Abstract
Transoral incisionless fundoplication (TIF) was introduced in 2006 as a concerted effort to produce a natural orifice procedure for reflux. Since that time, the device, as well as the procedure technique, has evolved. Significant research has been published during each stage of the evolution, and this has led to considerable confusion and a co-mingling of outcomes, which obscures the results of the current device and procedure. This report is intended to review the identified stages and literature associated with each stage to date and to review the current state of treatment outcomes.
Keywords: reflux, GERD, TIF, transoral incisionless fundoplication, hiatal hernia
Introduction
Reflux began as a surgical disease. The work of Allison and Barrett recognized the anatomic defects that lead to gastroesophageal reflux. They correlated this reflux with damage that occurred to the esophageal mucosa. They then surmised that this damage could lead to a full spectrum of complications to include stricture, contracture and foreshortening, and metaplasia progressing to carcinoma.1–4 Further work by Nissen, Belsey, Hill, Toupet and many others identified two defects to repair: approximation of the dilated hiatus to the esophagus, and restoration of the angle of HIS through invagination of the gastroesophageal junction into the gastric cardia.5–12
In the 1980s, a paradigm shift began to occur in the treatment of reflux disease. With the introduction of H2 antagonists, symptoms were partially controlled, but it took the introduction of proton pump inhibitors (PPIs) in 1988 to begin the swing from surgical therapy to medical therapy as the mainstay of reflux care. Even as open surgical procedures became less invasive, less morbid, and significantly easier to recover from, the era of surgery as a common therapy closed by 1998.13
In the next 20 years, two forces have begun to swing the pendulum back towards surgical therapy. It was always understood that medical therapy only treated the symptoms of reflux disease by significantly altering gastric physiology, and, subsequently, small and large bowel physiology by creating a non-acid gastric fluid. Eventually, the consequences of changing this physiology were understood as physicians began to recognize complications associated with prolonged acid suppression.14–27 At the same time, surgical innovators began to push the envelope from minimal incision surgery to no incision surgery through what has become known as natural orifice surgery.28
An obvious target of natural orifice surgery would be the gastroesophageal junction and its repair, thus treating reflux disease surgically again. Two routes of repair were pursued: gastroesophageal invagination through endoscopic tissue manipulation and suturing, and bulking of the gastroesophageal junction. Also, a new laparoscopic approach was developed to increase distal esophageal closing pressure, now called lower esophageal sphincter augmentation.28
Out of the tissue manipulation and suturing devices, only the EsophyX® device has gained acceptance in general practice.29 Tissue bulking has remained in use within concentrated circles. The initial devices for esophageal sphincter augmentation were, for the most part, removed due to complications of dysphagia, erosion, and migration, although a newer technique with a new device has gained some traction.30–42
The purpose of this report is to review how the EsophyX® device (EndoGastric Solutions, Inc. Redmond, WA) evolved. There are two evolutions that can be described. The beginning starts from the concept of an endoluminal device to manipulate and suture tissue. Then, initial Food and Drug Administration (FDA) clearance, and then additional major advancements to the device design occurred. In parallel, clinicians were developing the procedures we now use to control reflux disease. Like the evolution of many new ideas, several iterations of the device and the procedure have bred confusion in the broader medical community about which device and which procedure is now being used. Study results from one procedure are reported along with outcomes from other procedures, confusing patients, physicians, and reimbursement payers alike.43
Currently, there are three developmental steps to the device; the original EsophyX® device, EsophyX2®, EsophyX Z®. The improvements in design have created an easier to use, more automated device to ensure uniform, consistent, and reproducible fundoplication by each user.
Also, four procedures have been born out of the clinical application of the EsophyX® devices: Endoluminal fundoplication (ELF), transoral incisionless fundoplication 1.0 (TIF 1.0), transoral incisionless fundoplication 2.0 (TIF 2.0) and the combined laparoscopic hiatal hernia repair with transoral incisionless fundoplication 2.0 (HH-TIF). Each of these procedures are distinctly different and have markedly different clinical outcomes. However, again, confusion as to the differences have caused many authors to combine data sets in their analysis, leading to incorrect conclusions as to the effectiveness of the device in treating reflux disease.
The EsophyX® device
Although seemingly obvious, it is important to point out that the device is not the procedure. However, authors have compared different procedures performed by different iterations of the device to each other. Just as you would not compare suturing the colon to suturing the esophagus, despite using the same needle driver and suture, these outcomes cloud the value of the device and the procedure that is being performed.
The EsophyX® device was invented by Stephan Kramer while working with a group focused on achieving a natural orifice technique for reflux. He obtained a patent for the ideas behind the device in September 2004, and for the fasteners in December 2009. Initial functional testing, feasibility, efficacy, and safety studies were performed in Europe. The original device included an over-tube body that an endoscope would fit through, to be used for visualization of the device function. A cable with a helical screw was used to engage and hold one of the tissue planes while the end of the device folded upon itself to provide approximation and compression of the second tissue plane to the first just prior to suturing. The suturing function uses stylets on wires. An “H”-shaped suture material is snapped onto the wire. When ready to be delivered at various desired anatomical locations, the stylet is advanced through the tissue planes. The suture is then pushed out onto the stylet. The sutures are made of a polypropylene that closely approximates a prolene suture material, and is pre-formed into an “H” shape. One leg of the “H” has a groove that snaps onto the wire and then the rest of the “H” was folded down along the wire through the delivery channel. A pusher cable delivered the polypropylene “H” – now called a SerosaFuse® “fastener” – to the stylet, which provided full thickness penetration of the tissue and a passageway for the leading leg through the tissue. The trailing leg caught the innermost tissue plane and unfolded from the channel. In this way, the two planes of tissue were compressed together between the legs of the “H”. The initial web between the two legs had a length of 6.5 mm and were equivalent in strength to a 3-0 prolene suture.
These main components and their functionality are core to each of the devices further developed. In the initial EsophyX® device, the fasteners were loaded manually onto the stylets. Then, a separate pusher would advance each of the fasteners to deploy them individually. With EsophyX2®, a cartridge design was created to allow the fasteners to be snapped onto the stylet, although each fastener still had to be loaded on the stylet individually and then advanced manually – referred to as “musket loading”. At the end of the channel, the stylet was advanced. At the handle, the operator would use a pusher control to move the leading end of the fastener through the esophageal tissue and then the gastric tissue. This allowed the leading leg of the “H” to disengage from the stylet adjacent to the gastric tissue plane. The trailing leg was then released within the esophageal lumen. The “H” shape thereby approximates the two tissue planes until they develop serosal fusion. The initial “H” fastener was 6.5 mm wide. Clinicians using the device felt that there was too much compression on the tissue, leading to fastener pull-through, and the “H” fastener was widened to 7.5 mm, which remains the standard fastener today. With tension, the fastener web can elongate to 9 mm. Widening the fastener also had the effect of reducing the force necessary for deployment, improving the overall delivery of the fastener by avoiding intramural deployments. Other changes to the device were for differing endoscope sizes and a clear plastic visualization window to identify accurate loading of the fasteners onto the delivery stylet. These modifications improved use and functionality but did not change the application of the device. There were two important observations of the device. First, it took about 26 steps to complete a fastener cycle, so learning the procedure was a committed process. Second, the fundoplication formed by the device was significantly variable, so outcome varied depending on experience. The more you used the device, the more “tricks of the trade” you developed to get the fundoplication to look like and function like a fundoplication. The next development in the EsophyX2® device was a size adaptation to standard high-definition endoscopes. Also, the clamping pressure of the tissue mold to the chassis was increased to further secure tissue before suturing, increasing the accuracy of suture placement. Although changing to a high-definition endoscope may seem minor, the entire device had to be re-engineered due to limitations on the size of the device. The esophagus will accept only a certain diameter, and this change increased the overall device size from 58 to 60 on the French catheter scale.
The next significant upgrade to the device incorporated a change to the folding end. Previously, the distal end was secured to the endoscope with a silicone retaining cord. A molded plastic tongue projected to one side of the endoscope and is referred to as the “tissue mold”. To use the device, you backed the endoscope out of the retaining cord into the body of the device “chassis,” and actuated the folding mechanism. This folded the tissue mold to the chassis. Then, the endoscope was advanced behind the tissue mold into the gastric lumen and retroflexed to allow visualization. This configuration changed with the EsophyX Z® device, which tubularized the tissue mold. This would allow the endoscope to go through the tissue mold, streamlining the end of the device with the endoscope. This was felt to be a major safety feature in avoiding injury to the esophagus. Also, shields were added to protect surrounding tissue from the advancing stylet, which then allowed users to create a more fully rotated wrap. To further augment the device, a separate channel for the trailing leg of the fastener was created. This reduced the number of fasteners that would either not fully deploy or were be pushed fully through the tissue. This design change allowed the fastener deployment process to be mechanized. Now, two fasteners could be advanced simultaneously with the depression of a handle. An automated delivery further standardized the formation of the fundoplication. With this design, users were able to create a fundoplication that is reproducible in every patient (Figure 1).
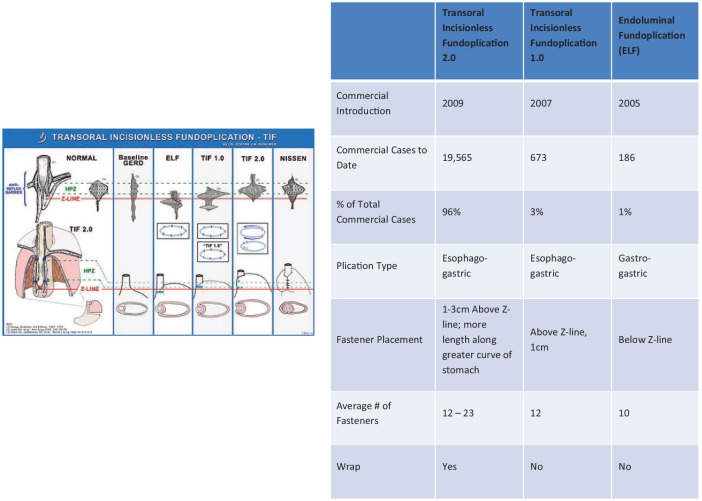
Figure 1.
EsophyX Device Iterations (EsophyX / EsophyX2 / EsophyX Z).
The procedures
Over the same timeline, four different procedures emerged. The initial device was used to perform the endoluminal gastro–gastric fundoplication, called “ELF”. This demonstrated the ability of the device to safely manipulate and suture gastric to gastric tissue but did not develop a significant clinical application. The second procedure was called TIF 1.0, and was a longitudinally oriented plication of gastric cardia onto the distal esophagus just proximal to the gastroesophageal junction. The third procedure was named TIF 2.0, and incorporated a rotational wrap of the cardia and fundus around the circumference of the distal esophagus in addition to providing a 2–4 cm length of the wrap over the intra-abdominal distal esophagus. It was this procedure that was identified as morphologically and physiologically most similar to the gold standard Nissen fundoplication. It was in this time period that expanded use of the device brought the treatment of reflux disease into the realm of possibility. Like the research performed by Belsey to identify the five tenants of reflux surgery,44 the TIF procedure developed with time to address each of these requirements.
The fundoplication must bring fundus over the esophagus and secure to the esophagus.
The fundoplication must be constructed without stricture.
The fundoplication is most effective when constructed 2–4 cm in length.
The fundoplication must remain below the diaphragm.
The diaphragmatic crura must be approximated to the esophagus.
The device improvements and transition from ELF to TIF 1.0 to TIF 2.0 allowed the creation of a standardized esophago-gastric fundoplication that conformed to the first four requirements. It was the addition of the laparoscopic hiatal repair just prior to the endoscopic fundoplication that identifies the fourth procedure in the developmental history of TIF 2.0. Combining laparoscopic hiatal hernia repair with TIF 2.0 allowed clinicians to fulfill all the criteria established by the 1990s for adequate control of reflux disease. The laparoscopic HH-TIF procedure, also called the “hybrid” procedure, was suggested at a user meeting in December of 2009 with the first publication of safety and efficacy being published in 2011.45 A review of the outcomes data will show that there is a gradual but definitive improvement of the control of reflux symptoms, esophagitis, and eventually distal esophageal acid exposure, as these devices and procedures developed.
Evolution of the procedures
Initial human trials began in Europe. In the hands of a few users, significant experience was obtained in how to engage and stabilize tissue, and then how to fold tissue upon itself and fix it to create a gastro–gastric plication below the Z-line. Additionally, the handloading of fasteners and the manual delivery technique had to be developed and standardized for the best delivery of fasteners. There was considerable concern over the seriousness of an esophageal injury. Although a gastric leak is a serious event, the gastric wall is three layers, much thicker and less prone to injury. A gastric leak is also more manageable with conservative therapy and fairly easy to address laparoscopically with suture repair. In contrast, an esophageal leak is a rapidly evolving event that is considerably more difficult to treat. In addition to mediastinitis, patients quickly develop a pleural effusion that will then loculate and create empyema. Treatment of this complication then requires a transthoracic approach. Patients with an esophageal leak become far more ill and require more extensive resources to treat if the leak is not repaired within the first 24 h. For these reasons, the initial device was developed around the idea of a gastro–gastric plication. The goal was to create a flap 6 cm below the z-line to form a bulk that would prevent the gastroesophageal junction from herniating through the hiatus, and to create an anti-reflux barrier by accentuating the angle of HIS. The initial procedures were studied to identify the safety of the device itself and the technique of endoluminal suturing. The first studies refer to the technique as ELF (endo-luminal fundoplication).
ELF
Studies of ELF were initiated with Guy Cadiere. His feasibility study was published in 2006 and laid the groundwork for using the device, initially in the canine model and then into human trials.46 This work allowed for introduction into the United States with FDA 510(k) clearance obtained in 2007. A total of 15 studies using the ELF technique alone have been published, documenting safety and efficacy in 181 patients with 7 (3.8%) serious adverse events (SAEs) identified.47–60 An additional five patients have been published in papers with mixed techniques; however, the ELF technique was otherwise abandoned once the safety of the esophageal fastener placement was shown. The ELF technique is only a gastro–gastric plication. Suturing is limited to a 3–5 cm gastrogastric fold. It did reduce the gastroesophageal junction below the diaphragm while providing a 200° to 310° wrap to recreate the angle of HIS. An average of 10 fasteners was used to create the wrap. These studies demonstrated an improvement in reflux symptoms and a reduction in PPI use despite not being a traditional fundoplication. 80% of patients were able to remain off PPIs at 6 months. Even more significant, and what eventually argues for the replacement of traditional fundoplication procedures, was the finding of less dysphagia, gas bloat, and flatulence. These are the primary issues that lead to traditional anti-reflux surgery being largely abandoned in the late 1990s and early 2000s.61
TIF 1.0
Introduction of the EsophyX device and ELF into the US was undertaken by Blair Jobe, Stephan Kraemer, and their teams. Two features distinguish the TIF 1.0 procedure. It was a marginally true esophagogastric fundoplication. It did not create the typical full invagination of the distal esophagus. Rather, it created a flap that was mainly gastrogastric, with the proximal fold sutured to the gastroesophageal junction. Their initial paper demonstrated the TIF 1.0 technique in the canine model, but also introduced the TIF 2.0 technique.62 This technique brought gastric fundus 2–4 cm over the distal esophagus, with fasteners well above the gastroesophageal junction. Comparisons performed in that study showed the superiority of the TIF 2.0 procedure in pH normalization and increased lower esophageal sphincter pressures. Vector volume analysis of the TIF 2.0 procedure showed that the pressure morphology was similar to a Nissen fundoplication, demonstrating a mechanism of action similar to the traditional fundoplication (Figure 2).
Figure 2.
Transoral incisionless fundoplication.
A number of centers began to use the TIF 1.0 and TIF 2.0 techniques and publications began to appear in 2010. After Jobe’s initial paper, an additional 21 publications evaluated outcomes between TIF 1.0 and TIF 2.0. A total of 673 unique patients have been recorded as undergoing the TIF 1.0 technique; however, the demonstrated superiority of TIF 2.0 lead to the discontinuation of the 1.0 procedure in favor of the 270° to 300° gastroesophageal fundoplication achieved with TIF 2.0.63–84
TIF 2.0
A report by Bell and Cadiere in 2011 is generally considered the start of the TIF 2.0 era.84 It marked the beginning of the adoption of TIF 2.0. Overall, the transition from TIF 1.0 to TIF 2.0 was fairly rapid, with two other reports published in 2010,85,86 and four additional reports published in 2011.45,87–89 The TIF 2.0 procedure fulfilled four of the five criteria for adequate anti-reflux surgery originally established with open, then laparoscopic, fundoplication. It was at this same time that the EsophyX2® device began to be used. There are three major studies that should be used to validate the use of TIF 2.0.
A multicenter registry of 100 patients published in 2012 demonstrated 80% off PPI therapy at 6 months, with normalization of quality-of-life scores in 73% of patients.90 A follow-up study at 24 months91 showed 66% of patients continued to have quality-of-life scores at least 50% improved from preoperative scores, and reflux symptom index scores were normalized in 65%. Daily PPI use decreased from 91% to 29%. Esophagitis was healed in 75% of patients, and 57% normalized their pH scores. De novo gas bloat, flatulence, and dysphagia were not associated with the TIF 2.0 technique in this study.
Two major level 1 trials were then initiated and published in the US, demonstrating continued improved outcomes. The TEMPO trial was designed to prospectively compare outcomes between incomplete responders to PPI therapy and TIF 2.0 therapy in an open-label crossover study.92 At 6 months, the fundoplication group reported esophagitis was healed in 100% of patients, symptoms were eliminated in 77%, and 82% were able to discontinue PPI use. After 6 months of high-dose PPI therapy, the control group was allowed to cross-over. Their initial 6-month outcomes post fundoplication and off PPI therapy demonstrated elimination of regurgitation and atypical symptoms in 65%; 75% of these patients further healed esophagitis, showing that fundoplication improved on outcomes after maximal medical therapy. This trial was carried out to 3 years, and demonstrated that 71% of the patients fully discontinued PPI therapy.93 Atypical symptoms were controlled, as demonstrated by normalization of the reflux symptom index (RSI) score in 87%. Quality-of-life scores remained normalized and 87% of patients were without esophagitis. Scores remained stable between the 12 months, 36 months, and a final 5-year report, demonstrating durability in the fundoplication for up to 5 years.29
A second level 1 trial involved TIF 2.0 with placebo versus a sham procedure with PPI therapy. In this study, designated RESPECT,94 the outcomes were reported at 6 months, then after crossover, at 12 months.95 Regurgitation was the endpoint of control, with 67% of fundoplication patients versus 45% of the PPI patients controlled at 6 months; 76% of the sham patients elected to crossover to fundoplication, and, at 12 months, 72% had control of regurgitation and 72% remained completely off PPI therapy.
These two trials occurred in the US, but there was also a randomized controlled sham trial published in Europe.96 Patients treated with TIF 2.0 were able to discontinue PPI therapy 59% of the time, compared with 9% of patients in the sham arm. Esophageal acid exposure was significantly improved from 8.89% to 3.73% of the time, demonstrating overall normalization. No sham patients showed statistical improvement in their acid exposure times.
Combined, these randomized controlled studies demonstrate that the TIF 2.0 procedure can reduce PPI use and control symptoms similar to current anti-reflux procedures, with a lower side effect profile and greater safety.97
Safety is demonstrated to be at least equivalent to laparoscopic fundoplication in literature outcomes but may be significantly lower. A review of industry-gathered data indicates that the SAE rate is markedly lower than laparoscopic fundoplication at 0.41%,98 with 91 serious events being reported to the database out of a total of approximately 22,000 procedures as of July 2019 (Figure 3).
Figure 3.
Commercial SAE rate of 0.43% (94 in 22,000 commercial cases); last SAE case reported July 2019; more than one harm reported in some cases.
SAE, serious adverse event.
The durability of the TIF 2.0 fundoplication at 5 years was demonstrated in the TEMPO trial, but two other European trials demonstrate 5-year and 10-year durability specifically in the TIF 2.0 technique as well, with non-significant changes in symptom control over the time of each study.96,99
Of the three procedures discussed, and, at the time of this writing, a total of 186 patients have undergone ELF, 673 patients have undergone TIF 1.0, and over 22,000 patients have undergone TIF 2.0.
Hiatal hernia repair with TIF
In 2011, a retrospective review suggested outcomes for TIF 2.0 would be improved if a hiatal hernia (HH) repair was performed just prior to the fundoplication.45 At the time, the FDA instructions for use (IFU) allowed for use of the EsophyX® device for hiatal hernias <2 cm in axial displacement, following the limit of sensitivity for detecting a hiatal hernia on barium swallow.
TIF 2.0 users were also adopting the Hill criteria for HH, and felt that only a Hill 4 rated hiatus demonstrated what would normally be considered a HH requiring repair.100 Following an initial 24 patients with TIF 2.0 only, 6-month outcomes showed significant improvement in the quality of life and atypical symptoms scores, and 76% of patients were off daily PPI use. However, follow-up endoscopy also showed there were intact fundoplications within a dilated hiatus.
Concern among users was whether a dilated hiatus was contributing to a return of symptoms after TIF 2.0, and a retrospective review confirmed that symptom outcomes and satisfaction scores improved in patients that received a hiatal repair prior to TIF 2.0 (Table 1). Other reports also began to reflect that a Hill 2 hiatus would do well after TIF 2.0, but a Hill 3 hiatus may be contributing to recurrent symptoms.64,87 A series of studies looked at the spectrum of hiatal dilation, and the appropriate assignment of Hill criteria, as well as the selection of when to repair the hiatus. It was determined that outcomes were affected when the hiatus was dilated to greater than 2 cm in transverse diameter.101–103 As users of the EsophyX device gained experience in identifying how the dilated hiatus affected outcomes, and as more data became available,68,96 the selection criteria for TIF-only patients matured. It is the process of this realization that may account for improved outcomes between the 2012 registry study and the TEMPO and RESPECT trials.
Table 1.
TIF 2.0 versus TIF 2.0 w/CC(median scores).
| Post TIF | Post TIF w/CC | ||
|---|---|---|---|
| • GERD-HRQL | 5 | • GERD-HRQL | 3 |
| • RSI | 5 | • RSI | 4 |
| • GERSS | 6 | • GERSS | 1 |
| • Regurgitation | 5 | • Regurgitation | 0 |
| • Satisfaction | 50% | • Satisfaction | 83% |
p < 0.001 for all changes.
CC, crural closure; GERD, gastroesophageal reflux disease; GERSS, gastroesophageal reflux symptom score; HRQL, health-related quality of life; RSI, reflux symptom index; TIF, transoral incisionless fundoplication.
Identifying and repairing a dilated hiatus fulfills the fifth criteria for effective anti-reflux surgery. In 2017, based on these studies, the FDA granted a modification of the IFU. This modification allowed TIF immediately after hiatal repair, similar to what was routinely performed in any other fundoplication.
Post procedure concerns
Postoperative care is similar to traditional fundoplication techniques. Patients will experience some substernal discomfort associated with irritation of the cura with or without hiatal repair. Shoulder discomfort associated with phrenic nerve irritation is common and resolves usually within a week. Some practitioners have opted to perform TIF 2.0 as an outpatient procedure but many observe the patient overnight. Risks include postoperative nausea, but patients are able to belch and vomit if necessary, although there is some risk to disruption of the fundoplication with heavy retching or vomiting. As discussed, postoperative dysphagia, bloat, gassiness, and flatulence is rare with the TIF 2.0 procedure; however, preoperative dysphagia may persist postoperatively. To moderate dysphagia, a graduated diet is prescribed. This consists of full liquids for 2 weeks, pureed foods for 1 week, soft foods for 1 week, and then a modified regular diet that avoids beef, chicken, bread, rice, and tortillas for a week. A regular diet is allowed in the 6th week. This is felt to improve esophageal muscular strength and peristaltic coordination in the postoperative period. Persistent dysphagia may be treated with bougie dilation as is typical with dysphagia due to reflux associated esophageal fibrosis.87,90,104
More serious complications, including pleural effusion, mediastitis, abscess, and esophageal perforation, have been reported.45,87,105 Observation overnight for manifestations of these complications is reasonable, and if white blood cell counts greater than 15K, and or tachycardia over 105 beats per minute, contrast study is recommended. Either esophagram or computed tomography with gastrograffin contrast is helpful.45,106,107 If a leak cannot be demonstrated, it would be prudent to continue on intravenous antibiotics until these issues resolve or a leak declares. A review of the manufacturer and user database reveals that complications were more common in the first few years after introduction of TIF 2.0. Changes in technique, device design, and the overall level of experience with the device have demonstrated a downward trend in the incidence of these complications.98
The spectrum of reflux treatment
The most common and most early presentation of reflux disease is heartburn. When symptoms initially present, lifestyle changes, a change in food choices and weight loss often will resolve symptoms. Many times, antacids will resolve the acute condition. If symptoms persist, a 6–8 week trial of H2 antagonists often will heal the underlying esophagitis, and, if not, a 6- to 8-week trial of PPI therapy is very effective. Patients that continue to have symptoms beyond a second 6- to 8-week trial may have a different etiology to their reflux that may be due to anatomic changes. These anatomic defects are amenable to repair using TIF with45,108,109 or without hiatal repair. With easy access to over-the-counter PPI therapy, many patients are on PPI therapy for several years before seeking alternative treatment. Studies indicate progression of the disease during the prolonged use of PPI therapy.14–27 Additionally, an increase in the incidence of esophageal adenocarcinoma has been identified during this period of PPI use.110
Based on the studies discussed, TIF 2.0 is a viable alternative to chronic medical therapy in patients who fail to resolve their reflux symptoms after 6 months of medical therapy. This provides ample time to allow for maximal medical care without subjecting patients to the risks associated with prolonged PPI therapy. Early disease without dilation of the hiatus can be treated with the TIF 2.0 technique alone, whereas patients with chronic and late disease often have a HH requiring repair. A recent study demonstrates that these patients can be identified with a technique that uses a retroflexed endoscope to reproducibly measure the hiatus for repair. When controlling for a hiatus less than the 3 cm in greatest transverse diameter limit, 95% of patients with an intact fundoplication were found to have normalized their pH score.109
A review of the studies presented suggests that, compared with traditional laparoscopic fundoplication, TIF 2.0 and possibly hiatal repair with TIF 2.0 offer greater safety and side effect profiles with equivalent outcomes and durability.29,91,96,97,99 This then suggests that TIF 2.0 procedure is poised to significantly alter the traditional spectrum of care. For patients that fail to get off of medical therapy after a 6-month trial of increasing medical care, TIF can restore the angle of HIS and improve the LES function necessary to control reflux symptoms, heal esophagitis, and allow discontinuation of medications. Patients that have a hiatus greater than 3 cm in diameter, as measured with a retroflexed endoscope,109 can undergo laparoscopic HH repair with TIF. The combined laparoscopic and endoscopic approach has fewer and less comprehensive studies to date, but, in available studies, appears to have similar outcomes in symptom control, safety, and normalization of esophageal pH without causing the bloat syndrome side effects that deterred patients from anatomic repair in the past.45,108,109
Acknowledgments
Thanks and acknowledgment to Debbie Donovan, Darren Crow, and Adrian Lobontiu. Debbie is the Senior Director, Corporate Marketing at EndoGastric Solutions and provided extensive up-to-date resources in order to provide accuracy in this report. Darren Crow is the vice president of research and development operations at EndoGastric Solutions, and was involved in the research and development of all EsophyX iterations. He kindly provided accurate information on the technical advancements of the device. Adrian Lobontiu is a French Board certified surgeon trained by Dr. Kraemer on the EsophyX device in 2005. Adrian is now located in the United States and is the chief medical officer at EndoGastric Solutions. Dr. Lobontiu has performed, proctored, and observed over 1500 procedures since 2005 – beginning before CE Mark and before FDA clearance and spanning all device and procedure iterations. He provided historical context and accuracy for this report.
Footnotes
Conflict of interest statement: Consultant for EndoGastric Solutions.
Funding: The author received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Glenn M. Ihde  https://orcid.org/0000-0001-8737-5152
https://orcid.org/0000-0001-8737-5152
Bibliography
- 1. Allison PR. Reflux esophagitis, sliding hiatal hernia, and the anatomy of repair. Surg Gyn Obst 1951; 92: 419. [PubMed] [Google Scholar]
- 2. Barrett NR. Hiatus hernia. Br Med J 1960; 2: 247–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Allison PR. Peptic ulcer of the esophagus. J Thorac Surg 1946; 15: 308–312. [PubMed] [Google Scholar]
- 4. Barret NR. Hiatus hernia: a review of some controversial points. Br J Surg 1954; 42: 231–238. [DOI] [PubMed] [Google Scholar]
- 5. Nissen R. Eine einfache operation zur beeinflussung der reflux oesophagitis. Schweiz Med Ochenschr 1956; 86: 590–592. [PubMed] [Google Scholar]
- 6. DeMeester TR, Ireland AP. Gastric pathology as an initiator and potentiator of gastroesophageal reflux disease. Dis Esoph 1997; 10: 1–8. [DOI] [PubMed] [Google Scholar]
- 7. Fein M, Ritter MP, DeMeester TR, et al. The role of the lower esophageal spincter and hiatal hernia in the pathogenesis of gastroesophageal reflux disease. J Gastrointest Surg 1999; 3: 405–410. [DOI] [PubMed] [Google Scholar]
- 8. Skinner DB, Belsey RHR. Surgical management of esophageal reflux and hiatus hernia: long term results with 1030 patients. J Thorac Cardiovasc Surg 1967; 53: 33–54. [PubMed] [Google Scholar]
- 9. Hill LD, Kozarek RA, Kraemer S, et al. The gastroesophageal flap valve: in vitro and in vivo observations. Gastro Endo 1996; 44: 541–547. [DOI] [PubMed] [Google Scholar]
- 10. Stylopoulos N, Rattner D. The history of hiatal hernia surgery. Ann Surg Jan 2005: 241: 185–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Turk R, Little A. The history of surgery for hiatal hernia and gastroesophageal reflux. In: Granderath F, Kamolz T, Pointner R. (eds) Gastroesophageal reflux disease, Chap 14. Austria: Springer, 2006, pp. 159–165. [Google Scholar]
- 12. Davis CS, Baldea A, Johns JR, et al. The evolution and long-term results of laparoscopic antireflux surgery for the treatment of gastroesophageal reflux disease. JSLS 2010; 13: 332–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Finks JF, Wei Y, Birkmeyer JD. The rise and fall of antireflux surgery in the United States. Surg Endosc 2006; 20: 1698–1701. [DOI] [PubMed] [Google Scholar]
- 14. Johnson D, Oldfield E. Reported side effects and complications of long-term proton pump inhibitor use: dissecting the evidence. Clin Gastroenterol Hepatol 2013; 11: 458–464. [DOI] [PubMed] [Google Scholar]
- 15. Demeester TR, Peters JH, Bremner CG, et al. Biology of gastroesophageal reflux disease: pathophysiology relating to medical and surgical treatment. Annu Rev Med 1999; 50: 469–506. [DOI] [PubMed] [Google Scholar]
- 16. Cahan MA, Baldof L, Colton K, et al. Proton pump inhibitors reduce gallbladder function. Surg Endosc 2006; 20: 1364–1367. [DOI] [PubMed] [Google Scholar]
- 17. Garcia Rodriguez LA, Ruigomez A, Panes J. Use of acid-suppressing drugs and the risk of bacterial gastroenteritis. Clin Gastroenterol Hepatol 2007; 5:1418–1423. [DOI] [PubMed] [Google Scholar]
- 18. Jalving M, Koornstra JJ, Wesseling J, et al. Increased risk of fundic gland polyps during long-term proton pump inhibitor therapy. Aliment Pharmacol Ther 2006; 24: 1341–1348. [DOI] [PubMed] [Google Scholar]
- 19. Targownik L, Lix L, Metge C, et al. Use of proton pump inhibitors and risk of steoporosis-related fractures. CMAJ 2008; 179: 319–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Geevasinga N, Colemna PL, Webster AC, et al. Proton pump inhibitors and acute interstitial nephritis. Clin Gastroenterol Hepatol 2006; 4: 597–604. [DOI] [PubMed] [Google Scholar]
- 21. Gomm W, von Holt K, Thome F, et al. Association of proton pump inhibitors with risk of Dementia: a pharmacoepidemiological claims data analsys. JAMA Neurol 2016; 73: 410–416. [DOI] [PubMed] [Google Scholar]
- 22. Sehested TS, Fosbol E, Hansen P, et al. Proton pump inhibitor use increases the associated risk of first-time ischemic stroke. A nationwide cohort study. Circulation 2016; 134: A18462. [Google Scholar]
- 23. Shiraev TP, Bullen A. Proton pump inhibitors and cardiovascular events: a systematic review. Heart, Lung and Circ 2018; 27: 443–450. [DOI] [PubMed] [Google Scholar]
- 24. Shah NH, LePuendu P, Bauer-Mehrne A, et al. Proton pump inhibitor usage and the risk of myocardial infarction in the general population. PLoS One 2015; 10: e0124653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Niu Q, Wang Z, Zhang Y, et al. Combination use of clopidogrel and proton pump inhibitors increases major adverse cardiovascular events in patients with coronary artery disease: a meta-analysis. J Cardiovasc Pharmacol Ther 2017; 22: 142–152. [DOI] [PubMed] [Google Scholar]
- 26. Lambert A, Lam J, Paik J, et al. Risk of community-acquired pneumonia with outpatient proton-pump inhibitor therapy. A systematic review and meta-analysis. PLoS One 2015; 10: e0128004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lazarus B, Chen Y, Wilson FP, et al. Proton pump inhibitor use and the risk of chronic kidney disease. JAMA Intern Med 2016; 176: 238–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shaheen N. The rise and fall and rise of endoscopic procedures. Gastro 2006; 131: 952–962. [DOI] [PubMed] [Google Scholar]
- 29. Trad KS, Barnes WE, Prevou ER, et al. The TEMPO trial at 5 years: transoral fundoplication (TIF 2.0) is safe, durable, and cost-effective. Surg Innov 2018; 25: 149–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jafri SM, Arora G, Triadafilopoulos G. What is left of the endoscopic antireflux devices? Curr Opin Gastroenterol 2009; 25: 352–357. [DOI] [PubMed] [Google Scholar]
- 31. Rothstein RI. Endoscopic therapy of gastroesophageal reflux disease: outcomes of the randomized-controlled trials done to date. J Clin Gastroenterol 2008; 42: 594–602. [DOI] [PubMed] [Google Scholar]
- 32. Reavis KM, Melvin WS. Advanced endoscopic technologies. Surg Endosc 2008; 22: 1533–1546. [DOI] [PubMed] [Google Scholar]
- 33. Pearl JP, Ponsky JL. Natural orifice translumenal endoscopic surgery: a critical review. J Gastrointest Surg 2008; 12: 1293–1300. [DOI] [PubMed] [Google Scholar]
- 34. Arts J, Sifrim D, Rutgeerts P, et al. Influence of radiofrequency energy delivery at the gastroesophageal junction (the stretta procedure) on symptoms, acid exposure, and esophageal sensitivity to acid perfusion in gastroesophageal reflux disease. Dig Dis Sci 2007; 52: 2170–2177. [DOI] [PubMed] [Google Scholar]
- 35. Kim MS, Holloway RH, Dent J, et al. Radiofrequency energy delivery to the gastric cardia inhibits triggering of transient lower esophageal sphincter relaxation and gastroesophageal reflux in dogs. Gastrointest Endosc 2003; 57: 17–22. [DOI] [PubMed] [Google Scholar]
- 36. Noar MD, Lotfi-Emran S. Sustained improvement in symptoms of GERD and antisecretory drug use: 4-year follow-up of the stretta procedure. Gastrointest Endosc 2007; 65: 367–372. [DOI] [PubMed] [Google Scholar]
- 37. Franciosa M, Triadafilopoulos G, Mashimo H. Stretta radiofrequency treatment for GERD: a safe and effective modality. Gastroenterol Res Pract 2013; 2013: 783–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Corley DA, Katz P, Wo JM, et al. Improvement of gastroesophageal reflux symptoms after radiofrequency energy: a randomized, sham-controlledtrial. Gastroenterology 2003; 125: 668–676. [DOI] [PubMed] [Google Scholar]
- 39. Smith RS, Chang F, Hayes K, et al. Complications of the angelchik antireflux prosthesis. A community experience. Am J Surg 1985; 150: 735–738. [DOI] [PubMed] [Google Scholar]
- 40. Stewart K, Urschel J, Hallgren R. Reoperation for complications of the angelchik antireflux prosthesis. Ann Thorac Surg 1994; 57: 1557–1558. [DOI] [PubMed] [Google Scholar]
- 41. Ganz RA, Peters JH, Horgan S, et al. Esophageal sphincter device for gastroesophageal reflux disease. N Engl J Med 2013; 368: 719–727. [DOI] [PubMed] [Google Scholar]
- 42. Katz P, DeVault K, Edmundowicz S, et al. Improvement in symptoms and QOL is sustained with minimal side effects 4 years after magnetic sphincter augmentation. Am J Gastroenterol 2014; 109: S34. [Google Scholar]
- 43. Stefanidis G, Viazis N, Kotsikoros N, et al. Long-term benefit of transoral incisionless fundoplication using the EsophyX device for the management of gastroesophageal reflux disease responsive to medical therapy. Dis Esophagus 2017; 30: 1–8. [DOI] [PubMed] [Google Scholar]
- 44. Horgan S, Pellegrini C. Surgical treatment of gastroesophageal reflux disease. Surg Clin North Am 1997; 77: 1063–1082. [DOI] [PubMed] [Google Scholar]
- 45. Ihde GM, Besancon K, Deljkich E. Short-term safety and symptomatic outcomes of transoral incisionless fundoplication with or without hiatal hernia repair in patients with chronic gastroesophageal reflux disease. Am J Surg 2011; 202: 740–747. [DOI] [PubMed] [Google Scholar]
- 46. Cadière GB, Rajan A, Rqibate M, et al. Endoluminal fundoplication (ELF)—evolution of EsophyX, a new surgical device for transoral surgery. Minim Invasive Ther Allied Technol 2006; 15: 348–355. [DOI] [PubMed] [Google Scholar]
- 47. Cadière GB, Rajan A, Germay O, et al. Endoluminal fundoplication by a transoral device for the treatment of GERD: a feasibility study. Surg Endosc 2008; 22: 333–342. [DOI] [PubMed] [Google Scholar]
- 48. Cadière GB, Buset M, Muls V, et al. Antireflux transoral incisionless fundoplication using EsophyX: 12-month results of a prospective multicenter study. World J Surg 2008; 32: 1676–1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bergman S, Mikami DJ, Hazey JW, et al. Endolumenal fundoplication with EsophyX: the initial North American experience. Surg Innov 2008; 15: 166–170. [DOI] [PubMed] [Google Scholar]
- 50. Cadière GB, Van Sante N, Graves JE, et al. Two-year results of a feasibility study on antireflux transoral incisionless fundoplication (TIF) using EsophyX. Surg Endosc 2009; 23: 957–964. [DOI] [PubMed] [Google Scholar]
- 51. Jafri SM, Arora G, Triadafilopoulos G. What is left of the endoscopic antireflux devices? Curr Opin Gastroenterol 2009; 25: 352–357. [DOI] [PubMed] [Google Scholar]
- 52. Eckardt AJ, Pinnow G, Pohl H, et al. Antireflux ‘barriers’: problems with patient recruitment for a new endoscopic antireflux procedure. Euro J Gastroenterol Hepatol 2009; 10: 1110–1118. [DOI] [PubMed] [Google Scholar]
- 53. Repici A, Fumagalli U, Malesci A, et al. Endoluminal fundoplication (ELF) for GERD using EsophyX: a 12-month follow-up in a single-center experience. J Gastrointest Surg 2010; 14: 1–6. [DOI] [PubMed] [Google Scholar]
- 54. Demyttenaere SV, Bergman S, Pham T, et al. Transoral incisionless fundoplication for gastroesophageal reflux disease in an unselected patient population. Surg Endosc 2010; 24: 854–858. [DOI] [PubMed] [Google Scholar]
- 55. Furnee EJB, Broeders AJL, Draaisma WA, et al. Laparoscopic Nissen fundoplication after failed EsophyX fundoplication. Br J Surg 2010; 97: 1051–1055. [DOI] [PubMed] [Google Scholar]
- 56. Romario U, Barbera R, Pepici A, et al. Nissen fundoplication after failure of endoluminal fundoplication: short term results. J Gastrointest Surg 2011; 15: 439–443. [DOI] [PubMed] [Google Scholar]
- 57. Zagol B, Mikami D. Advances in transoral fundoplication for oesophageal reflux. Dig Liver Dis 2011; 43: 361–364. [DOI] [PubMed] [Google Scholar]
- 58. Svoboda P, Kantorova I, Kozumplik L, et al. Our experience with transoral incisionless plication of gastroesophageal reflux disease: NOTES procedure. Hepatogastroenterology 2011; 58: 1208–1213. [DOI] [PubMed] [Google Scholar]
- 59. Frazzoni M, Conigliaro R, Manta R, et al. Reflux parameters as modified by EsophyX or laparoscopic fundoplication in refractory GERD. Aliment Pharmacol Ther 2011; 34: 67–75. [DOI] [PubMed] [Google Scholar]
- 60. Smeets FG, Keszthelyi D, Bouvy ND, et al. Does measurement of esophagogastric junction distensibility by endoFLIP predict therapy- responsiveness to endoluminal fundoplication in patients with gastroesophageal reflux disease? J Neurogastroenterol Motil 2015; 21: 255–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Galmiche J, Hatlebakk J, Attwood S. Laparoscopic antireflux surgery vs esomeprazole treatment for chronic GERD: the LOTUS randomized clinical trial. JAMA 2011; 305: 1969–1977. [DOI] [PubMed] [Google Scholar]
- 62. Jobe BA, O’Rourke RW, McMahon BP, et al. Transoral endoscopic fundoplication in the treatment of GERD: the anatomic and physiologic basis for reconstruction of the esophagogastric junction using a novel device. Ann Surg 2008; 248: 69–76. [DOI] [PubMed] [Google Scholar]
- 63. Velanovich V. Endoscopic, endoluminal fundoplication for gastroesophageal reflux disease: initial experience and lessons learned. Surgery 2010; 148: 646–653. [DOI] [PubMed] [Google Scholar]
- 64. Testoni PA, Corsetti M, Di Pietro S, et al. Effect of transoral incisionless fundoplication on symptoms, PPI use, and pH-impedance refluxes of GERD patients. World J Surg 2010; 34: 750–757. [DOI] [PubMed] [Google Scholar]
- 65. Ben-David K, Carreras J, Lopes J. Are incisionless fundoplication procedures a safer alternative to the laparoscopic Nissen for the treatment of chronic gastroesophageal reflux disease? J Gastrointest Surg 2011; 15: 885–890. [DOI] [PubMed] [Google Scholar]
- 66. Hoppo T, McMahon BP, Witteman BPL, et al. Functional lumen imaging probe to assess geometric changes in esophagogastric junction following endolumenal fundoplication. J Gastrointest Surg 2011; 15: 1112–1120. [DOI] [PubMed] [Google Scholar]
- 67. Testoni PA, Vailati C, Testoni S, et al. Transoral incisionless fundoplication (TIF 2.0) with EsophyX for gastroesophageal reflux disease: long-term results and findings affecting outcome. Surg Endosc 2012; 26: 1425–1435. [DOI] [PubMed] [Google Scholar]
- 68. Testoni PA, Vailati C. Transoral incisionless fundoplication with EsophyX® for treatment of gastro-oesphageal reflux disease. Dig Liver Dis 2012; 44: 631–635. [DOI] [PubMed] [Google Scholar]
- 69. Witteman BPL, Strijkers R, de Vries E, et al. Transoral incisionless fundoplication for treatment of gastroesophageal reflux disease in clinical practice. Surg Endosc 2012; 26: 3307–3315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Witteman BPL, Kessing BF, Snijders G, et al. Revisional laparoscopic antireflux surgery after unsuccessful endoscopic fundoplication. Surg Endosc 2013; 27: 2231–2236. [DOI] [PubMed] [Google Scholar]
- 71. Wendling MR, Melvin WS, Perry KA. Impact of transoral incisionless fundoplication (TIF) on subjective and objective GERD indices: a systematic review of the published literature. Surg Endosc 2013: 27: 3754–3761. [DOI] [PubMed] [Google Scholar]
- 72. Rinsma N, Smeets F, Bruls D, et al. Effect of transoral incisionless fundoplication on reflux mechanisms. Surg Endosc 2014; 28: 941–949. [DOI] [PubMed] [Google Scholar]
- 73. Maradey-Romero C, Kale H, Fass RC. Nonmedical therapeutic strategies for nonerosive reflux disease. J Clin Gastroenterol 2014; 48: 584–589. [DOI] [PubMed] [Google Scholar]
- 74. Ashfaq A, Rhee HK, Harold KL. Revision of failed transoral incisionless fundoplication by subsequent laparoscopic Nissen fundoplication. World J Gastroenterol 2014; 20: 17115–17119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Hopkins J, Switzer NJ, Karmali S. Update on novel endoscopic therapies to treat gastroesophageal reflux disease: a review. World J Gastrointest Endosc 2015; 7: 1039–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Testoni PA, Testoni S, Mazzoleni G, et al. Long-term efficacy of transoral incisionless fundoplication with EsophyX (TIF 2.0) and factors affecting outcomes in GERD patients followed for up to 6 years: a prospective single-center study. Surg Endosc 2015; 29: 2770–2780. [DOI] [PubMed] [Google Scholar]
- 77. Patti MG. An evidence-based approach to the treatment of gastroesophageal reflux disease. JAMA Surg 2016; 151: 73–78. [DOI] [PubMed] [Google Scholar]
- 78. Jain D, Singhal S. Transoral incisionless fundoplication for refractory gastroesophageal reflux disease: where do we stand? Clin Endosc 2016; 49: 147–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Testoni PA, Mazzoleni G, Testoni SG. Transoral incisionless fundoplication for gastro-esophageal reflux disease: techniques and outcomes. World J Gastrointest Pharmacol Ther 2016; 7: 179–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Huang X, Chen S, Zhao H, et al. Efficacy of transoral incisionless fundoplication (TIF) for the treatment of GERD: a systematic review with meta-analysis. Surg Endosc. Epub ahead of print 5 August 2016. DOI: 10.1007/s00464-016-5111-7. [DOI] [PubMed] [Google Scholar]
- 81. Fass R. An overview of transoral incisionless fundoplication and magnetic sphincter augmentation for GERD. Gastroenterol Hepatol (N Y) 2017; 13: 50–52. [PMC free article] [PubMed] [Google Scholar]
- 82. Thosani N, Goodman A, Manfredi M, et al. Endoscopic anti-reflux devices (with videos). Gastrointest Endosc. Epub ahead of print 18 October 2017. DOI: 10.1016/j.gie.2017.08.001. [DOI] [PubMed] [Google Scholar]
- 83. Richter JE, Kumar A, Lipka S, et al. Efficacy of laparoscopic Nissen fundoplication vs transoral incisionless fundoplication or proton pump inhibitors in patients with gastroesophageal reflux disease: a systematic review and network meta-analysis. Gastroenterology. Epub ahead of print 3 January 2018. doi: 10.1053/j.gastro.2017.12.021. [DOI] [PubMed] [Google Scholar]
- 84. Perretta S, Dallemagne B, Allemann P, et al. Multimedia manuscript. Heller myotomy and intraluminal fundoplication: a NOTES technique. Surg Endosc 2010; 24: 290–293. [DOI] [PubMed] [Google Scholar]
- 85. Bell RCW, Cadiere GB. Transoral rotation esophagogastric fundoplication: technical, anatomical, and safety considerations. Surg Endosc 2011; 25: 2387–2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Yushuva A, McMahon M, Goodman E. Transoral incision free fundoplication (TIF) - A new paradigm in the surgical treatment of GERD. J Surg Case Rep 2010; 2010: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Barnes WE, Hoddinott KM, Mundy S, et al. Transoral incisionless fundoplication offers high patient satisfaction and relief of therapy-resistant typical and atypical symptoms of GERD in community practice. Surg Innov 2011; 18: 119–129. [DOI] [PubMed] [Google Scholar]
- 88. Bell RCW, Freeman K. Clinical and pH-metric outcomes of transoral esophagogastric fundoplication for the treatment of gastroesophageal reflux disease. Surg Endosc 2011; 25: 1975–1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Nguyen A, Vo T, Nguyen XM, et al. Transoral incisionless fundoplication: initial experience in patients referred to an integrated academic institution. Am Surg 2011; 77: 1386–1389. [PubMed] [Google Scholar]
- 90. Bell RCW, Mavrelis PG, Barnes WE, et al. A prospective multicenter registry of patients with chronic gastroesophageal disease receiving transoral incisionless fundoplication. J Am Coll Surg 2012; 215: 794–809. [DOI] [PubMed] [Google Scholar]
- 91. Bell RC, Barnes WE, Carter BJ, et al. Transoral incisionless fundoplication: 2 year results from the prospective multicenter U.S. study. Am Surg 2014; 80: 1093–1105. [PubMed] [Google Scholar]
- 92. Trad KS, Barnes WE, Simoni G, et al. Transoral incisionless fundoplication effective in eliminating GERD symptoms in partial responders to proton pump inhibitor therapy at 6 months: the TEMPO randomized clinical trial. Surg Innov 2015; 22: 26–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Trad KS, Simoni G, Barnes WE, et al. Transoral fundoplication offers durable symptom control for chronic GERD: 3-year report from the TEMPO randomized trial with crossover arm. Surg Endosc 2017; 31: 2498–2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Hunter JG, Kahrilas PJ, Bell RC, et al. Efficacy of transoral fundoplication vs omeprazole for treatment of regurgitation in a randomized controlled trial. Gastroenterology 2015; 148: 324–333. [DOI] [PubMed] [Google Scholar]
- 95. Kahrilas PJ, Bell RC, Wilson EB, et al. Transoral esophagogastric fundoplication provides quality of life and sustained symptom and esophageal pH control: a randomized, sham-controlled, crossover trial. Poster session presented at the American College of Gastroenterology annual clinical meeting, October 16–21, 2015; Honolulu, Hawaii. [Google Scholar]
- 96. Håkansson B, Montgomery M, Cadiere GB, et al. Randomised clinical trial: transoral incisionless fundoplication vs. sham intervention to control chronic GERD. Aliment Pharmacol Ther 2015; 42: 1261–1270. [DOI] [PubMed] [Google Scholar]
- 97. Gerson L, Stouch B, Lobontiu A. Transoral incisionless fundoplication (TIF 2.0): a meta-analysis of three randomized, controlled clinical trials. Chirurgia 2018; 113: 173–184. [DOI] [PubMed] [Google Scholar]
- 98. U.S. Food and Drug Administration. MAUDE database, www.accessdata.fda.gov (accessed 29 July 2019).
- 99. Testoni PA, Testoni S, Distefano G, et al. Transoral incisionless fundoplication with EsophyX for gastroesophageal reflux disease: clinical efficacy is maintained up to 10 years. Endosc Int Open 2019; 7: E647–E654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Hill LD, Kozarek RA, Kraemer S, et al. The gastroesophageal flap valve: in vitro and in vivo observations. Gastro Endo 1996; 44: 541–547. [DOI] [PubMed] [Google Scholar]
- 101. Ihde GM, Dill LA, Lister DG, et al. Inter-rater agreement in evaluating the endoscopic appearance of the gastroesophageal junction in the community setting. Southwestern Surgical Congress 66th Annual Meeting, 13–16 April 2014, Scottsdale, AZ. [Google Scholar]
- 102. Ihde GM, Dill LA, Lister DG, et al. Type of anesthesia affects the assessment of the gastroesophageal junction in patients evaluated for anti-reflux. Southwestern Surgical Congress 66th Annual Meeting, 13–16 April 2014, Scottsdale, AZ. [Google Scholar]
- 103. Ihde GM, Dill LA, Lister DG, et al. A comparison of the endoscopic and laparoscopic view of the gastroesophageal junction in the use of transoral fundoplication. Am J Surg Dec 2015; 210: 1018–1023; discussion 1022–1023. [DOI] [PubMed] [Google Scholar]
- 104. Hoppo T, Immanuel A, Schuchert M, et al. Transoral incisionless fundoplication 2.0 procedure using EsophyX™ for gastroesophageal reflux disease. J Gastrointest. Surg 2010; 14: 1895–1901. [DOI] [PubMed] [Google Scholar]
- 105. Peterson RP, Filippa L, Wassenaar E, et al. Comprehensive evaluation of endoscopic fundoplication using the EsophyX™ device. Surg Endosc 2012; 26: 1021–1027. [DOI] [PubMed] [Google Scholar]
- 106. Trad K S, Turgeon D G, Deljkich E. Long-term outcomes after transoral incisionless fundoplication in patients with GERD and LPR symptoms. Surg Endosc 2012; 26: 650–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Lundell L. Complications after anti-reflux surgery. Best Pract Res Clin Gastroenterol 18: 935–945. [DOI] [PubMed] [Google Scholar]
- 108. Janu P, Shughoury A, Venkat K, et al. Laparoscopic hiatal hernia repair followed by transoral incisionless fundoplication with EsophyX device (HH + TIF): efficacy and safety in two community hospitals. Surg Innov 2019; 26: 675–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Ihde G, Pena C, Scitern C, et al. pH scores in hiatal repair with transoral incisionless fundoplication. JSLS 2019; 23: e2018.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Pohl H, Welch HG. The role of overdiagnosis and reclassification in the marked increase of esophageal adenocarcinoma incidence. J Natl Cancer Inst 2005; 97: 142–146. [DOI] [PubMed] [Google Scholar]