Abstract
Oral microbiome research has moved from asking “Who’s there?” to “What are they doing?” Understanding what microbes “do” involves multiple approaches, including obtaining genomic information and examining the interspecies interactions. Recently we isolated a human oral Saccharibacteria (TM7) bacterium, HMT-952, strain TM7x, which is an ultrasmall parasite of the oral bacterium Actinomyces odontolyticus. The host-parasite interactions, such as phage-bacterium or Saccharibacteria–host bacterium, are understudied areas with large potential for insight. The Saccharibacteria phylum is a member of Candidate Phyla Radiation, a large lineage previously devoid of cultivated members. However, expanding our understanding of Saccharibacteria-host interactions requires examining multiple phylogenetically distinct Saccharibacteria-host pairs. Here we report the isolation of 3 additional Saccharibacteria species from the human oral cavity in binary coculture with their bacterial hosts. They were obtained by filtering ultrasmall Saccharibacteria cells free of other larger bacteria and inoculating them into cultures of potential host bacteria. The binary cocultures obtained could be stably passaged and studied. Complete closed genomes were obtained and allowed full genome analyses. All have small genomes (<1 Mb) characteristic of parasitic species and dramatically limited de novo synthetic pathway capabilities but include either restriction modification or CRISPR-Cas systems as part of an innate defense against foreign DNA. High levels of gene synteny exist among Saccharibacteria species. Having isolates growing in coculture with their hosts allowed time course studies of growth and parasite-host interactions by phase contrast, fluorescence in situ hybridization, and scanning electron microscopy. The cells of the 4 oral Saccharibacteria species are ultrasmall and could be seen attached to their larger Actinobacteria hosts. Parasite attachment appears to lead to host cell death and lysis. The successful cultivation of Saccharibacteria species has significantly expanded our understanding of these ultrasmall Candidate Phyla Radiation bacteria.
Keywords: human, mouth, microbiota, parasite, culture technique, genomics
Introduction
Molecular methods such as 16S rRNA gene sequencing and, more recently, metagenomic analyses have revealed the vast diversity of microbes on Earth. Only a fraction of microbes have been cultured, and a majority of these are from a limited number of well-known, easy-to-culture phyla (Stewart 2012). The inability of microbiologists to culture organisms from major segments of microbial diversity is a key impediment to advancing the field, as it prevents investigators from performing detailed scientific studies, such as examining microbe-microbe or microbe-host interactions, characterizing lipids and other small molecules, engineering genetic modifications, and exploiting industrial potential. Here we report the isolation and characterization of Saccharibacteria strains representing 3 previously uncultured human microbial taxa (Dewhirst et al. 2010; Escapa et al. 2018).
Early studies of microbial diversity with 16S rRNA methods identified many novel bacterial phyla, such as TM7 (Hugenholtz et al. 1998), currently named Saccharibacteria. The Candidate Phyla Radiation (CPR) is a recently discovered major lineage of previously uncultured phyla (Brown et al. 2015) whose genetic potential is being studied intensively (Danczak et al. 2017). The CPR is predicted to comprise roughly one-quarter of bacterial diversity on Earth and to contain >73 individual phyla, including the Saccharibacteria. Using genome comparison methods, Parks et al. (2018) proposed a standardized bacterial taxonomy that places the CPR lineage in a single phylum. Regardless of how taxonomic issues are resolved, the CPR lineage is an important subdivision of Bacteria. Saccharibacteria species are routinely detected in many natural environments (Hugenholtz et al. 2001) and in the microbiome of several mammals, including human (Ouverney et al. 2003; Marcy et al. 2007; Camanocha and Dewhirst 2014). CPR organisms share small genomes and ultrasmall cell sizes (Miyoshi et al. 2005; Brown et al. 2015; Luef et al. 2015). The genomes of CPR bacteria often lack numerous biosynthetic pathways, such as those for the synthesis of fatty acids, nucleotides, amino acids, and vitamin cofactors (Brown et al. 2015).
To date, strains representing species in the phylum Saccharibacteria are the only CPR bacteria to be cultured. The first isolate, Saccharibacteria bacterium HMT-952, strain TM7x (He et al. 2015), was recently designated Nanosynbacter lyticus (McLean et al. 2018). With an ultrasmall cell size (200 to 300 nm) and highly reduced genome of 699 genes (705 kb), TM7x cannot synthesize any amino acids, vitamins, or cell wall precursors, and it displays an unconventional lifestyle as an obligate epiparasite (Baker et al. 2017; Bor et al. 2019). TM7x is parasitic in nature and grows on its Actinomyces hosts, forming stable long-term binary cocultures in vitro. It can be separated from its host and transferred to infect new host strains and phylogenetically related species in the genus Actinomyces (Bor et al. 2018). Strains representing 3 additional human oral taxa of Saccharibacteria have recently been described by Podar’s laboratory (Cross et al. 2019).
The primary goal of this research was to isolate and culture novel Saccharibacteria from the human oral cavity based on insights from the first cultivation of the strain, TM7x. A second goal was to characterize the novel Saccharibacteria strains in coculture with their previously unknown hosts and obtain biological information not predictable from simple in silico genome analyses. This work reports the isolation of strains of 3 novel species of human oral Saccharibacteria and provides preliminary phenotypic and genotypic descriptions.
Materials and Methods
Approach and Rationale
On the basis of the knowledge gained from examining Saccharibacteria strain TM7x (He et al. 2015), we attempted to isolate novel human oral Saccharibacteria from clinical samples by filtering the ultrasmall Saccharibacteria cells free from other bacteria and inoculating them into cultures of potential host or “bait” species. Success was defined as establishing a coculture of novel Saccharibacteria species with a host species that could be stably passaged.
Bacterial Media and Culture Conditions
Potential host bacteria were revived and passaged on brain heart infusion agar (Becton, Dickinson), trypticase soy agar (Becton, Dickinson), or trypticase soy agar and brain heart infusion (1:1) with yeast extract (TSBY; 10 g/L). Sheep’s blood (5%) was routinely added to TSBY agar plates. Hemin (5 mg/L), the vitamin K precursor 1,4-dihydroxy-2-naphthoic acid (DHNA; 50 µg/L), nicotinamide adenine dinucleotide (NAD+; 1 mg/L), and hog gastric mucin (1 g/L; Sigma-Aldrich) were added as noted for culture of some fastidious hosts. Broth media—namely, brain heart infusion broth, TSBY broth, or TSBY mixed 1:1 with RPMI 1640 (Gibco)—were used for coculture of Saccharibacteria and hosts (see Appendix Table 1).
Anaerobic culture was performed in an anaerobic chamber (Coy) at 37 °C with an atmosphere of 5% H2, 10% CO2, and 85% N2. Microaerophilic culture was performed in a hypoxic chamber (Coy or Don Whitely) at 37 °C with an atmosphere of 2% O2, 5% CO2, and 93% N2. Aerobic culture was performed in a warm room (37 °C) in air.
Subjects and Sampling
Forty-four subjects aged ≥18 y were recruited to provide oral samples for bacterial cultivation. Sex balance and racial diversity were sought; the study had Institutional Review Board approval (13-14, Forsyth; 13-001075, University of California Los Angeles); and all subjects provided informed consent. Supragingival plaque samples were collected with Gracey curettes and dispersed in 5 mL of Maximum Recovery Diluent buffer (Sigma-Aldrich). Two milliliters of unstimulated saliva was collected by having the subject drool into a sterile 15-mL screw cap centrifuge tube. Saliva samples were diluted 2-fold with sterile phosphate-buffered saline before being subject to filtration.
Isolation of Saccharibacteria Strains in Coculture with Host Bacteria
Because Saccharibacteria cells are known to be ultrasmall (<0.2 µm; Miyoshi et al. 2005; He et al. 2015) they were separated from other bacteria in clinical samples by filtration. Saliva samples were filtered through 0.45-µm polyvinylidene fluoride membranes and plaques samples through 0.2-µm track-etched polycarbonate filters (Millipore). The filtered Saccharibacteria cells were pelleted by ultracentrifugation (saliva, 80,000g; plaque, 60,000g), an approach modified from a reported method for extracellular vesicle enrichment (Théry et al. 2006). The collected and resuspended Saccharibacteria cells were added to overnight broth monocultures of several potential host or bait species, and the resultant cocultures were passaged every 24 to 48 h for at least 5 passages. The presence of Saccharibacteria cells in the final cultures was confirmed by microscopic examination and specific polymerase chain reaction for Saccharibacteria (see Appendix).
Bacterial Imaging
Detailed imaging methods can be found in the Appendix. In brief, bacterial monocultures or binary cocultures were grown to an early stationary phase and processed for imaging. For fluorescence in situ hybridization (FISH), cells were fixed and followed by DNA probe labeling in hybridization buffer (Bor et al. 2016). Stained cells were imaged with fluorescence confocal microscope. For scanning electron microscopy (SEM) imaging, cells were seeded on a glass coverslip, followed by treatment with fixing reagent overnight. Cells were washed, with critical points dried and coated with platinum-palladium before imaging.
Additional Methods
In addition to imaging methods, the Appendix contains detailed methods for polymerase chain reaction detection of Saccharibacteria, genome sequencing, genome assembly and annotation, acquisition and analysis of growth curves, and testing of Saccharibacteria species host ranges.
Results
Isolation and Cultivation of Novel Saccharibacteria Species
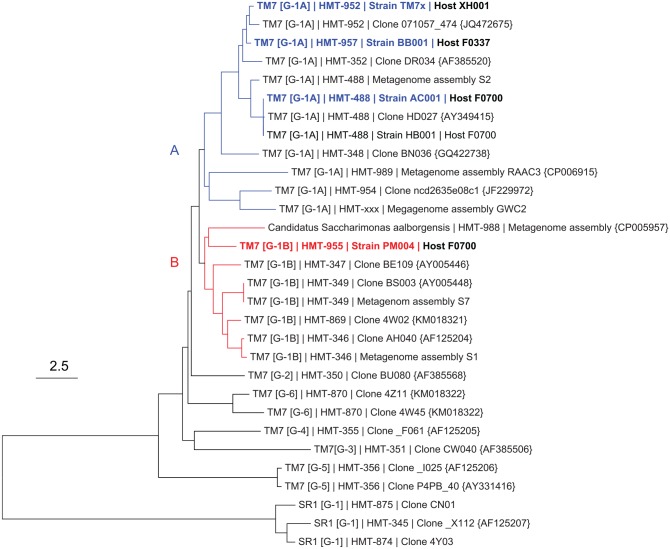
Several potential host or bait species were used in experiments that led to the successful isolation of 3 novel Saccharibacteria species. The 18 potential host bacteria species tried in the isolation experiments, including medium and atmosphere conditions, are presented in Appendix Table 1. Of 18 bait species, 2 were successful: 1) Saccharibacteria HMT-957, strain BB001, was isolated on Actinomyces sp. HMT-171, strain F0337; 2) Saccharibacteria HMT-488, strain AC001, and Saccharibacteria HMT-955, strain PM004, were isolated on Pseudopropionibacterium propionicum HMT-739, strain F0230. A maximum likelihood 16S rRNA phylogenetic tree showing the relationship of these species to other Saccharibacteria is shown in Figure 1. Note that strains TM7x, BB001, and AC001 are closely related and fall in G-1 subgroup A (blue), while PM004 was in the phylogenetically distinct G-1 subgroup B (red). The percentage similarity among these 4 species is presented in Appendix Table 2.
Figure 1.
Neighbor-joining 16S rRNA phylogenetic gene tree for candidate division Saccharibacteria. Blue (group G-1A) and red (group G-1B) are separately rooted monophyletic clades that both fall into group 1 (Camanocha and Dewhirst 2014). Blue and red bold strains are currently isolated strains and used in this study. The scale is 2.5 substitutions per site.
Genome Sequencing, Description, and Comparative Genomics
Complete closed genomes were obtained for 3 newly isolated Saccharibacteria. Three environmental Saccharibacteria genomes (RAAC3 and GWC2 from groundwater and Saccharimonas aalborgensis from sludge bioreactor) were available as closed assemblies at the time of this study (Albertsen et al. 2013; Kantor et al. 2013) and were included in genome comparisons across the G1 group.
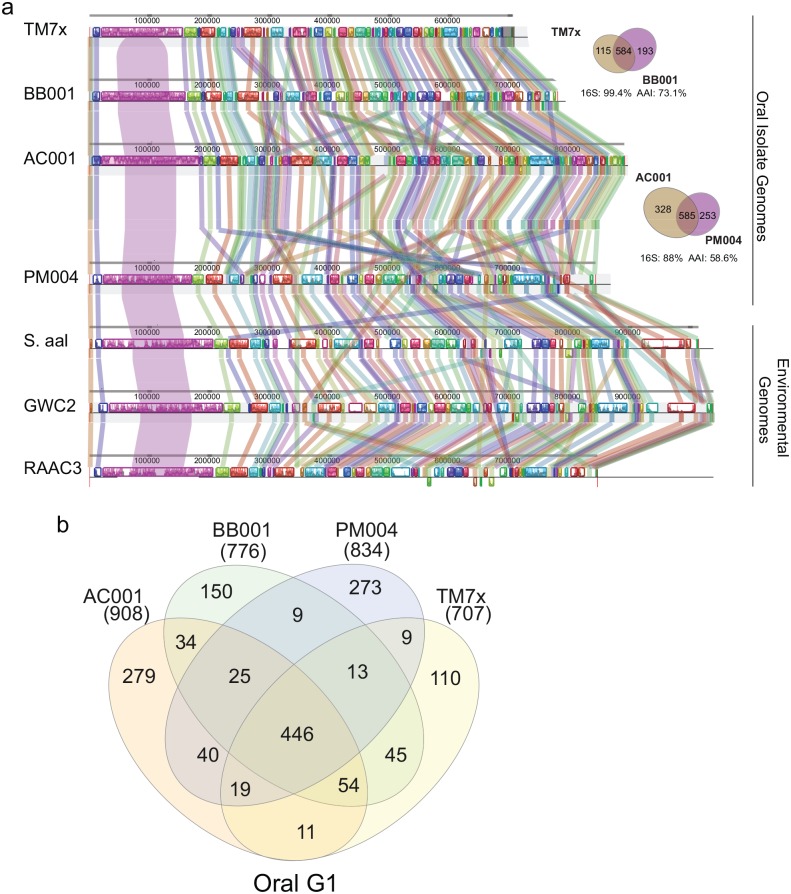
All 7 complete genomes were small, with most being <1 Mb. Three environmental Saccharibacteria genomes had relatively larger genome sizes (845, 1,013, 1,039 kb) with a mean length of 966 kb and 1,003 open reading frames, while the 4 oral strains had smaller genomes (705, 780, 890, 842 kb) with a mean size of 804 kb and 806 open reading frames (Fig. 2A, Appendix Table 3). After the start position for all genomes was reassigned with dnaA, the whole genomes were aligned. Remarkably, the maintenance of gene order and large syntenic blocks of genes across 500 kb between the oral and environmental genomes, previously seen with just a few Saccharibacteria genomes (He et al. 2015; McLean et al. 2018), was again very evident across this new set (Fig. 2A).
Figure 2.
Saccharibacteria genome comparisons. (A) Closed contigs for 4 oral (top) and 3 environmental (bottom) genomes were aligned after reassigning the start position for all genomes using dnaA with progressive Mauve (Darling et al. 2004). Syntenic blocks are colored to locate regions in each genome that are similar. The bar chart within the blocks shows the percentage of Blastn identity. (B) Venn diagram shows unique and shared genes of 4 oral Saccharibacteria strains. See Appendix Tables 4 and 6 for specific gene list.
The 4 oral Saccharibacteria isolates shared 446 core genes (Fig. 2B, Appendix Table 4), of which 129 (28.9%) cannot be assigned a specific biological function (hypothetical) and the remaining 317 were annotated (71.1%) and placed into a specific functional group (Appendix Fig. 1A, Appendix Table 5). Four groups accounted for 69% of the core genes—specifically, hypothetical, translation, DNA, and RNA metabolism genes. Consistent with previous studies (McLean et al. 2018), many of the biosynthetic pathways were incomplete, such as lipid, nucleotide, and essential amino acid biosynthesis pathways. In addition, when the core functional categories were compared with that of the synthetic minimal genome (531 kb, 473 genes) of independently growing Mycoplasma mycoides JCVI-syn3.0 (Appendix Fig. 1B; Hutchison et al. 2016), it was evident that JCVI-syn3.0 had significantly more functionality in the categories of biosynthesis and processing of macromolecules such as lipids, nucleotides, and cofactors/vitamins (Appendix Fig. 1C). TM7x, BB001, AC001, and PM004 had 110, 150, 279, and 273 unique genes, respectively (Fig. 2B, Appendix Table 6). Unfortunately, the majority of the unique genes (70% to 77%) cannot be assigned a specific biological function.
Differential Defense Systems against Foreign DNA
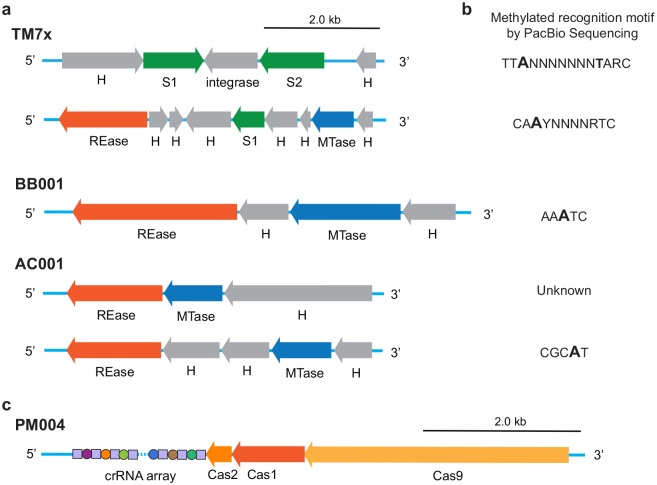
One of the more prominent features of the unique genes among the 4 strains were defense systems against invasive foreign DNA. Restriction-modification (RM) systems are prominent defenses that utilize 1) a restriction endonuclease, which cuts inappropriately methylated DNA sequences on foreign DNA within a specific target motif, and 2) a methyltransferase, which protects the same motif on the host genome through the addition of a methyl group (m6A/m4C/m5C). These systems can be differentiated into 4 types (types I to IV) based on their recognized target, subunit composition, and substrate specificity. RM systems were found encoded in all strains except for PM004 (Fig. 3A). PacBio sequencing enabled single-base resolution of methylated bases across genomes (Flusberg et al. 2010), and in combination with the Restriction Enzyme Database, we confirmed the activity status of each system (Appendix Table 7). TM7x contained 2 type I systems (modified motifs: TTAN7TARC and CAAYN4RTC); AC001 contained a type II system (predicted to modify a currently unknown m5C-based target motif) and type III system (modified motif: CCGAT); and BB001 contained a single type III system (modified motif: AAATC; Fig. 3B). In each strain, despite the diversity in RM systems found, all strains contained a single RM system/mobile genetic element within a conserved genetic locus, flanked by a 5′ 1.7-kb region containing a peptidoglycan-N-acetylmuramic acid deacetylase gene and a 3′ 5.2-kb region containing genes associated with ferredoxin reductase and an anaerobic ribonucleoside-triphosphate reductase (Appendix Fig. 2), perhaps indicating an integrative hotspot for such a mobile genetic element.
Figure 3.
Restriction-modification (RM) and CRISPR-Cas system of cultivated Saccharibacteria strains. (A) Schematic diagram of TM7x (Type I/Type I), BB001 (Type III), and AC001 (Type II/Type III) RM systems. Hypothetical (H) and non-RM system proteins are shown in gray. The MTase (blue) and specificity subunit S (green) form an active multisubunit protein that methylates bipartite DNA recognition sequence. In the presence of REase subunit (orange), the complex can also act as an endonuclease. (B) Predicted recognition sequences of each RM system types shown in panel A. In each motif, base modifications that were detected during PacBio SMRT sequencing are shown in bold. (C) Gene cluster organization of PM004 CRISPR-Cas loci includes genes for the Cas9, Cas1, and Cas2 proteins with the CRISPR array.
PM004 was the only strain to have CRISPR-Cas genes. The CRISPR-Cas system is an RNA-guided adaptive immune system against invasive genetic elements (Jinek et al. 2012) and is detected in various CPR organisms, including the oral Saccharibacteria (Burstein et al. 2017; McLean et al. 2018). PM004 CRISPR-Cas loci consisted of cas9, cas1, cas2, and CRISPR RNA array in a type IIC CRISPR system arrangement (Fig. 3C). Of all the CRISPR-Cas systems, type IIC is the simplest, consisting of the fewest cas genes (Chylinski et al. 2013). The PM004 CRISPR RNA array consists of 24 unique spacers flanked by the same 25-repeating sequence (Fig. 3C, Appendix Table 8). Blasting the spacer sequences in the Actinobacteriophage database or other phage databases did not yield confident identification of the target phage. The cas9 endonuclease was 1,119 amino acids in length and most closely related to other Saccharibacteria cas9 proteins in the G-1 phylogenetic group, followed by Clostridium and Geobacillus cas9 genes (NCBI blast).
Characterization of Saccharibacteria Species and Their Hosts by Microscopy
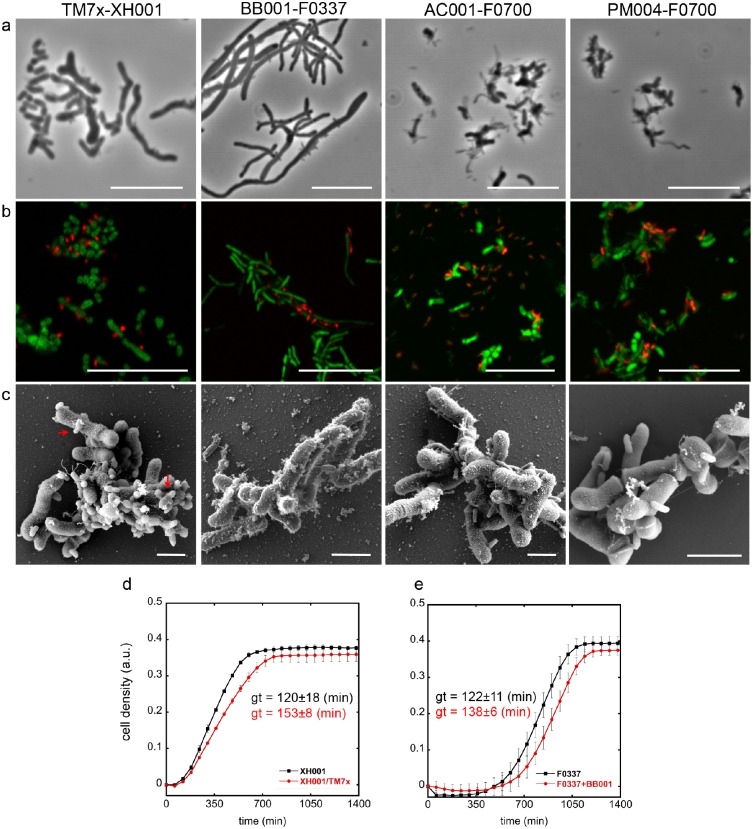
The cocultivation of Saccharibacteria with its bacterial host allowed a detailed characterization of cell morphology with various imaging techniques. All images were taken at the early stationary phase. Phase contrast and FISH imaging showed that the new strains lived on the surface of their host bacteria. While TM7x and BB001 appeared mainly as a small cocci, AC001 and PM004 were a mix of small cocci and rods (Fig. 4A, B; Appendix Fig. 3A, B). SEM images largely supported these findings, although TM7x and BB001 appeared to be small rods rather than cocci (Fig. 4C, Appendix Fig. 3C), suggesting that the resolution of phase contrast and FISH imaging does not allow accurate distinction. Infected host cells were observed with 1 to >10 Saccharibacteria attached. TM7x morphology was distinct from the other Saccharibacteria species in that they were often “bowling pin” shaped, which may represent budding (Fig. 4C). Furthermore, all 3 newly isolated Saccharibacteria strains were shown to induce elongation and swelling of their host bacteria (Fig. 4A, Appendix Fig. 3A) as previously seen with TM7x (Bedree et al. 2018; Bor et al. 2018). Cells experiencing stress often show elongation and swelling, and it is not surprising for cells being parasitized to exhibit symptoms of stress.
Figure 4.
Phenotypic characterization of isolated Saccharibacteria strains. (A) Phase contrast, (B) fluorescence in situ hybridization (FISH), and (C) scanning electron microscope images were taken for each Saccharibacteria strain–host binary coculture and compared with that of the monocultures (see Appendix Fig. 3). In the FISH images, red and green represent Saccharibacteria and host bacteria, respectively. Scale bars are 10 µm for phase contrast and FISH images and 1 µm for the scanning electron microscope images. Red arrows point out the “bowling pin”–like morphology of TM7x. (D, E) Growth curve of 2 Saccharibacteria–host binary cocultures (TM7x, BB001) and host monocultures were acquired with image-based cell density measurement by oCelloScope. Generation time (gt) for each mono- and coculture was determined (see Methods and Appendix Table 9) and presented next to the line graph in matching color. Values are means of 3 independent generation times with SD as an error bar.
Impact of Parasitism on Host Growth
Parasitism did slow the growth of host species as determined by monitoring the cell density through image-based oCelloScope measurements (Fig. 4D, E). Binary cocultures of TM7x/XH001 and BB001/F0337 had slight but significantly increased generation/doubling times, 153 ± 8 and 138 ± 6 min (mean ± SD), respectively, as compared with the monocultures of XH001 and F0337, 120 ± 18 and 122 ±11 min (Fig. 4D, E; Appendix Table 9), while their maximum cell density at the stationary phase was comparable. Growth curves for AC001 and PM004 with Pseudopropionibacterium propionicum F0700 were precluded by clumping of host cells. The binary coculture of AC001/F0700 could be passaged indefinitely by 1:10 dilution into fresh medium. Binary coculture of PM004/F0700 required addition of fresh host at each passage, as essentially all host cells were killed at 48 h. Consistent with growth characteristics, live-dead staining of the stationary phase cocultures demonstrated that cocultures of TM7x/XH001 and BB001/F0337 had marginally more dead cells than their monoculture hosts (Appendix Fig. 4A, B). In contrast, AC001/F0700 and PM004/F0700 cocultures displayed drastically increased cell death when compared with F0700 monoculture (Appendix Fig. 4C), as reflected by the increased red signals from the membrane-compromised dead cells.
Host Range of Saccharibacteria Strains
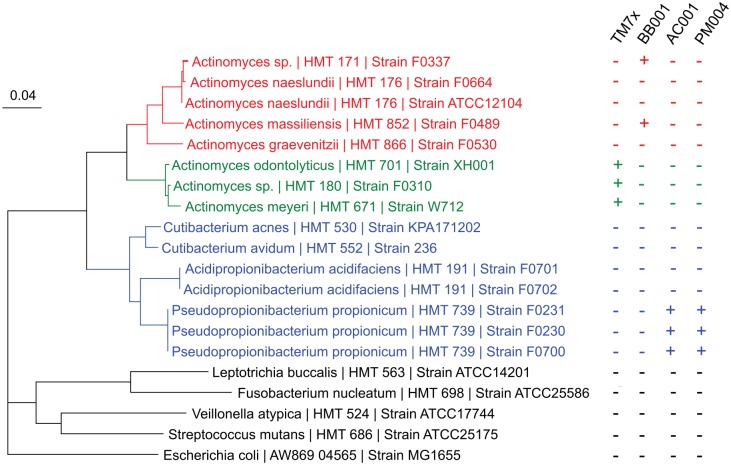
The results of studies to examine the host range of the 4 isolated Saccharibacteria species is shown in Figure 5. Saccharibacteria HMT-952 (TM7x) and Saccharibacteria HMT-957 (BB001), while closely related by 16S rRNA sequence identity (99.4%; Fig. 2) and shared genomic amino acid identity (73.1%), preferred different sets of Actinomyces species from different clades (Fig. 5; red vs. green clade). However, Saccharibacteria HMT-488 (AC001) and Saccharibacteria HMT-955 (PM004), which are phylogenetically distant by 16S rRNA (88%; Fig. 2) and share low amino acid identity (58.6%), parasitized strains of the single species P. propionicum (Fig. 5, blue clade). All 4 oral strains failed to infect and stably grow with other common oral bacterial strains tested (Fig. 5, dark clade).
Figure 5.
Host range of cultivated isolates. Phylogenetic tree was created with the 16S rRNA sequences of different Actinomyces sp. (red and green), Pseudopropionibacterium sp. (blue), and common oral bacteria (black). Attempts were made to infect each bacterium with each of the 4 isolated Saccharibacteria species. Those that supported and did not support the growth of each Saccharibacteria are denoted by plus (+) and minus (–) signs, respectively. The scale equals 0.04 substitutions per site. TM7x is a strain of Saccharibacteria HMT-952; BB001 is a strain of Saccharibacteria HMT-957; AC001 is a strain of Saccharibacteria HMT-488; and PM004 is a strain of Saccharibacteria HMT-955.
Discussion
The human oral cavity is reported to have at least 16 species of Saccharibacteria (Escapa et al. 2018). Including the initial isolate, TM7x (He et al. 2015), the 3 species isolated in this work, and the 4 species reported by Podar’s laboratory (Cross et al. 2019), half of the human oral Saccharibacteria species have been cultured by oral microbiologists. The approach of taking complex microbial samples from oral sites, filtering to remove all but ultrasmall bacteria, and then inoculating them into broth culture of potential host or bait bacterial species has been validated by this work.
Having Saccharibacteria species in binary coculture with their hosts has allowed isolation of significant biomass, production of tens of micrograms of DNA, and highly accurate sequencing to produce closed and complete genomes. Comparative genomics demonstrates that the 3 novel species have greatly reduced genomes and lack many pathways for synthesis of fatty acids, amino acids, nucleotides, and vitamin cofactors. This is fully consistent with these bacteria being obligate parasites. The remarkable degree of genomic synteny across genomes may reflect strong selection for functional relationships among genes within highly streamlined genomes. The smaller size of Saccharibacteria species genomes in species living in the human oral microbiome versus those in the environment may reflect the stability of the microbial consortia in the oral niche.
While restriction-modification systems are common to CPR bacteria (Westra et al. 2015), CRISPR-Cas systems are uncommon in environmental CPR (Burstein et al. 2016) but have been found in Saccharibacteria species associated the human and mammalian oral cavity (McLean et al. 2018). The presence of a CRISPR-Cas system in Saccharibacteria HMT-955, strain PM004, growing in stable coculture with its host should facilitate future molecular characterization studies. By working with the new isolates, it should be possible to examine parasite (or symbiont)–host relationships at 4 levels: phage, Saccharibacteria, bacterial host, and human host (or animal model).
Establishing 4 Saccharibacteria species in stable long-term binary coculture with hosts has allowed us to perform longitudinal microscopy and growth studies of host parasite interactions. While species differences exist, the 4 Saccharibacteria species all eventually killed host cells to which they attached, prevented division of infected cells, and slowed the overall growth rate of infected host cultures. Human oral Saccharibac-teria seem to infect specific host bacteria, possibly through binding to specific cell surface structures that are shared by only closely related hosts. It is interesting that 2 closely related Saccharibacteria have phylogenetically distinct hosts, while 2 distantly related Saccharibacteria have the same host bacteria. As we learn more about the attachment of Saccharibacteria to their host bacteria, it will be interesting to compare and contrast attachment mechanisms used by Saccharibacteria and phage. Much remains to be learned about the full life cycle of Saccharibacteria and other CPR bacteria and their interactions with hosts, but having multiple human oral Saccharibacteria in binary coculture with their hosts provides an excellent foundation for future studies.
Author Contributions
B. Bor, A.J. Collins, P.P. Murugkar, J.S. McLean, contributed to conception, design, data acquisition, analysis, and interpretation, drafted and critically revised the manuscript; S. Balasubramanian, contributed to data acquisition, critically revised the manuscript; T.T. To, E.L. Hendrickson, contributed to data analysis, critically revised the manuscript; J.K. Bedree, contributed to data analysis, drafted and critically revised the manuscript; F.B. Bidlack, contributed to data acquisition and analysis, drafted and critically revised the manuscript; C.D. Johnston, contributed to design, data acquisition, analysis, and interpretation, drafted and critically revised the manuscript; W. Shi, contributed to conception and data interpretation, drafted the manuscript; X. He, F.E. Dewhirst, contributed to conception, design, data analysis, and interpretation, drafted and critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
Supplemental Material
Supplemental material, DS_10.1177_0022034520905792 for Insights Obtained by Culturing Saccharibacteria With Their Bacterial Hosts by B. Bor, A.J. Collins, P.P. Murugkar, S. Balasubramanian, T.T. To, E.L. Hendrickson, J.K. Bedree, F.B. Bidlack, C.D. Johnston, W. Shi, J.S. McLean, X. He and F.E. Dewhirst in Journal of Dental Research
Acknowledgments
We thank Susan Yost, Molly Murnane, and Jessica Woods for microbial technical assistance. We thank Sean Cotton and Alexis Kokaras for their assistance in obtaining PacBio genomes for Saccharibacteria and their hosts. We thank Carolyn Marks for help with sample preparation and SEM imaging at the Harvard Center for Nanoscale Structures. This work was performed in part at the Center for Nanoscale Systems, Harvard University. We thank Bruce Paster, Anne Tanner, and Margaret Duncan for helpful discussion of this research project. We thank Heike Boisvert for initial work demonstrating that Saccharibacteria bacterium HMT-488 could be passaged in complex coculture.
Footnotes
A supplemental appendix to this article is available online.
W.S. is an employee of C3J Therapeutics, Inc., which has licensed technologies from the University of California Regents that could be indirectly related to this research project. The remaining authors declare no further potential conflicts of interest with respect to the authorship and/or publication of this article.
Research in this publication was supported by the National Institute of Dental and Craniofacial Research of the National Institutes of Health under awards 1R01DE023810, 1R01DE020102, 1R01DE026186 (to X.H., J.S.M., and W.S.); R37DE016937, DE024468, and R01DE 027850 (to F.E.D and C.D.J); F31DE026057 (to J.K.B.); and F32DE025548-01 and 1K99DE027719-01 (to B.B.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
ORCID iDs: B. Bor  https://orcid.org/0000-0002-1797-1730
https://orcid.org/0000-0002-1797-1730
References
- Albertsen M, Hugenholtz P, Skarshewski A, Nielsen KL, Tyson GW, Nielsen PH. 2013. Genome sequences of rare, uncultured bacteria obtained by differential coverage binning of multiple metagenomes. Nat Biotechnol. 31(6):533–538. [DOI] [PubMed] [Google Scholar]
- Baker JL, Bor B, Agnello M, Shi W, He X. 2017. Ecology of the oral microbiome: beyond bacteria. Trends Microbiol. 25(5):362–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedree JK, Bor B, Cen L, Edlund A, Lux R, McLean JS, Shi W, He X. 2018. Quorum sensing modulates the epibiotic-parasitic relationship between Actinomyces odontolyticus and its saccharibacteria epibiont, a Nanosynbacter lyticus strain, TM7x. Front Microbiol. 9:2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bor B, Bedree JK, Shi W, McLean JS, He X. 2019. Saccharibacteria (TM7) in the human oral microbiome. J Dent Res. 98(5):500–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bor B, McLean JS, Foster KR, Cen L, To TT, Serrato-Guillen A, Dewhirst FE, Shi W, He X. 2018. Rapid evolution of decreased host susceptibility drives a stable relationship between ultrasmall parasite TM7x and its bacterial host. Proc Natl Acad Sci U S A. 115(48):12277–12282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bor B, Poweleit N, Bois JS, Cen L, Bedree JK, Zhou ZH, Gunsalus RP, Lux R, McLean JS, He XS, et al. 2016. Phenotypic and physiological characterization of the epibiotic interaction between TM7x and its basibiont Actinomyces. Microbial Ecology. 71(1):243–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown CT, Hug LA, Thomas BC, Sharon I, Castelle CJ, Singh A, Wilkins MJ, Wrighton KC, Williams KH, Banfield JF. 2015. Unusual biology across a group comprising more than 15% of domain Bacteria. Nature. 523(7559):208–211. [DOI] [PubMed] [Google Scholar]
- Burstein D, Harrington LB, Strutt SC, Probst AJ, Anantharaman K, Thomas BC, Doudna JA, Banfield JF. 2017. New CRISPR-Cas systems from uncultivated microbes. Nature. 542(7640):237–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burstein D, Sun CL, Brown CT, Sharon I, Anantharaman K, Probst AJ, Thomas BC, Banfield JF. 2016. Major bacterial lineages are essentially devoid of CRISPR-Cas viral defence systems. Nat Commun. 7:10613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camanocha A, Dewhirst FE. 2014. Host-associated bacterial taxa from Chlorobi, Chloroflexi, GN02, Synergistetes, SR1, TM7, and WPS-2 Phyla/candidate divisions. J Oral Microbiol. 6:1. doi: 10.3402/jom.v6.25468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chylinski K, Le Rhun A, Charpentier E. 2013. The tracrRNA and Cas9 families of type II CRISPR-Cas immunity systems. RNA Biol. 10(5):726–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross KL, Campbell JH, Balachandran M, Campbell AG, Cooper SJ, Griffen A, Heaton M, Joshi S, Klingeman D, Leys E, et al. 2019. Targeted isolation and cultivation of uncultivated bacteria by reverse genomics. Nat Biotechnol. 37(11):1314–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danczak RE, Johnston MD, Kenah C, Slattery M, Wrighton KC, Wilkins MJ. 2017. Members of the candidate phyla radiation are functionally differentiated by carbon- and nitrogen-cycling capabilities. Microbiome. 5(1):112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darling AC, Mau B, Blattner FR, Perna NT. 2004. Mauve: multiple alignment of conserved genomic sequence with rearrangements. Genome Res. 14(7):1394–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewhirst FE, Chen T, Izard J, Paster BJ, Tanner AC, Yu WH, Lakshmanan A, Wade WG. 2010. The human oral microbiome. J Bacteriol. 192(19):5002–5017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escapa IF, Chen T, Huang Y, Gajare P, Dewhirst FE, Lemon KP. 2018. New insights into human nostril microbiome from the expanded Human Oral Microbiome Database (eHOMD): a resource for the microbiome of the human aerodigestive tract. mSystems. 3(6):e00187-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flusberg BA, Webster DR, Lee JH, Travers KJ, Olivares EC, Clark TA, Korlach J, Turner SW. 2010. Direct detection of DNA methylation during single-molecule, real-time sequencing. Nat Methods. 7(6):461–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X, McLean JS, Edlund A, Yooseph S, Hall AP, Liu SY, Dorrestein PC, Esquenazi E, Hunter RC, Cheng G, et al. 2015. Cultivation of a human-associated TM7 phylotype reveals a reduced genome and epibiotic parasitic lifestyle. Proc Natl Acad Sci U S A. 112(1):244–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugenholtz P, Pitulle C, Hershberger KL, Pace NR. 1998. Novel division level bacterial diversity in a Yellowstone hot spring. J Bacteriol. 180(2):366–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugenholtz P, Tyson GW, Webb RI, Wagner AM, Blackall LL. 2001. Investigation of candidate division TM7, a recently recognized major lineage of the domain bacteria with no known pure-culture representatives. Appl Environ Microbiol. 67(1):411–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison CA, 3rd, Chuang RY, Noskov VN, Assad-Garcia N, Deerinck TJ, Ellisman MH, Gill J, Kannan K, Karas BJ, Ma L, et al. 2016. Design and synthesis of a minimal bacterial genome. Science. 351(6280):aad6253. Erratum in: ACS Chem Biol; 2016;11(5):1463. [DOI] [PubMed] [Google Scholar]
- Jinek M, Chylinski K, Fonfara I, Hauer M, Doudna JA, Charpentier E. 2012. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science. 337(6096):816–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantor RS, Wrighton KC, Handley KM, Sharon I, Hug LA, Castelle CJ, Thomas BC, Banfield JF. 2013. Small genomes and sparse metabolisms of sediment-associated bacteria from four candidate phyla. mBio. 4(5):e00708–e00713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luef B, Frischkorn KR, Wrighton KC, Holman HY, Birarda G, Thomas BC, Singh A, Williams KH, Siegerist CE, Tringe SG, et al. 2015. Diverse uncultivated ultra-small bacterial cells in groundwater. Nat Commun. 6:6372. [DOI] [PubMed] [Google Scholar]
- Marcy Y, Ouverney C, Bik EM, Losekann T, Ivanova N, Martin HG, Szeto E, Platt D, Hugenholtz P, Relman DA, et al. 2007. Dissecting biological “dark matter” with single-cell genetic analysis of rare and uncultivated TM7 microbes from the human mouth. Proc Natl Acad Sci U S A. 104(29):11889–11894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean JS, Bor B, To TT, Liu Q, Kerns KA, Solden L, Wrighton KC, He X, Shi W. 2018. Evidence of independent acquisition and adaption of ultra-small bacteria to human hosts across the highly diverse yet reduced genomes of the phylum Saccharibacteria. bioRxiv. doi: 10.1101/258137. [DOI] [Google Scholar]
- Miyoshi T, Iwatsuki T, Naganuma T. 2005. Phylogenetic characterization of 16s rRNA gene clones from deep-groundwater microorganisms that pass through 0.2-micrometer-pore-size Filters. Appl Environ Microbiol. 71(2):1084–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouverney CC, Armitage GC, Relman DA. 2003. Single-cell enumeration of an uncultivated TM7 subgroup in the human subgingival crevice. Appl Environ Microbiol. 69(10):6294–6298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks DH, Chuvochina M, Waite DW, Rinke C, Skarshewski A, Chaumeil PA, Hugenholtz P. 2018. A standardized bacterial taxonomy based on genome phylogeny substantially revises the tree of life. Nat Biotechnol. 36(10):996–1004. [DOI] [PubMed] [Google Scholar]
- Stewart EJ. 2012. Growing unculturable bacteria. J Bacteriol. 194(16):4151–4160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Théry C, Amigorena S, Raposo G, Clayton A. 2006. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr Protoc Cell Biol. Chapter 3:Unit 3.22. doi: 10.1002/0471143030.cb0322s30. [DOI] [PubMed] [Google Scholar]
- Westra ER, van Houte S, Oyesiku-Blakemore S, Makin B, Broniewski JM, Best A, Bondy-Denomy J, Davidson A, Boots M, Buckling A. 2015. Parasite exposure drives selective evolution of constitutive versus inducible defense. Curr Biol. 25(8):1043–1049. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, DS_10.1177_0022034520905792 for Insights Obtained by Culturing Saccharibacteria With Their Bacterial Hosts by B. Bor, A.J. Collins, P.P. Murugkar, S. Balasubramanian, T.T. To, E.L. Hendrickson, J.K. Bedree, F.B. Bidlack, C.D. Johnston, W. Shi, J.S. McLean, X. He and F.E. Dewhirst in Journal of Dental Research