzsch, et al., 2015, Jahan, et al., 2010b, Muniak, et al., 2016) even if such nuclei do not form (Elliott, et al., 2017). Additional work using the Npr2-cn mouse mutant that lacks activity due to a point mutation in the guanylyl cyclase domain (Tsuji and Kunieda, 2005) has implicated Npr2 in some aspects of cochleotopic sorting of spiral ganglion (auditory) afferents leading to a ‘blurred tonotopic organization’ (Goodrich, 2016, Lu, et al., 2014) and confirmed branching defects of Npr2 mutants (Lu, et al., 2011, Schmidt, et al., 2009, Schmidt, et al., 2007). Surprisingly, this reported ‘blurred projection of cochleotopic afferents’ had little effect on multiple physiological properties in the Npr2-cn mutant (Lu, et al., 2014) implying that in the auditory system the mapping of the primary organization axes does not profoundly influence physiological properties of higher order neurons. Likewise, physiological analysis of Npr2 null mutant mice show only very limited effect with a slightly altered auditory brainstem response (ABR) and distortion product otoacoustic emission [DPOAE (Wolter, et al., 2018)]. This is in sharp contrast to a recent report showing that a massively disorganized cochleotopic projection in mutants with a conditional deletion of Neurod1 causes loss of tuning in higher order neurons (Macova, et al., 2019).
In contrast to previous work (Lu, et al., 2014), we here use the well-established Npr2 null mouse model (Ter-Avetisyan, et al., 2014) to investigate in more detail the development of the cochleotopic central projections of the ear with an emphasis on the development over a larger range of stages compared to previous reports (Lu, et al., 2014) to better elucidate how lack of bifurcation branching influences tonotopic and polarity specific projections. Notably, a blurred cochleotopic projection was only reported for 2 out 4 mutants at E16.5 implying incomplete penetrance of the point mutation. Moreover, we also like to better understand how Npr2 and other guanylyl cyclase signaling intersects with the Wnt/planar cell polarity (PCP) pathway recently implicated in massive reorganization of some otherwise cochleotopically organized spiral ganglion neuron projections (Fritzsch, et al., 2019, Yang, et al., 2017). Our data reveal near normal cochleotopic and vestibular projections in Npr2-deficient mutants suggesting limited influence of lack of bifurcation on cochleotopic or vestibulotopic projections. Surprisingly, we find that Npr2 alters anterior cochlear nucleus afferent projections by partially removing blocks that prevent auditory afferents to grow beyond rhombomere 2 to enter the cerebellum, like vestibular fibers. Later stages show an unexpected reduction of afferents to the antero-ventral cochlear nucleus and a bias toward the postero ventral and dorsal cochlear nucleus as well as an unusual cochleovestibular anastomosis interconnecting vestibular organs with the apex of the cochlea and the most ventral cochlear nucleus through unusual bifurcations in the ear.
Material and Methods
Mice:
We used the previously described (Ter-Avetisyan, et al., 2014) Npr2-LacZ (B6.129P2-Npr2tm1.1(NLS-LacZ)Fgr) mouse line to generate homozygotic Npr2-LacZ/LacZ mice, here referred to as Npr2 null mice. Timed pregnancies were initiated by placing males and females together and time of detection of a vaginal plug was designated as embryonic day (E) 0.5 Litters were collected at E13.5, E16.5 and E18.5. Toes of embryos were clipped and used for genotyping as previously described (Ter-Avetisyan, et al., 2014). Embryos were immersion fixed in 4% paraformaldehyde (PFA) in 0.1 M phosphate buffer with 0.3 M sucrose added (Fritzsch, et al., 2016). Fixed heads were transferred to 0.4 % PFA in the same buffer and shipped from Germany to the US for analysis. All procedures were performed in accordance with the relevant ethical regulations and had been approved by the local animal use and care authority (LaGeSo, Berlin: No. T0370/97 abd G0370/13).
Expression analysis:
Heterozygous Npr2-LacZ [B6.129P2-Npr2tm1.1(nlsLacZ)/Fgr] (Ter-Avetisyan, et al., 2014) and heterozygous CNP-LacZ [B6;129P2-Nppctm1.1(nlsLacZ)/Fgr] (Schmidt, et al., 2009) mice were used to monitor the expression of Npr2 and CNP, respectively, in embryonic brains and sensory neurons. Both transgenic reporter lines encode a β-galactosidase expression cassette containing a nuclear localization signal.
For whole mount β-galactosidase (β-gal) staining embryos were fixed in Zamboni’s fixative (Stefanini, et al., 1967): PBS containing 2% paraformaldehyde and 15% picric acid, pH 7.3 at room temperature for 15–30 min according to the developmental stage of the specimen. After 3 incubations for 10 minutes each in β-gal wash solution (2 mM MgCl2, 0.02% Nonidet-P40, 0.01% sodium deoxycholate in PBS) tissues were transferred to β-gal wash solution containing 0.5 mg/ml 5-bromo-4-chloro-3-indolyl-β-D galactopyranoside (X-Gal; Life Technologies, Germany), 5 mM potassium ferrocyanide and 5 mM potassium ferricyanide. Tissues were incubated at 37°C under light agitation until development of a blue color. The staining reaction was then terminated by rinsing the embryos 3 times for 10 minutes each in ice-cold PBS containing 2 mM MgCl2. Subsequently, the tissues were postfixed in PBS containing 4% paraformaldehyde and shipped to University of Iowa for further analysis.
Following X-Gal staining, whole heads were dissected and either the whole mounted brain (CNP-Z/+) or the ganglia (Npr2-Z/+) were imaged using a compound Nikon E800 microscope to validate expression changes in particular in rhombomere4, the entry point of the Vlllth cranial nerve (Maklad, et al., 2010), as well as the sensory neurons delaminating from the developing ear (Elliott, et al., 2017) as previously described (Matei, et al., 2006).
Dye tracing:
Heads of Npr2 mutants and control animals were transferred to 4% PFA in 0.1 M phosphate buffer with 0.3 M sucrose added and stored at 4°C until tracing was initiated after shipment to the University of Iowa. For tracing, heads were split to allow insertion of dyes into the CNS and the ear (Fritzsch, et al., 2016, Maklad, et al., 2010). We previously had established that long wavelength lipophilic dyes allow for better signal to noise ratio due to lack of background fluorescence that is particularly high in the 488nm excitation range and also established that the use of dye soaked waivers provides a more reliable way of dye application compared to crystals or blind injections (Duncan, et al., 2011, Duncan, et al., 2015, Tonniges, et al., 2010). Specifically, we used the NeuroVue dyes Maroon (NVM; 635/650-700 excitation/emission), Red (NVR; 543/565-615) and Jade (NVJ; 488/500-540) we had previously developed in collaboration with Molecular Targeting Technologies (Duncan, et al., 2011, Duncan, et al., 2015, Fritzsch, et al., 2016, Tonniges, et al., 2010) that are sold through Polyscience (http://www.polysciences.com/default/catalog-products/life-sciences/molecular-biology/neuronal-tracing-neurovue-sup-r-sup-dyes). NeuroVue wavers were cut into appropriately sized and shaped pieces and inserted into either the cochlea, vestibular organs or different parts of the brain as previously described (Elliott, et al., 2017, Fritzsch, et al., 2016, Macova, et al., 2019). We always inserted NeuroVue Maroon (NVM) into the apex and false colored it green and NeuroVue Red (NVR) into the base and false colored it red. NeuroVue Jade (NVJ) was used in addition to reveal in some stages the vestibular afferents through waver application into the anterior and horizontal canal as well as the utricle. Insertion of appropriately sized slivers of the dye-soaked waver was for the base transverse to the basal turn hook region through the round window. For apex we used a lateral approach that inserted the dye filter near the apical tip into the modiolus. For the vestibular insertion we removed the otic wall near the cristae of the anterior and horizontal canal using the pigment epithelium surrounding these organs as a guide. Care was taken to apply dyes as identical as possible to control and mutant animals to reveal differences in a direct comparison of littermates with identical diffusion times. Analysis was mostly done in whole mounted ears and brains to generate overviews of most profound qualitative differences. Diffusion of dyes was initiated at 60°C for 2-7 days in 4% PFA with sucrose added as previously described (Fritzsch, et al., 2016).
Brainstem analysis:
After diffusion, the cochlear and vestibular nerves were cut close the cochlear nuclei and the half brainstem was trimmed to better show cochlear and vestibular nuclei and the cerebellum in younger stages. Diffusion timing was optimized for a given age of the embryos. We previously had shown that lipophilic dye diffusion leads to unreliable results in older stages due to massive dye uptake in myelin (Duncan, et al., 2011) and low osmolality of the carrier leads to swelling of neurons, membrane disruption and unspecific dye leaking. We therefore terminate our investigation at P7, the oldest stage we previously showed leads to reliable results showing single labeled fibers using lipophilic dye tracing for the above outlined reasons (Maklad and Fritzsch, 2003a, Maklad and Fritzsch, 2003b). For each preparation, the microdissected ear and the cochlear/vestibular nuclei were mounted on a glass slide in glycerol with spacers added to avoid compression. Preparations were imaged within an hour after mounting to avoid excessive washout effects of lipids and, by logical extension, lipophilic dyes with them. Preparations were viewed on either a Leica SP5 or SP8. Stacks of images were taken covering the entire area of cochlear nuclei or the microdissected inner ear. Using the intensity settings of the Leica system, laser power and gain was set to stay within the linear dynamic range of the system. Stacks were taken at 8-12 μm (lower power lenses such as 10×; 0.5 NA) or at 6 μm (higher power lenses such as 20×; 0.75NA or 40×l 1.35 NA). Resolution was set at 1026×1026 pixels. Using Leica software the image stacks were rendered into a single 2 D image. Since our previous work had demonstrated that NVM is the fastest diffusing NeuroVue dye (Fritzsch, et al., 2005, Jensen-Smith, et al., 2007) we always used this dye to label the apex. Unfortunately, the 635nm excitation also stimulates hemoglobin and thus shows all remaining red blood cells in particular in younger stages. Only preparations in which the labeling in the ear showed that only intended parts were labeled were used for the central projection analysis.
Ear analysis:
In several cases we injected dye into the brainstem and cerebellum to label afferents to the vestibular part of the ear as previously described (Maklad and Fritzsch, 2003a, Maklad and Fritzsch, 2003b, Maklad, et al., 2010). As before, we verified the intended insertion and accepted only preparations confirming to the intent. After appropriate diffusion of 2-5 dyes at 60°C the nerves connecting the ear with brain were cut with micro-scissors, the ears were microdissected and mounted with the brainstem in glycerol using spacers and viewed as described above.
An initial assessment of projections was conducted in a blinded fashion to ensure that the data generated by the tracing could identify the mutants without knowing which of the traced projections belonged to a mutant. Only after it become obvious that only Npr2 mutant embryos had fiber projections passing beyond the rostral boundaries of the cochlear nucleus to reach the cerebellum (Figs. 1–3) were the genotypes disclosed prior to the tracing experiment. For each data point we analyzed at least 3 Npr2 null mutants and control wild type littermates for a total of six half head preparations per each stage and genotype analyzed.
Fig. 1.
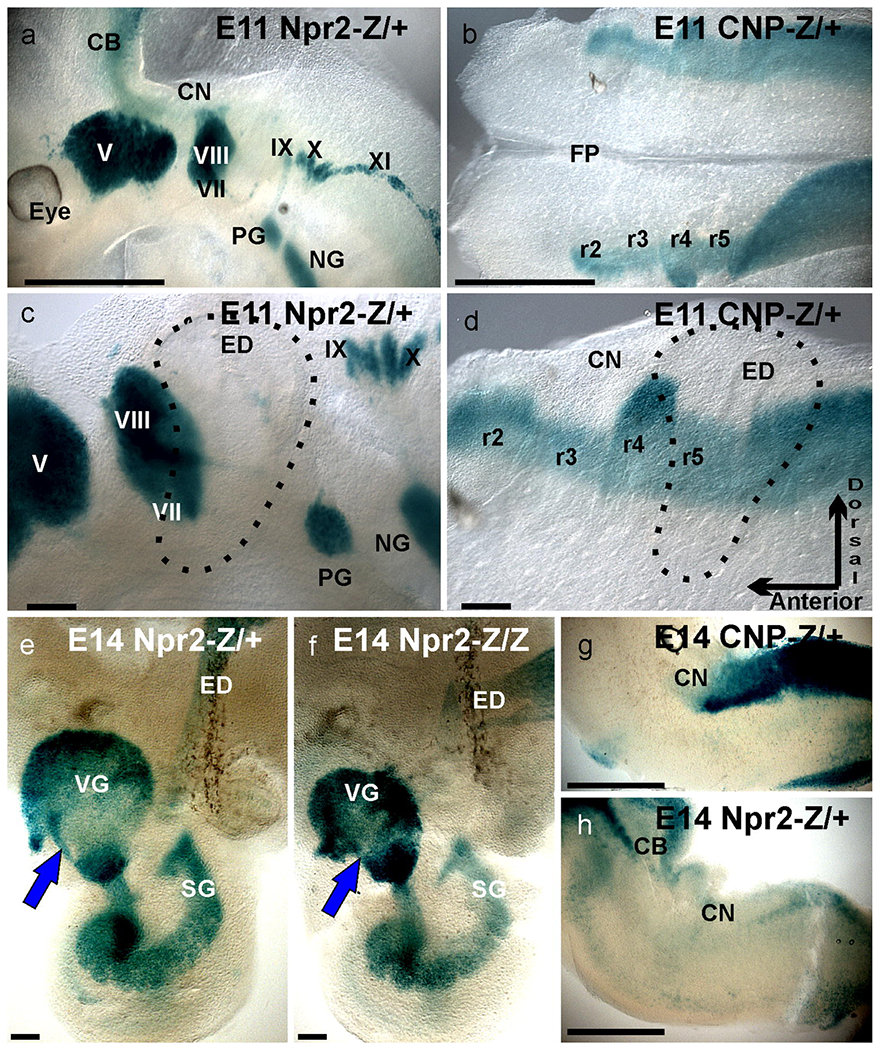
X-Gal staining of tissue from Npr2- or CNP-lacZ reporter mice to monitor expression of the receptor Npr2 and its ligand CNP. Whole mounted hemisected heads (a), peripheral ganglia (c) and whole mounted brains (b,d) show the β-galactosidase reaction product indicating expression of Npr2 in all cranial ganglia (a,c) and expression of CNP in all rhombomeres (b,d). Note neurons delaminating from the otocyst already express Npr2 and the ligand CNP is especially highly expressed dorsal in rhombomere 4(r4) where the VIIIth cranial nerve enters. Later stages show continued expression of Npr2 in inner ear sensory neurons (e,f), hindbrain (h) and of CNP in the hindbrain (g). Comparing Npr2 mutant lacZ expression (f) with mice heterozygotic for Npr2 deletion show only minor size reduction of vestibular ganglion neurons (e,f). Note the Npr2 positive population of cells near the anterior margin of the vestibular ganglion (blue arrowhead). CNP expression remains high in the caudal hindbrain but is not detected in the cochlear nuclei (g). Bar indicates 1mm in a,b,g,h and 100 μm in c,d,e,f.
Fig. 3. All 4 Npr2 mutants show different cochlear projections to the cochlear nucleus complex.
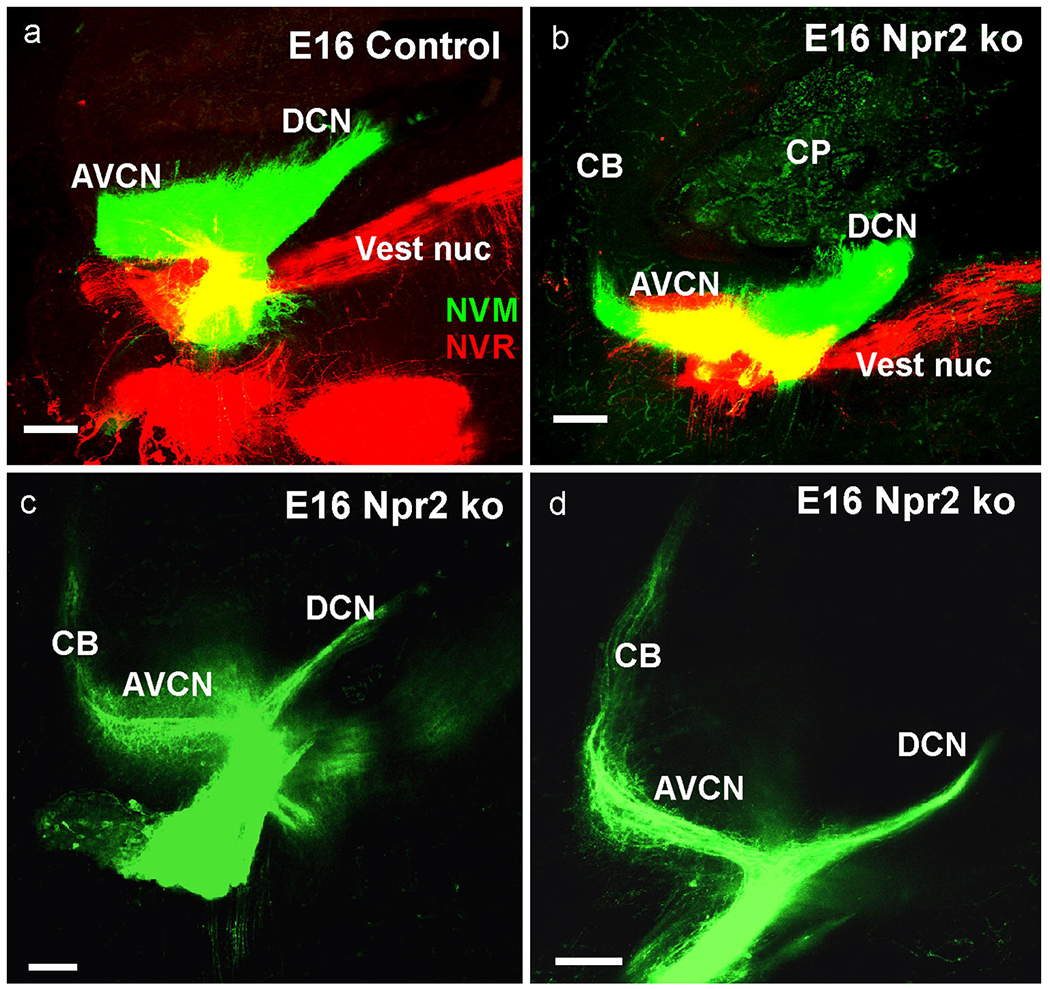
At E16.5 labeling of nearly all cochlear afferents by large insertions (a) resulted in fiber distributions to all parts of the cochlear nuclei. At this stage the control shows more massive projections to the AVCN compared to DCN (a) whereas projections to AVCN and DCN in Npr2 ko were more profound to DCN (b). Small applications to the cochlea revealed that the origin of cochlear afferents to the cerebellum is predominantly from the apex (c,d). Bar indicates 100 μm.
Statistics:
We used the Wilcoxon-Mann-Whitney U-test (Mann and Whitney, 1947) to establish at one stage (E18.5) the anterior extend of cochlear afferents labeled from base and apex in Npr2 mutant and control animals. For this measurement started at the anterior edge of the entering basal turn bundle to provide a uniform landmark for rostral fiber extension of both apical and basal turn spiral ganglion neuron afferents. A line was drawn along this rostral bundle and another line at 90° was extended to the most rostral area that was showing above background label. This likely overrates the mutant extension as it tended to be more gradual compared to the sharper end of the of horizontal fibers in the control animals. We accepted a one-tailed difference as significant at p>0.05.
Results:
Expression of Npr2 and CNP as revealed by X-Gal staining.
Previous work has demonstrated the expression of Npr2 and CNP in developing whole heads, demonstrating initial expression of CNP selectively in the even rhombomeres r2, r4, r6 as early as E8.5 followed by expansion at E9.5 into the odd rhombomeres ahead of afferent growth from the entry points at even rhombomeres into odd rhombomeres (Ter-Avetisyan, et al., 2018). Here we expand this initial finding to E11.5 and E14.5 (Fig. 1). At E11.5 the first afferents of spiral ganglion neurons enter the hindbrain to form a distinct cochlear projection as early as E12.5 (Fritzsch, et al., 2015) and this selective projection of spiral ganglion neurons to cochlear nuclei has been verified using Gata3-tauLacZ (Fritzsch, et al., 2006, Karis, et al., 2001). At this stage all inner ear derived ganglia are strongly Npr2 positive (Fig. 1a,c). CNP is particularly strongly expressed in the most dorsal part of r4 that is the entry point of vestibular and spiral afferents (Fig. 1b.d). Later stages show the CNP expression to be limited to the caudal part of the hindbrain with no expression in the cochlear nuclei (Fig. 1g). Npr2 expression remains strong in all sensory neurons of the ear and the endolymphatic duct (Fig. 1e). Comparison of mice heterozygous or homozygous for the LacZ reading frame replacement showed overall similar expression but somewhat reduced vestibular ganglia size (Fig. 1e,f). A particularly intensely stained population of seemingly vestibular neurons interconnected the vestibular and cochlear nerve foramina (arrowhead, Fig. 1e,f). In the brain, Npr2 expression was detected in the cerebellum and the caudal hindbrain but not in the cochlear nuclei (Fig. 1h). Overall, these data demonstrate that there is a somewhat complementary distribution of Npr2 and its ligand CNP suggesting an instructive function of CNP/Npr2 signaling for Npr2-expressing neurons.
Dye tracing data.
E13.5
Inner ear vestibular afferents reach the brain around E10.5 (Fritzsch, 2003) and the cochlear spiral ganglion neurons reach the cochlear nucleus by E12.5 (Fritzsch, et al., 2015, Fritzsch, et al., 2006, Karis, et al., 2001, Yang, et al., 2011). Afferent development follows with a 1-2 day delay the cell cycle exit of vestibular and spiral ganglion neurons (Matei, et al., 2005, Ruben, 1966), in line with progressive ventral to dorsal projection development of other cranial afferents over time (Fritzsch and Elliott, 2017). At E13.5 vestibular and spiral afferents reach the entire rostro-caudal targets in the cochlear nucleus and vestibular nuclei, including the cerebellum for vestibular fibers (Fig. 2). Control animals show a sharp stop at the r1/2 boundary (Fig. 2) with no fiber extending beyond this rostral boundary of the cochlear nucleus complex (Farago, et al., 2006, Fritzsch, et al., 2006, Maricich, et al., 2009). In contrast, all Npr2 mutant animals had fibers labeled from the cochlea apex extending beyond this boundary to reach nearly as far to the midline of the cerebellum as vestibular afferents (Fig. 2d–d”). There was also a notable projection difference in vestibular fibers reaching far more medial across the cerebellum in Npr2 mutants compared to control animals (Fig. 2). Combined these data suggest that in the absence of Npr2 embryonic afferent growth is expanded toward r1 (Glover, et al., 2018) with both vestibular and cochlear afferents projecting more extensive to the cerebellum. Whether this enhanced anterior expansion is a consequence of reduced branching allowing remaining fibers to extend further or indicates an impairment of axonal guidance remains unclear and requires future analysis using sparingly labeled individual fibers in later stages (Ter-Avetisyan, et al., 2014). It should be noted that in Npr2 and CNP mutants some TrkA positive neurites prematurely entered the spinal cord (Schmidt, et al., 2009, Schmidt, et al., 2002) suggesting a loss of responsivity to repulsive guidance cues such as semaphorins known to play such function in the ear (Gu, et al., 2003). Previous work in a point mutation of Npr2 using limited sparing spiral ganglion label analysis at E12.5 (Lu, et al., 2011) and more extensive analysis at E16.5 (Lu, et al., 2014) did not report such fiber extensions. This could be due to a failure of labeling these fibers or due to the differences between the Npr2-cn mutant (Tsuji and Kunieda, 2005) they used known to cause premature axonal ingrowth of some DRG neurons into the spinal cord compared to the Npr2 null mutant (Ter-Avetisyan, et al., 2014) used here.
Fig. 2. Cerebellar afferent projections studied in 3 Npr2 mutants.
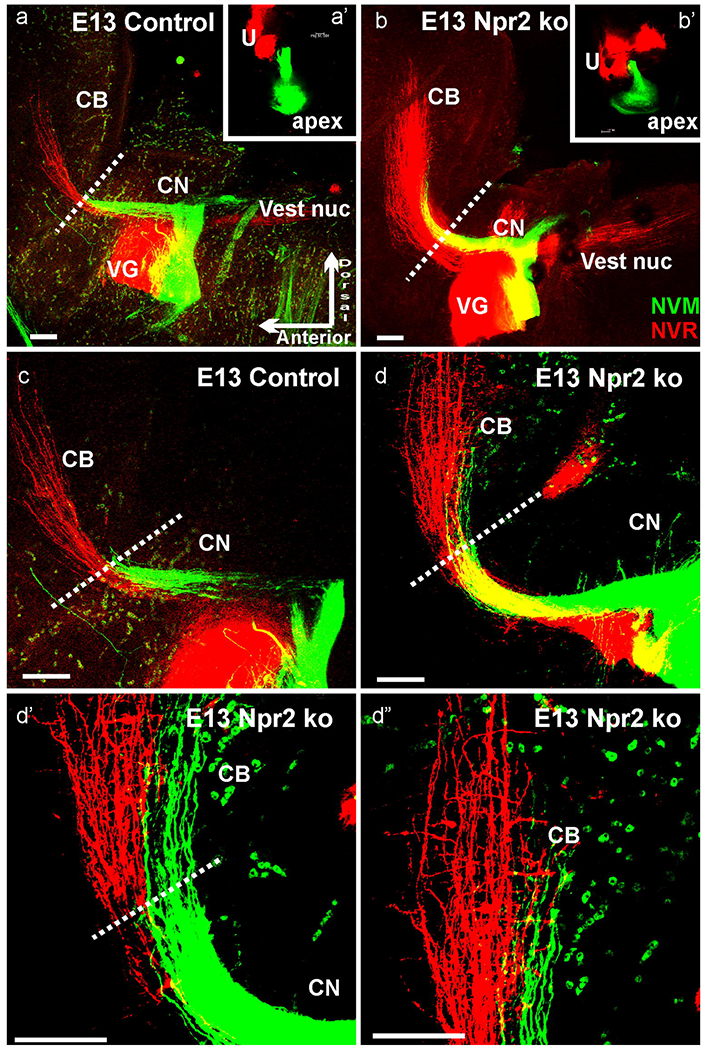
E13.5 Npr2 mutants (b,d) showed more vestibular (NCR, red) and cochlear (NVM, green) afferents in compared to littermate control animals (a,c) after comparable applications of dyes to the cochlea and vestibular organs (a’,b’). Higher power shows that many, but not all cochlear afferents go past the r1/2 border (indicated by dotted lines) in Npr2 mutants (d,d’,d”) but not in control animals (a,c). Cochlear afferents project at this stage almost as far medial as vestibular afferents (d”). CB, cerebellum; CN, cochlear nucleus. Red blood cells (green dots) are highly excited by the 635nm used for NVM. Orientation shown in (a) is identical for all images with dorsal up and anterior to the left. Bar indicates 100 μm.
E16.5
We next investigated whether the cerebellar projections of cochlear spiral ganglion afferents were transient or persisted by investigating the afferent organization in later stage animals. At E16.5 we found similar expansion of cochlear afferents to the cerebellum in Npr2 mutants only (Fig. 3b,c,d). In addition, we noted an overall asymmetry of cochlear afferent distribution to the different parts of the cochlear nuclei. Afferent projections were overall more profound to the large anteroventral cochlear nucleus in all control animals (Fig. 3a) whereas the proportion of afferent distribution was equal to or even inverse with more afferents projecting to the posterior part of the cochlear nucleus complex with occasionally enlarged afferents to the dorsal cochlear nucleus (Fig. 3b). In an attempt to localize the origin of the cochleo-cerebellar fibers we inserted very small amounts of dye into the extreme apex or base and obtained consistent labeling of cochlear afferents extending to the cerebellum only from the apex (Fig. 3c,d). These data suggest that perhaps more spiral ganglion afferents turn caudal, reducing the projection to the anteroventral cochlear nucleus. Those cochlear afferents projecting anteriorly from the apex expand beyond the r1/2 boundary into the cerebellum.
We next injected different colored dyes into the cerebellum and brainstem, respectively, to label afferents to the ear that project to these different parts of the hindbrain as previously described (Maklad, et al., 2010). Such injections consistently labeled differential afferent distribution to vestibular organs, indicating that different hair cell polarities in the utricle and saccule (not shown) project differentially to the brain. Overall this segregation was maintained in the Npr2 mutants with more fibers projecting to the cerebellum (Fig. 4a,c,d). These data suggest that there is not a specific vestibular ganglion population targeted by the loss of Npr2 but either population reaching the distinct polarities of the gravistatic sensory organs (Jiang, et al., 2017) project nearly normal to their rostral and caudal targets as previously described (Maklad and Fritzsch, 2003b, Maklad, et al., 2010).
Fig. 4. All 4 Npr2 mutants have altered cerebellar projections.
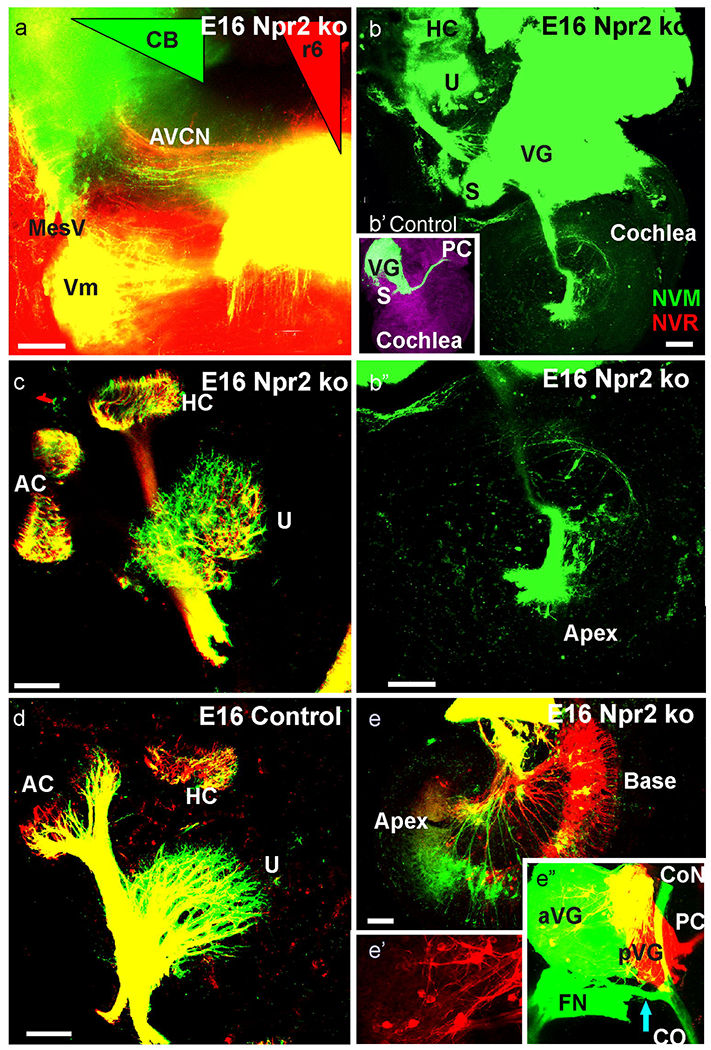
These E16.5 Npr2 mutant and control data show the injection of different colored dyes in the cerebellum (NVM, green triangle in a) and rhombomere 6 (NVR, red triangle in a). Cochlear afferents (labeled green in AVCN) became retrogradely labeled from the cerebellum and r6 cochlear afferents (labeled red) from the r6 injection only in mutants. These dye insertions labeled vestibular fibers to the vestibular organs in Npr2 mutants (c) and control animals (d). While the overall segregation was comparable, fibers from the cerebellum occupied a larger area in the utricle (compare c,d). Only Npr2 mutants had spiral ganglion cells labeled from r6 or cerebellar injections (b,b’) whereas no such fibers to the cochlea are found in control animals (b,b’). Cerebellar labeling resulted in prominent apical labeling (e) that was exclusive from the apex in more medial cerebellar injections (b,d). In contrast, r6 injections labeled only basal turn spiral ganglion neurons in some animals (e). Injections into vestibular endorgans labeled the posterior vestibular ganglion (pVG) from the posterior canal crista (PC) and anterior vestibular ganglion (aVG) from the utricle. Only mutants showed massive labeling of a fiber bundle expanding from the aVG to the modiolus to bifurcate towards the cochlear nuclei and the cochlea (e’). Note the distributed and highly branched vestibular neurons in the aVG (e”). Bar indicates 100 μm.
Consistent with cochlear spiral ganglion afferents reaching the cerebellum in particular from the apex (Fig. 3c,d), we also found afferents labeled from the cerebellum to pass through the ventral part of the cochlear nucleus complex but also labeled cochlear nucleus afferents from injections into the r6 area that spares cochlear afferents entirely in control animals (Fig. 4a). As suggested by the cochlear tracing (Fig. 3c,d), we could only label fibers in the ventral part of the cochlear nucleus complex after cerebellar injections. These data suggest that at least some spiral ganglion afferents develop collaterals that project past the rostral and caudal boundaries of the cochlear nucleus and some caudally projecting spiral ganglion afferents have at this stage developed collaterals that expand to the anteroventral cochlear nucleus as also reported for other Npr2 mutants and control animals (Goodrich, 2016, Lu, et al., 2014, Muniak, et al., 2016).
We next investigated the origin of these unusual cochlear nucleus projection by flat mounting the cochlea after lateral cerebellar as well as r6 dye insertions. While such injections do not label any cochlear afferents in control animals (Fig. 4b,b’), we found many basal turn spiral ganglion neurons to be labeled after r6 dye insertions in the Npr2 null mutant (Fig. 4b) whereas more apical spiral ganglion neurons were labeled after cerebellar injections (Fig. 4e). Importantly, spiral ganglion neurons projecting either rostrally or caudally past the cochlear nucleus complex were never double labeled, suggesting that each of these afferents is likely forming a single unbranched central fiber consistent with our analysis on initial afferent projections (Ter-Avetisyan, et al., 2014).
We next aimed to investigate whether the most medial cerebellar projections are even more restricted in their origin or derive from widespread distributed spiral ganglion neurons. Such near midline cerebellar dye injections revealed an unusual projection of only a limited number of a very apical population of spiral ganglion neurons that projected to the apex of the cochlea (Fig. 4b,b’). This filling of only apical spiral ganglion neurons is consistent with data after point applications to the apex showing more profound anterior cochlear nucleus projections that always extended far into the cerebellum (Fig. 3c,d). Given that we found some spiral ganglion neuron labeling after cerebellar applications (Fig. 4e), we next investigated if perhaps vestibular neurons project to the cochlea as recently reported in Neurod1 (Macova, et al., 2019) and Foxg1 mutant mice (Pauley, et al., 2006). Our data show both an unusual distribution of vestibular ganglion neurons across the vestibular ganglion complex and a bundle of fibers passing ventral to the vestibular ganglion to bifurcate upon reaching the auditory afferents to extend toward the cochlear nucleus and the cochlea (Fig. 4e,e’). Such additional branching was also obvious in vestibular ganglion neurons labeled from the posterior canal crista application in the anterior vestibular ganglion (Fig. 4e,e’,e”).
In summary, tracing data show that mostly apical spiral ganglion neurons project to the cerebellum and have a bifurcation to reach also anterior vestibular sensory epithelia.
E18.5
At this stage the cochlear nucleus could be fully analyzed in terms of cochleotopic projections using differently colored dye injections into the base and apex as previously described (Fritzsch, et al., 2016, Macova, et al., 2019, Xiang, et al., 2003, Yang, et al., 2017). These tracings revealed identical cochleotopic trajectories of basal and apical fiber bundles within the cochlear nerve as previously described (Macova, et al., 2019, Xiang, et al., 2003) but also revealed profound differences between the anterior projections to the AVCN and the posterior projections to the PVCN and DCN. Whereas the projections to the PVCN and, to a lesser degree the DCN were enlarged relative to that of control mice (Fig. 5a,b) the anterior extend of the projection to AVCN in Npr2 null mice was reduced (Table 1): Instead of extending for 333 (±25) pm into the dorsal part of the AVCN, only very few fibers passed anteriorly for only 63 (±7) μm (Fig. 5a”, b”). Furthermore, the base and apex showed differences with apical fibers projecting more profoundly to the DCN with expansions across the basal fibers beyond the limited expansion found in control animals at this stage (Fig. 5a’,b’). Interestingly, the apical insertions resulted in many more fibers to the AVCN compared to basal insertions but fibers extended only about half as far anteriorly with a sharp reduction in longitudinal extent in more dorsally located fibers (Table 1). Quantification of rostral fibers showed a clear and significant difference between control and mutant for both basal and apical projections (N=6 for control and Npr2 null mice; p>0.05) indicating that anterior growth of spiral ganglion axons was reduced in Npr2 null mice at this stage. In contrast, projections to the postero-ventral cochlear nucleus was more profound in all preparations (Fig. 5). We could not trace fibers at this stage reaching the cerebellum and injections into the cerebellum failed to label any spiral ganglion neurons suggesting that all of these fibers had either withdrawn, or the parental neurons had died.
Fig. 5. Cochleotopic projections reveal profound differences at E18.5 in all six Npr2 mutants studied.
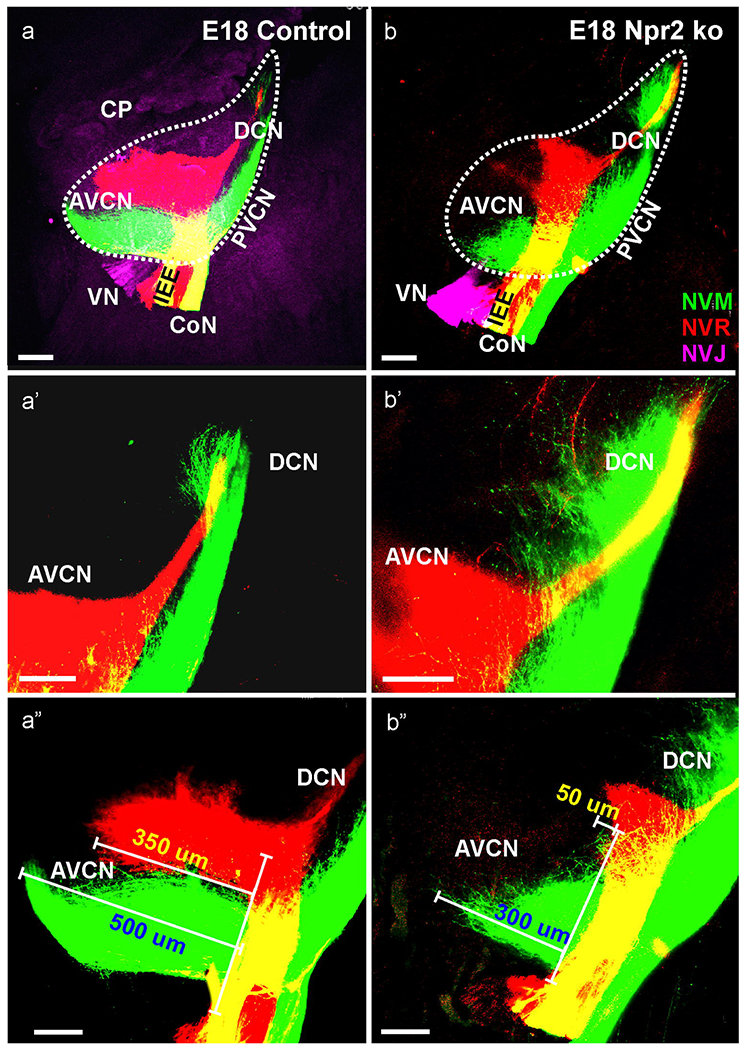
Triple dye injections into the vestibular organs (NVJ, lilac), apex of the cochlea (NVM, green) and base of the cochlea (NVR, red) show profound afferent distribution differences between control (a,a’,a”) and Npr2 null mutants (b,b’,b”) but show no effect on overall cochleotopic dorsoventral patterning. Most notable were profoundly reduced basal projections to the AVCN (a,b,a”,b”) and profoundly expanded projections of apex to the PVCN and DCN (a’,b’). Measuring the extension of afferents showed a significant reduction in the length of apical or basal cochlear projections in Npr2 ko mice to the AVCN (Table 1). NVJ is excited at 488nm and thus results in background fluorescence that was used to outline the cochlear nuclei (a) and also labels the choroid plexus (CP in a). Bar indicates 100 μm.
Table 1.
Rostral extent of spiral ganglion afferents from apical and basal turn bundle (length in μm).
| control | Mean + SD | Npr2 ko | Mean + SD | |
|---|---|---|---|---|
| apex | 468, 512, 488, 532, 490, 500 | 498 + 23 | 285, 300, 412, 375, 423, 328 | 354 + 30 |
| base | 325, 346, 385, 350, 304, 290 | 333 + 25 | 75, 55, 50, 65, 45, 85 | 63 + 7 |
We next wanted to verify if the unusual fibers interconnecting vestibular endorgans with the cochlea remained and injected dye into the cochlear apex, base and vestibular organs (Fig. 6a,c;green, red, lilac, respectively). In most animals we found comparable small projections emanating from cochlear labeling toward the vestibular ganglion (Fig. 6a,c). However, in two of two mutant mice we labeled more excessive fibers and cells in this cochlear vestibular anastomosis (Fig. 6e; CVA). Preparing the vestibular organs in one of these animals revealed several fibers emanating from a ganglion along this anastomosis to reach the three anterior vestibular endorgans, the utricle, horizontal and anterior canal (Fig. 6f). These data suggest that many but not all of the unusual vestibulo-cochlear connections are eliminated in older embryos. We also investigated the collaterals reaching from the PVCN/DCN to the AVCN by injecting dye into the DCN and cerebellum (Fig. 6b,d). This approach labeled expectedly some fibers in the restiform body adjacent to the cochlear nucleus in both control and mutant mice but no cochlear nucleus afferents suggesting that the expansion to the cerebellum is reduced or eliminated at this stage. Most importantly, such an approach labels multiple parallel fibers covering all of AVCN in control animals but shows only limited expansion to the more posterior half of the AVCN in mutants (compare Fig. 6b,d) consistent with our labeling from cochlear injections (Fig. 5).
Fig. 6. Triple labeling of cochlear apex (NVM, green, a,c,e,f,g,h) cochlear base (NVR, red, a,c,e,f,g,h) and utricle (NVJ, lilac a,c) at E18.5 show a cochlea-vestibular anastomosis of fibers.
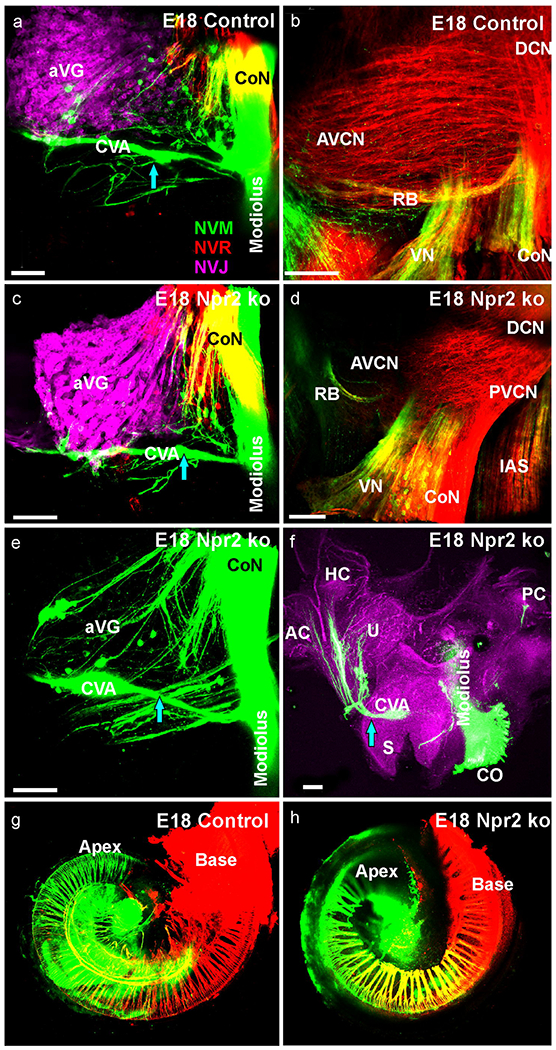
This anastomosis extend from the apex to the anterior vestibular nerve foramen and is in three cases of Npr2 mutants more profound (c,e) compared to littermate control (a). In all three mutants fibers can be traced to utricle, anterior and horizontal canal crista (f) not found in control littermates. Dye injection into the cerebellum (green in b,d) and DCN (red, b,d) labels most fibers in the entire AVCN in control (b) but not Npr2 mutant mice (d). Afferents in the restiform body (RB) are equally labeled and so are vestibular afferents exiting the brainstem. Dye injections into the cochlea reveal a comparable distribution of labeled neuronal profiles in control (g) and Npr2 mutant (h). Lilac in (f) is the background fluorescence. Bar indicates 100 μm in all images.
We also investigated the insertion sites in the cochlea not only for precision of injections as intended but also for unusual interconnections of spiral ganglion neurons between the base and the apex as recently reported in a mutant with a near complete loss of cochleotopic projections (Macova, et al., 2019). Our data showed virtually identical labeling of a gradual blending of basal and apical labeled neurons and fibers between control and Npr2 mutant mice (Fig. 6g,h) giving no indication of either differential migration or multiple connections of a given spiral ganglion neuron within the cochlea.
P2
After birth, the differences in control (Fig. 7a,b) and mutant animals became more obvious. Neither small (Fig. 7a) nor very large (Fig. 7b) injections in control animals showed any deviation from the cochleotopic projection pattern as previously reported in neonates of a mutant with a disorganized cochleotopic projection (Macova, et al., 2019). While Npr2 mutants also showed well organized fiber stratification after large (Fig. 7c,d) or small (Fig. 7e) dye applications, there were noticeable differences in the labeling of afferents and their rostro-caudal extent. All mutants showed a profound projection to the PVCN from the apex that was nearly absent in the basal turn projections. Conversely, the projection to the AVCN was reduced in number of fibers and their anterior extend (Fig. 7c,d,e). In fact, in some cases we obtained, despite massive PVCN labeling from the apex, hardly any fibers to the AVCN (Fig. 7e). Importantly, fibers did not show the parallel fiber bundles of cochleotopic projections (Muniak, et al., 2016) but showed a more random distribution as in Neurod1 mutant mice (Macova, et al., 2019) and as was previously reported based on single fiber tracings (Lu, et al., 2014). While afferents in control animals showed multiple parallel fibers with short collaterals (Fig. 7a), fibers in the Npr2 null mutant were erratic and random with many branches suggesting them to be collaterals, comparable to those expanding to the ventral part of the DCN (Fig. 7c,d,e) instead of primary afferents. In three (out of six cases) with anterior vestibular injections we obtained labeling not only of vestibular afferents entering with the vestibular nerve but also of some afferents entering with the cochlear nerve (Fig. 7f), consistent with the vestibulo-cochlear anastomosis shown in younger animals (Figs. 4,6).
Fig. 7. Small (a) or large (b) dye application to the base (NVR, red) and apex (NVM, green) show cochleotopic projections expanding the entire length of the cochlear nucleus complex in control animals.
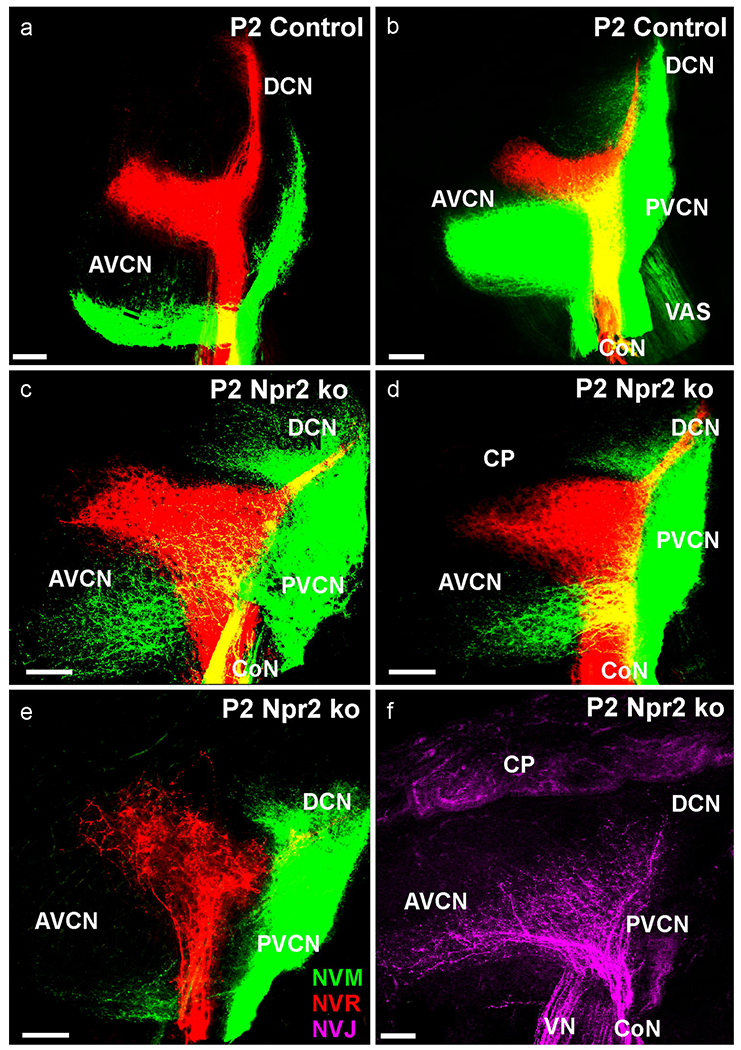
All 3 mutants show here display a more restricted fiber expansion of apparent collaterals to the AVCN (c,d,e) but a more profound projection of apical cochlear afferents to the PVCN, Only in 2 of 3 mutants could we trace vestibular fibers from the utricle not only through the vestibular nerve to the vestibular nucleus but also through the cochlear nerve to the most ventral aspect of the cochlear nucleus complex. Note the branches off the stem fibers in vestibular fibers entering in the ventral part of the AVCN. A single optical plane reveals a highly disorganized fiber projection that is not organized in parallel lines (e). Bar indicates 100 μm.
P7
Some Npr2 mutants can survive for some time (Wolter, et al., 2018) and we harvested two mutants at P7 to investigate late stages in cochleotopic projections. The data collected on 4 half brains show features mostly consistent with the earlier data as far as apical projections are concerned. Clearly, expansion of anterior directed fibers is truncated to about 2/3 the length of control animals (Fig. 8). Both, control and Npr2 mutants, show comparable expansion to the DCN and PVCN. In particular the projection of the apex to the most ventral part of the DCN showed a comparable expansion at this stage (Fig. 8a’,b’) with fibers reaching the area of the octopus cells (Malmierca, 2015, Osen, 1969) which were occasionally transcellularly labeled by the lipophilic dye. The more profound projection of the apex to the PVCN as compared to the AVCN found in earlier stages (Figs. 5, 7) remained, suggesting that caudal branches of spiral ganglion neurons are more prominent in Npr2 mutant mice. Beyond the obvious reduction of the apical projection to the AVCN was a blurring of the dorsal boundary of the projection area. In control animals there is space left between the basal (red) and apical (green fibers; Fig. 8a,a”). In contrast, in the mutants there is expansion of branches from primary apical fibers to fill in the gap to basal fibers with the most profound expansion in the posterior part of the AVCN (Fig. 8b,b”). These data imply a differential overlap of cochleotopic projection by branches mostly from apical fibers in the AVCN that could affect in a subtle way tonotopic aspects of hearing.
Fig. 8. Detailed analysis of two P7 Npr2 mutant (b,b’b”) and control littermate (a,a’a”) show overall cochleotopic matching projections with apical fibers (NVM, green) projecting ventral and basal fibers (NVR, red) projecting dorsal in the antero-ventral cochlear nucleus (AVCN).
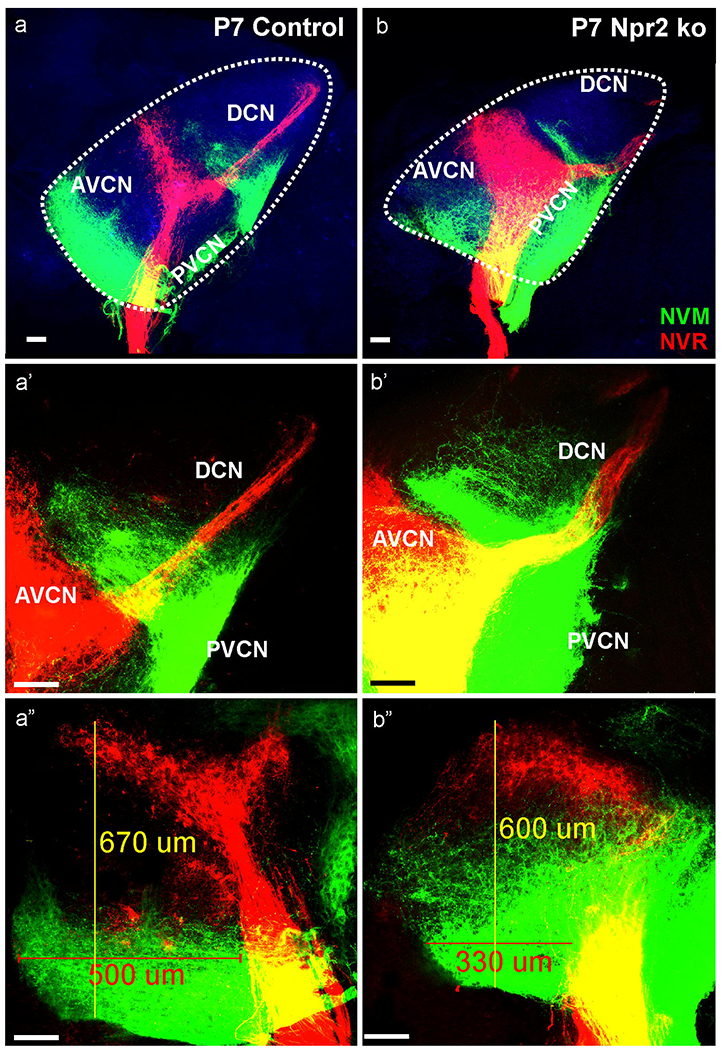
However, while fibers in control animals remain restricted to narrow bands, in particular cochlear afferents from the apex expand to reach almost the basal turn projection in the mutant (compare a”,b”). Projection of the basal turn reaches unbranched to the dorsal cochlear nucleus (DCN; b’) whereas basal turn neurons show a profound collateral expansion along the ventral border of the DCN with the PVCN which is comparable in Npr2 mutant (b,b’) and control littermates (a,a’). In addition to the AVCN dorsal expansion, the anterior expansion of the basal turn is truncated to about 2/3 of the rostral extend (in the presented case from 500 um to 300 um). Note that branches emerge from the apical fibers to nearly fill the gap between apical and basal tonotopic tracts (a”, b”). Blue indicates the 488 background fluorescence that was used to outline with a dotted line the cochlear nuclei complex. Bar indicates 100 μm.
Discussion
Our data extend previous descriptions of the Npr2 phenotype of inner ear and in particular cochlear afferent projections (Lu, et al., 2011, Lu, et al., 2014, Ter-Avetisyan, et al., 2014) that focused on erroneous single fiber branching with little data provided on the development of the cochleotopic projection (4 Npr2 point mutants each at E16.5 and P14) in addition to physiological work (Lu, et al., 2014). Auditory physiology was recently extended to the genetically targeted Npr2 null mutants we analyzed here (Wolter, et al., 2018). Our data show for the first time an unusual cochlear afferent expansion past the rostral and caudal boundaries of the cochlear nuclei in early embryos followed by a base to apex progressing reduction of AVCN projections and expansion of PVCN projection (Figs. 2,3,4,9). Our data show that these expansions form early and suggest that extension of the single unbranched afferent in Npr2 null mutants may drive the extensive longitudinal growth that normally may be more truncated due to the initial branching, assuming comparable growth potential in control and Npr2 null spiral ganglion neurons. Alternatively, cochlear afferents of Npr2 mutants might have lost the ability to read a r1/2 stop signal that normally restricts anterior expansion of cochlear afferents unless the Hox code of rostral rhombomeres has been manipulated (Oury, et al., 2006). However, given that we also obtained caudal expansion of a different set of spiral ganglion neuron afferents, we suggest that the expansions reflect mostly additional longitudinal growth possibly due to the absence of primary branching.
Fig. 9.
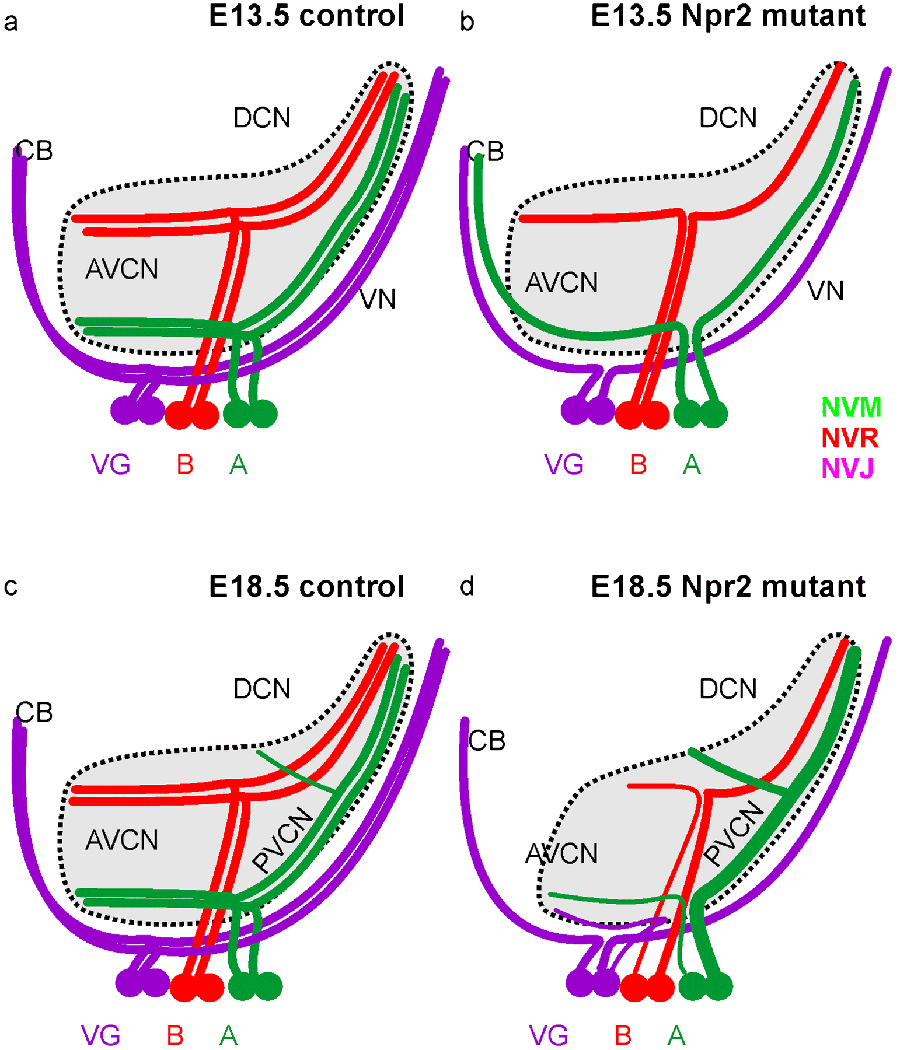
This summary diagram shows the differences in afferent projections of spiral and vestibular ganglion neurons in control and Npr2 mutant mice at two different stages (E13.5,a,b; E18.5, c,d). For each projection we depict only two representative neurons for the vestibular ganglion (VG), the base (B) and the apex (A) of the spiral ganglion. Color coding is consistent with color rendering of the dye tracing. All neurons project in parallel and cochleotopic to both anterior and posterior aspects of the vestibular and cochlear nuclei in control animals with a small, late forming branch of only the apical fibers to the octopus region. In mutants there is an initial overshooting of projections from apical spiral ganglion neurons to the cerebellum whereas in later mutants the projection of the apex is expanded to the PVCN and DCN (thick green fiber) and reduced in rostro-caudal extent and projection size (thin fibers) to the anteroventral cochlear nucleus. Note also the expansion of vestibular fibers to the most ventral aspect of the cochlear nucleus complex only in mutants. Thus, while the overall cochleotopic projection is retained in Npr2 mutants in particular the apical afferents show unusual expansions and contractions to different parts of the cochlear nuclei.
The differential origin of caudally projecting versus extensively rostrally projecting spiral ganglion neurons is a novel finding. Obviously, only the most apical spiral ganglion neurons project the most like vestibular afferents suggesting that these fibers behave without Npr2 as a mix of spiral ganglion neurons (cochlear nucleus projection) and vestibular neurons (projection to the cerebellum). We here provide for the first time evidence for an unusual connection between the auditory afferents and vestibular endorgans, the cochleo-vestibular anastomosis (Figs. 1,4,6,7). Past work on Neurod1 mutant mice has shown that a spiro-vestibular ganglion forms in these mutants due to migration of spiral ganglion neurons to mix with vestibular neurons (Jahan, et al., 2010a, Macova, et al., 2019). These mixed ganglia also have mixed peripheral fibers in the cochlea and a mixed central projection of vestibular and spiral ganglion neurons. In contrast, our data on Npr2 null mutants do not show such mixing of spiral and vestibular ganglia and limited projection of some vestibular fibers to the cochlear nuclei (Fig. 7). Importantly, beyond this limited overlap our data show no blurring of basal and apical projections within the cochlear nerve as recently shown for the Npr2-cn mouse mutant (Lu, et al., 2014) but remained always discrete within the nerve (Figs. 3,5,6–8). In addition, we never found basal turn afferents to be most ventro-caudal relative to apical afferents in the PVCN as reported (Lu, et al., 2014). Whether these differences between our findings and previous work are due to technical issues (overlapping injections in base and apex, co-labeling of vestibular fibers after basal turn injection) or relate to the different genotype (Npr2 deletion versus Npr2 point mutation) remains to be resolved. It should be mentioned that the previous work published no evidence that the injection was accomplished as intended by showing the injected cochlea as we provide here (Fig. 6). Interestingly, the added schematics show segregated fibers in the modiolus and cochlear nerve, identical to our findings.
Our data go beyond previous findings on other mutants by demonstrating a unique connection between the two ear foramina for the anterior vestibular nerve and the posterior vestibular/cochlear nerve (Figs. 4,6), also it appears that many spiro-vestibular afferents in Neurod1 mutants project along that pathway (Macova, et al., 2019). It remains unclear if Neurod1 regulates transcription of Npr2, and whether absence of Npr2 alters interactions with neural crest derived Schwann cells known to affect peripheral afferent distribution (Mao, et al., 2014). Clearly, data on cGMP null mice are needed to fully reveal the signal/receptor deletion effects in the cochleotopic projection development.
While early embryonic stages show overshooting projections, later stages show a mix of reduction and expansion of different longitudinal spiral ganglion populations to different parts of cochlear nuclei. Basal fibers show reduced growth to the AVCN combined with normal DCN projection whereas apical fibers show reduced AVCN combined with expanded PVCN and premature DCN projections. These differences in behavior of apical and basal fibers has previously been noted for the DCN projection in another Npr2 mutant line (Lu, et al., 2014) and is particularly obvious in Prickle 1 mutants (Yang, et al., 2017). In the latter mutants, the basal projections are nearly normal whereas apical afferents develop collaterals that expand across the entire cochlear nucleus complex (Yang, et al., 2017) indicating radically different effects of the same mutation to different spiral ganglion neurons coding different frequencies in line with the observed expansion of apical fibers in the caudal part of the AVCN we show here (Fig. 7,8). This expansion of apical afferents is particularly obvious in the consistent posterior projection expansion but variably reduced anterior projection (Fig. 6; Table 1). Somewhat similar effects were previously reported as intensity differences that were claimed to be more profound for the apex compared to the base (Lu, et al., 2014). Since no data were provided in this work for the stage we analyzed here we do not know how the dynamics of fiber expansion and retraction compares to our mutant (Fig. 9).
Cochleotopic projection is essential for normal function of frequency specific hearing (Muniak, et al., 2016) and complete loss of tonotopic organization results in blurred tuning that cannot be corrected by activity (Macova, et al., 2019). Previous work has indicated that some blurring of this frequency specific projection happens in certain areas of the cochlear nucleus complex in mice harboring the Npr2-cn point mutation (Lu, et al., 2014). Our data do not confirm such a blurring in Npr2 knock out mutant primary afferents (Fig. 9) but show a much more dynamic effect of early fiber overshooting followed by later retraction of some branches to some parts of the cochlear nucleus combined with expansion of other branches. These data imply that Npr2 does not only exert a profound initial effect on ingrowing afferents (Schmidt, et al., 2007, Ter-Avetisyan, et al., 2014) but may play a second role in differential modeling of fibers to different parts of their targets. The secondary expansion of apical afferents seemingly has little effect on tonotopic performance (Lu, et al., 2014) compared to the massive tuning distortion resulting from disorganization afferents of Neurod1 conditional deletion mutants (Macova, et al., 2019). Further work is needed to reconcile the minor physiological differences of the two Nrp2 mutants (Lu, et al., 2014, Wolter, et al., 2018) with the more obvious tonotopic differences we found here compared to the Npr2 point mutant.
Finally, while Npr2 regulates bifurcation of afferents upon entering the brainstem or spinal cord (Ter-Avetisyan, et al., 2014, Tröster, et al., 2018) we here describe unusual peripheral branching at the ear that may represent an exaggeration of a transient natural branching, possibly regulated by Neurod1 (Macova, et al., 2019). Whether other developing sensory processes also exhibit not only truncation of central branches but exaggeration of peripheral branches remains to be seen. It is also unclear what other factor(s) may compensate for Npr2 in some inner ear neurons (Booth, et al., 2018) and why there is a graded effect of anterior versus posterior branch expansion in base versus apical spiral ganglion neurons (Fig. 7, 9). Obviously, the overshooting and retraction in anterior projecting branches combined with more profound posterior projections correlates with the differential distribution of the Npr2 ligand CNP that is more profound in caudal rhombomeres at later stages but initially expands to r2 (Fig. 1B,D,G). Investigating inner ear projections in CNP mutants could reveal causality of this observation if an unknown CNP receptor is expressed in inner ear sensory neurons that can read that gradient. Clearly, more data on quantitative expression variation on possible co-factors in spiral ganglia is needed to mechanistically explain this continued variation using quantifiable in situ hybridization approaches (Kersigo, et al., 2018) combined with fiber tracing.
Comparative implications:
Previous mutant analysis has shown that cochlear apex and base respond differentially to the loss of Lmx1a (Nichols, et al., 2008), N-Myc (Kopecky, et al., 2011) and Neurod1 (Jahan, et al., 2010b) implying that the apex is part of an ancestral vestibular sensory organ, the lagena of tetrapods (Schultz, et al., 2017), incorporated into the expanding eutherian organ of Corti (Fritzsch, et al., 2013). The data presented here show that only the most apical spiral ganglion neurons can behave nearly identical to vestibular afferents by projecting to the cerebellum (Figs. 3,4) and form an anastomosis with fibers that apparently branch to reach the apex of the cochlea and project to the most ventral aspect of the cochlear nuclei. We assume that these mixed spiro-vestibular neurons are the ones that form the projection to the cerebellum indicating that loss of Npr2, possibly regulated by Neurod1 (Pataskar, et al., 2016), may unmask an ancient vestibular-auditory connection. It should be noted that no such anastomosis nor cochlear nucleus projections were described in chicken lagena projections (Kaiser and Manley, 1996, Mahmoud, et al., 2013) but more refined connectional analysis in developing chicken, and in particular monotremes as the only mammal that retain the lagena (Schultz, et al., 2017) with the above information in mind, are clearly warranted.
In summary (Fig. 9) our data on Npr2 knockout mutants are in line with physiological data (Lu, et al., 2014, Wolter, et al., 2018) and reveal no major tonotopic disorganization as previously claimed based on limited cochleotopic tracing experiments (Lu, et al., 2014). However, we reveal a surprising dynamics of cochlear afferent branching to various subnuclei, confirming and extending previous indications of reduced anteroventral projection and expanded posteroventral projection (Lu, et al., 2014). In addition, we find unusual connections of only the apical spiral ganglion neurons with the cerebellum as well as limited projection of vestibular neurons to the cochlear nuclei.
Funding Acknowledgements:
The authors acknowledge support by the NIH (R01 AG060504 to BF) and by the Deutsche Forschungsgemeinschaft (Grant FOR 2060 project SCHM 2371/1 to HS).
Abbreviations (text and figures):
- AH
anterior canal crista
- AVCN
anteroventral cochlear nucleus
- CB
cerebellum
- CN
cochlear nucleus
- CP
choroid plexus
- CO
cochlea
- CoN
cochlear nerve
- CVA
cochleo-vestibular anastomosis
- DCN
dorsal cochlear nucleus
- ED
endolymphatic duct
- FN
facial nerve
- HC
horizontal canal crista
- MesV
mesencephalic trigeminal projection
- NG
nodose ganglion
- NVJ
NeuroVue Jade
- NVM
NeuroVue Maroon
- NVR
NeuroVue Red
- PC
posterior canal crista
- PG
petrosal ganglion
- PVCN
posteroventral cochlear nucleus
- RB
restiform body
- S
saccule
- SG
spiral ganglion
- VAS
ventral acoustic stria
- (a,p)VG
(anterior, posterior) vestibular ganglion
- VN
vestibular nerve
- V
trigeminal ganglion
- Vm
trigeminal motor neurons
- VIII
octaval ganglion
- VII
facial (geniculate) ganglion
- IX
proximal glossopharyngeal ganglion
- X
proximal vagal ganglion
- XI
transient accessory ganglia
Footnotes
Conflict of interest statement: On behalf of all authors, the corresponding author states that there is no conflict of interest.
Ethical approval: All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Literature:
- Booth KT, Azaiez H, Jahan I, Smith RJ, Fritzsch B (2018) Intracellular Regulome Variability Along the Organ of Corti: Evidence, Approaches, Challenges, and Perspective. Frontiers in genetics 9:158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chagnaud BP, Engelmann J, Fritzsch B, Glocr JC, Straka H (2017) Sensing external and self-motion with hair cells, a comparison of the lateral line and vestibular systems from a developmental and evolutionary perspective. Brain Behav Evol 90:98–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumoulin A, Ter-Avetisyan G, Schmidt H, Rathjen FG (2018) Molecular analysis of sensory axon branching unraveled a cGMP-dependent signaling cascade. International journal of molecular sciences 19:1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan J, Kersigo J, Gray B, Fritzsch B (2011) Combining lipophilic dye, in situ hybridization, immunohistochemistry, and histology. Journal of visualized experiments: JoVE 2451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan JS, Elliott KL, Kersigo J, Gray B, Fritzsch B (2015) Combining whole-mount in situ hybridization with neuronal tracing and immunohistochemistry In: (eds) HG (ed) In Situ Hybridization Methods, vol 99 New York, Human Press, pp 339–352 [Google Scholar]
- Elliott KL, Kersigo J, Pan N, Jahan I, Fritzsch B (2017) Spiral Ganglion Neuron Projection Development to the Hindbrain in Mice Lacking Peripheral and/or Central Target Differentiation. Front Neural Circuits 11:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farago AF, Awatramani RB, Dymecki SM (2006) Assembly of the brainstem cochlear nuclear complex is revealed by intersectional and subtractive genetic fate maps. Neuron 50:205–218 [DOI] [PubMed] [Google Scholar]
- Fritzsch B (2003) Development of inner ear afferent connections: forming primary neurons and connecting them to the developing sensory epithelia. Brain research bulletin 60:423–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzsch B, Duncan JS, Kersigo J, Gray B, Elliott KL (2016) Neuroanatomical Tracing Techniques in the Ear: History, State of the Art, and Future Developments Sokolowski B, Ed: Auditory and Vestibular Research: Methods and Protocols, vol 1427 Springer Science+Business Media; New York, pp 243–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzsch B, Elliott KL (2017) Gene, cell, and organ multiplication drives inner ear evolution. Developmental Biology 431:3–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzsch B, Elliott KL, Pavlinkova G (2019) Primary sensory map formations reflect unique needs and molecular cues specific to each sensory system. F1000Research 8:345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzsch B, López-Schier H (2014) Evolution of polarized hair cells in aquatic vertebrates and their connection to directionally sensitive neurons. Flow Sensing in Air and Water. Springer, pp 271–294 [Google Scholar]
- Fritzsch B, Muirhead K, Feng F, Gray B, Ohlsson-Wilhelm B (2005) Diffusion and imaging properties of three new lipophilic tracers, NeuroVue™ Maroon, NeuroVue™ Red and NeuroVue™ Green and their use for double and triple labeling of neuronal profile. Brain research bulletin 66:249–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzsch B, Pan N, Jahan I, Duncan JS, Kopecky BJ, Elliott KL, Kersigo J, Yang T (2013) Evolution and development of the tetrapod auditory system: an organ of Corti-centric perspective. Evolution & development 15:63–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzsch B, Pan N, Jahan I, Elliott KL (2015) Inner ear development: building a spiral ganglion and an organ of Corti out of unspecified ectoderm. Cell and tissue research 361:7–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzsch B, Pauley S, Feng F, Matei V, Nichols D (2006) The molecular and developmental basis of the evolution of the vertebrate auditory system. International Journal of Comparative Psychology 19:1–25 [Google Scholar]
- Glover JC, Elliott KL, Erives A, Chizhikov VV, Fritzsch B (2018) Wilhelm His’ lasting insights into hindbrain and cranial ganglia development and evolution. Developmental Biology 444:S14–S24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodrich LV (2016) Early development of the spiral ganglion In: Dabdoub A, Fritzsch B, Fay R, Popper A (eds) The Primary Auditory Neurons of the Mammalian Cochlea. Springer, pp 11–48 [Google Scholar]
- Gu C, Rodriguez ER, Reimert DV, Shu T, Fritzsch B, Richards LJ, Kolodkin AL, Ginty DD (2003) Neuropilin-1 conveys semaphorin and VEGF signaling during neural and cardiovascular development. Developmental cell 5:45–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahan I, Kersigo J, Pan N, Fritzsch B (2010a) Neurod1 regulates survival and formation of connections in mouse ear and brain. Cell and tissue research 341:95–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahan I, Pan N, Kersigo J, Fritzsch B (2010b) Neurod1 suppresses hair cell differentiation in ear ganglia and regulates hair cell subtype development in the cochlea. PloS one 5:e11661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen-Smith H, Gray B, Muirhead K, Ohlsson-Wilhelm B, Fritzsch B (2007) Long-distance three-color neuronal tracing in fixed tissue using NeuroVue dyes. Immunological investigations 36:763–789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang T, Kindt K, Wu DK (2017) Transcription factor Emx2 controls stereociliary bundle orientation of sensory hair cells. Elife 6:e23661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser A, Manley GA (1996) Brainstem cconnections of the macula lagenae in the chicken. Journal of Comparative Neurology 374:108–117 [DOI] [PubMed] [Google Scholar]
- Karis A, Pata I, van Doorninck JH, Grosveld F, de Zeeuw CI, de Caprona D, Fritzsch B (2001) Transcription factor GATA-3 alters pathway selection of olivocochlear neurons and affects morphogenesis of the ear. Journal of Comparative Neurology 429:615–630 [DOI] [PubMed] [Google Scholar]
- Kersigo J, Pan N, Lederman JD, Chatterjee S, Abel T, Pavlinkova G, Silos-Santiago I, Fritzsch B (2018) A RNAscope whole mount approach that can be combined with immunofluorescence to quantify differential distribution of mRNA. Cell and tissue research 374:251–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopecky B, Santi P, Johnson S, Schmitz H, Fritzsch B (2011) Conditional deletion of N-Myc disrupts neurosensory and non-sensory development of the ear. Developmental Dynamics 240:1373–1390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu CC, Appler JM, Houseman EA, Goodrich LV (2011) Developmental profiling of spiral ganglion neurons reveals insights into auditory circuit assembly. Journal of Neuroscience 31:10903–10918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu CC, Cao X-J, Wright S, Ma L, Oertel D, Goodrich LV (2014) Mutation of Npr2 leads to blurred tonotopic organization of central auditory circuits in mice. PLoS genetics 10:e1004823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macova I, Pysanenko K, Chumak T, Dvorakova M, Bohuslavova R, Syka J, Fritzsch B, Pavlinkova G (2019) Neurod1 is essential for the primary tonotopic organization and related auditory information processing in the midbrain. Journal of Neuroscience 39:984–1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmoud A, Reed C, Maklad A (2013) Central projections of lagenar primary neurons in the chick. Journal of Comparative Neurology 521:3524–3540 [DOI] [PubMed] [Google Scholar]
- Maklad A, Fritzsch B (2003a) Development of vestibular afferent projections into the hindbrain and their central targets. Brain research bulletin 60:497–510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maklad A, Fritzsch B (2003b) Partial segregation of posterior crista and saccular fibers to the nodulus and uvula of the cerebellum in mice, and its development. Brain research Developmental brain research 140:223–236 [DOI] [PubMed] [Google Scholar]
- Maklad A, Kamel S, Wong E, Fritzsch B (2010) Development and organization of polarity-specific segregation of primary vestibular afferent fibers in mice. Cell and tissue research 340:303–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malmierca MS (2015) Auditory system The Rat Nervous System (Fourth Edition). Elsevier, pp 865–946 [Google Scholar]
- Mann HB, Whitney DR (1947) On a test of whether one of two random variables is stochastically larger than the other. The annals of mathematical statistics 50–60 [Google Scholar]
- Mao Y, Reiprich S, Wegner M, Fritzsch B (2014) Targeted deletion of Sox10 by Wnt1-cre defects neuronal migration and projection in the mouse inner ear. PloS one 9:e94580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maricich SM, Xia A, Mathes EL, Wang VY, Oghalai JS, Fritzsch B, Zoghbi HY (2009) Atoh1-lineal neurons are required for hearing and for the survival of neurons in the spiral ganglion and brainstem accessory auditory nuclei. J Neurosci 29:11123–11133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matei V, Feng F, Pauley S, Beisel K, Nichols M, Fritzsch B (2006) Near-infrared laser illumination transforms the fluorescence absorbing X-Gal reaction product BCI into a transparent, yet brightly fluorescent substance. Brain research bulletin 70:33–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matei V, Pauley S, Kaing S, Rowitch D, Beisel K, Morris K, Feng F, Jones K, Lee J, Fritzsch B (2005) Smaller inner ear sensory epithelia in Neurog1 null mice are related to earlier hair cell cycle exit. Developmental Dynamics 234:633–650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muniak MA, Connelly C, Suthakar K, Milinkeviciute G, Ayeni FE, Ryugo DK (2016) Central Projections of Spiral Ganglion Neurons. The Primary Auditory Neurons of the Mammalian Cochlea. Springer, pp 157–190 [Google Scholar]
- Nichols DH, Pauley S, Jahan I, Beisel KW, Millen KJ, Fritzsch B (2008) Lmx1a is required for segregation of sensory epithelia and normal ear histogenesis and morphogenesis. Cell and tissue research 334:339–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osen KK (1969) Cytoarchitecture of the cochlear nuclei in the cat. Journal of Comparative Neurology 136:453–483 [DOI] [PubMed] [Google Scholar]
- Oury F, Murakami Y, Renaud J-S, Pasqualetti M, Charnay P, Ren S-Y, Rijli FM (2006) Hoxa2-and rhombomere-dependent development of the mouse facial somatosensory map. Science 313:1408–1413 [DOI] [PubMed] [Google Scholar]
- Pataskar A, Jung J, Smialowski P, Noack F, Calegari F, Straub T, Tiwari VK (2016) NeuroD1 reprograms chromatin and transcription factor landscapes to induce the neuronal program. The EMBO journal 35:24–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauley S, Lai E, Fritzsch B (2006) Foxg1 is required for morphogenesis and histogenesis of the mammalian inner ear. Developmental dynamics: an official publication of the American Association of Anatomists 235:2470–2482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruben RJ (1966) Development of the inner ear of the mouse: a radioautographic study of terminal mitoses. Acta oto-laryngologica Suppl 220: 221–244 [PubMed] [Google Scholar]
- Schmidt H, Stonkute A, Jüttner R, Koesling D, Friebe A, Rathjen FG (2009) C-type natriuretic peptide (CNP) is a bifurcation factor for sensory neurons. Proceedings of the National Academy of Sciences 106:16847–16852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt H, Stonkute A, Jüttner R, Schäffer S, Buttgereit J, Feil R, Hofmann F, Rathjen FG (2007) The receptor guanylyl cyclase Npr2 is essential for sensory axon bifurcation within the spinal cord. J Cell Biol 179:331–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt H, Werner M, Heppenstall PA, Henning M, Moré Ml, Kühbandner S, Lewin GR, Hofmann F, Feil R, Rathjen FG (2002) cGMP-mediated signaling via cGKI< is required for the guidance and connectivity of sensory axons. The Journal of cell biology 159:489–498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz JA, Zeller U, Luo ZX (2017) Inner ear labyrinth anatomy of monotremes and implications for mammalian inner ear evolution. Journal of morphology 278:236–263 [DOI] [PubMed] [Google Scholar]
- Stefanini M, de Martino C, Zamboni L (1967) Fixation of ejaculated spermatozoa for electron microscopy. Nature 216:173. [DOI] [PubMed] [Google Scholar]
- Ter-Avetisyan G, Dumoulin A, Herrel A, Schmidt H, Strump J, Afzal S, Rathjen FG (2018) Loss of axon bifurcation in mesencephalic trigeminal neurons impairs the maximal biting force in Npr2-deficient mice. Frontiers in Cellular Neuroscience 12:153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ter-Avetisyan G, Rathjen FG, Schmidt H (2014) Bifurcation of axons from cranial sensory neurons is disabled in the absence of Npr2-induced cGMP signaling. Journal of Neuroscience 34:737–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonniges J, Hansen M, Duncan J, Bassett M, Fritzsch B, Gray B, Easwaran A, Nichols MG (2010) Photo-and bio-physical characterization of novel violet and near-infrared lipophilic fluorophores for neuronal tracing. Journal of microscopy 239:117–134 [DOI] [PubMed] [Google Scholar]
- Tröster P, Haseleu J, Petersen J, Drees O, Schmidtko A, Schwaller F, Lewin GR, Ter-Avetisyan G, Winter Y, Peters S (2018) The absence of sensory axon bifurcation affects nociception and termination fields of afferents in the spinal cord. Frontiers in molecular neuroscience 11:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuji T, Kunieda T (2005) A loss-of-function mutation in natriuretic peptide receptor 2 (Npr2) gene is responsible for disproportionate dwarfism in cn/cn mouse. Journal of Biological Chemistry 280:14288–14292 [DOI] [PubMed] [Google Scholar]
- Wolter S, Möhrle D, Schmidt H, Pfeiffer S, Zelle D, Eckert P, Krämer M, Feil R, Pilz PK, Knipper M (2018) GC-B Deficient Mice With Axon Bifurcation Loss Exhibit Compromised Auditory Processing. Frontiers in neural circuits 12:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang M, Maklad A, Pirvola U, Fritzsch B (2003) Brn3c null mutant mice show long-term, incomplete retention of some afferent inner ear innervation. BMC neuroscience 4:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang T, Kersigo J, Jahan I, Pan N, Fritzsch B (2011) The molecular basis of making spiral ganglion neurons and connecting them to hair cells of the organ of Corti. Hearing research 278:21–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang T, Kersigo J, Wu S, Fritzsch B, Bassuk AG (2017) Prickle1 regulates neurite outgrowth of apical spiral ganglion neurons but not hair cell polarity in the murine cochlea. PloS one 12:e0183773. [DOI] [PMC free article] [PubMed] [Google Scholar]


