Abstract
Background
Understanding local epidemiology and etiologies of community-acquired pneumonia in hospitalized patients is crucial for determining the appropriateness of treatment guidelines. We aim to determine the etiologies, severity, and outcomes in adults hospitalized for community-acquired pneumonia and to study the impact of empirical antibiotic therapy on patient outcomes.
Methods
We performed a prospective observational cohort study involving adults hospitalized for community-acquired pneumonia in Hong Kong. Sputum, nasopharyngeal aspirate, blood, and urine were collected for bacterial culture, molecular tests for detection of viruses and atypical pathogens, and antigen tests. Multivariable logistic regression model and Cox proportional hazard models were performed to determine independent factors associated with prolonged hospitalization and mortality.
Results
From February 2017 to July 2018, 258 patients were enrolled. The median age was 73 (interquartile range, 61–80) years, 66% were male, 57% had underlying chronic illnesses, 13% had CURB-65 score ≥3, and 10% had higher 1-year mortality. Pathogens were identified in 45% of patients; 20% had viral, 15% had bacterial, and 9% had polymicrobial pneumonia. Streptococcus pneumoniae (12%), influenza virus (12%), and Mycoplasma pneumoniae (1.2%) were the most common bacterial, viral, and atypical pathogens, respectively. Nonadherence to local empirical antibiotic treatment guidelines (primarily recommending beta-lactam and doxycycline) was observed in 25% and was independently associated with prolonged hospitalization (≥7 days) and higher mortality, after adjustment for age, underlying chronic illness, and disease severity.
Conclusions
Adherence to treatment guidelines was associated with shorter hospitalization and improved survival. We provided evidence for the use of doxycycline for coverage of atypical pathogens in nonsevere pneumonia.
Keywords: community-acquired pneumonia, length of stay, mortality, treatment guidelines
In this prospective study involving adults hospitalized with community-acquired pneumonia, adherence to treatment guidelines was independently associated with shorter hospitalization and improved survival. Our findings provided evidence for atypical pathogen coverage with doxycycline, which was less evaluated in other cohorts.
Pneumonia and lower respiratory tract infections caused 2.6 million deaths in 2017 globally, and it is the fourth leading cause of death worldwide [1]. The annual incidence of community-acquired pneumonia (CAP) in the United States was 25 cases per 10 000 adults, with the incidence rising to 63 and 164 per 10 000 population in those older than 65 and 80 years, respectively [2].
The epidemiology, aetiologies, and antibiotic resistance patterns of pathogens causing CAP may differ in Asia from other regions of the world [3]. Local surveillance data on the aetiologies and susceptibility patterns of the most common pathogens causing CAP is crucial in formulating treatment guidelines in guiding empirical antibiotics choices. This is particularly important for management of CAP, because in clinical practice, no pathogens can be identified for the majority of patients, precluding pathogen-guided therapy [4]. Application of newer diagnostic tools that are currently widely available in research and clinical settings allow a better understanding of contemporary causes of CAP in Asia.
In Hong Kong, a new bundle of treatment guidelines for the management of various common infectious diseases was formulated and published in 2017 [5]. It would be important to determine the latest epidemiology of CAP, clinicians’ adherence to these guidelines, and the impact of guideline adherence on patients’ outcomes. Therefore, we aim to determine the aetiologies, severity, and immediate and long-term outcomes in adults hospitalized for CAP and to study the impact of empirical antibiotic therapy on patient outcomes.
MATERIALS AND METHODS
Study Design
We performed a prospective, observational study involving adult patients admitted for CAP to an acute care hospital in Hong Kong. Disease severity, antibiotic treatment, and outcomes were documented. Sputum, nasopharyngeal aspirate (NPA), and urine were collected within 72 hours from admission for microbiological studies. The study was approved by the Joint Chinese University of Hong Kong–New Territories East Cluster Clinical Research Ethics Committee.
Subjects
All adult patients with an admission diagnosis of CAP during the period from February 2017 to July 2018 were screened for eligibility. Inclusion criteria included (1) age ≥18 years, (2) presence of respiratory symptoms with or without fever, and (3) evidence consistent with pneumonia on chest radiograph. Patients were excluded if they had one of the following: (1) hospital-acquired pneumonia (occurring ≥48 hours after admission; (2) recent hospitalization in the previous 28 or 90 days for immunocompetent and immunocompromised patients, respectively; (3) resided in nursing home and dependent in activities of daily living; (4) conditions predisposing to noncommunity-acquired pathogens (including tracheostomy, percutaneous gastrostomy tube, cancer with neutropenia, solid or hematopoietic stem cell transplant within the previous 90 days, or human immunodeficiency virus infection with CD4 <200 cells/mm3); or (5) clear alternative diagnosis. These criteria were reported to be associated with drug-resistant pathogens for pneumonia [2, 4]. Informed consent was obtained from each patient or next of kin.
Study Procedures
Demographic and clinical features were documented. The CURB-65 score was determined, with 1 point for each of confusion, urea >7 mmol/L, respiratory rate >30/minute, low systolic (<90 mmHg) or diastolic (<60 mmHg) blood pressure, and age >65 years [6]. Empirical antibiotics prescribed on the day of admission and use of corticosteroid (for exacerbation of chronic obstructive pulmonary disease or asthma) were documented. Clinical outcomes, including the 30-day, 60-day, and 1-year all-cause mortality and duration of hospitalization, were captured from an electronic platform that records all death and hospitalization information in all public hospitals in Hong Kong.
Microbiologic investigations were performed as described with modifications [7]. Blood culture (BacT/Alert; bioMerieux, Marcy l’Etoile, France) was obtained and expectorated sputum showing >25 white blood cells on high-power field with heavy/predominant bacterial growth was included in the analysis. Acid-fast smear with culture of Mycobacterium tuberculosis was performed if pulmonary tuberculosis was suspected. In-house multiplex real-time polymerase chain reactions (PCRs) were performed on nasopharyngeal aspirate for the detection of respiratory viruses (influenza A, B, parainfluenza 1, 2 and 3, respiratory syncytial virus, adenovirus, human metapneumovirus, and enterovirus/rhinovirus), Mycoplasma pneumoniae, and Chlamydophila pneumoniae using primers and conditions as described [8, 9]. Nasopharyngeal aspirate samples positive for enterovirus/rhinovirus were further genotyped using nested PCR of VP4/2 region (nucleotides 616–1004 numbered according to GenBank accession no. EF582385 for RV-C4) followed by Sanger sequencing. Genotype was determined by similarity >89.5%, 90.0%, and 89.5% in RV-A, -B and -C, respectively, with the alignment length of ≥355 base pairs, which covered ≥90% of the prototype [10]. Urine samples were tested for Streptococcus pneumoniae and Legionella pneumophila serogroup 1 using urinary antigen tests (Alere BinaxNOW; Abbott, Chicago, IL).
Definitions
Bacterial pneumonia was defined as isolation of clinically significant pathogen from sputum or blood bacterial cultures, positive urine streptococcal and legionella antigen tests, or positive Mycoplasma or Chlamydophila PCR tests. Viral pneumonia was defined as positive respiratory viruses detected via PCR. Polymicrobial pneumonia was defined as the presence of 2 or more bacterial, viral, and/or mycobacterial pathogens. Severe pneumonia was defined as CURB-65 score ≥3 [6], and/or requirement of intensive care, or mechanical or noninvasive ventilation.
Adherent empirical antibiotics was defined as the choice of antibiotics in adherence to local recommendations [5]. In brief, amoxicillin-clavulanate, or ceftriaxone, with or without doxycycline, are the recommended regimens for CAP requiring hospitalization. For patients with bronchiectasis, piperacillin-tazobactam with a macrolide is recommended. For patients with severe pneumonia, ceftriaxone or piperacillin-tazobactam, with a macrolide, are recommended. Empirical fluoroquinolone is only recommended for patients with documented allergy to beta-lactam antibiotics.
Statistical Analysis
Continuous variables were presented as mean ± standard deviation, or median (interquartile range [IQR]), depending on data distribution. Comparisons of categorical variables between groups were performed using χ 2 or Fisher exact tests, as appropriate. Comparisons of continuous variables between groups were performed using Student’s t test, one-way analysis of variance, or Kruskal-Wallis test, as appropriate. Multivariable binary logistic regression and Cox proportional hazards models were used to determine independent variables associated with length of stay ≥7 days and 1-year mortality, respectively. Variables with P < .05 observed in univariate analyses were included in these multivariable models. Assuming that 1-year mortality is 13%, and approximately 84% of patients would receive adherent empirical antibiotics, which would result in 20% reduction in mortality, we estimated a sample size of 292 would be required to achieve a statistical power of 80%, at a Type I error of 5% (PS: Power and Sample Size calculation software, version 3.0) [11–13].
RESULTS
Baseline Characteristics, Disease Severity, and Microbiological Aetiologies
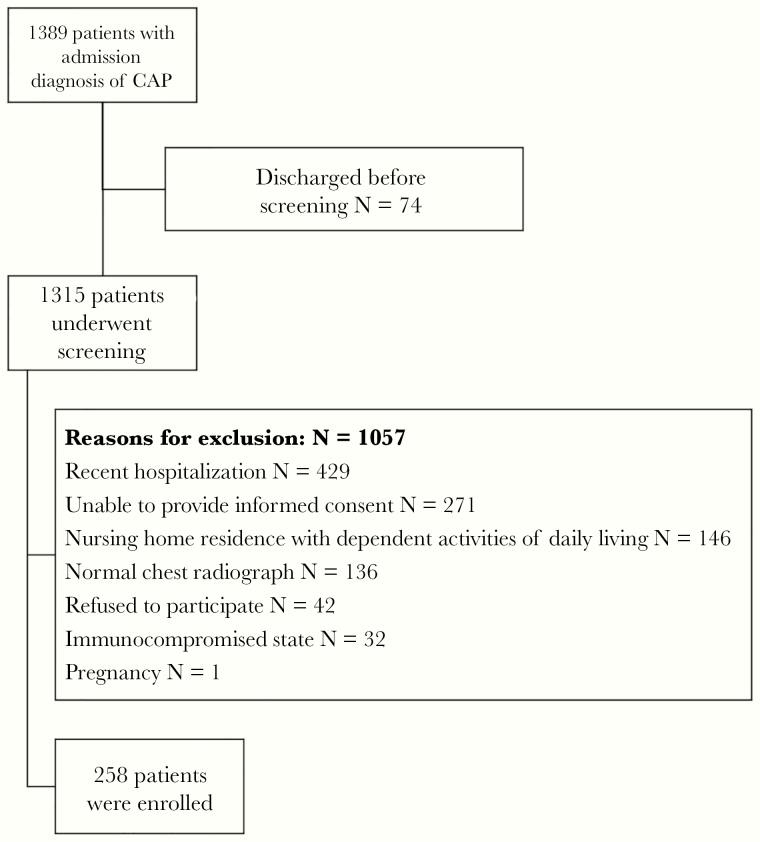
From February 2017 to July 2018, 1315 patients were screened for eligibility, and 258 patients were enrolled (Figure 1). Baseline clinical characteristics and microbiological aetiologies were presented in Table 1. One or more pathogens were identified in 116 (45%) patients (Table 1, Supplementary Figure 1). Fifty-two patients (20%) had viral pneumonia (most commonly influenza viruses [12%] and rhinovirus [7%]), 38 (15%) had bacterial pneumonia (S pneumoniae [12%], Hemophilus influenzae [7%], and M pneumoniae [1%], and 23 (9%) had polymicrobial pneumonia. Among those with a viral pathogen identified (N = 73), 20 (27%) had a bacterial copathogen (S pneumoniae 13, H influenzae 6, and Legionella 1). Bacterial pathogens in 93% of patients with bacterial pneumonia (excluding atypical pathogens) were susceptible to amoxicillin-clavulanate.
Figure 1.
Screening and enrollment of patients hospitalized with community-acquired pneumonia (CAP).
Table 1.
Baseline Demographic, Clinical Characteristics, Disease Severity, Treatment and Microbiological Aetiologies
| Age Group (years) | |||||
|---|---|---|---|---|---|
| Variables | Whole Cohort | 18–49 | 50–74 | ≥75 | P |
| Number | N = 258 | N = 30 | N = 115 | N = 113 | |
| Age, years | 73 (61–80) | 37 (31–43) | 66 (60–70) | 81 (78–85) | <.001 |
| Male | 169 (65.5%) | 12 (40.0%) | 73 (63.5%) | 84 (74.3%) | .002 |
| Nursing home resident | 4 (1.6%) | 0 (0%) | 2 (1.7%) | 2 (1.8%) | .765 |
| Chronic illness, any | 147 (57.0%) | 3 (10.0%) | 68 (59.1%) | 76 (67.3%) | <.001 |
| Respiratory disease | 54 (20.9%) | 1 (3.3%) | 23 (20.0%) | 30 (26.5%) | .02 |
| Diabetes | 53 (20.5%) | 1 (3.3%) | 23 (20.0%) | 29 (25.7%) | .026 |
| Coronary artery disease | 35 (13.6%) | 0 (0%) | 13 (11.3%) | 22 (19.5%) | .014 |
| Stroke | 24 (9.3%) | 1 (3.3%) | 8 (7.0%) | 15 (13.3%) | .127 |
| Malignancies | 21 (8.1%) | 1 (3.3%) | 14 (12.2%) | 6 (5.3%) | .098 |
| Congestive heart failure | 18 (7.0%) | 0 (0%) | 2 (1.7%) | 16 (14.2%) | <.001 |
| Chronic kidney disease | 14 (5.4%) | 0 (0%) | 4 (3.5%) | 10 (8.8%) | .076 |
| Pneumococcal vaccine | 87 (34.8%) | 0 (0%) | 30 (26.8%) | 57 (52.3%) | <.001 |
| Influenza vaccine | 114 (44.7%) | 1 (3.4%) | 41 (36.0%) | 72 (64.3%) | <.001 |
| Antibiotics before admission | 37 (16.4%) | 7 (25.9%) | 19 (18.3%) | 11 (11.6%) | .16 |
| Symptom duration, days | 1.1 ± 1.2 | 1.1 ± 0.3 | 1.1 ± 0.2 | 1.2 ± 1.8 | .528 |
| CURB-65 score | |||||
| CURB-65 = 0–1 | 166 (64.3%) | 30 (100%) | 85 (73.9%) | 51 (45.1%) | <.001 |
| CURB-65 = 2 | 59 (22.9%) | 0 (0%) | 16 (13.9%) | 43 (38.1%) | |
| CURB65 ≥3 | 33 (12.8%) | 0 (0%) | 14 (12.2%) | 19 (16.8%) | |
| Supplementary oxygen | 150 (58.1%) | 7 (23.3%) | 67 (58.3%) | 76 (67.3%) | <.001 |
| Noninvasive ventilation | 14 (5.4%) | 0 (0%) | 5 (4.3%) | 9 (8.0%) | .183 |
| Mechanical ventilation | 2 (0.8%) | 0 (0%) | 1 (0.9%) | 1 (0.9%) | .876 |
| Intensive care | 4 (1.6%) | 0 (0%) | 3 (2.6%) | 1 (0.9%) | .440 |
| Use of corticosteroid | 50 (19.4%) | 2 (6.7%) | 18 (15.7%) | 30 (26.5%) | .020 |
| Empirical antibiotics | |||||
| Amoxicillin-clavulanate ± doxycycline | 204 (79.1%) | 26 (86.7%) | 85 (73.9%) | 93 (82.3%) | .165 |
| Cephalosporin ± doxycycline | 21 (8.1%) | 1 (3.3%) | 10 (8.7%) | 10 (8.8%) | .591 |
| Amoxicillin-clavulanate or cephalosporin with macrolide | 8 (3.1%) | 2 (6.7%) | 3 (2.6%) | 3 (2.7%) | .487 |
| Piperacillin-tazobactam or carbapenem ± doxycycline or macrolide | 15 (5.8%) | 0 (0%) | 13 (11.3%) | 2 (1.8%) | .003 |
| Fluoroquinolone | 7 (2.7%) | 0 (0%) | 4 (3.5%) | 3 (2.7%) | .579 |
| Empirical coverage for atypical pathogensa | 141 (54.7%) | 24 (80.0%) | 65 (56.5%) | 52 (46.0%) | .003 |
| Nonadherent empirical antibiotics | 64 (24.8%) | 4 (13.3%) | 30 (26.1%) | 30 (26.5%) | .301 |
| Type of pneumoniab | |||||
| Bacterial | 38 (14.7%) | 9 (30.0%) | 16 (13.9%) | 13 (11.5%) | .037 |
| Viral | 52 (20.2%) | 4 (13.3%) | 28 (24.3%) | 20 (17.7%) | .280 |
| Mycobacterial | 3 (1.2%) | 0 (0%) | 3 (2.6%) | 0 (0%) | .152 |
| Polymicrobialc | 23 (8.9%) | 4 (13.3%) | 12 (10.4%) | 7 (6.2%) | .354 |
| No aetiology identified | 142 (55.0%) | 13 (43.3%) | 56 (48.7%) | 73 (64.6%) | .021 |
| Pathogens | |||||
| Streptococcus pneumoniaed | 31 (12.0%) | 6 (20.0%) | 13 (11.3%) | 12 (10.6%) | .355 |
| Hemophilus influenzae | 17 (6.6%) | 2 (6.7%) | 11 (9.6%) | 4 (3.5%) | .186 |
| Staphylococcus aureus | 3 (1.2%) | 1 (3.3%) | 2 (1.7%) | 0 (0%) | .236 |
| Other Gram-negative bacteria | 5 (1.9%) | 1 (3.3%) | 1 (0.9%) | 3 (2.7%) | .188 |
| Mycoplasma pneumoniae | 3 (1.2%) | 2 (6.7%) | 0 (0%) | 1 (0.9%) | .009 |
| Legionella pneumophila | 2 (0.8%) | 0 (0%) | 2 (1.7%) | 0 (0%) | .286 |
| Influenza virus | 32 (12.4%) | 4 (13.3%) | 18 (15.7%) | 10 (8.8%) | .293 |
| Rhinoviruse | 19 (7.4%) | 2 (6.7%) | 13 (11.3%) | 4 (3.5%) | .080 |
| Parainfluenza virus | 10 (3.9%) | 0 (0%) | 4 (3.5%) | 6 (5.3%) | .390 |
| Respiratory syncytial virus | 7 (2.7%) | 0 (0%) | 3 (2.6%) | 4 (3.5%) | .567 |
| Human metapneumovirus | 3 (1.2%) | 1 (3.3%) | 1 (0.9%) | 1 (0.9%) | .499 |
| Adenovirus | 3 (1.2%) | 1 (3.3%) | 0 (0%) | 2 (1.8%) | .229 |
| Mycobacterium tuberculosis | 5 (1.9%) | 1 (3.3%) | 4 (3.5%) | 0 (0%) | .137 |
aDoxycycline (n = 124, 48.1%), macrolide (n = 10, 3.9%), or fluoroquinolone (n = 7, 2.7%).
bExpectorated sputum and blood samples were obtained for bacterial culture in 181 (70.2%) and 173 (67.1%) of patients, respectively. All patients had nasopharyngeal aspirates and urine samples obtained for microbiological testing as described above. The diagnostic yield among patients with the respective specimens submitted for testing were as follows: 19.9% (36 of 181) for sputum bacterial culture, 1.8% (3 of 173) for blood culture, 28.3% (73 of 258) for respiratory virus polymerase chain reaction (PCR), 1.2% (3 of 258) for Mycoplasma pneumoniae PCR, 0% (0 of 258) for Chlamydophila pneumoniae, 8.5% (22 of 258) for urinary Streptococcus pneumoniae antigen, and 0.8% (2 of 258) for Legionella pneumophila antigen.
cEight patients had rhinovirus and S pneumoniae or Hemophilus influenzae, 7 had influenza and S pneumoniae or H influenzae or Legionella, 3 had parainfluenza and S pneumoniae or H influenzae, 2 had respiratory syncytial virus and S pneumoniae, 1 had influenza and mycobacterial, 1 had S pneumoniae and mycobacterial, and 1 had 2 bacterial pathogens identified.
d Streptococcus pneumoniae was detected solely by a positive urinary antigen test in 54.8% and solely by positive bacterial culture in sputum samples in 29.0%.
eEight rhinovirus A, 1 rhinovirus B, and 10 rhinovirus C were detected in the whole cohort.
The aetiologic type of pneumonia varied by age group (Table 1). Younger patients had the highest incidence of bacterial pneumonia, predominantly due to higher incidence of M pneumoniae and S pneumoniae. Patients 75 years or older were less likely to have a pathogen identified. The different types of pneumonia were not associated with disease severity.
Empirical Antibiotic Treatment
The majority of patients were prescribed empirical amoxicillin-clavulanate (79%) or ceftriaxone (8%) with or without doxycycline. Empirical coverage for atypical pathogens was more common in younger age groups (Table 1).
Nonadherent empirical antibiotic treatment was observed in 64 (25%) patients. Forty-four patients with severe pneumonia/bronchiectasis were prescribed amoxicillin-clavulanate or ceftriaxone with or without doxycycline, or piperacillin-tazobactam monotherapy without macrolide. Among the 20 patients with nonsevere pneumonia given nonadherent antibiotics, 7 received piperacillin-tazobactam or meropenem, 8 received amoxicillin-clavulanate/ceftriaxone, with a macrolide, 2 received fluoroquinolone in the absence of documented allergy to beta-lactam antibiotics, and 3 were not prescribed any antibiotics on the first day of admission.
Prescription of nonadherent empirical antibiotics was more common in patients with underlying chronic illnesses (70% vs 53%, P = .013) and more severe disease, including CURB-65 ≥3 (44% vs 3%, P < .001), use of supplementary oxygen (78% vs 52%, P < .001), and requirement of intensive care or noninvasive ventilation (17% vs 4%, P = .001). Nonadherent empirical antibiotics was associated with longer duration of hospitalization (9 [IQR, 7–14] vs 5 (3–10) days, P < .001) and higher 1-year mortality (19% vs 7%, P = .005).
Short- and Long-Term Outcomes
The median (IQR) length of stay in hospital was 7 (3–13) days. Length of stay ≥7 days was associated with older age groups, male sex, underlying chronic illnesses, absence of pathogen identified, more severe disease, as well as nonadherent empirical antibiotics and use of corticosteroid (Supplementary Table 1). After adjustment for age, underlying chronic illness, and disease severity, length of stay ≥7 days was independently associated with nonadherent empirical antibiotics (adjusted odds ratio, 2.27; 95% confidence interval, 1.02–5.06) and the absence of pathogen identified (adjusted odds ratio, 2.11; 95% confidence interval, 1.17–3.79) (Supplementary Table 1).
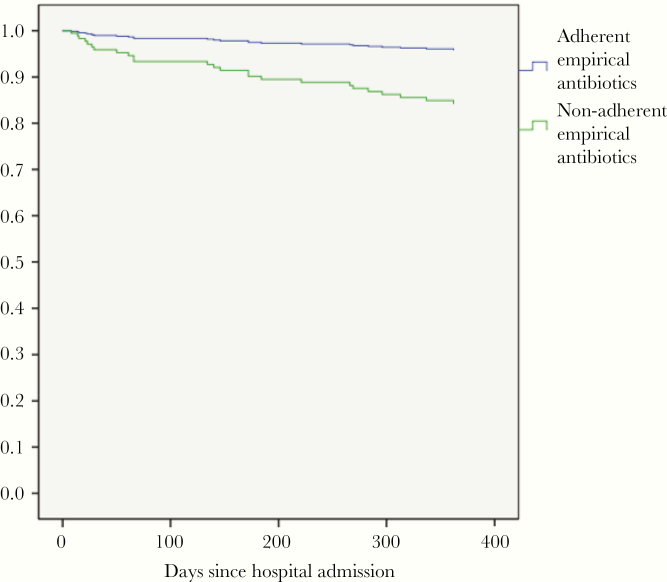
Thirty-day, 60-day, and 1-year mortality were 3%, 3%, and 10%, respectively. Mortality at 1 year was associated with older age, underlying chronic illness, more severe disease, as well as nonadherent empirical antibiotics (Table 2). After adjustment for age, underlying chronic illness, and disease severity, nonadherent empirical antibiotics was independently associated with 1-year mortality (adjusted hazard ratio, 3.88; 95% confidence interval, 1.60–9.41) (Table 2, Figure 2).
Table 2.
Variables Associated With Mortality at One Year
| Variables | Survived | Died | P | Adjusted Hazard Ratio (95% confidence interval) | P |
|---|---|---|---|---|---|
| Number | 233 | 25 | |||
| Age Group | |||||
| 18–49 years | 30 (12.9%) | 0 (0%) | .002 | ||
| 50–74 years | 109 (46.8%) | 6 (24.0%) | |||
| ≥75 years | 94 (40.3%) | 19 (76.0%) | 5.09 (1.98–13.09) | .001 | |
| Male | 151 (64.8%) | 18 (72.0%) | .472 | ||
| Nursing home resident | 3 (1.3%) | 1 (4.0%) | .336 | ||
| Underlying chronic illness | 128 (54.9%) | 19 (76.0%) | .043 | ||
| Pneumococcal vaccine | 76 (33.6%) | 11 (45.8%) | .233 | ||
| Influenza vaccine | 100 (43.3%) | 14 (58.3%) | .158 | ||
| Symptom duration, days | 1.0 ± 0.2 | 1.8 ± 3.8 | .323 | ||
| Antibiotics before admission | 36 (17.5%) | 1 (5.0%) | .211 | ||
| Type of Pneumonia | |||||
| Bacterial | 33 (14.2%) | 5 (20.0%) | .386 | ||
| Viral | 50 (21.5%) | 2 (8.0%) | .111 | ||
| Mycobacterial | 3 (1.3%) | 0 (0%) | 1.000 | ||
| Polymicrobial | 21 (9.0%) | 2 (8.0%) | 1.000 | ||
| No etiology | 126 (54.1%) | 16 (64.0%) | .343 | ||
| CURB65 ≥3 | 29 (12.4%) | 4 (16.0%) | .539 | ||
| Supplementary oxygen | 129 (55.4%) | 21 (84.0%) | .006 | ||
| Noninvasive ventilation, or intensive care | 12 (5.2%) | 6 (24.0%) | .004 | 5.52 (1.97–15.45) | .001 |
| Empirical coverage for atypical pathogens | 127 (54.5%) | 14 (56.0%) | .887 | ||
| Nonadherent empirical antibiotics | 52 (22.3%) | 12 (48.0%) | .005 | 3.88 (1.60–9.41) | .003 |
| Use of steroid | 43 (18.5%) | 7 (28.0%) | .286 |
Figure 2.
Kaplan-Meier curves showing survival of patients prescribed empirical antibiotics adherent and nonadherent to local treatment guidelines.
DISCUSSION
In this prospective cohort study, viral pneumonia was the most common cause of CAP. The most common bacterial and atypical pathogens were S pneumoniae, H influenzae, and M pneumoniae, supporting our current treatment guidelines, in which amoxicillin-clavulanate, with or without doxycycline, are recommended for hospitalized patients with pneumonia. Adherence to treatment guidelines was associated with better short- and long-term outcomes.
Respiratory viruses are increasingly recognized as important, or even predominant, causes of CAP in adult and elderly populations [4]. This is partly due to improved detection rates of a wide spectrum of respiratory viruses using molecular tests performed on upper respiratory tract samples [14]. In our cohort, influenza and rhinovirus are the predominant viral pathogens, similar to other studies of CAP [2]. In previous studies, researchers questioned the role of rhinoviruses in lower respiratory tract infections. More recent studies demonstrated a high rate of detection of rhinovirus from lower respiratory tract samples in patients with severe pneumonia, supporting its contributory role in the pathogenesis of CAP [15].
In our study and others, up to >20% patients with viral pneumonia had a bacterial copathogen [4]. This increases the difficulty in identifying patients not requiring antibacterial coverage in patients with confirmed viral pneumonia. Adopting more sensitive diagnostic tools for detection of bacterial pathogens, or using serum biomarkers such as procalcitonin, may guide optimal treatment decisions [4, 15].
Younger age groups had a higher proportion of bacterial pneumonia, largely due to higher detection rates of M pneumoniae and S pneumoniae. Prevalence of M pneumoniae was 2 to 12 times higher in younger versus older adult populations [16, 17]. According to our treatment guideline, coverage for atypical pathogens is optional for patients with nonsevere disease [5]. Such coverage should be strongly recommended for younger patients. On the other hand, a lower rate of pathogen detection was observed in elderly patients. In a recent study, dysbiosis of the normal flora in the respiratory tract, which allows overgrowth of 1 or more of the resident flora, was observed to be a cause of CAP. This may explain the high rates of culture-negativity in elderly patients with CAP [4, 14].
The findings of our study supported our current treatment guidelines [5]. First, 93% of bacterial pathogens were susceptible to amoxicillin-clavulanate, and M pneumoniae was the most common atypical pathogen, supporting the recommendation of amoxicillin-clavulanate and doxycycline for hospitalized nonsevere CAP. Macrolide resistance secondary to A2063G mutation among M pneumoniae isolates was present in 47% to 80% of isolates in Hong Kong and China in recent studies [16], whereas no resistance to tetracyclines had yet been reported.
Second, adherence to treatment guidelines was associated with better short- and long-term outcomes. This finding is reassuring, because no pathogens were identified in more than 50% of patients hospitalized with CAP in most studies using contemporary methods of pathogen identification [2, 4]. In the absence of pathogen-guided therapy, good clinical outcomes would heavily rely on appropriate guideline-based recommendation in antibiotic treatment. In both the United States and Europe, adherence to local guidelines for patients hospitalized with CAP was associated with decreased time to clinical stability, shorter duration of hospitalization, lower in-hospital mortality, lower costs, and improved quality of life compared with over- and undertreatment [18–20]. We further demonstrated the benefit of guideline adherence in Asia, as well as impact on long-term outcomes. Possible mechanisms include the impact of antibiotics on the risk of recurrent infections due to resistant pathogens and level of persistent inflammation [11].
In our cohort, nonadherence to local guidelines involved both “overtreatment” (broad-spectrum antibiotics prescribed in the absence of relevant indications) and “undertreatment” (inadequate coverage for potential drug-resistant pathogens in patients with severe pneumonia or underlying bronchiectasis). In patients at low risk of infection due to drug-resistant pathogens, overtreatment with broad-spectrum antibiotics (with antipseudomonal or methicillin-resistant Staphylococcus aureus coverage) was also associated with higher mortality in other studies [4, 12, 13].
On the other hand, undertreatment was observed in patients with severe pneumonia or bronchiectasis who were prescribed beta-lactam antibiotics in the absence of macrolide combination therapy. Because only 7% of bacterial pathogens were nonsusceptible to amoxicillin-clavulanate in our cohort, the lack of macrolide was possibly the major factor accounting for worse outcomes. Multiple studies have shown better outcomes in moderate-to-severe pneumonia with the combination of beta-lactam and macrolide, compared with beta-lactam monotherapy or combinations without a macrolide [4].
In our cohort, among those with empirical coverage for atypical pathogens, 88% were given doxycycline. The use of doxycycline as part of a combination therapy for CAP for coverage of atypical pathogens was less well evaluated, compared with macrolide or respiratory fluoroquinolones [4]. In a retrospective study in San Francisco, empirical ceftriaxone and doxycycline reduced in-patient and 30-day mortality in patients hospitalized for CAP, compared with other antibiotic regimens [21]. In another retrospective study in Australia, doxycycline was shown to have comparable effects on outcomes compared with macrolide, both of which were used in combination with beta-lactam therapy in patients hospitalized with CAP [22].
Doxycycline is recommended for coverage of certain atypical pathogens, such as macrolide-resistant M pneumoniae or Coxiella burnetii, and where macrolide or fluoroquinolone resistance in S pneumoniae [23, 24] or tuberculosis are prevalent [25]. Our results and others supported that doxycycline-based combination therapy is a feasible first-line recommended empirical therapy for nonsevere CAP in regions with high macrolide resistance in atypical pathogens. However, the role of doxycycline in severe pneumonia is uncertain and needs further evaluation.
There are some limitations of our study. The inability to obtain informed consent was one of the major reasons for exclusion in our screening process. Therefore, some of the critically ill patients would have been excluded from our analyses. We did not perform PCR testing for some atypical pathogens, including Legionella species and C burnetii; thus, we may have underestimated the proportions of patients with atypical pathogens. This study was conducted over an 18-month period, and it might not be representative of some pathogens, such as influenza, which varies in activity from year to year, and M pneumoniae, which typically causes epidemics every 3 to 7 years [16]. The relatively short duration did not allow us to evaluate seasonality of various pathogens in our locality, which might have a bearing on the choice of empirical antibiotic treatment when patients present at different times of the year. Because this was an observational study, we might not have been able to adjust for potential confounders that might have affected physicians’ choice of empirical antibiotics as well as mortality.
CONCLUSIONS
In conclusion, using molecular diagnostics and antigen tests in addition to traditional culture methods, pathogens were identified in 45% of patients hospitalized with CAP in Hong Kong. Viral pneumonia was most common. Influenza virus, S pneumoniae, and M pneumoniae were the most common viral, bacterial, and atypical pathogens, respectively, supporting our treatment guidelines. Almost all bacterial pathogens were susceptible to amoxicillin-clavulanate, supporting its role as empirical treatment. Adherence to treatment guidelines was associated with shorter duration of hospitalization and improved survival.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
We thank the Li Ka Shing Institute of Health Sciences for providing technical support in conducting this research.
Financial support. This work was funded by a research grant from the Health and Medical Research Fund Commissioned Research on Control of Infectious Diseases (CU-17-A16).
Potential conflicts of interest. M. I. has received funds for self-initiated projects or consultancy/advisory boards from Pfizer Corp., MSD, and GSK. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Roth GA, Abate D, Abate KH, et al. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018; 392:1736–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jain S, Self WH, Wunderink RG, et al. Community-acquired pneumonia requiring hospitalization among U.S. adults. N Engl J Med 2015; 373:415–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Song JH, Huh K, Chung DR. Community-acquired pneumonia in the Asia-Pacific region. Semin Respir Crit Care Med 2016; 37:839–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wunderink RG, Waterer G. Advances in the causes and management of community acquired pneumonia in adults. BMJ 2017; 358:j2471. [DOI] [PubMed] [Google Scholar]
- 5. Ho PL, Wu TC, Chao DVK, et al. Reducing Bacterial Resistance with IMPACT. 5th ed Hong Kong: Centre; for Health Protection, Hong Kong SAR; Available at: http://www.chp.gov.hk/files/pdf/reducing_bacterial_resistance_with_impact.pdf. Accessed 17 January 2020. [Google Scholar]
- 6. Lim WS, van der Eerden MM, Laing R, et al. Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax 2003; 58:377–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ip M, Rainer TH, Lee N, et al. Value of serum procalcitonin, neopterin, and C-reactive protein in differentiating bacterial from viral etiologies in patients presenting with lower respiratory tract infections. Diagn Microbiol Infect Dis 2007; 59:131–6. [DOI] [PubMed] [Google Scholar]
- 8. Winchell JM, Thurman KA, Mitchell SL, et al. Evaluation of three real-time PCR assays for detection of Mycoplasma pneumoniae in an outbreak investigation. J Clin Microbiol 2008; 46:3116–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mitchell SL, Budhiraja S, Thurman KA, et al. Evaluation of two real-time PCR chemistries for the detection of Chlamydophila pneumoniae in clinical specimens. Mol Cell Probes 2009; 23:309–11. [DOI] [PubMed] [Google Scholar]
- 10. McIntyre CL, Savolainen-Kopra C, Hovi T, Simmonds P. Recombination in the evolution of human rhinovirus genomes. Arch Virol 2013; 158:1497–515. [DOI] [PubMed] [Google Scholar]
- 11. Guertler C, Wirz B, Christ-Crain M, et al. Inflammatory responses predict long-term mortality risk in community-acquired pneumonia. Eur Respir J 2011; 37:1439–46. [DOI] [PubMed] [Google Scholar]
- 12. Shindo Y, Ito R, Kobayashi D, et al. Risk factors for drug-resistant pathogens in community-acquired and healthcare-associated pneumonia. Am J Respir Crit Care Med 2013; 188:985–95. [DOI] [PubMed] [Google Scholar]
- 13. Pereira JM, Gonçalves-Pereira J, Ribeiro O, et al. Impact of antibiotic therapy in severe community-acquired pneumonia: data from the Infauci study. J Crit Care 2018; 43:183–9. [DOI] [PubMed] [Google Scholar]
- 14. Karhu J, Ala-Kokko TI, Vuorinen T, et al. Lower respiratory tract virus findings in mechanically ventilated patients with severe community-acquired pneumonia. Clin Infect Dis 2014; 59:62–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Torres A, Lee N, Cilloniz C, et al. Laboratory diagnosis of pneumonia in the molecular age. Eur Respir J 2016; 48:1764–78. [DOI] [PubMed] [Google Scholar]
- 16. Ho PL, Law PY, Chan BW, et al. Emergence of macrolide-resistant mycoplasma pneumoniae in Hong Kong is linked to increasing macrolide resistance in multilocus variable-number tandem-repeat analysis type 4-5-7-2. J Clin Microbiol 2015; 53:3560–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kothe H, Bauer T, Marre R, et al. Outcome of community-acquired pneumonia: influence of age, residence status and antimicrobial treatment. Eur Respir J 2008; 32:139–46. [DOI] [PubMed] [Google Scholar]
- 18. Arnold FW, LaJoie AS, Brock GN, et al. Improving outcomes in elderly patients with community-acquired pneumonia by adhering to national guidelines: community-acquired pneumonia organization international cohort study results. Arch Intern Med 2009; 169:1515–24. [DOI] [PubMed] [Google Scholar]
- 19. Dambrava PG, Torres A, Vallès X, et al. Adherence to guidelines’ empirical antibiotic recommendations and community-acquired pneumonia outcome. Eur Respir J 2008; 32:892–901. [DOI] [PubMed] [Google Scholar]
- 20. Menéndez R, Reyes S, Martínez R, et al. Economic evaluation of adherence to treatment guidelines in nonintensive care pneumonia. Eur Respir J 2007; 29:751–6. [DOI] [PubMed] [Google Scholar]
- 21. Flanders SA, Dudas V, Kerr K, et al. Effectiveness of ceftriaxone plus doxycycline in the treatment of patients hospitalized with community-acquired pneumonia. J Hosp Med 2006; 1:7–12. [DOI] [PubMed] [Google Scholar]
- 22. Teh B, Grayson ML, Johnson PD, Charles PG. Doxycycline vs. macrolides in combination therapy for treatment of community-acquired pneumonia. Clin Microbiol Infect 2012; 18:E71–3. [DOI] [PubMed] [Google Scholar]
- 23. Charles PG, Whitby M, Fuller AJ, et al. The etiology of community-acquired pneumonia in Australia: why penicillin plus doxycycline or a macrolide is the most appropriate therapy. Clin Infect Dis 2008; 46:1513–21. [DOI] [PubMed] [Google Scholar]
- 24. Ho PL, Yung RW, Tsang DN, et al. Increasing resistance of Streptococcus pneumoniae to fluoroquinolones: results of a Hong Kong multicentre study in 2000. J Antimicrob Chemother 2001; 48:659–65. [DOI] [PubMed] [Google Scholar]
- 25. Lui G, Wong RY, Li F, et al. High mortality in adults hospitalized for active tuberculosis in a low HIV prevalence setting. PLoS One 2014; 9:e92077. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.