Abstract
Calciphylaxis is a rare and severe complication characterized by calcification of arterioles and capillaries in the dermis and subcutaneous adipose tissue that leads to ischemia, necrosis, and painful skin lesions in patients with end-stage renal disease (ESRD). It is also known as calcific uremic arteriolopathy. Calciphylaxis occurs most commonly with the ESRD with skin ulceration as a predominant presenting feature. Calcium-phosphorus dysregulation in dialysis patients are traditionally considered as a risk factor for the development of calciphylaxis. The involvement of an integrated interdisciplinary and multifaceted approach is key to the success of the calciphylaxis treatment. We present a case of a 51-year-old female with ESRD on home hemodialysis who developed calciphylaxis, which was successfully managed with increasing dialysis prescription, diligent wound care, and sodium thiosulfate infusion. Management of calciphylaxis in a patient receiving home hemodialysis has never been reported as per the review of the literature. Calciphylaxis is a sporadic disease, frequently encountered in the patients undergoing hemodialysis and carries a very grave prognosis. Current treatment is rarely effective, so preventive strategies play an important role by modifying the risk factors that promote the development of calciphylaxis.
Keywords: calciphylaxis, home hemodialysis, calcific uremic arteriolopathy, end-stage renal disease, skin ulcers, sodium thiosulfate
Introduction
Calciphylaxis is a rare and severe complication characterized by calcification of arterioles and capillaries in the dermis and subcutaneous adipose tissue that leads to ischemia, necrosis, and painful skin lesions in patients with end-stage renal disease (ESRD). It is also known as calcific uremic arteriolopathy. The term calciphylaxis is known to modern medicine for more than 50 years, initially described by Selye in 1961, based on his experience of promoting vascular calcification in rodents.1 Although what was described by Selye as “calciphylaxis” in rodents does not fit precisely that was observed in patients, “calcific uremic arteriolopathy” is a more accurately descriptive term. Calciphylaxis occurs most commonly with ESRD. It has also been described in patients with normal renal function, and chronic kidney disease, which is termed as non-uremic calciphylaxis.2 Nonetheless, due to the sparse incidence, poor understanding of pathogenesis, lack of standardized treatment guidelines, and the relentless clinical course makes the management of calciphylaxis very challenging. It is a lethal disease with high morbidity and mortality, with an estimated 6-month survival of approximately 50%.3 As per the US Renal Data System, the mortality of calciphylaxis patients on long-term hemodialysis is 3-fold higher than the patients without calciphylaxis.4 We present a case of calciphylaxis successfully managed in the patient who has ESRD on home hemodialysis.
Case Presentation
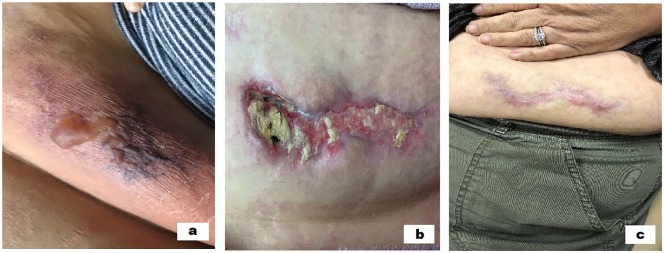
A 51-year-old female with the history of ESRD on home hemodialysis, antiphospholipid syndrome on warfarin, systemic lupus erythematosus, diabetes mellitus, hyperparathyroidism, and bowel obstruction followed by the surgical repair in the past admitted to the hospital with severe pain and hardness in the abdominal wall as shown in Figure 1a. The patient was initially diagnosed with abdominal wall hematomas and was advised to stop the warfarin. Even with warfarin on hold, the lesions increased in size, became more painful, and started to ulcerate as showed in Figure 1b. The patient has been on home hemodialysis for 2 years prior to the onset of symptoms and undergoes 5 times a week dialysis treatment. The medications include calcitriol 0.25 µg daily, ergocalciferol 50 000 IU weekly, omeprazole 40 mg daily, oxycodone-acetaminophen 10-325 mg every 6 hours as needed for pain, ferric citrate 210 mg 2 tablets with meals and 1 tablet with snack, pentoxifylline 400 mg daily, prednisone 5 mg daily, febuxostat 80 mg daily, hydroxychloroquine 200 mg daily, warfarin 2 mg daily, insulin glargine 25 units every day, and insulin sliding scale. The patient has been on warfarin for 10 years because of antiphospholipid syndrome.
Figure 1.
(a) The blistering of the abdominal wall. (b) Ulceration with purulence on the abdominal wall. (c) Completely healed ulcer on the abdominal wall at 4 months with treatment.
The patient was seen at home dialysis clinic 2 weeks after the hospital admission, and clinical diagnosis of calciphylaxis was made. The vital signs on presentation were temperature of 97°F, pulse rate of 96 beats per minute, respiratory rate of 18 breaths per minute, and blood pressure of 145/82 mm Hg. Patient was in distress from pain, abdominal examination revealing an eschar of 13 cm × 6 cm on right lower abdomen with purulent drainage and surrounding erythema as shown in Figure 1b. Firm subcutaneous lumps were felt bilaterally on the lower abdomen, which are tender to palpation. Rest of the examination was nonsignificant.
The laboratory data revealed white blood cell count 10.9 × 103 cells/µL, hemoglobin 10.6 g/dL, sodium 135 mEq/L, potassium 4.5 mEq/L, carbon dioxide 23 mEq/L, blood urea nitrogen 69 mg/dL, creatinine 7.7 mg/dL, parathyroid hormone 333 pg/mL, calcium 9 mg/dL, phosphorus 5.7 mg/dL, calcium phosphorus product elevated at 51.3, vitamin D level 16.4 ng/mL, and albumin level 3.1 g/dL. Patient was meeting the goal of dialysis adequacy. The laboratory data before, during, and after presentation are summarized in Table 1. Home hemodialysis prescription is summarized in Table 2.
Table 1.
Laboratory Data Before, During, and After presentation.
| Laboratory | 2 Months Before Presentation | 1 Month Before Presentation | On Presentation | 1 Month After Presentation | 2 Months After Presentation | 3 Months After Presentation | 4 Months After Presentation | 5 Months After Presentation | 6 Months After Presentation |
|---|---|---|---|---|---|---|---|---|---|
| Calcium, mg/dL | 8.7 | 8.7 | 8.3 | 8.5 | 8.8 | 9.6 | 9.7 | 9.8 | 9.9 |
| Phosphorus, mg/dL | 6.7 | 6.5 | 5.7 | 3.9 | 2.5 | 4.4 | 4.7 | 5.4 | 4.1 |
| Parathyroid hormone, pg/mL | 368 | 441 | 333 | 155 | 49 | 46 | 82 | 125 | 129 |
| Calcium-phosphorus product | 58.3 | 56.6 | 47.3 | 33.2 | 22 | 42.2 | 45.6 | 52.9 | 40.6 |
Table 2.
Dialysis Prescription.
| Treatment time: 3 hours, 5 times a week |
| Dialysis bath sodium 140 mEq/L, potassium 1 mEq/L, calcium 3 mEq/L, lactate 40 mEq/L |
| 20 L solution, flow fraction 35% |
| Blood flow rate (QB) 400 mL/min |
| Dialysate flow rate 200 mL/min |
The patient’s calcium supplements were stopped. Patient was started on sodium thiosulfate 3 times a week with home hemodialysis. The home dialysis frequency was increased to 6 times a week. Patient was referred to the dermatology, hematology, endocrine surgery, and wound care. The patient was resumed back on warfarin as the benefits of being anticoagulated outweighed the risks. Hematology agreed with plan to continue warfarin. Dermatology confirmed the diagnosis of calciphylaxis and deferred the biopsy because of extensive necrosis. The patient was managed meticulously with wound care with frequent dressing changes and periodic debridement. The course of wound healing was described in images with timeline (Figure 1a, b, and c). Endocrine surgery deferred parathyroidectomy as the parathyroid hormone was in acceptable range. The ulcers completely resolved in 4 months (Figure 1c), wound care was discontinued, sodium thiosulfate infusions were stopped, and the patient’s home dialysis frequency was decreased to 5 times a week.
Discussion
Calciphylaxis is known to modern medicine for a long time but remains a poorly understood entity due to its rarity. The most recent estimate places the incidence of calciphylaxis at 3.5 new cases/1000 patient-years among patients with ESRD on hemodialysis.5 It is possible that misdiagnosis or subclinical disease may be attributed to lower than the actual incidence.
Various case-controlled studies have proposed different risk factor associations for the calciphylaxis, but they are limited by sample size being small and experience from single center only. Nigwekar et al described 62 cases and identified risk factors for the development of calciphylaxis, such as hypercalcemia, calcitriol therapy, warfarin therapy, and hypoalbuminemia.6 Weenig et al identified increased calcium-phosphorus product, obesity, liver disease, increased albumin levels, and systemic corticosteroid use as risk factors in 49 patients.7 Fine and Zacharias described 36 patients who are predominantly on peritoneal dialysis (78%) and identified calcium binder therapy, high serum phosphorus, peritoneal dialysis, diabetes mellitus, and vitamin D analogs as risk factors for calciphylaxis.8 Hayashi et al studied 28 Japanese hemodialysis patients and identified that warfarin therapy, elevated calcium, and low serum albumin are associated with calciphylaxis.9 Mazhar et al studied 19 patients with calciphylaxis and majority were on hemodialysis and there was 8-fold independent increase in the death rate.10 Most of the patients in the above-mentioned studies were females and had a mean age of 54 to 59 years.6-10
Calciphylaxis is more common in Caucasians, female preponderance, and commonly reported in the fifth decade of life.6-10 Calcium-phosphorus dysregulation in dialysis patients is traditionally considered as a risk factor for the development of calciphylaxis but has been described in patients without any significant bone mineral abnormalities.11 Other comorbid conditions that are associated with calciphylaxis are diabetes, obesity, autoimmune conditions like lupus, rheumatoid arthritis, antiphospholipid antibody syndrome, hypercoagulable conditions, and liver disorders.12 Obesity is considered as a risk factor for proximal calciphylaxis in areas like trunk, thighs, and breasts.11 Patients who are on dialysis for more than 6 years are considered at risk of developing calciphylaxis.13 Active vitamin D analogs, calcium-based binders, calcium supplements, iron therapy, warfarin, methotrexate, corticosteroids, and ultraviolet light can potentially trigger the development of calciphylaxis.6-9,12,14
The skin lesions associated with calciphylaxis are quite variable in appearance.15 The firm calcified subcutaneous lesions, which are tender to touch in patients on dialysis, should raise the possibility of calciphylaxis.15,16 Clinically, patients have skin lesions that are severely painful with poor healing and ulcerations, which are complicated by superimposed infections.15,16 Black eschar is demonstrated in the ulcerated lesions.17 Patients with calciphylaxis also have systemic vascular calcifications, although skin condition is the predominating feature, and hence, it is regarded as a systematic disease process.18
Identification of risk factors, as well as the factors promoting the progression of lesion, should be addressed. Many clinical conditions like vasculitis, nephrogenic systemic fibrosis, atherosclerotic vascular disease, warfarin necrosis, and purpura fulminans can clinically mimic calciphylaxis.17 Careful elicitation of history, characteristic clinical findings, area of distribution, and biopsy findings will differentiate these conditions from calciphylaxis. Warfarin necrosis mainly occurs within the first 10 days after starting warfarin therapy due to the imbalance between anticoagulant and procoagulant pathways creating hypercoagulable state paradoxically.17 This condition improves after discontinuation of warfarin. Our patient was on long-term anticoagulation on warfarin, but the timeline did not fit into the diagnosis of warfarin necrosis.
Skin biopsy establishes the diagnosis of calciphylaxis and excludes the mimickers.17 The risks associated with skin biopsy like ulceration, bleeding, and infection should be taken into account when skin biopsy is considered.17 Skin biopsy by an experienced dermatologist or surgeon will maximize the yield.17 A punch biopsy is preferred and safer than incisional biopsy even though the yield is better with the latter.19 The biopsy in the necrotic areas and central part of an ulcer may provide dismal results.17
The initial event implicated in the pathogenesis of calciphylaxis is arteriolar calcification, followed by thrombosis and ischemia of skin.20,21 Calcification, microthrombi, and fibrointimal hyperplasia in subcutaneous arteries and arterioles is the histological hallmark leading to ischemia.22-24 The media of the small arteries and arterioles is involved by calcification, and intimal layer involvement has been described, thus leading to vascular endothelial injury and dysfunction.16 Alizarin red and von Kossa are the special stains used to detect microcalcification, and the yield is better when they are used together.24
The laboratory tests should evaluate the risk factors for calciphylaxis comprehensively as outlined in Table 3.17
Table 3.
Laboratory Tests to Evaluate the Risk Factors for Calciphylaxis.
| Serum calcium, phosphorus, intact parathyroid hormone, 25 hydroxy vitamin D, and alkaline phosphatase |
| Serum creatinine, blood urea nitrogen, serum sodium, potassium, magnesium, and urinalysis |
| Serum transaminases, alkaline phosphatase, and serum albumin |
| White blood count with differential, hemoglobin and hematocrit, and platelet count |
| Prothrombin time, international normalized ratio, and partial thromboplastin time |
| Antiphospholipid antibody, protein C deficiency, protein S deficiency, and antithrombin III deficiency panels |
| C-reactive protein and erythrocyte sedimentation rate |
| Complete malignancy and autoimmune disease workup |
No radiological tests or biomarkers have been comprehensively evaluated and clinically recommended, even though they may aid in the diagnosis of calciphylaxis.17
The involvement of an integrated interdisciplinary and multifaceted approach is key to the success of the calciphylaxis treatment.25 A comprehensive management plan should be formulated as soon as the diagnosis of calciphylaxis is suspected with the involvement of various specialties like nephrology, dermatology, pathology, pain management, endocrine surgery, general surgery, nutrition, and wound care. No randomized controlled trials have been published on the management of calciphylaxis, and the treatment recommendations are based on case reports, case series, and retrospective cohort studies. The key aspects of management are summarized in Table 4.
Table 4.
Key Aspects of Management.
| Calcium, phosphorus, and calcimimetic management |
| Meticulous wound care |
| Antibiotic therapy |
| Pain management |
| Avoidance of triggers |
| Nutritional management |
| Specific medical therapy |
The wound care management goal is to facilitate wound healing, keep wound bed free of necrotic tissue, and prevention of wound infection. An experienced burn center or wound care team involvement is critical for the wound healing process. Surgical management like wound debridement is very controversial and must be considered only on a case-by-case basis. Chemical debridement is preferable than surgical debridement when the wound is not infected, with limited tissue involvement and dry eschar.26,27 The aim is to remove the necrotic tissue without hampering with healthy healing tissue. In a retrospective analysis from the Mayo Clinic involving 63 patients with calciphylaxis, the 1-year survival rate was 61.6% in patients who underwent surgical debridement compared with 27.4% who did not. However, the disease severity was not matched among the patients.7 Distal calciphylaxis was managed with a combined approach of deep ulcer shaving and split-thickness skin transplantation.28 Hyperbaric oxygen therapy is recommended as second-line therapy for recalcitrant calciphylaxis wounds.29 Very few cases reports mentioned treatment with sterile maggot therapy.30 Antibiotic therapy should be instituted promptly when the features of infection are suspected.
The pain originates from the ischemia with a possible contribution from the neuropathic component and can be very challenging. Opioids are the key in the management of pain in calciphylaxis, and potential side effects like hypotension, and altered mental status from the accumulation of neurotoxic metabolites are encountered in patients with renal insufficiency, so close monitoring is warranted.31 The involvement of the pain management team is an effective strategy in addressing this issue.
We need to maintain calcium and phosphorus within the normal reference range along with the parathyroid hormone level between 150 to 300 ng/mL. The patients should avoid calcium-based supplements, calcium-based binders, as well as high-calcium dialysis bath.32 Cinacalcet should be used in the management of secondary hyperparathyroidism compared with vitamin D analogs.33 There is a reduced risk of calciphylaxis in cinacalcet arm compared with placebo in the Evaluation of Cinacalcet Hydrochloride Therapy to Lower Cardiovascular Events (EVOLVE) trial, which includes 3883 dialysis patients, but the event occurrence was low in the study.34 Cinacalcet minimizes the risk of adynamic bone disease, hungry bone syndrome, and accompanying surgical wound infection compared with parathyroidectomy.35
Warfarin therapy, iron, and trauma from insulin injections are linked to the development of calciphylaxis in epidemiological data.17 The risk-benefit impact of continuing warfarin has to be taken into account, depending on the indication of using warfarin, and the safety of alternative anticoagulants is not established in patients with renal impairment. Other approaches would be switching the immunosuppressive medications that do not interfere with wound healing and rotation of the injection sites from subcutaneous insulin or heparin to minimize trauma.17
The optimization of dialysis prescription plays a critical role in the management of calciphylaxis. Approaches like increasing dialysis duration and frequency have been described, but there is no conclusive data available to support this approach.36 Peritoneal dialysis has been attributed to the development of calciphylaxis compared with hemodialysis in the literature.8 Transitioning patients from peritoneal dialysis to hemodialysis is not routinely recommended.
Nutritional consult should be obtained to prevent the protein-energy malnutrition. There is a lack of evidence to support alternative approaches like enteral and parenteral nutrition when oral intake is inadequate.37
The most commonly used intervention in the management of calciphylaxis is administering intravenous sodium thiosulfate, which is an off-label use.38 Its use was first reported in a case report in 2004, and the possible mechanism is through acting as a reducing agent that forms water-soluble complexes with minerals and metals.39 There are no prospective studies done with sodium thiosulfate; the available data are in 53 patients from a multicenter cohort study through a physician survey. It was administered as 25 g intravenously in 100 mL normal saline over the last 30 minutes of hemodialysis in a 70-kg person 3 times a week.38 Nausea, vomiting, hypotension, fluid overload, and metabolic acidosis are the most common side effects that sometimes warrants in dose reduction or drug discontinuation.38 The calciphylaxis improved in 70% of the patients, and the study has limitations of survey bias. The optimal duration of the therapy is unknown, but the response in pain and lesion improvement in the first 2 weeks will predict the long-term outcome. The possible mechanism of action is through an inhibitory effect on direct vascular calcification, antioxidation, and vasodilatation. Sodium thiosulfate, when administered intralesionally, results in the resolution of calciphylaxis lesions as described in 4 patients.40 Sodium thiosulfate results in chemical peritonitis when administered intraperitoneally and should be avoided.41
There has been literature on different treatment modalities like bisphosphonates, vitamin K, low-density lipoprotein apheresis, low-dose tissue plasminogen activator infusion, but these should be considered on an individual basis.42-45 The data from a large nationwide registry in management of calciphylaxis are summarized in Table 5.46 The wound healing based on case reports and case series in cohort studies using different strategies as discussed above are summarized in Table 6.7,38,47
Table 5.
Management of Calciphylaxis Based on Data From Large Nationwide Registry From Germany.
| Surgical wound management | 29% |
| Increase dialysis duration or frequency | 17% |
| Sodium thiosulfate | 21% |
| Antibiotics | 16% |
| Initiate cinacalcet | 11% |
| Stopping warfarin | 25% |
| Decreasing or stopping calcium containing phosphate binders | 24% |
| Stopping or decreasing the dose of vitamin D | 16% |
Table 6.
Wound Healing Based on Case Reports, Case Series, and Cohort Studies.
| Sodium thiosulfate | Complete resolution = 26.4%, marked improvement = 18.9%, improvement = 28.3%, No improvement = 5.7%, unknown = 20.8%38
Complete/partial wound healing of 80% in case report and case series and 72% in cohort studies |
| Surgical debridement of wound | One-year survival rate was 61.6% in patients who underwent surgical debridement compared with 27.4% who did not (retrospective analysis from the Mayo Clinic)7 |
| Bisphosphonates therapy | Complete/partial wound healing of 77% in case report and case series and 87% in cohort studies |
| Hyperbaric oxygen therapy | Complete/partial wound healing of 65% in case report and case series and 62% in cohort studies |
| Cinacalcet therapy | Complete/partial wound healing of 87% in case report and case series and 78% in cohort studies |
| Surgical parathyroidectomy | Complete/partial wound healing of 67% in case report and case series and 64% in cohort studies |
Conclusion
Calciphylaxis is a sporadic disease, frequently encountered in the patients undergoing hemodialysis and carries a very grave prognosis. Current treatment is rarely effective, so preventive strategies play an important role by modifying the risk factors that promote the development of calciphylaxis.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethics Approval: Our institution does not require ethical approval for individual case reports.
Informed Consent: Verbal informed consent from the patient has been obtained for their anonymized information to be published in this article.
ORCID iDs: Sreedhar Adapa  https://orcid.org/0000-0001-5608-5654
https://orcid.org/0000-0001-5608-5654
Vijay Gayam  https://orcid.org/0000-0001-5194-9134
https://orcid.org/0000-0001-5194-9134
Venu Madhav Konala  https://orcid.org/0000-0003-1953-8815
https://orcid.org/0000-0003-1953-8815
References
- 1. Selye H, Gentile G, Prioreschi P. Cutaneous molt induced by calciphylaxis in the rat. Science. 1961;134:1876-1877. [DOI] [PubMed] [Google Scholar]
- 2. Nigwekar SU, Wolf M, Sterns RH, Hix JK. Calciphylaxis from nonuremic causes: a systematic review. Clin J Am Soc Nephrol. 2008;3:1139-1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. McCarthy JT, El-Azhary RA, Patzelt MT, et al. Survival, risk factors, and effect of treatment in 101 patients with calciphylaxis. Mayo Clin Proc. 2016;91:1384-1394. [DOI] [PubMed] [Google Scholar]
- 4. Nigwekar SU, Solid CA, Ankers E, et al. Quantifying a rare disease in administrative data: the example of calciphylaxis. J Gen Intern Med. 2014;29(suppl 3):S724-S731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhang Y, Corapi KM, Luongo M, Thadhani R, Nigwekar SU. Calciphylaxis in peritoneal dialysis patients: a single center cohort study. Int J Nephrol Renovasc Dis. 2016;9:235-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nigwekar SU, Bhan I, Turchin A, et al. Statin use and calcific uremic arteriolopathy: a matched case-control study. Am J Nephrol. 2013;37:325-332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Weenig RH, Sewell LD, Davis MD, McCarthy JT, Pittelkow MR. Calciphylaxis: natural history, risk factor analysis, and outcome. J Am Acad Dermatol. 2007;56:569-579. [DOI] [PubMed] [Google Scholar]
- 8. Fine A, Zacharias J. Calciphylaxis is usually non-ulcerating: risk factors, outcome and therapy. Kidney Int. 2002;61:2210-2217. [DOI] [PubMed] [Google Scholar]
- 9. Hayashi M, Takamatsu I, Kanno Y, Yoshida T, Abe T, Sato Y; Japanese Calciphylaxis Study Group. A case-control study of calciphylaxis in Japanese end-stage renal disease patients. Nephrol Dial Transplant. 2012;27:1580-1584. [DOI] [PubMed] [Google Scholar]
- 10. Mazhar AR, Johnson RJ, Gillen D, et al. Risk factors and mortality associated with calciphylaxis in end-stage renal disease. Kidney Int. 2001;60:324-332. [DOI] [PubMed] [Google Scholar]
- 11. Bleyer AJ, Choi M, Igwemezie B, de la Torre E, White WL. A case control study of proximal calciphylaxis. Am J Kidney Dis. 1998;32:376-383. [DOI] [PubMed] [Google Scholar]
- 12. Lee JL, Naguwa SM, Cheema G, Gershwin ME. Recognizing calcific uremic arteriolopathy in autoimmune disease: an emerging mimicker of vasculitis. Autoimmun Rev. 2008;7:638-643. [DOI] [PubMed] [Google Scholar]
- 13. Angelis M, Wong LL, Myers SA, Wong LM. Calciphylaxis in patients on hemodialysis: a prevalence study. Surgery. 1997;122:1083-1090. [DOI] [PubMed] [Google Scholar]
- 14. James LR, Lajoie G, Prajapati D, Gan BS, Bargman JM. Calciphylaxis precipitated by ultraviolet light in a patient with end-stage renal disease secondary to systemic lupus erythematosus. Am J Kidney Dis. 1999;34:932-936. [DOI] [PubMed] [Google Scholar]
- 15. Brewster UC. Dermatological disease in patients with CKD. Am J Kidney Dis. 2008;51:331-344. [DOI] [PubMed] [Google Scholar]
- 16. Daudén E, Oñate MJ. Calciphylaxis. Dermatol Clin. 2008;26:557-568. [DOI] [PubMed] [Google Scholar]
- 17. Nigwekar SU, Kroshinsky D, Nazarian RM, et al. Calciphylaxis: risk factors, diagnosis, and treatment. Am J Kidney Dis. 2015;66:133-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Moe SM, Chen NX. Calciphylaxis and vascular calcification: a continuum of extra-skeletal osteogenesis. Pediatr Nephrol. 2003;18:969-975. [DOI] [PubMed] [Google Scholar]
- 19. Ng AT, Peng DH. Calciphylaxis. Dermatol Ther. 2011;24:256-262. [DOI] [PubMed] [Google Scholar]
- 20. Au S, Crawford RI. Three-dimensional analysis of a calciphylaxis plaque: clues to pathogenesis. J Am Acad Dermatol. 2002;47:53-57. [DOI] [PubMed] [Google Scholar]
- 21. Hayden MR, Kolb LG, Khanna R. Calciphylaxis and the cardiometabolic syndrome. J Cardiometab Syndr. 2006;1:76-79. [DOI] [PubMed] [Google Scholar]
- 22. Reiter N, El-Shabrawi L, Leinweber B, Berghold A, Aberer E. Calcinosis cutis: part I. Diagnostic pathway. J Am Acad Dermatol. 2011;65:1-12. [DOI] [PubMed] [Google Scholar]
- 23. Essary LR, Wick MR. Cutaneous calciphylaxis: an underrecognized clinicopathologic entity. Am J Clin Pathol. 2000;113:280-287. [DOI] [PubMed] [Google Scholar]
- 24. Mochel MC, Arakaki RY, Wang G, Kroshinsky D, Hoang MP. Cutaneous calciphylaxis: a retrospective histopathologic evaluation. Am J Dermatopathol. 2013;35:582-586. [DOI] [PubMed] [Google Scholar]
- 25. Vedvyas C, Winterfield LS, Vleugels RA. Calciphylaxis: a systematic review of existing and emerging therapies. J Am Acad Dermatol. 2012;67:e253-e260. [DOI] [PubMed] [Google Scholar]
- 26. Sato T, Ichioka S. How should we manage multiple skin ulcers associated with calciphylaxis? J Dermatol. 2012;39:966-968. [DOI] [PubMed] [Google Scholar]
- 27. Zitt E, König M, Vychytil A, et al. Use of sodium thiosulphate in a multi-interventional setting for the treatment of calciphylaxis in dialysis patients. Nephrol Dial Transplant. 2013;28:1232-1240. [DOI] [PubMed] [Google Scholar]
- 28. Wollina U, Helm C, Hansel G, et al. Deep ulcer shaving combined with split-skin transplantation in distal calciphylaxis. Int J Low Extrem Wounds. 2008;7:102-107. [DOI] [PubMed] [Google Scholar]
- 29. Podymow T, Wherrett C, Burns KD. Hyperbaric oxygen in the treatment of calciphylaxis: a case series. Nephrol Dial Transplant. 2001;16:2176-2180. [DOI] [PubMed] [Google Scholar]
- 30. Picazo M, Bover J, de la Fuente J, Sans R, Cuxart M, Matas M. Sterile maggots as adjuvant procedure for local treatment in a patient with proximal calciphylaxis [in Spanish]. Nefrologia. 2005;25:559-562. [PubMed] [Google Scholar]
- 31. Dean M. Opioids in renal failure and dialysis patients. J Pain Symptom Manage. 2004;28:497-504. [DOI] [PubMed] [Google Scholar]
- 32. Russell R, Brookshire MA, Zekonis M, Moe SM. Distal calcific uremic arteriolopathy in a hemodialysis patient responds to lowering of Ca × P product and aggressive wound care. Clin Nephrol. 2002;58:238-243. [DOI] [PubMed] [Google Scholar]
- 33. Velasco N, MacGregor MS, Innes A, Mackay IG. Successful treatment of calciphylaxis with cinacalcet—an alternative to parathyroidectomy? Nephrol Dial Transplant. 2006;21:1999-2004. [DOI] [PubMed] [Google Scholar]
- 34. Perkovic V, Neal B. Trials in kidney disease—time to EVOLVE. N Engl J Med. 2012;367:2541-2542. [DOI] [PubMed] [Google Scholar]
- 35. Headley CM. Hungry bone syndrome following parathyroidectomy. ANNA J. 1998;25:283-291. [PubMed] [Google Scholar]
- 36. Baldwin C, Farah M, Leung M, et al. Multi-intervention management of calciphylaxis: a report of 7 cases. Am J Kidney Dis. 2011;58:988-991. [DOI] [PubMed] [Google Scholar]
- 37. Don BR, Chin AI. A strategy for the treatment of calcific uremic arteriolopathy (calciphylaxis) employing a combination of therapies. Clin Nephrol. 2003;59:463-470. [DOI] [PubMed] [Google Scholar]
- 38. Nigwekar SU, Brunelli SM, Meade D, Wang W, Hymes J, Lacson E., Jr. Sodium thiosulfate therapy for calcific uremic arteriolopathy. Clin J Am Soc Nephrol. 2013;8:1162-1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cicone JS, Petronis JB, Embert CD, Spector DA. Successful treatment of calciphylaxis with intravenous sodium thiosulfate. Am J Kidney Dis. 2004;43:1104-1108. [DOI] [PubMed] [Google Scholar]
- 40. Strazzula L, Nigwekar SU, Steele D, et al. Intralesional sodium thiosulfate for the treatment of calciphylaxis. JAMA Dermatol. 2013;149:946-949. [DOI] [PubMed] [Google Scholar]
- 41. Gupta DR, Sangha H, Khanna R. Chemical peritonitis after intraperitoneal sodium thiosulfate. Perit Dial Int. 2012;32:220-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Musso CG, Enz PA, Guelman R, et al. Non-ulcerating calcific uremic arteriolopathy skin lesion treated successfully with intravenous ibandronate. Perit Dial Int. 2006;26:717-718. [PubMed] [Google Scholar]
- 43. Levy R. Potential treatment of calciphylaxis with vitamin K(2): comment on the article by Jacobs-Kosmin and DeHoratius. Arthritis Rheum. 2007;57:1575-156. [DOI] [PubMed] [Google Scholar]
- 44. Iwagami M, Mochida Y, Ishioka K, et al. LDL-apheresis dramatically improves generalized calciphylaxis in a patient undergoing hemodialysis. Clin Nephrol. 2014;81:198-202. [DOI] [PubMed] [Google Scholar]
- 45. Sewell LD, Weenig RH, Davis MD, McEvoy MT, Pittelkow MR. Low-dose tissue plasminogen activator for calciphylaxis. Arch Dermatol. 2004;140:1045-1048. [DOI] [PubMed] [Google Scholar]
- 46. Brandenburg VM, Kramann R, Rothe H, et al. Calcific uraemic arteriolopathy (calciphylaxis): data from a large nationwide registry. Nephrol Dial Transplant. 2017;32:126-132. [DOI] [PubMed] [Google Scholar]
- 47. Udomkarnjananun S, Kongnatthasate K, Praditpornsilpa K, Eiam-Ong S, Jaber BL, Susantitaphong P. Treatment of calciphylaxis in CKD: a systematic review and meta-analysis. Kidney Int Rep. 2018;4:231-244. [DOI] [PMC free article] [PubMed] [Google Scholar]