Abstract
Objective
Some patients have been found to develop intraoperative amaurosis under sub-Tenon’s anesthesia. We explored whether these patients have poor surgical outcomes during mid- to long-term postoperative follow-up.
Methods
In this case series, 74 of 85 patients with macular diseases who underwent phacoemulsification combined with vitrectomy under sub-Tenon’s anesthesia developed intraoperative amaurosis. The surgical outcomes at the 2- and 4-month follow-ups in these patients were investigated and compared with the outcomes in patients without amaurosis using best-corrected visual acuity (BCVA), optical coherence tomography (OCT), and pattern visual evoked potential (PVEP).
Results
Both BCVA and the OCT-based macular structure in patients with intraoperative amaurosis showed significant postoperative improvement comparable with that of patients without amaurosis. The presence of intraoperative amaurosis was not associated with either macular hole closure or macular edema regression. PVEP revealed no significant changes in the wave latency or amplitude before and after surgery.
Conclusion
Intraoperative amaurosis following sub-Tenon’s block is commonly seen but does not predict a poor surgical prognosis. When a patient develops amaurosis during surgery, the surgeon should increase patient comfort through verbal communication rather than perform an additional intervention to help relieve the patient’s anxiety.
Keywords: Intraoperative amaurosis, intraocular surgery, sub-Tenon’s anesthesia, macular diseases, surgical outcome, case series
Introduction
Local anesthesia is widely used in ophthalmic procedures, and commonly used techniques include peribulbar anesthesia, retrobulbar anesthesia, and sub-Tenon’s anesthesia. Among these techniques, sub-Tenon’s anesthesia is of particular advantage because the anesthetic is administered into Tenon’s capsule using a blunt cannula, which can avoid serious complications such as ocular penetrating injury and optic nerve damage.1,2
Clinically, some patients have been found to develop transient amaurosis under sub-Tenon’s anesthesia. According to published case-based studies, the incidence of intraoperative amaurosis during vitreoretinal surgery under sub-Tenon’s anesthesia ranges from 6.7% to 53.8%.3–7 Interestingly, we previously found that the contralateral eye, although routinely draped with cloths during surgery, can sense the microscope light, and this may affect the evaluation of light perception.8 One study showed that after tightly covering the contralateral eye and removing the photo-bleaching effect on the operative eye, more than 85% of patients under sub-Tenon’s anesthesia developed intraoperative amaurosis in at least one of the surgical steps,9 indicating that amaurosis is a relatively common event.
The occurrence of intraoperative amaurosis may cause anxiety in patients and stress in surgeons. More importantly, although several case reports of intraoperative visual loss have been described, no studies with a larger sample size have focused on the visual outcomes in patients who develop intraoperative amaurosis. Whether the presence of intraoperative amaurosis predicts irreversible damage of the optic nerve and a poor surgical prognosis remains unknown. In this study, we explored the anatomical and functional surgical outcomes in patients undergoing treatment of an idiopathic macular hole (IMH) or epimacular membrane (EMM), paying particular attention to the correlation between intraoperative amaurosis and the visual prognosis.
Patients and methods
This study was conducted according to the guidelines of the Declaration of Helsinki and was approved by the Joint Shantou International Eye Center Ethics Committee. Written informed consent was obtained from all participants. The case series included consecutive patients diagnosed with IMH or EMM, some of whom developed intraoperative amaurosis under sub-Tenon’s anesthesia. The patients’ follow-up data were collected and analyzed to determine whether intraoperative amaurosis predicts poor anatomical and functional outcomes.
Sub-Tenon’s anesthesia and all surgical procedures were performed as previously described.9 Amaurosis was defined as the inability to see any light with the operative eye at one or more surgical steps after tightly covering the nonsurgical eye and removing the photo-bleaching effect.
Optical coherence tomography
All patients in the IMH group had been diagnosed with a full-thickness IMH by optical coherence tomography (OCT) before surgery. The central hole diameter and height were measured under OCT imaging. Repair of the IMH after surgery was categorized into three patterns based on a previous study:10 U-type, in which sealing of the hole occurs with the foveal morphology close to that of normal people; V-type, in which healing of the hole occurs with a steep foveal contour and coverage of the retinal pigment epithelium and choriocapillaris layers by moderately backscattering layers with a notch; and W-type, in which there is a foveal defect of the neurosensory retina but the edge of the hole is attached to the retinal pigment epithelial layer. Failure of healing was defined as non-closure of the hole and detachment of its edge from the retinal pigment epithelial layer.
In the EMM group, the macular fovea thickness was measured according to three-dimensional OCT imaging before and after surgery. Regression of macular edema was calculated as follows: preoperative macular fovea thickness − macular fovea thickness at the 4-month follow-up. The rate of macular edema regression was calculated as follows: regression of macular edema / preoperative macular fovea thickness.
Statistical analysis
The patients’ data were compared between amauosis and non-amaurosis in the IMH and EMM groups, respectively. Student’s t test and repeated-measures analysis of variance were used for the statistical analysis. A P value of <0.05 was considered statistically significant.
Results
Eighty-five consecutive patients diagnosed with IMH (n = 50) and EMM (n = 35) were enrolled in our case series, and 74 (87.1%) of these patients developed intraoperative amaurosis under sub-Tenon’s anesthesia. There was no significant difference in the incidence of intraoperative amaurosis between the IMH group (88.0%, 44/50) and EMM group (85.7%, 30/35).
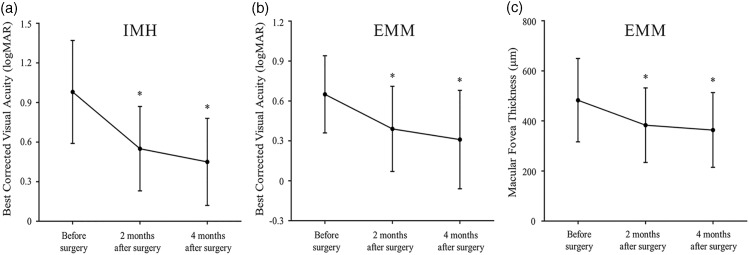
The best-corrected visual acuity (BCVA) in the IMH group showed significant improvement after surgery (0.98 ± 0.39 logMAR before surgery and 0.55 ± 0.32 and 0.45 ± 0.33 logMAR at the 2- and 4-month follow-up, respectively; P < 0.0001) (Figure 1(a)). At the 4-month follow-up, 96% of patients had gained visual improvement in BCVA, with 82% of them showing an increase of more than 2.0 (4.1 ± 2.3) Snellen lines. The IMH closure rate was 100.0%, including 38 with a U-type pattern, 10 with a V-type pattern, and 2 with a W-type pattern based on OCT images (Figure 2, Table 1). The base diameter (at the level of the retinal pigment epithelium) and minimum diameter (the minimal extent of the hole) were 865 ± 307 µm and 457 ± 179 µm, respectively. The IMH closure pattern was significantly correlated with the preoperative hole diameter (P < 0.0001). A hole with a smaller diameter was more likely to exhibit a U-type configuration after surgery. Two W-type closures were caused by a large hole diameter (656 and 601 µm, respectively). None of the patients developed recurrence of the IMH throughout the 4-month follow-up.
Figure 1.
Visual outcomes in patients with IMH and EMM before and after surgery. (a) Best-corrected visual acuity (BCVA) showed significant improvement in patients with IMH at both the 2- and 4-month follow-up compared with preoperatively. (b) BCVA showed significant improvement in patients with EMM at both the 2- and 4-month follow-up. (c) The macular foveal thickness in patients with EMM showed a significant decrease at the 4-month follow-up compared with preoperatively. *Paired-samples t test, P < 0.0001. IMH, idiopathic macular hole; EMM, epimacular membrane.
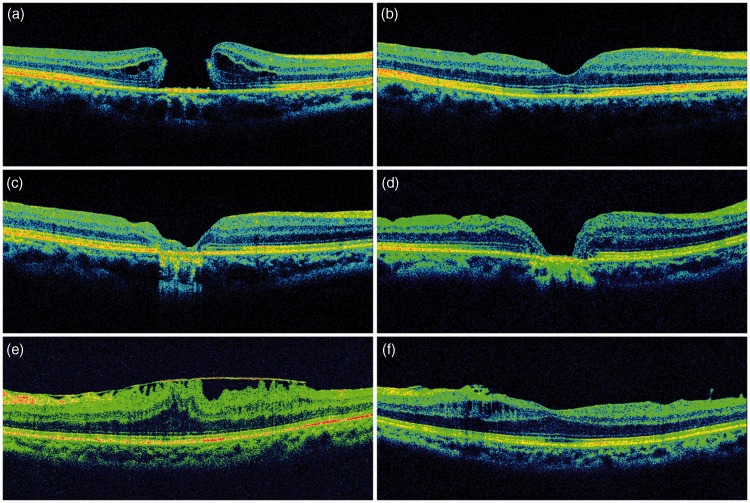
Figure 2.
Representative optical coherence tomography (OCT) images in patients with IMH and EMM who developed intraoperative amaurosis. (a) Preoperative OCT imaging showing full-thickness IMH in a patient with intraoperative amaurosis. IMH closure in (b) U-type, (c) V-type, and (d) W-type configuration after surgery in patients with IMH who developed intraoperative amaurosis. (e) Preoperative OCT image in a patient with EMM. (f) OCT demonstrated removal of the EMM and improvement of the macular fovea structure in the same patient after surgery. IMH, idiopathic macular hole; EMM, epimacular membrane.
Table 1.
Types of macular hole closure in patients with and without intraoperative amaurosis.
| U-type | V-type | W-type | Total | P* | |
|---|---|---|---|---|---|
| Intraoperative amaurosis | 34 | 8 | 2 | 44 | 0.620 |
| No intraoperative amaurosis | 4 | 2 | 0 | 6 | |
| Total | 38 | 10 | 2 | 50 | / |
*Pearson chi-square (α = 0.05).
The IMH group also showed no significant difference in the improvement of the postoperative BCVA between patients with and without amaurosis. The BCVA in patients with IMH was correlated with the time effect (P < 0.0001) rather than the grouping effect. Additionally, there was no significant difference in the IMH closure types between patients with and without amaurosis (Table 1).
In the EMM group, the BCVA also showed notable improvement after surgery (0.65 ± 0.29 logMAR before surgery and 0.39 ± 0.32 and 0.31 ± 0.37 logMAR at the 2- and 4-month follow-up, respectively; P < 0.0001) (Figure 1(b)). OCT revealed a decreased thickness of the macular fovea after surgery (482.9 ± 166.6 µm preoperatively, 383.1 ± 149.1 µm at 2 months, and 363.8 ± 149.3 µm at 4 months). A significant difference was found in the fovea thickness at both the 2- and 4-month follow-ups compared with that before surgery (P = 0.004 and 0.001, respectively) (Figure 1(c)). The rate of macular edema regression was 20.7% at 2 months and 24.7% at 4 months after surgery.
The EMM group also consistently showed no significant difference in the improvement of the postoperative BCVA between patients with and without amaurosis. The BCVA was correlated with a time effect (P = 0.005) rather than a grouping effect, suggesting no correlation between intraoperative amaurosis and the visual prognosis. Whether intraoperative amaurosis occurred had no impact on the reduction of macular edema or rate of macular edema regression (Table 2).
Table 2.
Postoperative regression of macular edema in patients with EMM who did and did not develop intraoperative amaurosis.
| Follow-up time | Intraoperative amaurosis | No intraoperative amaurosis | P* | |
|---|---|---|---|---|
| Macular edema regression (µm)† | 2 months | 84.9 ± 149.5 | 190.2 ± 372.8 | 0.262 |
| 4 months | 104.4 ± 139.0 | 232.3 ± 418.8 | 0.651 | |
| Rate of macular edema regression (%) | 2 months | 13.1 | 9.7 | 0.863 |
| 4 months | 19.0 | 11.7 | 0.779 |
*Independent-samples t test (α = 0.05)
†Data are presented as mean ± standard deviation.
EMM, epimacular membrane.
In addition, 49 patients with intraoperative amaurosis underwent pattern visual evoked potential (PVEP) examination before and 2 months after surgery, including 35 with IMH and 14 with EMM. The PVEP revealed no significant change in the P100 wave latency or amplitude, indicating that the presence of intraoperative amaurosis had no long-term impact on optic nerve transmission (Table 3). Finally, seven other patients who failed to attain a PVEP waveform preoperatively showed a significant P100 wave amplitude after surgery, implicating improvement of visual function.
Table 3.
Preoperative and postoperative PVEP examination in patients with intraoperative amaurosis.
| Patients | Preoperatively | 2 months postoperatively | ||
|---|---|---|---|---|
| n = 49* | P100 latency (ms)† | P100 amplitude (μV)† | P100 latency (ms)† | P100 amplitude (μV)† |
| 118.27 ± 10.28 | 13.20 ± 5.68 | 118.14 ± 10.62P = 0.930 | 12.97 ± 6.61P = 0.780 | |
*Forty-nine patients with intraoperative amaurosis underwent a pattern visual evoked potential (PVEP) examination preoperatively and 2 months postoperatively, including 35 with an idiopathic macular hole and 14 with an epimacular membrane.
†Data are presented as mean ± standard deviation
Discussion
The reported IMH closure rate using current standard surgical procedures ranges from 69% to 100%.11–14 In the present study, we attained a closure rate of 100%, which is comparable with previous studies. In addition, the BCVA in previous studies12–15 showed an average increase of two to three Snellen lines after surgery. In our study, 96% (48/50) of patients showed visual improvement after surgery, with 82% (41/50) increasing by more than two Snellen lines. The visual improvement in this study may have been partially due to the combination of phacoemulsification and intraocular lens implantation, which avoids the development of cataract that often occurs after simple PPV surgery. In addition, removal of the EMM or inner limiting membrane without indocyanine green staining can avoid potential toxicity to the macula.
The BCVA of patients with EMM increased from 0.65 ± 0.29 to 0.31 ± 0.37 logMAR, which is comparable with a previous study16 that showed a BCVA of 0.5 ± 0.2 logMAR and 0.43 logMAR before and 6 months after surgery, respectively. Another study17 showed a BCVA improvement of 0.14 ± 0.28 logMAR during the 4-month follow-up. Our study demonstrated regression in macular edema by 119.0 ± 185.0 µm after surgery, which is comparable with a previous study that showed a decrease of 87 µm in the macular thickness.18
In this study, 87.1% of patients developed intraoperative amaurosis. Loss of light perception during surgery can lead to anxiety and fear in patients and was once considered a surgical complication. We recently demonstrated that intraoperative amaurosis is common and occurs perhaps more often than expected in intraocular surgeries under sub-Tenon’s anesthesia.9 Although intraoperative amaurosis has been previously reported in individual patients, few studies with large sample sizes have focused on the mid- to long-term visual outcomes in these patients. Whether the intraoperative visual loss results from retinal ischemia or nerve transmission blockade and whether it indicates a poor prognosis for visual function after surgery remain to be further investigated. In this study, all patients recovered to at least light perception on the first postoperative day. Using OCT and PVEP, we found that the improvement of visual function at 2 and 4 months after surgery was similar between patients who did and did not develop intraoperative amaurosis. Additionally, closure of the IMH and regression of macular edema in patients with EMM in our study were consistent with the results of other studies. These results show that the presence of intraoperative amaurosis is transient and reversible and does not predict a poor surgical outcome.
The mechanism of intraoperative amaurosis under local anesthesia is controversial. Some researchers have considered that anesthetic injection increases the intraocular pressure, resulting in ischemia of the retina and optic nerve.19,20 However, studies have shown that the intraocular pressure only mildly increases without a significant difference after anesthetic injection.21,22 In this study, because the anesthetic administered via sub-Tenon’s injection could quickly enter the muscle pyramid and because patients received sufficient topical anesthesia before sub-Tenon’s block, we usually started the operation immediately after sub-Tenon’s anesthesia. The intraocular pressure was thus well maintained at a level of about 30 mmHg after the incision and vitreoperfusion were performed. During surgery, paleness of the optic disc and retina and pulsation of blood vessels (changes that indicate ocular ischemia) were not observed in all patients. Furthermore, visual function is difficult to restore if retinal ischemia lasts for more than 90 minutes; in the present study, however, visual improvement was observed in 96% of patients, and OCT showed no defect in the inner layer of the retina, which does not support the hypothesis of ischemia-based amaurosis. On the other side, some researchers believe that intraoperative amaurosis is caused by transient blockade of optic nerve transmission to the brain by the anesthetic.23–25 This assumption seems credible because all cases of intraoperative amaurosis in this study were reversible, and patients who developed amaurosis showed visual improvement comparable with that of patients who did not report loss of light perception. In addition, there was no decrease in the amplitude or latent phase prolongation in the PVEP pattern several months after surgery, indicating no damage to optic nerve transmission. Moreover, our previous study using VEP in New Zealand rabbits demonstrated significantly prolonged N1 latency and decreased P1 amplitude at 5 and 15 minutes after Sub-Tenon’s anesthesia, and both the latency and amplitude recovered to the baseline level 5 days afterwards.26 These data together with the findings in the present study provide some evidence for a transient visual transmission block as a reasonable explanation for anesthesia-induced amaurosis.
The incidence of intraoperative amaurosis in this case series was 87.1%, which is higher than that in previous studies (6.7% to 53.8%).3–7 One possible explanation for the difference seems to be that the nonsurgical eye was not completely covered during the investigation. Under routine eye draping, the nonsurgical eye, when opened, achieves light perception from the operative illumination, leading to a low detection rate of intraoperative amaurosis.8 This may explain why the incidence of lost light perception was low and widely varying; most studies did not clearly mention whether the nonsurgical eye was tightly covered during visual evaluation.
The main limitation of this study is the low number of patients who did not develop intraoperative amaurosis, making the statistical analysis slightly less persuasive. The difference in surgical outcomes between patients with and without intraoperative amaurosis needs further investigation with larger sample sizes and longer follow-up periods.
In summary, intraoperative amaurosis is a common event during sub-Tenon’s anesthesia-based intraocular surgery. The presence of amaurosis is not associated with structural changes in the macula and does not predict a poor surgical prognosis in patients, either functionally or anatomically. When a patient reports loss of light perception during surgery, the surgeon should relieve his or her anxiety through verbal communication rather than perform a special intervention.
Declaration of conflicting interests
The authors declare that there is no conflict of interest.
Funding
This research was supported by grants from the Foundations of Innovation and Strong School Project of Shantou University Medical College and Medical and Health Technology Plan of Shantou (No. 180726214011420) and the Science and Technology Planning Project of Guangdong Province of China (No. 2011B031800369) to Weiqi Chen.
ORCID iD
Zijing Huang https://orcid.org/0000-0003-2909-2538
References
- 1.Guise P. Sub-Tenon’s anesthesia: an update. Local Reg Anesth 2012; 5: 35–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kumar CM, Eid H, Dodds C. Sub-Tenon’s anaesthesia: complications and their prevention. Eye (Lond) 2011; 25: 694–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tan CS, Mahmood U, O'Brien PD, et al. Visual experiences during vitreous surgery under regional anesthesia: a multicenter study. Am J Ophthalmol 2005; 140: 971–975. [DOI] [PubMed] [Google Scholar]
- 4.Sugisaka E, Shinoda K, Sano RY, et al. Mechanism of visual sensations experienced during pars plana vitrectomy under retrobulbar anesthesia. Ophthalmologica 2010; 224: 103–108. [DOI] [PubMed] [Google Scholar]
- 5.Sugisaka E, Shinoda K, Ishida S, et al. Patients’ descriptions of visual sensations during pars plana vitrectomy under retrobulbar anesthesia. Am J Ophthalmol 2007; 144: 245–251. [DOI] [PubMed] [Google Scholar]
- 6.Vohra SB, Anya C, Farooq T, et al. Subjective visual perceptions during vitreoretinal surgery under local anaesthesia. Eye (Lond) 2009; 23: 1831–1835. [DOI] [PubMed] [Google Scholar]
- 7.Tan CSH. Subjective visual perceptions during vitreoretinal surgery under local anaesthesia. Eye (Lond) 2010; 24: 1417–1418. [DOI] [PubMed] [Google Scholar]
- 8.Zheng D, Huang Z, Zhang Q, et al. The impact of contralateral eye in intraoperative visual assessment during ophthalmic surgery. Int J Clin Exp Med 2019; 12: 800–804. [Google Scholar]
- 9.Zheng D, Huang Z, Zhang G, et al. Incidence and impact factors of intraoperative loss of light perception under sub-Tenon’s anesthesia in patients with macular diseases. Eye (Lond) 2019; 33: 1784–1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Imai M, Iijima H, Gotoh T, et al. Optical coherence tomography of successfully repaired idiopathic macular holes. Am J Ophthalmol 1999; 128: 621–627. [DOI] [PubMed] [Google Scholar]
- 11.Freeman WR, Azen SP, Kim JW, et al. Vitrectomy for the treatment of full-thickness stage 3 or 4 macular holes. Results of a multicentered randomized clinical trial. The Vitrectomy for Treatment of Macular Hole Study Group. Arch Ophthalmol 1997; 115: 11–21. [DOI] [PubMed] [Google Scholar]
- 12.Margherio RR, Margherio AR, Williams GA, et al. Effect of perifoveal tissue dissection in the management of acute idiopathic full-thickness macular holes. Arch Ophthalmol 2000; 118: 495–498. [DOI] [PubMed] [Google Scholar]
- 13.Ip MS, Baker BJ, Duker JS, et al. Anatomical outcomes of surgery for idiopathic macular hole as determined by optical coherence tomography. Arch Ophthalmol 2002; 120: 29–35. [DOI] [PubMed] [Google Scholar]
- 14.Brooks HL., Jr. Macular hole surgery with and without internal limiting membrane peeling. Ophthalmology 2000; 107: 1939–1948. [DOI] [PubMed] [Google Scholar]
- 15.Thompson JT, Smiddy WE, Glaser BM, et al. Intraocular tamponade duration and success of macular hole surgery. Retina 1996; 16: 373–382. [DOI] [PubMed] [Google Scholar]
- 16.Frisina R, Pinackatt SJ, Sartore M, et al. Cystoid macular edema after pars plana vitrectomy for idiopathic epiretinal membrane. Graefes Arch Clin Exp Ophthalmol 2015; 253: 47–56. [DOI] [PubMed] [Google Scholar]
- 17.Laban KG, Scheerlinck LM, Van Leeuwen R. Prognostic factors associated with visual outcome after pars plana vitrectomy with internal limiting membrane peeling for idiopathic epiretinal membrane. Ophthalmologica 2015; 234: 119–126. [DOI] [PubMed] [Google Scholar]
- 18.Shahzadi B, Rizvi SF, Latif K, et al. Visual and anatomical outcomes following idiopathic macular epiretinal membrane surgery. J Coll Physicians Surg Pak 2016; 26: 971–974. [PubMed] [Google Scholar]
- 19.Fry RA, Ring P. Cilioretinal artery occlusion associated with Sub-Tenon’s regional blockade. Clin Exp Ophthalmol 2008; 36: 196–197. [DOI] [PubMed] [Google Scholar]
- 20.Pianka P, Weintraub-Padova H, Lazar M, et al. Effect of sub-Tenon’s and peribulbar anesthesia on intraocular pressure and ocular pulse amplitude. J Cataract Refract Surg 2001; 27: 1221–1226. [DOI] [PubMed] [Google Scholar]
- 21.Patton N, Malik TY, Aslam TM, et al. Effect of volume used in sub-Tenon’s anaesthesia on efficacy and intraocular pressure: a randomized clinical trial of 3 mL versus 5 mL. Clin Exp Ophthalmol 2004; 32: 488–491. [DOI] [PubMed] [Google Scholar]
- 22.Alwitry A, Koshy Z, Browning AC, et al. The effect of sub-Tenon’s anaesthesia on intraocular pressure. Eye (Lond) 2001; 15: 733–735. [DOI] [PubMed] [Google Scholar]
- 23.Talks SJ, Chong NH, Gibson JM, et al. Visual acuity and pupillary reactions after peribulbar anaesthesia. Br J Ophthalmol 1994; 78: 41–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ramsay AS, Ray-Chaudhuri N, Dayan M, et al. Quantification of relative afferent pupillary defects induced by posterior sub-Tenon’s, peribulbar, and retrobulbar anaesthetics. Br J Ophthalmol 2001; 85: 1445–1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoffman RS, Fine IH. Transient no light perception visual acuity after intracameral lidocaine injection. J Cataract Refract Surg 1997; 23: 957–958. [DOI] [PubMed] [Google Scholar]
- 26.Wu X, Jhanji V, Chen H, et al. Change in flash visual evoked potentials in New Zealand albino rabbits after sub-tenon’s anesthesia. Cutan Ocul Toxicol 2017; 36: 118–124. [DOI] [PubMed] [Google Scholar]