Abstract
Metastatic cancer that involves the structures of the heart is a rare complication and most commonly diagnosed during postmortem examination. Classically, the development of secondary tumors involves invasion of the pericardium or the myocardium and may disrupt the cardiac conduction system, causing new arrhythmias and heart failure. In this article, we present the case of a 58-year-old female with new diagnosis of ventricular bigeminy, and evidence of cardiac tamponade physiology from direct compression of the right ventricular outflow tract from high-grade carcinoma of the left breast. As oncologic therapies advance and provide more life-prolonging options to patients, recognition of the mass effect of large tumors should be recognized.
Keywords: RVOT, oncocardiology, extrinsic tumor compression, metastatic breast cancer
Case Presentation
A 58-year-old female with a past medical history notable for the recent diagnosis of Stage IVB pT4bN2aM1 estrogen (+), progesterone (+), and HER2 (+) poorly differentiated left breast adenocarcinoma presented to the emergency department (ED) of our hospital. She was scheduled for a palliative mastectomy. Family members volunteered a concern of increased lethargy, confusion, and dyspnea on exertion, worsening over the past week. On evaluation in the ED, her vitals included a temperature of 36.8°C, a blood pressure that ranged from 119/58 to 143/75 mm Hg, a pulse of 113 to 142 beats per minute, and a respiratory rate of 12 to 22 breaths per minute, with an oxygen saturation of 92% to 95% on room air. Bedside evaluation revealed a nonverbal and extremely somnolent patient. She was tachycardic with a right ventricular heave and an elevated jugular venous pressure. Auscultation revealed a III/VI systolic murmur at the cardiac apex. She had decreased breath sounds bilaterally, a normal abdominal examination, and purposeful movements noted with attempts to examine the left lower extremity. Admission laboratory tests were notable for leukocytosis (white blood cell = 14 300/µL; normal range = 4000-11 000/µL), anemia (hemoglobin = 9.2 g/dL; normal range = 12.6-17.1 g/dL), thrombocytosis (platelets = 552 000/µL; normal range = 140 000-440 000/µL), hypercalcemia (10.2 mg/dL; normal range = 8.4-10.5 mg/dL), and hyperammonemia (ammonia = 95 µg/dL; normal range = 19-65 µg/dL). Serial measurements of high-sensitivity cardiac troponin (T) assay (fifth generation) returned with levels of 23, 82, and 107 ng/mL (normal range: <14 ng/mL).
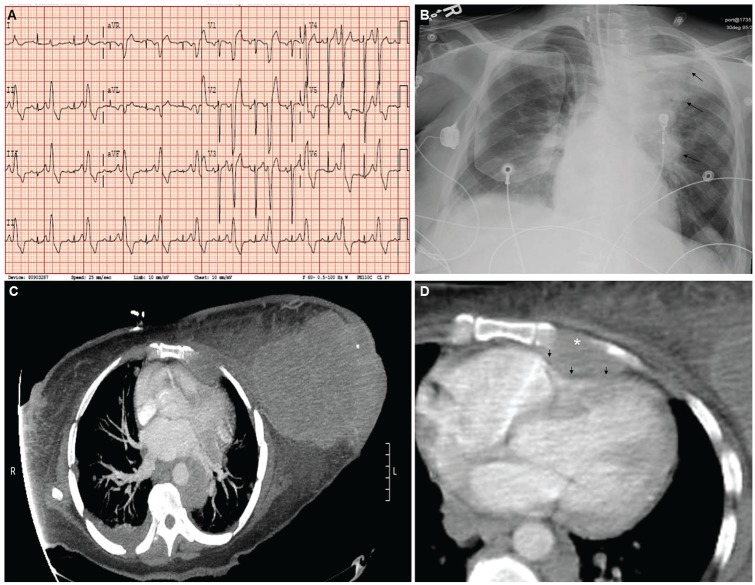
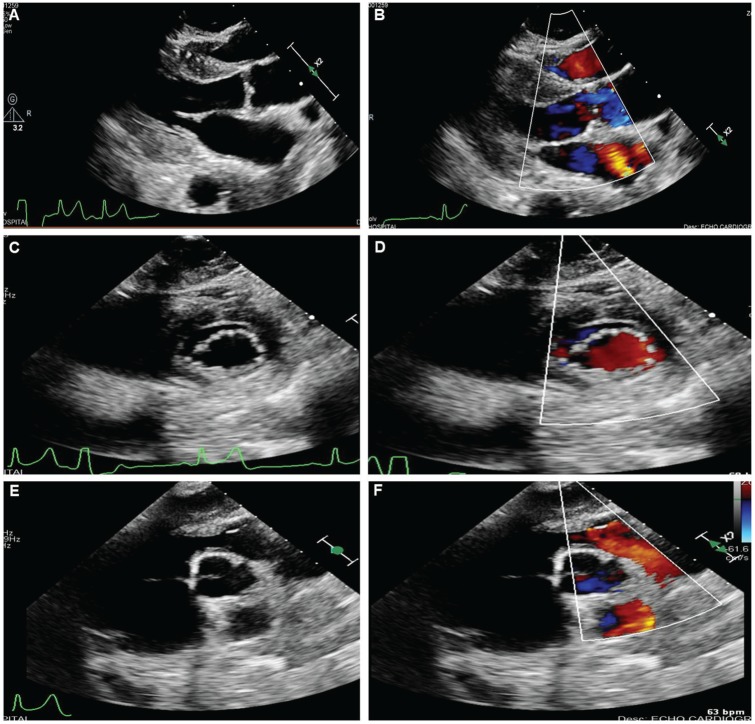
An electrocardiogram performed on presentation revealed sinus tachycardia, ventricular bigeminy, low voltage in the extremity leads, and abnormal R-wave transition, which were new findings from an electrocardiogram on file from the year prior (Figure 1A). Chest radiograph revealed cardiomegaly, with mediastinal and hilar opacities of the left chest (Figure 1B). Computed tomography (CT) of the chest showed a disproportionately large left breast mass with left axillary metastatic disease, extensive pulmonary metastatic disease, mediastinal and hilar adenopathy, left internal mammary adenopathy, and osseous metastatic disease involving the sternum with encroachment on the right ventricle of the heart (Figure 1C and D). CT of the head revealed several circumscribed areas of metastasis surrounded by vasogenic edema, with the largest ~2 cm in size and located in the left parietal lobe, with osseus involvement of the left skull and left mastoid. There was no evidence of bleeding into the brain metastases. Transthoracic echocardiogram was notable for a hyperdynamic ejection fraction of 70%, the presence of an anterior extracardiac structure causing compression of the right ventricular outflow tract (Figure 2) with an increased right ventricular outflow tract (RVOT) peak flow velocity of 150 cm/s, moderate dilation of the inferior vena cava with decreased inspiratory collapse, and elevated right ventricular systolic pressure of 52 mm Hg, with mild tricuspid regurgitation and mild pulmonic valve regurgitation. X-ray of the lower extremity revealed pathologic fracture of the left femur and peripheral vascular ultrasound demonstrated an acute occlusive deep venous thrombosis of the left mid-femoral vein.
Figure 1.
Prior electrocardiogram (A), chest radiograph on admission (B), and computed tomography of the chest (C and D).
*denotes osseus involvement of the sternum; black arrows demonstrate extrinsic compression.
Figure 2.
Transthoracic echocardiogram demonstrating right ventricular outflow tract compression.
During the course of her ED evaluation, she became progressively more altered and was subsequently intubated for airway protection. She was taken to the operating theater for successful femoral fixation by the orthopedics service, and then transferred to the medical intensive care unit for ongoing management. Shortly after arrival to the medical intensive care unit, the patient became hypotensive with a mean arterial pressure of 40 mm Hg with supraventricular tachycardia and heart rates between 160 and 200 beats per minute. She required direct current cardioversion, which returned the patient to ventricular bigeminy and was subsequently started on an amiodarone infusion. A heparin infusion was initiated, and in coordination with radiation oncology, a course of brain irradiation was initiated.
Outcome and Follow-up
On hospital day 1, new infiltrates of the left lower lobe were evident on the patient’s chest radiograph, and piperacillin/tazobactam was initiated for presumed aspiration pneumonia. The patient remained in ventricular bigeminy for the remainder of her hospital stay. Despite maximum medical therapy, the patient deteriorated further over the course of the following week, with lack of purposeful movements, inability to wean from the ventilator, and loss of all brain stem reflexes. In coordination with palliative care, the patient’s family made the decision to proceed with a comfort care approach, and the patient died peacefully on hospital day 11.
Materials and Methods
Consent for this report was unable to be obtained from the patient as they did not survive. Every effort was undertaken to maintain patient privacy and security, both during the review of this patient’s data and in creating this manuscript.
Discussion
The multidisciplinary field of oncology and cardiology has only recently evolved to meet the unique cardiovascular needs of oncology patients.1,2 Secondary involvement of the heart in patients with distant metastasis is a relatively rare phenomenon, and often not diagnosed either as a contributory cause of death or as an incidental finding, until the postmortem examination.3,4 However, with advancements in life-prolonging oncologic therapies, this is expected to change considerably,1,2 requiring a heightened clinical suspicion from the provider in any known oncologic patient with new cardiovascular-related complaints or diagnosis.
We present here the case of a 58-year-old female with recent diagnosis of widely metastatic and poorly differentiated adenocarcinoma originating from the left breast. At the time of her admission to our facility, diagnostic imaging revealed the presence of tumor spread to the brain, the lungs, the liver, and the long bones of the lower extremities. Additionally, review of the patient’s CT chest revealed osseous involvement of the sternum and of the left aspect of the mediastinum, causing extrinsic compression of the RVOT, with elevated right heart pressures seen on echocardiography. The RVOT is a known arrhythmogenic source of many ventricular arrhythmias, such as premature ventricular contractions, ventricular bigeminy, and sustained ventricular tachyarrhythmias,5,6 and a site of involvement identified in early case reports with extrinsic compression from anterior chest involvement from lymphoma and new diagnosis of ventricular arrhythmia.7,8
In patients with advanced tumor burden and either extrinsic or intrinsic cardiac involvement, there are no current consensus guidelines for selecting an ideal candidate for transcatheter or minimally invasive management, though it seems a reasonable consideration for patients who may receive a direct palliative benefit from intervention, or in those with a stable tumor burden and related cardiovascular symptoms. Given the successes of treating RVOT arrhythmias in patients without cancer, more research is needed to develop therapies unique to oncology patients.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethics Approval: Our institution does not require ethical approval for reporting individual cases or case series.
Informed Consent: Informed consent for patient information to be published was not obtained as the patient passed away during the course of her hospitalization. Every effort was made to protect patient anonymity and privacy.
References
- 1. Petek BJ, Greenman C, Herrmann J, Ewer MS, Jones RL. Cardio-oncology: an ongoing evolution. Future Oncol. 2015;11:2059-2066. [DOI] [PubMed] [Google Scholar]
- 2. Donisan T, Balanescu DV, Palaskas N, et al. Cardiac interventional procedures in cardio-oncology patients. Cardiol Clin. 2019;37:469-486. [DOI] [PubMed] [Google Scholar]
- 3. Hilal T, Anthony LB, Sorrell VL. Unexpected cardiac masses. JAMA Oncol. 2015;1:1343-1344. [DOI] [PubMed] [Google Scholar]
- 4. Sarjeant JM, Butany J, Cusimano RJ. Cancer of the heart: epidemiology and management of primary tumors and metastases. Am J Cardiovasc Drugs. 2003;3:407-421. [DOI] [PubMed] [Google Scholar]
- 5. Lavalle C, Mariani MV, Piro A, et al. Electrocardiographic features, mapping and ablation of idiopathic outflow tract ventricular arrhythmias. J Interv Card Electrophysiol. 2020;57:207-218. doi: 10.1007/s10840-019-00617-9 [DOI] [PubMed] [Google Scholar]
- 6. Lin T, Conti S, Cipolletta L, et al. Arrhythmias: benign or early stage arrhythmogenic right ventricular cardiomyopathy/dysplasia? J Atr Fibrillation. 2014;7:1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ren J, Rich MW, Makan M. Right ventricular outflow tract obstruction by lymphoma: case series and review of the literature. Echocardiography. 2011;28:1164-1167. [DOI] [PubMed] [Google Scholar]
- 8. Putterman C, Gilon D, Uretzki G, Bar-Ziv J, Polliack A. Right ventricular outflow tract obstruction due to extrinsic compression by non-Hodgkin’s lymphoma: importance of echocardiographic diagnosis and follow up. Leuk Lymphoma. 1992;7:211-215. [DOI] [PubMed] [Google Scholar]