Abstract
BACKGROUND
Frailty is a geriatric syndrome thought to identify the most vulnerable older adults, and morbidity and mortality has been reported to be higher for frail patients after cardiac surgery compared to nonfrail patients. However, the cognitive consequences of frailty after cardiac surgery have not been well described. In this study, we examined the hypothesis that baseline frailty would be associated with postoperative delirium and cognitive change at 1 and 12 months after cardiac surgery.
METHODS
This study was nested in 2 trials, each of which was conducted by the same research team with identical measurement of exposures and outcomes. Before surgery, patients were assessed with the validated “Fried” frailty scale, which evaluates 5 domains (shrinking, weakness, exhaustion, low physical activity, and slowed walking speed) and classifies patients as nonfrail, prefrail, and frail. The primary outcome was postoperative delirium during hospitalization, which was assessed using the Confusion Assessment Method, Confusion Assessment Method for the Intensive Care Unit, and validated chart review. Neuropsychological testing was a secondary outcome and was generally performed within 2 weeks of surgery and then 4–6 weeks and 1 year after surgery, and the outcome of interest was change in composite Z-score of the test battery. Associations were analyzed using logistic and linear regression models, with adjustment for variables considered a priori (age, gender, race, education, and logistic European System for Cardiac Operative Risk Evaluation). Multiple imputation was used to account for missing data at the 12-month follow-up.
RESULTS
Data were available from 133 patients with baseline frailty assessments. Compared to nonfrail patients (13% delirium incidence), the incidence of delirium was higher in prefrail (48% delirium incidence; risk difference, 35%; 95% CI, 10%–51%) and frail patients (48% delirium incidence; risk difference, 35%; 95% CI, 7%–53%). In both univariable and multivariable models, the odds of delirium were significantly higher for prefrail (adjusted odds ratio, 6.43; 95% CI, 1.31–31.64; P = .02) and frail patients (adjusted odds ratio, 6.31; 95% CI, 1.18–33.74; P = .03) compared to nonfrail patients. The adjusted decline in composite cognitive Z-score was greater from baseline to 1 month only in frail patients compared to nonfrail patients. By 1 year after surgery, there were no differences in the association of baseline frailty with change in cognition.
CONCLUSIONS
Compared to nonfrail patients, both prefrail and frail patients were at higher risk for the primary outcome of delirium after cardiac surgery. Frail patients were also at higher risk for the secondary outcome of greater decline in cognition from baseline to 1 month, but not baseline to 1 year, after surgery.
Frailty is a geriatric syndrome thought to identify the most vulnerable older adults, who have reduced reserve when faced with stressors.1 In community-dwelling populations of adults >65 years old, the prevalence of frailty has been estimated to be 7%.2 However, in patients undergoing cardiac surgery, frailty is much more common, with a reported prevalence of 20%–46%.3
In multiple studies, morbidity and mortality has been reported to be higher for frail patients after cardiac surgery compared to nonfrail patients.3–5 However, the cognitive consequences of frailty after cardiac surgery have not been well described. In 2 prior studies,6,7 early neurocognitive dysfunction manifest as delirium was more common in frail patients after cardiac surgery. However, these studies were limited by the frailty and delirium assessments. Delirium appears to be a strong risk factor for cognitive decline in the months to years after surgery,8–10 so frail patients with delirium may also be at disproportionate risk for cognitive decline after cardiac surgery. Because preserving cognition after surgery is an important patient-centered goal, understanding the potential contribution of frailty on postoperative cognitive function is important both for risk stratification and optimization of perioperative management. Further, the anesthesia specialty has embraced the goal of optimizing perioperative brain health through the American Society of Anesthesiologists Brain Health Initiative.
In this cohort study nested within 2 trials of patients undergoing cardiac surgery, the primary objective was to examine the association of baseline frailty with rigorously assessed postoperative delirium during hospitalization. The secondary objectives were to examine the association of baseline frailty with 1- and 12-month change in cognition from baseline. The primary hypothesis was that frailty would be associated with an increased risk of delirium during hospitalization. The secondary hypotheses were that frailty would be associated with a decline in a composite measure of cognition and that delirium would mediate the association of frailty with cognitive decline after cardiac surgery.
METHODS
Study procedures were approved by the Institutional Review Board at Johns Hopkins School of Medicine (jhmeirb@jhmi.edu). Written informed consent was obtained from all patients in this study. This manuscript adheres to the applicable STrengthening the Reporting of OBservational studies in Epidemiology guidelines.
Study Design and Patients
This observational study was nested in 2 trials, each of which was conducted by the same research team with identical measurement of frailty and neuropsychological outcomes. Data from 2 trials were combined in this study. The primary aim of the first trial was to determine if targeted mean arterial pressure during cardiopulmonary bypass (CPB) based on cerebral autoregulation monitoring data reduced the incidence of a composite outcome (stroke, ischemic lesions, cognitive change) compared with standard blood pressure management. This trial was registered as NCT00981474. Patients were enrolled from August 2014 to May 2016. Inclusion criteria were coronary artery bypass and/or valve surgery that required CPB and who were at high risk for neurologic complications (stroke or encephalopathy) as determined by a Johns Hopkins risk score composed of history of stroke, presence of carotid bruit, hypertension, diabetes, and age that generally excluded patients in the lowest quartile of risk.11 Exclusion criteria were contraindications to magnetic resonance imaging (eg, pacemaker), hepatic dysfunction (aspartate or alanine transaminase or alkaline phosphatase elevated to twice the upper limit of normal), chronic renal failure requiring dialysis, inability to attend outpatient visits, non-English speaking, and emergency surgery. Data on these patients have been published separately, in a manuscript examining the association of delirium and cognitive change after cardiac surgery.10
The primary aim of the second trial was to determine if remote ischemic preconditioning could reduce the incidence of delirium. This trial was registered as NCT02587039. Patients were enrolled from July 2014 to December 2015. Inclusion criteria were age ≥65 years old and undergoing coronary artery bypass graft and/or valve operation that required CPB. Exclusion criteria were baseline delirium, Mini-Mental State Examination12 score <23, inability to speak/read English, severe hearing impairment, inability to tolerate upper extremity tourniquet, hemoglobinopathy, or intraoperative ketamine use.
Frailty Assessment
Frailty assessments were performed using the validated scale from Fried et al2 evaluating 5 domains: (1) “shrinking,” defined as unintentional weight loss of ≥10 pounds in the last year; (2) “weakness,” determined by grip-strength, adjusted for gender and body mass index; (3) “exhaustion,” determined by 2 questions from the modified 10-item Center for Epidemiological Studies Depression scale13; (4) “low physical activity,” determined by the modified Minnesota Leisure Time Activities Questionnaire14; and (5) “slowed walking speed,” as measured at normal pace over 15 feet. Each of the 5 domains yielded a score of 0 or 1 based on cutoffs previously described.2 Patients who refused to walk were scored as “1” for gait speed. Nonfrail, prefrail, and frail patients were defined as total scores of 0, 1–2, and 3–5, respectively, as previously described.
Delirium Assessment
Delirium was assessed using rigorous methodologies, including the Confusion Assessment Method15 and Confusion Assessment Method for the Intensive Care Unit.16 The Confusion Assessment Method assessment was performed in person by formally trained research assistants and included a structured cognitive examination (minimental state examination, digit span forward/backward, and timed months of the year backward). Research assistants also queried the patient, nurses, families, and medical records for evidence of delirium. Findings from this overall assessment were used to determine the diagnosis of delirium. For intubated patients in the intensive care unit, the Confusion Assessment Method for the Intensive Care Unit was used. Delirium was assessed in person on 3 of the first 4 postoperative days. For days on which patients were not assessed in person, a validated chart review was used (sensitivity of 74% and specificity of 83%).17 To further classify delirium episodes, information from each assessment was used to evaluate delirium severity with the Delirium Rating Scale-Revised-98.18 Coma was assessed using the Richmond Agitation Sedation Scale, with a score of −4 or −5 indicating coma. Patients who were comatose on all assessments (regardless of sedation medication) were classified as having coma in this analysis. The once-daily delirium assessments were limited to the first 4 postoperative days because of evidence that >90% of delirium occurs within this time.19 For the analysis, delirium was defined as any Confusion Assessment Method, Confusion Assessment Method for the Intensive Care Unit, or chart-review positive assessment during hospitalization.
Delirium assessors underwent formal training by a psychiatrist (K.J.N.), who is an expert in delirium diagnosis. Training included readings, videos, and delirium assessments of 10 patients with subsequent discussion. During the study, delirium assessors and the psychiatrist team member conducted coratings of patients every 2 weeks. Finally, research assistants met with delirium experts 1–2 times/mo to discuss delirium assessments of nonstudy patients, to ensure consistent methods and judgment. During the study, we measured agreement among researchers and κ statistics were between 0.7 and 0.8, which is consistent with substantial agreement.
Neuropsychological Testing
Neuropsychological testing was generally performed within 2 weeks of surgery and then 4–6 weeks and 1 year after surgery. The tests assessed a number of cognitive domains known to be affected by cardiac surgery.20,21 The test battery consisted of the Rey Auditory Verbal Learning Test,22 Rey Complex Figure Test,23 Controlled Oral Word Association Test,24 Symbol Digits Modalities Test,25 Trail Making Tests A and B,26 and Grooved Pegboard Test.27
Perioperative Management
Patients received standard institutional monitoring, including radial arterial blood pressure monitoring. General anesthesia was induced with fentanyl, midazolam, and/or propofol and was maintained with isoflurane and a nondepolarizing muscle relaxant. CPB was performed with a nonocclusive roller pump and a membrane oxygenator, and the circuit included a 40 μm or smaller arterial line filter. Nonpulsatile flow was maintained between 2.1 and 2.4 L/min/m2. Patients were managed using alpha-stat pH management. Rewarming was based on institutional standards with a goal of maintaining nasal pharyngeal temperature <37°C. After surgery, patients were sedated with a propofol infusion until they qualified for tracheal extubation or for 24 hours after surgery. Patients requiring >24 hours of mechanical ventilation could receive an infusion of fentanyl and/or midazolam.
Statistical Analysis
The primary exposure was baseline category of frailty, defined before this study. The primary outcome was defined before the initiation of the study design and was any delirium assessment that was positive (Confusion Assessment Method, Confusion Assessment Method for the Intensive Care Unit, or chart review). The secondary outcomes were also defined before study design and were change in a composite cognitive Z-score from baseline to 1 month and 1 year after surgery, as described and used previously by our group.28,29 The composite cognitive Z-score was obtained by first calculating Z-scores for individual tests at each testing time point, using the mean and SD of baseline tests of all patients in the parent study. Timed tests were multiplied by “−1” so that higher scores represented better performance. Next, individual test Z-scores were averaged at each time point and renormalized to generate a composite cognitive Z-score. Finally, the difference in composite Z-scores was calculated for each interval of interest.
Baseline patient characteristics were compared using Student t tests, Wilcoxon rank sum tests, and Fisher exact tests. The association of frailty and delirium was examined using a logistic regression model, with frailty status included as a categorical variable (nonfrail, prefrail, frail) and the delirium outcome defined as any positive delirium assessment during hospitalization. Delirium severity was categorized into quintiles because it was highly skewed with the outcome of interest being quintile of severity. Cognitive change was examined using linear regression. As advocated by others,30 we did not account for learning effect or surgery because we were interested in the difference between 2 groups of patients, both of whom underwent surgery and had the opportunity for learning effect. Variables for which to adjust were considered a priori and included age, gender, race, education, and logistic European System for Cardiac Operative Risk Evaluation. Of note, the logistic European System for Cardiac Operative Risk Evaluation includes several variables which were thought to differ by frailty status, such as age, comorbidities (ie, left ventricle dysfunction), and type and urgency of surgery. We accounted for missing 1-year follow-up cognitive data with multiple imputation using PROC MI in Statistical Analysis Software (SAS Institute, Cary, NC). Missing data (10 datasets) were imputed using age, gender, race, education, log European System for Cardiac Operative Risk Evaluation, and baseline and 1-month cognitive data. The regression model was fit using PROC MIANALYZE. Mediation was examined using the product method by Baron & Kenny, and the Sobel test was used to test the significance of the mediation effect (indirect effect). The exposure was frailty status (nonfrail, prefrail, and frail), the putative mediator was any delirium, and the outcome was change in cognition from baseline to 1 month. Interaction was tested by examining significance of an interaction term in the adjusted regression model. In exploratory analysis, we examined the association of each individual component of the frailty scale with postoperative delirium. This analytic plan was based on prior methodology used by our research group28 and was agreed on before accessing the data. A P value of <0.05 was considered significant for the primary outcome of delirium and the secondary outcomes of change in cognition at 1 month and 1 year from baseline. We did not adjust for multiple comparisons.
The sample size for this nested cohort study was determined by the number of patients with available frailty, delirium, and cognitive assessments. However, we had also determined that a sample size of 108 patients would be necessary to detect a difference in delirium incidence between both nonfrail and prefrail patients and between nonfrail and frail patients, using a χ2 test with a type I error of 0.05 and a power of >80%, assuming a nonfrailty prevalence of approximately 18%, a prefrailty prevalence of 40%, a frailty prevalence of 40%, and a delirium incidence of 12% in the nonfrail patients, 50% in the prefrail patients, and 50% in the frail patients.
RESULTS
Patient Characteristics
Data were available on 133 patients with baseline frailty measurement, and a flow diagram is shown in Supplemental Digital Content, Appendix A, http://links.lww.com/AA/C673. Characteristics of patients by frailty status are presented in Table 1. Compared to nonfrail patients, frail patients had higher log European System for Cardiac Operative Risk Evaluation, were more likely to undergo surgery involving valves, and had more congestive heart failure (the latter 2 variables being considered in the log European System for Cardiac Operative Risk Evaluation).
Table 1.
Patient and Surgical Characteristics
| Nonfrail (N = 15) | Prefrail (N = 74) | Frail (N = 44) | P Value | |
|---|---|---|---|---|
| Age (years), mean (SD) | 69.33 (7.90) | 71.72 (6.93) | 73.48 (8.09) | .156a |
| Male, n (%) | 12 (80) | 57 (77) | 28 (64) | .230b |
| Race, n (%) | ||||
| Caucasian | 13 (87) | 62 (84) | 35 (80) | .490b |
| African American | 1 (7) | 7 (9) | 8 (18) | |
| Other | 1 (7) | 5 (7) | 1 (2) | |
| Education, n (%) | ||||
| High school or below | 4 (27) | 27 (38) | 21 (48) | .330b |
| Above high school | 11 (73) | 45 (63) | 23 (52) | |
| Comorbidities, n (%) | ||||
| Prior stroke | 1 (7) | 11 (15) | 7 (16) | .823b |
| Hypertension | 13 (87) | 71 (96) | 38 (86) | .103b |
| Congestive heart failure | 0 (0) | 13 (18) | 12 (27) | .042b |
| Peripheral vascular disease | 4 (27) | 7 (9) | 9 (20) | .087b |
| COPD | 0 (0) | 5 (7) | 2 (5) | .869b |
| Tobacco (prior) | 9 (69) | 36 (61) | 24 (63) | .886b |
| Diabetes | 4 (27) | 26 (35) | 22 (50) | .166b |
| Logistic European System for Cardiac Operative Risk Evaluation, median (interquartile range) | 2.52 (1.56, 4.00) | 3.64 (2.38, 7.38) | 6.29 (3.29, 9.47) | .003c |
| Surgery, n (%) | .028b | |||
| Coronary artery bypass | 13 (87) | 31 (42) | 18 (41) | |
| Coronary artery bypass + Valve | 0 (0) | 12 (16) | 8 (18) | |
| Valve | 1 (7) | 28 (38) | 17 (39) | |
| Other | 1 (7) | 3 (4) | 1 (2) | |
| Cardiopulmonary bypass duration (min), median (interquartile range) | 118 (54, 130) | 116 (84, 157) | 128 (80, 154) | .439c |
Abbreviation: COPD, chronic obstructive pulmonary disease.
Comparison with Student t test.
Comparison with Fisher exact test.
Comparison with Wilcoxon rank sum test.
Delirium (Primary Outcome)
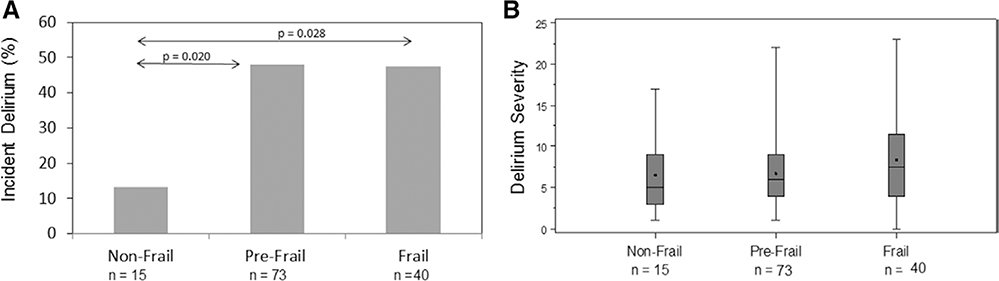
The overall incidence of delirium was 43.8%, 95% CI, 35%–52% (56/128), with delirium assessments not available in 5 patients (staff unavailable [n = 4] and coma at all assessments [n = 1]). As shown in Figure 1A, the incidence of delirium was higher in prefrail (35/73 [48%], P = .02) and frail (19/40 [48%], P = .03) patients, compared to nonfrail patients (2/15 [13%]). In both univariable models and multivariable models adjusted for age, gender, education, and log European System for Cardiac Operative Risk Evaluation (Table 2), the odds of delirium were significantly higher for prefrail (adjusted odds ratio, 6.43; 95% CI, 1.31–31.64; P = .02) and frail patients (adjusted odds ratio, 6.31; 95% CI, 1.18–33.74; P = .03) compared to nonfrail patients. Because the patients in this analysis were combined from 2 different trials, we also separately adjusted for study and intervention and found no difference in inferences, with greater statistical significance. In exploratory analysis, we examined individual components of the frailty phenotype and found that the association between individual components of the frailty scale and delirium was strongest for exhaustion (36% delirium in no-exhaustion group versus 55% delirium in exhaustion group, P = .04).
Figure 1.
The incidence of delirium is higher in prefrail and frail patients compared with nonfrail patients. There is no difference in median delirium severity scores (Delirium Rating Scale-Revised-98) by frailty status for prefrail and frail patients.
Table 2.
Odds of Incident Delirium, Increasing Number of Days of Delirium, and Increasing Delirium Severity for Prefrail and Frail Patients Compared With Nonfrail Patients
| Unadjusted |
Adjusteda |
|||
|---|---|---|---|---|
| Odds Ratio (95% CI) | P Value | Odds Ratio (95% CI) | P Value | |
| Delirium incidenceb | ||||
| Nonfrail | Ref | Ref | ||
| Prefrail | 5.99 (1.26–28.43) | .024 | 6.43 (1.31–31.64) | .022 |
| Frail | 5.88 (1.17–29.51) | .031 | 6.31 (1.18–33.74) | .031 |
| Number of days of deliriumc | ||||
| Nonfrail | Ref | Ref | ||
| Prefrail | 2.09 (0.88–5.00) | .096 | 2.16 (0.90–5.18) | .086 |
| Frail | 2.58 (1.06–6.25) | .036 | 2.76 (1.11–6.82) | .028 |
| Delirium severity (by quintile)d | ||||
| Nonfrail | Ref | Ref | ||
| Prefrail | 1.09 (0.74–1.97) | .676 | 1.04 (0.69–1.55) | .853 |
| Frail | 1.29 (0.85–1.97) | .233 | 1.25 (0.80–1.96) | .322 |
Adjusted for age, gender, education, and logistic European System for Cardiac Operative Risk Evaluation.
Odds ratio refers to the odds of delirium for prefrail or frail patients compared with nonfrail patients.
Odds ratio refers to the odds of 1 more day of delirium for prefrail or frail patients compared with nonfrail patients.
Odds ratio refers to the odds of being in a higher quintile of delirium severity for prefrail or frail patients compared with nonfrail patients.
The median (interquartile range) number of days of delirium for nonfrail patients was 0 (interquartile range, 0–0), for prefrail patients was 0 (interquartile range, 0–1), and for frail patients was 0 (interquartile range, 0–2; P = .05). As shown in Table 2, odds of having 1 more day of delirium were higher for frail patients (but not prefrail patients) compared to nonfrail patients in both unadjusted and adjusted models. Delirium severity scores by frailty status are shown in Figure 1B. Median delirium severity scores were 5 (interquartile range, 3–9) in nonfrail patients, 6 (interquartile range, 4–9) in prefrail patients, and 7.5 (interquartile range, 4–11.5) in frail patients. There was no difference in the odds of being in a higher quintile of delirium severity score by frailty status (Table 2).
Cognitive Change (Secondary Outcomes)
Cognitive data were available from 76 patients at baseline, 80 patients at 1 month, and 54 patients at 1 year after surgery. The composite cognitive Z-score for the entire study cohort at baseline was 0.12 ± 0.58, at 1 month was 0.0008 ± 0.65, and at 1 year was −0.043 ± 0.46. The change in composite cognitive Z-score from baseline to 1 month was −0.082 ± 0.39 (eg, a decline in cognition), and from baseline to 1 year was −0.23 ± 0.37 (secondary outcomes). Follow-up composite cognitive Z-score was higher than baseline in 39% of participants at 1 month and in 21% of participants at 1 year.
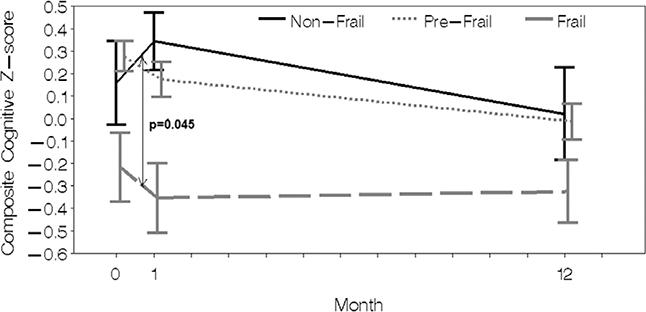
Cognitive scores at each time point, as well as the trajectory of cognitive change after surgery by frailty status, are shown in Figure 2 and Table 3. The decline in composite cognitive Z-score was marginally greater from baseline to 1 month in prefrail and frail patients compared to nonfrail patient in unadjusted models. In adjusted models, the change for prefrail compared to nonfrail patients was attenuated slightly and no longer significant, while the change for frail compared to nonfrail patients remained significant. The likelihood of being in the worst tertile of cognitive decline from baseline to 1 month also differed by frailty status (nonfrail 0/9 [0%], prefrail 15/42 [36%], frail 10/23 [43%]; P = .045). By 1 year after surgery, there were no differences in the association of baseline frailty with mean change in cognition. The likelihood of being in the worst tertile of cognitive decline from baseline to 1 year was also not different by frailty status (nonfrail 3/7 [43%], prefrail 11/31 [35%], frail 4/14 [29%]; P = .84). The results of a sensitivity analysis to account for potential effects of study intervention were similar to the main adjusted model.
Figure 2.
Composite cognitive Z-scores are shown at baseline, 1 month, and 1 year after surgery. Missing values at 1 month and 1 year are imputed.
Table 3.
Composite Cognitive Z-Scores and Interval Changes in Scores at Baseline, 1 Month, and 1 Year After Surgery by Frailty Status
| Difference in Prefrail Compared to Nonfrailb |
Difference in Frail Compared to Nonfrailb |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Unadjusted |
Adjustedc |
Unadjusted |
Adjustedc |
||||||||
| Nonfraila | Prefraila | Fraila | β-Coefficient (95% CI) | P Value | β-Coefficient (95% CI) | P Value | β-Coefficient (95% CI) | P Value | β-Coefficient (95% CI) | P Value | |
| Cognitive Z-score, mean (SD) | |||||||||||
| Baseline (n = 76) | 0.16 (0.56) | 0.28 (0.44) | −0.15 (0.75) | 0.12 (−0.31 to 0.54) | .575 | 0.20 (−0.19 to 0.60) | .308 | −0.36 (−0.82 to 0.088) | .109 | −0.13 (−0.57 to 0.31) | 553 |
| 1 mo (n = 80) | 0.34 (0.39) | 0.17 (0.50) | −0.31 (0.75) | −0.17 (−0.62 to 0.28) | .442 | −0.068 (−0.50 to 0.37) | .749 | −0.70 (−1.17 to −0.22) | .006 | −0.48 (−0.96 to 0.002) | .051 |
| 1 y (n = 54) | 0.08 (0.66) | −0.06 (0.47) | −0.06 (0.34) | −0.017 (−0.45 to 0.42) | .936 | 0.097 (−0.32 to 0.51) | .631 | −0.33 (−0.80 to 0.14) | .161 | −0.13 (−0.58 to 0.32) | .573 |
| Change in cognitive Z–score, mean (SD) | |||||||||||
| Baseline to 1 month (n = 74) | 0.19 (0.29) | −0.09 (0.30) | −0.14 (0.51) | −0.29 (−0.57 to −0.0003) | .0498 | −0.27 (−0.57 to 0.032) | .078 | −0.33 (−0.65 to −0.015) | .041 | −0.35 (−0.69 to −0.0079) | .045 |
| Baseline to 1 year (n = 52) | −0.21 (0.48) | −0.31 (0.33) | −0.13 (0.35) | −0.13 (−0.44 to 0.17) | .367 | −0.11 (−0.39 to 0.19) | .460 | −0.11 (−0.29 to 0.37) | .804 | 0.004 (−0.32 to 0.33) | .979 |
Cognitive scores at each time point are presented without imputation of missing values.
The unadjusted and adjusted regression models use imputed missing values for the 1-year time point. A (–) value for the β-coefficient indicates decline in cognition.
Adjusted for age, gender, education, and logistic European System for Cardiac Operative Risk Evaluation.
Interaction of Frailty, Delirium, and Cognitive Change
We examined whether delirium might mediate the association between baseline frailty and cognitive decline at 1 month. There did not appear to be such an effect, with the main model of frailty and cognitive change minimally changed with the addition of delirium (<10% change in β-coefficient for both prefrailty and frailty with the addition of delirium to the model). Similarly, there was no interaction of delirium and frailty (P interaction for prefrail = .67 and for frail = .92), suggesting that the association between frailty and cognitive change was not different according to delirium status.
DISCUSSION
The results of this study demonstrate that, compared to nonfrail patients, both prefrail and frail patients were at higher risk for the primary outcome of delirium after cardiac surgery. In terms of secondary outcomes, frail patients were also at higher risk for greater decline in cognition from baseline to 30 days postoperatively. By 1 year after surgery, there were no differences in cognitive decline by frailty status.
These results add further clarity to the role of frailty in preoperative risk stratification. Both prefrail and frail patients are at higher risk for delirium and short-term cognitive decline, but reassuringly, the changes in cognition are attenuated at 1 year. Patients who are nonfrail can expect a low incidence of delirium and preservation of cognition even at 30 days after surgery. Nonfrail patients who are particularly worried about postoperative cognitive function should be reassured by these findings.
After cardiac surgery, frailty has been consistently identified as an important predictor of morbidity and mortality.5 Frailty also appears to add predictive value to established risk scores, such as the Society of Thoracic Surgeons Predicted Risk of Mortality or Major Morbidity.3 However, there are few studies that have examined cognitive sequelae among frail patients. Two prior studies found an association between baseline frailty and delirium after cardiac surgery.6,7 The first study from our group was limited by the delirium assessment (chart review only) and found a low incidence of delirium in the nonfrail and prefrail patients (2.6% in total).6 Our findings of a 48% incidence of delirium in the prefrail patients suggest that a more sensitive in-person delirium assessment likely diagnosed less obvious cases of delirium in prefrail patients. A second study incorporated baseline cognitive status into the definition of frailty,7 thus potentially confounding the association between frailty and delirium, because cognitive impairment is such a strong risk factor for delirium. Indeed, prediction models of delirium heavily weigh brain-based variables, including stroke, cognitive status, and depression.31 Our results suggest that non–brain-based factors are also associated with vulnerability to postoperative delirium.
Importantly, the association of frailty and posthospitalization cognitive outcomes has not been well described. Our results show a decline in cognition in the month after surgery in prefrail and frail patients, but not nonfrail patients, with no difference in cognitive outcomes at 1 year. Although frailty is commonly seen as a marker of vulnerability and the results at 1 month may be expected, our results demonstrate that prefrail patients may also be an important group to target for brain health preservation. Interestingly, delirium did not mediate the association of frailty and cognitive decline, and thus, mechanisms of cognitive change by frailty status and delirium status may be different.
These results demonstrate the importance of further research to determine how to optimize perioperative strategies for frail older adults undergoing cardiac surgery, especially in the weeks to months after surgery. Unfortunately, the evidence base to support targeted strategies for frail patients is lacking. Isolated studies have supported a role for structured exercise before cardiac surgery.32 However, safety concerns, logistical considerations, and lack of a strong evidence base have limited uptake. At least 1 current study is enrolling frail patients with a goal of optimization before cardiac surgery.33 Although it is clear that frailty identifies patients at high risk before surgery, further research is needed for clinicians to know how to act on this information.
Strengths of this study include rigorous assessment of exposures and outcomes. Frailty was assessed using the well-validated Fried scale. Delirium was assessed using state-of-the-art methods by a research group experienced in delirium assessment after cardiac surgery, and cognitive outcomes were obtained up to 1 year after surgery. Nevertheless, there are several weaknesses to consider. Although the frailty and outcome assessments were similar, these results do reflect a combination of 2 studies, with different enrollment criteria and goals. There were missing cognitive assessments at 1 year in a substantial number of patients, which might contribute to bias and lead to a Type II error, although multiple imputation was used to account for this missingness. There is a possibility of residual confounding given baseline differences in comorbidity by frailty status, even though we adjusted for several variables that were determined a priori. These results also reflect patients only at 1 academic medical center. Finally, the cognitive changes observed may not be clinically significant, with some suggesting that a difference of 0.5 SD is clinically significant.
In conclusion, the primary outcome of delirium is more common both in prefrail and frail patients compared to nonfrail patients after cardiac surgery. In terms of secondary outcomes, frail patients also have greater cognitive decline at 1 month; however, at 1 year, cognitive change is similar by frailty status. Further research is needed to determine how to incorporate frailty assessment into perioperative management to optimize brain health early after cardiac surgery.
Supplementary Material
KEY POINTS.
Question: What is the association of baseline frailty with delirium and cognitive change at 1 month and 1 year after cardiac surgery?
Findings: Both frail and prefrail patients had a higher risk of delirium (primary outcome) compared to nonfrail patients, while frail patients had greater cognitive decline at 1 month but not 1 year after surgery (secondary outcomes) compared to nonfrail patients.
Meaning: Frailty identifies patients at risk for delirium and cognitive decline in the month after cardiac surgery, but there were no differences in cognitive change by frailty status at 1 year.
ACKNOWLEDGMENTS
The authors thank Michelle Parish, RN, Elizabeth White, RN, and Mirinda Anderson, RN (from Department of Anesthesiology and Critical Care Medicine, Clinical Research Core, Johns Hopkins University School of Medicine, Baltimore, MD 21287).
Funding: This work was supported by Older Americans Independence Center Research Career Development Core Award (P30 AG021334). C.H.B. was supported by National Institutes of Health (NIH) K76 AG057020, International Anesthesia Research Society, Johns Hopkins Clinician Scientist Award, and Magic That Matters Grant. C.W.H. was supported by NIH RO1 HL092259.
DISCLOSURES
Name: Yohei Nomura, MD.
Contribution: This author helped acquire data, revise the manuscript for critical intellectual content, approve the final version, and agree to be accountable for the work.
Conflicts of Interest: None.
Name: Mitsunori Nakano, MD.
Contribution: This author helped design the study, revise the manuscript for critical intellectual content, approve the final version, and agree to be accountable for the work.
Conflicts of Interest: None.
Name: Brian Bush, MD.
Contribution: This author helped design the study, revise the manuscript for critical intellectual content, approve the final version, and agree to be accountable for the work.
Conflicts of Interest: None.
Name: Jing Tian, MS.
Contribution: This author helped design the study, revise the manuscript for critical intellectual content, approve the final version, and agree to be accountable for the work.
Conflicts of Interest: None.
Name: Atsushi Yamaguchi, MD, PhD.
Contribution: This author helped design the study, revise the manuscript for critical intellectual content, approve the final version, and agree to be accountable for the work.
Conflicts of Interest: None.
Name: Jeremy Walston, MD, PhD.
Contribution: This author helped design the study, revise the manuscript for critical intellectual content, approve the final version, and agree to be accountable for the work.
Conflicts of Interest: None.
Name: Rani Hasan, MD, MHS.
Contribution: This author helped design the study, revise the manuscript for critical intellectual content, approve the final version, and agree to be accountable for the work.
Conflicts of Interest: None.
Name: Kenton Zehr, MD.
Contribution: This author helped design the study, revise the manuscript for critical intellectual content, approve the final version, and agree to be accountable for the work.
Conflicts of Interest: None.
Name: Kaushik Mandal, MD.
Contribution: This author helped design the study, revise the manuscript for critical intellectual content, approve the final version, and agree to be accountable for the work.
Conflicts of Interest: None.
Name: Andrew LaFlam, BS.
Contribution: This author helped acquire data, design the study, revise the manuscript for critical intellectual content, approve the final version, and agree to be accountable for the work.
Conflicts of Interest: None.
Name: Karin J. Neufeld, MD, MPH.
Contribution: This author helped design the study, revise the manuscript for critical intellectual content, approve the final version, and agree to be accountable for the work.
Conflicts of Interest: K. J. Neufeld has received grant funding from Hitachi Inc and Ornim Inc.
Name: Vidyulata Kamath, PhD.
Contribution: This author helped design the study, revise the manuscript for critical intellectual content, approve the final version, and agree to be accountable for the work.
Conflicts of Interest: None.
Name: Charles W. Hogue, MD.
Contribution: This author helped design the study, revise the manuscript for critical intellectual content, approve the final version, and agree to be accountable for the work.
Conflicts of Interest: C. W. Hogue is a consultant and provides lectures for Medtronic/Covidien, Inc (Boulder, CO). He is a consultant to Merck, Inc (Kenilworth, NJ).
Name: Charles H. Brown IV, MD, MHS.
Contribution: This author helped design the study, draft and revise the manuscript for critical intellectual content, approve the final version, and agree to be accountable for the work.
Conflicts of Interest: C. H. Brown has consulted for and received grant funding from Medtronic
This manuscript was handled by: Robert Whittington, MD.
Footnotes
Conflicts of Interest: See Disclosures at the end of the article.
Clinical trial registration numbers: NCT00981474 and NCT02587039.
Reprints will not be available from the authors.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (www.anesthesia-analgesia.org).
REFERENCES
- 1.Walston J, Hadley EC, Ferrucci L, et al. Research agenda for frailty in older adults: toward a better understanding of physiology and etiology: summary from the American Geriatrics Society/National Institute on aging research conference on frailty in older adults. J Am Geriatr Soc. 2006;54:991–1001. [DOI] [PubMed] [Google Scholar]
- 2.Fried LP, Tangen CM, Walston J, et al. ; Cardiovascular Health Study Collaborative Research Group. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. [DOI] [PubMed] [Google Scholar]
- 3.Afilalo J, Mottillo S, Eisenberg MJ, et al. Addition of frailty and disability to cardiac surgery risk scores identifies elderly patients at high risk of mortality or major morbidity. Circ Cardiovasc Qual Outcomes. 2012;5:222–228. [DOI] [PubMed] [Google Scholar]
- 4.Afilalo J, Lauck S, Kim DH, et al. Frailty in older adults undergoing aortic valve replacement: the FRAILTY-AVR study. J Am Coll Cardiol. 2017;70:689–700. [DOI] [PubMed] [Google Scholar]
- 5.Sepehri A, Beggs T, Hassan A, et al. The impact of frailty on outcomes after cardiac surgery: a systematic review. J Thorac Cardiovasc Surg. 2014;148:3110–3117. [DOI] [PubMed] [Google Scholar]
- 6.Brown CH IV, Max L, LaFlam A, et al. The association between preoperative frailty and postoperative delirium after cardiac surgery. Anesth Analg. 2016;123:430–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jung P, Pereira MA, Hiebert B, et al. The impact of frailty on postoperative delirium in cardiac surgery patients. J Thorac Cardiovasc Surg. 2015;149:869.e1–875.e1. [DOI] [PubMed] [Google Scholar]
- 8.Saczynski JS, Marcantonio ER, Quach L, et al. Cognitive trajectories after postoperative delirium. N Engl J Med. 2012;367:30–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Inouye SK, Marcantonio ER, Kosar CM, et al. The short-term and long-term relationship between delirium and cognitive trajectory in older surgical patients. Alzheimers Dement. 2016;12:766–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brown CH IV, Probert J, Healy R, et al. Cognitive decline after delirium in patients undergoing cardiac surgery. Anesthesiology. 2018;129:406–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McKhann GM, Grega MA, Borowicz LM Jr, et al. Encephalopathy and stroke after coronary artery bypass grafting: incidence, consequences, and prediction. Arch Neurol. 2002;59:1422–1428. [DOI] [PubMed] [Google Scholar]
- 12.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. [DOI] [PubMed] [Google Scholar]
- 13.Orme JG, Reis J, Herz EJ. Factorial and discriminant validity of the Center for Epidemiological Studies Depression (CES-D) scale. J Clin Psychol. 1986;42:28–33. [DOI] [PubMed] [Google Scholar]
- 14.Taylor HL, Jacobs DR Jr, Schucker B, Knudsen J, Leon AS, Debacker G. A questionnaire for the assessment of leisure time physical activities. J Chronic Dis. 1978;31:741–755. [DOI] [PubMed] [Google Scholar]
- 15.Inouye SK, van Dyck CH, Alessi CA, Balkin S, Siegal AP, Horwitz RI. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113:941–948. [DOI] [PubMed] [Google Scholar]
- 16.Ely EW, Margolin R, Francis J, et al. Evaluation of delirium in critically ill patients: validation of the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU). Crit Care Med. 2001;29:1370–1379. [DOI] [PubMed] [Google Scholar]
- 17.Inouye SK, Leo-Summers L, Zhang Y, Bogardus ST Jr, Leslie DL, Agostini JV. A chart-based method for identification of delirium: validation compared with interviewer ratings using the confusion assessment method. J Am Geriatr Soc. 2005;53:312–318. [DOI] [PubMed] [Google Scholar]
- 18.Trzepacz PT, Mittal D, Torres R, Kanary K, Norton J, Jimerson N. Validation of the Delirium Rating Scale-revised-98: comparison with the delirium rating scale and the cognitive test for delirium. J Neuropsychiatry Clin Neurosci. 2001;13:229–242. [DOI] [PubMed] [Google Scholar]
- 19.Robinson TN, Raeburn CD, Tran ZV, Angles EM, Brenner LA, Moss M. Postoperative delirium in the elderly: risk factors and outcomes. Ann Surg. 2009;249:173–178. [DOI] [PubMed] [Google Scholar]
- 20.van Dijk D, Keizer AM, Diephuis JC, Durand C, Vos LJ, Hijman R. Neurocognitive dysfunction after coronary artery bypass surgery: a systematic review. J Thorac Cardiovasc Surg. 2000;120:632–639. [DOI] [PubMed] [Google Scholar]
- 21.Stump DA. Selection and clinical significance of neuropsychologic tests. Ann Thorac Surg. 1995;59:1340–1344. [DOI] [PubMed] [Google Scholar]
- 22.Powell JB, Cripe LI, Dodrill CB. Assessment of brain impairment with the Rey Auditory Verbal Learning Test: a comparison with other neuropsychological measures. Arch Clin Neuropsychol. 1991;6:241–249. [PubMed] [Google Scholar]
- 23.Meyers JE, Meyers KR. Rey complex figure test under four different administration procedures. Clin Neuropsychol 1995;9:63–67. [Google Scholar]
- 24.Lezak M Neuropsychological Assessment. New York, NY: Oxford University Press; 1983. [Google Scholar]
- 25.Spreen O, Strauss E. A Compendium of Neuropsychological Tests: Administration, Norms, and Commentary. New York, NY: Oxford University Press; 1998. [Google Scholar]
- 26.Reitan R, Wolfson D. The Halstead-Reitan Neuropsychological Test Battery. Tucson, AZ: Neuropsychology Press; 1985. [Google Scholar]
- 27.Costa LD, Vaughan HG Jr, Levita E, Farber N. Purdue Pegboard as a predictor of the presence and laterality of cerebral lesions. J Consult Psychol. 1963;27:133–137. [DOI] [PubMed] [Google Scholar]
- 28.Brown CH IV, Morrissey C, Ono M, et al. Impaired olfaction and risk of delirium or cognitive decline after cardiac surgery. J Am Geriatr Soc. 2015;63:16–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Selnes OA, Grega MA, Borowicz LM Jr, Royall RM, McKhann GM, Baumgartner WA. Cognitive changes with coronary artery disease: a prospective study of coronary artery bypass graft patients and nonsurgical controls. Ann Thorac Surg. 2003;75:1377–1384. [DOI] [PubMed] [Google Scholar]
- 30.Nadelson MR, Sanders RD, Avidan MS. Perioperative cognitive trajectory in adults. Br J Anaesth. 2014;112:440–451. [DOI] [PubMed] [Google Scholar]
- 31.Rudolph JL, Jones RN, Levkoff SE, et al. Derivation and validation of a preoperative prediction rule for delirium after cardiac surgery. Circulation. 2009;119:229–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arthur HM, Daniels C, McKelvie R, Hirsh J, Rush B. Effect of a preoperative intervention on preoperative and postoperative outcomes in low-risk patients awaiting elective coronary artery bypass graft surgery. A randomized, controlled trial. Ann Intern Med. 2000;133:253–262. [DOI] [PubMed] [Google Scholar]
- 33.Stammers AN, Kehler DS, Afilalo J, et al. Protocol for the PREHAB study-pre-operative rehabilitation for reduction of hospitalization after coronary bypass and valvular surgery: a randomised controlled trial. BMJ Open. 2015;5:e007250. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.