Abstract
Medication-related osteonecrosis of the jaw (MRONJ) is a rare intraoral lesion that occurs in patients undergoing long-term and/or high-dose therapy with nitrogen-containing bisphosphonates, a RANKL inhibitor, antiangiogenic agents, or mTOR inhibitors. The presence of pathogenic bacteria is highly associated with advanced stages of MRONJ lesions; however, the exact role of indigenous microbes in MRONJ development is unknown. Here, we report that the normal oral flora in mice protects against inflammation-induced osteonecrosis. In mice that developed osteonecrosis following tooth extraction, there was increased bacterial infiltration when compared with healed controls. Antibiotic-mediated oral dysbiosis led to a local inhibition of bone resorption in the presence of ligature-induced periodontitis (LIP). There was no significant difference in empty lacunae, necrotic bone formation, osteoclast number, and surface area in antibiotic-treated as compared with conventionally colonized mice following extraction of healthy teeth after zoledronic acid infusions. However, extraction of LIP teeth led to increased empty lacunae, necrotic bone, and osteoclast surface area in antibiotic- and zoledronic acid–treated mice as compared with conventionally colonized mice. Our findings suggest that the presence of the indigenous microbiota protects against LIP-induced osteonecrosis.
Keywords: osteoclast(s), medication-related osteonecrosis of the jaw/MRONJ, ligature-induced periodontitis/periodontal disease, wound healing, bone, antibiotic(s)
Introduction
Antiresorptive therapies, including nitrogen-containing bisphosphonates such as zoledronic acid (ZOL), are the first-line treatment of choice for preventing bone loss in osteoporosis and suppressing skeletal metastatic events in cancers (Chen and Sambrook 2011). In patients undergoing antiresorptive therapy for prolonged periods or at high doses, a rare but serious oral complication called medication-related osteonecrosis of the jaw (MRONJ) can occur following dental interventions (Marx et al. 2005). MRONJ clinically manifests as exposed bone that has persisted for >8 wk without history of radiation exposure to the head and neck area (Ruggiero et al. 2014).
According to clinical studies, 84% of patients with MRONJ presented with periodontal disease as a dental comorbidity, and in 78% of the cases, the precipitating event that led to osteonecrosis of the jaw (ONJ) development was an extraction or dental surgery (Marx et al. 2005). Consistent with this, the etiologic role of periodontal disease and dentoalveolar surgery in MRONJ pathogenesis has been corroborated in multiple preclinical animal models (Aghaloo et al. 2011; Kim et al. 2018; Soundia et al. 2018). Because periodontal disease is driven by local microbial dysbiosis and extraction sites are in intimate contact with oral bacteria during healing, several groups have investigated the association between the oral microbiome and MRONJ lesion development. Scanning electron microscope analysis from MRONJ lesions has revealed microbial biofilm formation on sequestered bone (Sedghizadeh et al. 2008). Metagenomic analyses revealed altered bacterial diversity in patients with MRONJ, with some studies reporting an increase in Actinomyces spp. accumulation on MRONJ lesions (Marx et al. 2005; Hansen et al. 2006; Kos, Brusco, et al. 2010). Moreover, a decrease in MRONJ incidence in patients with cancer who improved their oral hygiene suggests a critical role for bacterial infections in MRONJ pathogenesis (Ripamonti et al. 2009). Together these findings suggest that antiresorptive therapy, dental surgery, and local inflammation are each a risk factor for MRONJ development and further raise the question of whether the microbiota plays a role in the etiopathogenesis of MRONJ.
The oral mucosal barrier comprises the epithelium—which separates a diverse community of microorganisms that exert pleiotropic effects on host function (Shreiner et al. 2015)—from numerous host immune, endothelial, and mesenchymal cells that form intricate networks for nutrient and antigen exchange (Chang et al. 2014). The lack of tight junctions between teeth and oral mucosa is unique to the oral cavity and makes this area particularly susceptible to bacterial infiltration (Bosshardt and Lang 2005). When bacteria accumulate at sites directly adjacent to this interface, they can become pathobionts that cause local inflammation leading to host-mediated destruction of alveolar bone, clinically known as periodontitis (Darveau 2010). Periodontitis is driven by local microbial dysbiosis, and extraction sites are inevitably colonized by oral bacteria during healing. Therefore, we hypothesize that disruption of the microbiota may influence the development of MRONJ.
In this study, we investigate the role of indigenous bacteria in MRONJ pathogenesis by assessing bone mass, osteonecrosis development, and osteoclast number in the maxillary second molar region. These phenotypes are examined in response to microbiota depletion with broad-spectrum antibiotic (Abx) treatment and subsequent extraction of healthy or periodontally diseased teeth in vehicle (Veh)– or ZOL-treated mice.
Materials and Methods
Animals
Six-week-old female C57BL/6J mice were purchased from the Jackson Laboratories (stock No. 000664) and housed in a specific pathogen–free (SPF) environment with 12-h light/dark cycle managed by the UCLA Division of Laboratory and Animal Medicine. All experimental protocols were approved by institutional guidelines from the Chancellor’s Animal Research Committee (2011-062).
Commensal Microbiota Reduction and Ligature-Induced Periodontitis
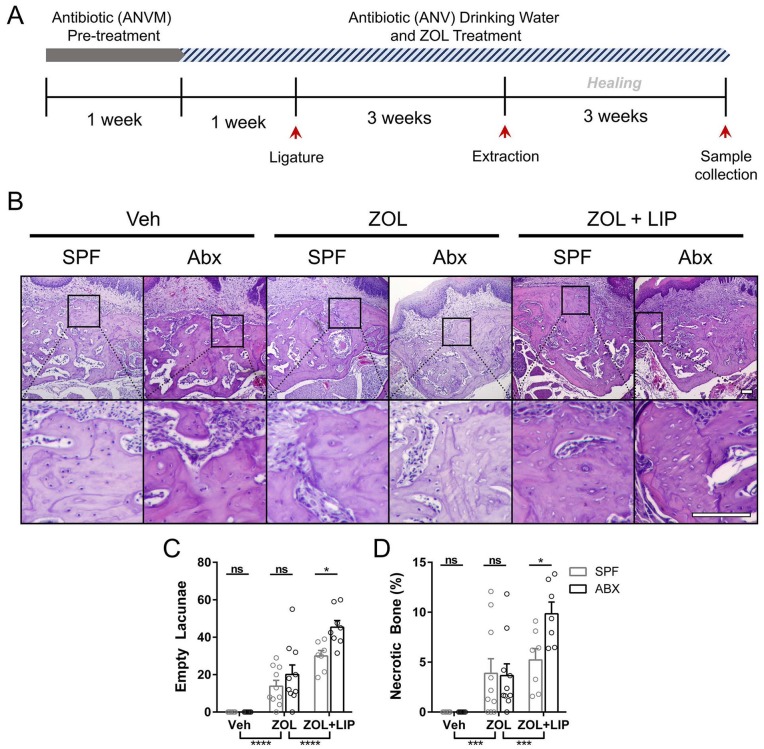
SPF mice were randomly assigned into 4 groups: SPF + no ligature control (NLC), SPF + LIP, Abx treated + NLC, and Abx + LIP (n = 8 mice each). All Abx animals received a mixture of intragastric and intraoral gavage of vancomycin (50 mg/kg), metronidazole (100 mg/kg), and neomycin (100 mg/kg) twice daily and ampicillin (0.5 mg/mL) in the drinking water ad libitum for the first 7 d of the study. For the control groups, sterile saline gavage and unaltered water were provided. After 7 d, a 6-0 silk suture was aseptically placed on the right and left second maxillary molar (M2) of the Abx + LIP and SPF + LIP groups. Dysbiosis in Abx animals was maintained by delivering a mixture of vancomycin (0.5 mg/mL), ampicillin (1 mg/mL), and neomycin (1 mg/mL) in drinking water ad libitum for the remainder of the study. Three weeks after ligature placement, animals were sacrificed. A schematic illustrating the timeline of the study is presented in Figure 3A.
Figure 3.
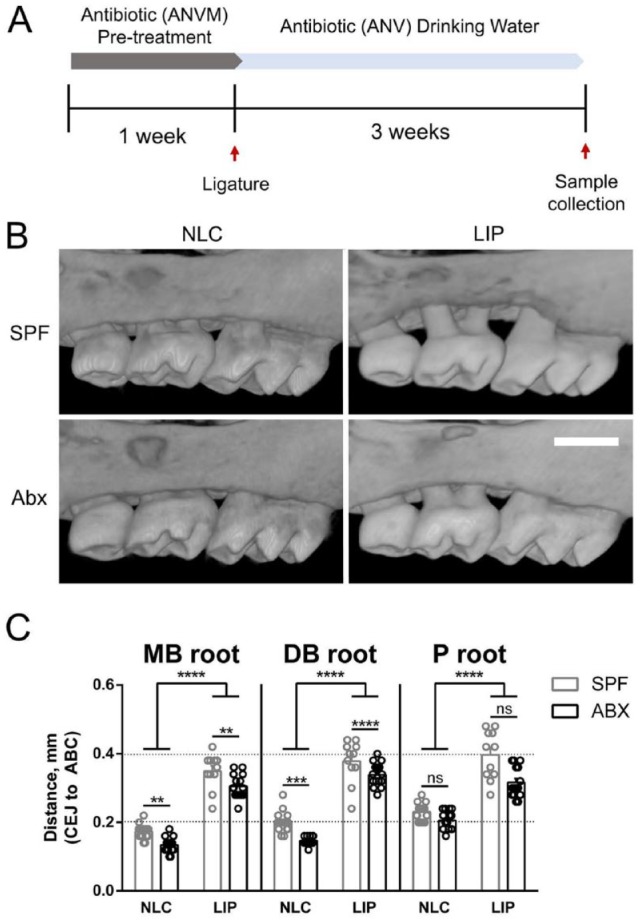
Systemic dysbiosis reduces alveolar bone loss and bone mass reduction following LIP. (A) Schematic of mouse model. For a detailed description, see Materials and Methods (Commensal Microbiota Reduction and Ligature-Induced Periodontitis). (B) Representative buccal views of micro–computed tomography reconstructed maxillae from specific pathogen–free (SPF) or antibiotic (Abx) animals with or without ligature-induced periodontitis. Scale bar: 500 μm. (C) Quantification total distance from the cementoenamel junction (CEJ) to the alveolar bone crest (ABC) measured from mesiobuccal (MB), distobuccal (DB), and palatal (P) roots on the second maxillary molar. All quantified data represent mean ± SEM. **P < 0.01. ***P < 0.001. ****P < 0.0001. ns, not statistically significant. LIP, ligature induced periodontitis; NLC, no-ligature control.
Commensal Microbiota Reduction and Osteonecrosis Development
SPF mice were randomly assigned into 6 groups: SPF/Veh/NLC, SPF/ZOL/NLC, SPF/ZOL/LIP, Abx/Veh/NLC, Abx/ZOL/NLC, and Abx/ZOL/LIP (n = 7 to 10 mice each). Animals in the SPF and Abx groups received unaltered water or Abx gavage/water as described earlier for the entire study. Animals in the Veh and ZOL groups received biweekly intravenous injections of 0.9% NaCl or 125 μg/kg ZOL (Sagent Pharmaceuticals) starting 1 wk after the gavage administration and continuing throughout the study. Two weeks after the gavage administration, animals in the LIP groups received a ligature as previously described. Three weeks after ligature placement, all animals underwent maxillary M2 extraction. Prior to sacrifice, oral swabs and fecal samples were aseptically obtained. Following sacrifice, spleens and ceca were harvested, and weights were compared per total body weight. A schematic illustrating the timeline of the study is presented in Figure 4A.
Figure 4.
Histologic assessment of osteonecrosis formation in dysbiotic mice. (A) Schematic of mouse model. For a detailed description, see Materials and Methods (Commensal Microbiota Reduction and Osteonecrosis Development). (B) Hematoxylin and eosin section staining at the site of extraction. Representative images from each animal cohort are shown at 2 magnifications. Scale bar: 100 μm. (C) The number of osteocyte lacunae without nuclear stain were quantified as empty lacunae. (D) Areas containing >3 empty lacunae were quantified as necrotic and are presented as a percentage of the total bone present. All quantified data represent mean ± SEM. *P < 0.05. ***P < 0.001. ****P < 0.0001. ns, not statistically significant. Abx, antibiotic; LIP, ligature-induced periodontitis; SPF, specific pathogen–free; Veh, vehicle; ZOL, zoledronic acid.
Micro–computed tomography
Whole maxillae and femurs were scanned with a voxel size of 10 μm3 and a 1.0-mm aluminum filter at 60 kVp and 166 μA (SkyScan 1275; Bruker). See Appendix for detailed reconstruction and analysis methods.
Histomorphometric and Histochemical Staining
Hematoxylin/eosin and tartrate-resistant acid phosphatase (TRAP; 387A-1KT, Millipore Sigma) staining was performed following decalcification and paraffin embedding as described previously (Williams et al. 2014). Quantifications were performed with ImageJ and Histomorph (van ’t Hof et al. 2017).
16S Sequencing and Quantitative Real-time Polymerase Chain Reaction
Bacterial DNA was extracted from fecal and oral samples before sacrifice with the DNeasy PowerSoil Kit (12888-100; Qiagen). Total bacterial load was performed as described previously (Dutzan et al. 2018). DNA underwent library preparation as described (Tong et al. 2014) with uniquely barcoded primers targeting the V4 region of the 16S rRNA gene (Caporaso et al. 2012). Demultiplexed sequencing results were analyzed with the QIIME2 pipeline (Bolyen et al. 2019). See Appendix for detailed 16S sequencing, analysis, and polymerase chain reaction methods.
Fluorescent In Situ Hybridization
Archived MRONJ samples (Williams et al. 2014) were deparaffinized in a 60 °C oven and rehydrated in serial dilutions of xylene and ethanol. Bacterial fluorescent in situ hybridization was performed with a universal bacterial probe (5′-[Cy3]GCTGCCTCCCGTAGGAGT-3′; MilliporeSigma) as previously described (Fung et al. 2019).
Statistical Analysis
Data are shown as mean ± SEM unless otherwise noted. Differences among groups were compared by 1- or 2-way analysis of variance with Tukey’s post hoc test, unless otherwise noted.
Results
MRONJ Lesions Display Increased Local Bacterial Infiltrate
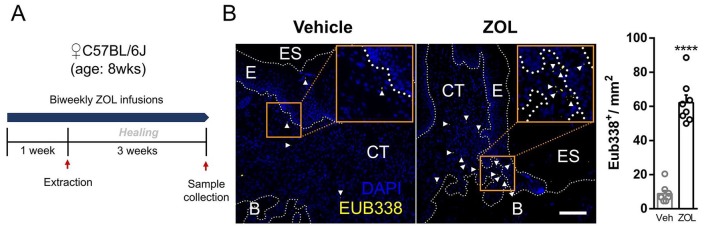
MRONJ lesions are barrier defects that involve bone exposure in the maxillofacial region that inevitably becomes colonized by the oral microbiota. To determine the extent of microbial infiltration in murine MRONJ lesions, we used archived Veh- and ZOL-treated tissue samples from our previous study (Fig. 1A; Williams et al. 2014) and performed fluorescent in situ hybridization with a universal bacterial 16S rRNA gene probe. We found significantly increased bacterial staining in the connective tissue and epithelium in ZOL-treated tissue with exposed bone and unhealed epithelium as compared with Veh-treated controls that had complete epithelial closure and no exposed bone (Fig. 1B). These data indicate that bacteria are present in ONJ-like lesions in mice.
Figure 1.
Increased microbial infiltrate in mouse osteonecrosis of the jaw–like lesions. (A) Experimental schematic of the previously published study. (B) Representative images (left) and quantification (right) of fluorescence in situ hybridization of 16S rDNA with the universal bacterial probe Eub338 conjugated to Cy3. Positive stain is indicated by white arrowheads. Scale bar: 100 μm. Values are presented as mean ± SEM. ****P < 0.0001. B, bone; CT, connective tissue; E, epithelium; ES, extraction site; Veh, vehicle; ZOL, zoledronic acid.
Broad-Spectrum Abx Treatment Alters Spleen and Cecal Weight and Oral Microbiota Composition
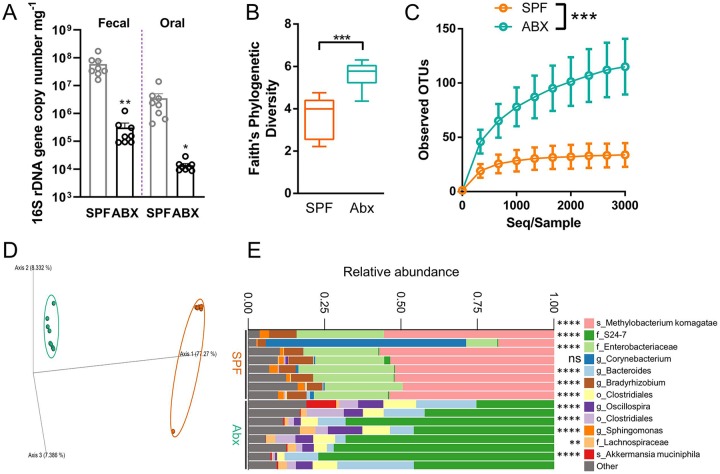
To understand the role of the microbiota in MRONJ pathogenesis, we adopted a model of systemic dysbiosis that utilized broad-spectrum Abx to deplete oral and gastrointestinal (GI) microbiota (Reikvam et al. 2011). Mice treated with Abx for 4 wk showed significant decreases in oral and fecal 16S rDNA gene expression as compared with SPF controls (Fig. 2A). Spleen size as a percentage of body weight was decreased by 40% in Abx mice versus SPF mice (Appendix Fig. 1A). Conversely, Abx mice developed enlarged ceca that weighed an average of 4 times more than ceca from SPF controls (Appendix Fig. 1B). 16S sequencing of oral swabs revealed significantly increased alpha diversity in Abx as compared with SPF groups (Fig. 2B, C), while Bray-Curtis principal coordinate analysis (q < 0.001) and taxonomic bar plots showed that SPF and Abx samples comprised significantly different bacterial taxa (Fig. 2D, E). Taken together, these data demonstrate that broad-spectrum Abx sufficiently depleted the oral and GI microbiota and altered oral microbial composition.
Figure 2.
Broad-spectrum antibiotic (Abx) treatment alters oral microbiota composition in mice. (A) 16S rDNA quantitative real-time polymerase chain reaction was performed from genomic DNA extracted from fecal and oral samples collected prior to sacrifice and normalized to the sample weight in milligrams. Significance was calculated with unpaired t tests. The alpha (B, C) and beta (D) diversity of the microbial communities found at the site of ligature placement were calculated by Faith’s phylogenetic diversity, alpha rarefaction plot, and Bray-Curtis dissimilarity principal component analysis, respectively. (E) Microbiota composition is shown at the amplicon sequence variant level. The top 12 variants are classified at the highest taxonomic level identified by sequencing. f_, family; g_, genus; o_, order; s_, species. Significant differences in microbes between specific pathogen–free (SPF) and Abx were calculated with discrete false-discovery rate. Quantified data from panel A are presented as mean ± SEM. Data from panel B are presented as a minimum/maximum box and whisker plot. Data from panel C are presented as mean ± SD. *P < 0.05. **P < 0.01. ***P < 0.001. ****P < 0.0001. ns, not statistically significant.
Microbiota Depletion Inhibits Alveolar Bone Loss and Bone Mass Reduction Following LIP
To determine how the microbiota influences maxillary bone homeostasis in the absence of antiresorptive therapy or tooth extraction, we compared alveolar bone height and maxillary bone mass following LIP in SPF and Abx mice (Fig. 3A). Micro–computed tomography imaging and cementoenamel junction (CEJ)–alveolar bone crest (ABC) distance revealed that LIP induced significant alveolar bone loss in SPF and Abx mice as compared with NLC mice, and there was a significant increase in CEJ-ABC distance in SPF versus Abx animals with LIP, which was associated with dysbiosis at the ligature site (Fig. 3B, C; Appendix Fig. 2). We also observed a significant increase in CEJ-ABC distance on buccal roots of SPF + NLC animals as compared with Abx + NLC animals (Fig. 3B, C).
Gut microbiota perturbation has been shown to regulate long bone mass in Abx mice, which was replicated in our cohort (Appendix Fig. 3; Yan et al. 2016). In our model of microbiota depletion, bone loss on buccal but not palatal roots was significantly reduced in Abx animals relative to SPF controls (Fig. 3C). In contrast to long bone, maxillary bone mass and trabecular structure were not significantly altered by Abx treatment (Appendix Fig. 4, NLC groups). In addition, though buccal bone mass and trabecular bone structure were significantly altered in SPF + LIP mice, these parameters were unaltered in the Abx + LIP group (Appendix Fig. 4, LIP groups) even though bone loss occurred in both cohorts (Fig. 3C). This is evident qualitatively by observing porous buccal bone in SPF + LIP animals and smooth buccal bone in Abx + LIP animals (Fig. 3B). Together, these findings suggest that the microbiota regulates maxillary bone mass and structure in periodontal health and disease.
Reduction of Commensal Microbiota Exacerbates Inflammation-Induced Osteonecrosis Development
A histologic hallmark of ZOL-induced osteonecrosis is an increase in empty lacunae, which is associated with reduced bone remodeling (Williams et al. 2014; Kim et al. 2017). To test whether the microbiota regulates the development of ZOL-induced osteonecrosis, we examined the number of empty lacunae and amount of necrotic bone in SPF and Abx mice treated with ZOL and LIP (Fig. 4A). ZOL treatment without LIP did not affect the number of empty lacunae or amount of necrotic bone in Abx as compared with SPF animals (Fig. 4B–D). However, ZOL-treated mice with LIP prior to tooth extraction developed significantly increased empty lacunae and necrotic bone in Abx versus SPF animals. This is consistent with previous findings demonstrating that LIP exacerbates osteonecrosis development in ZOL-treated mice (Kim et al. 2018). These data suggest that microbiota depletion combined with local inflammation (LIP) preceding a tooth extraction potentiates the development of osteonecrosis during ZOL therapy.
Abx-Induced Dysbiosis Increases Osteoclast Number and Surface during Inflammation-Induced Osteonecrosis
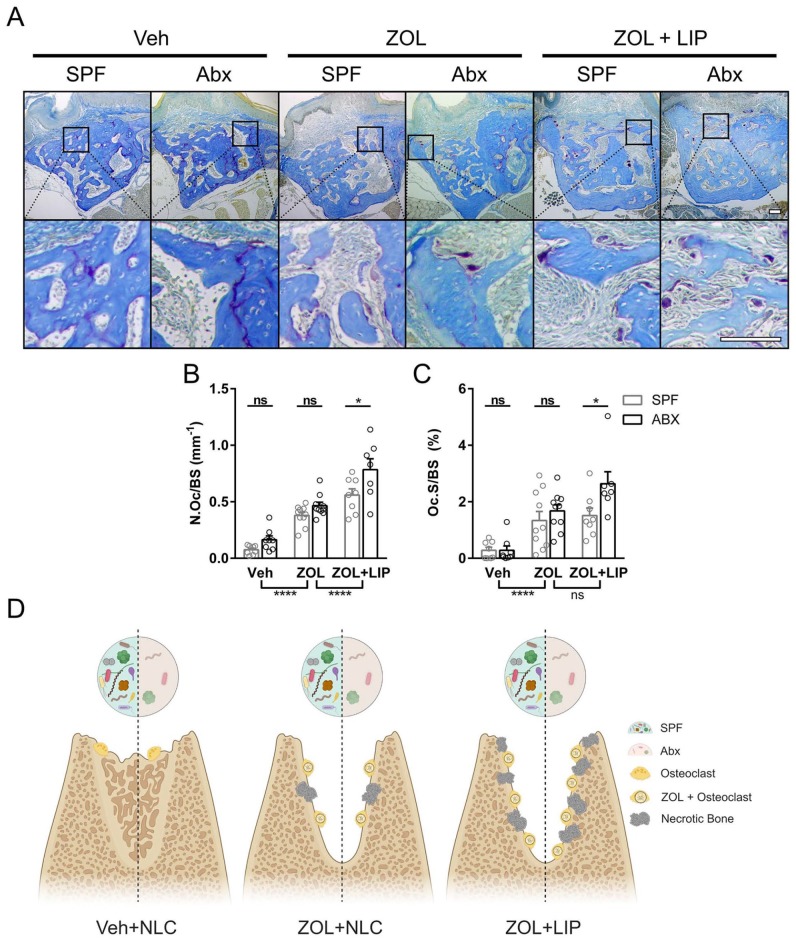
As demonstrated previously, biweekly ZOL infusions increase TRAP-positive osteoclasts 3 wk after healthy or diseased tooth extraction occurs (Williams et al. 2014; Kim et al. 2018). To examine whether microbiota depletion affects resorptive cell numbers and surface area, we utilized enzyme histochemistry to identify TRAP-positive osteoclasts at the site of extraction in Abx and SPF animals (Fig. 5A). ZOL infusions increased osteoclast number (Fig. 5B) and surface (Fig. 5C) in Abx and SPF tissues following healthy tooth extraction. Interestingly, Abx tissues from LIP groups showed an increase in osteoclast number and surface as compared with SPF tissues. When we examined the differentiation and bone-resorptive functions of osteoclasts from Abx-treated mice, we found that the number of osteoclasts increased but were smaller and showed suppressed resorptive function when compared with osteoclasts from SPF mice (Appendix Fig. 5). Cumulatively, our data suggest that in the setting of bisphosphonate treatment and periodontal inflammation, the commensal microbiota dampens osteonecrosis formation and the number of active osteoclasts (Fig. 5D).
Figure 5.
Histologic assessment of TRAP-positive osteoclasts in dysbiotic mice. (A) TRAP staining (purple) with aniline blue counterstain at the site of extraction. Representative images from each animal cohort are shown at 2 magnifications. Scale bar: 100 μm. The number (B) and surface (C) of TRAP+ osteoclasts per bone surface were quantified. (D) Proposed model. Antibiotic (Abx) perturbation of the oral microbiota does not alter the number of osteoclasts or necrotic bone formation in periodontal health (left) or periodontal health with zoledronic acid (ZOL) treatment (center). When periodontal disease is combined with ZOL therapy, Abx-induced dysbiosis leads to increased necrotic bone and osteoclast number at the extraction site (right). All quantified data represent mean ± SEM. *P < 0.05. ****P < 0.0001. ns, not statistically significant. LIP, ligature-induced periodontitis; SPF, specific pathogen–free; TRAP, tartrate-resistant acid phosphatase; Veh, vehicle.
Discussion
Pathogenesis of MRONJ is still incompletely understood, although the first formal report appeared more than 15 y ago (Marx 2003). Several hypotheses have been proposed that could explain its etiology, including inflammation and bacterial infection (Aghaloo et al. 2015). In this study, we examined the role of bacteria in osteonecrosis development by using a microbiota depletion model and demonstrated for the first time a protective role for indigenous bacteria in inflammation-induced osteonecrosis development. Our data reveal that host-microbe interactions are important for oral-maxillofacial bone physiology.
To delineate the role of the commensal microbiota in LIP-induced bone loss and subsequent osteonecrosis development, we significantly depleted bacteria indigenous to the oral and GI microbiota with an Abx treatment regimen modified from Reikvam et al. (2011), which includes Abx with activity against gram-positive, gram-negative, and anaerobic bacteria. Such a regimen yields significant reduction in oral and gut microbes in mice as measured by 16S rDNA expression (Fig. 2A). Reduced spleen weights and enlarged ceca were also confirmed (Appendix Fig. 1), which are important physiologic features observed in germ-free and Abx-treated mice (Gordon and Pesti 1971; Kennedy et al. 2018). Furthermore, taxonomic analysis and Bray-Curtis principal coordinate analysis revealed that Abx treatment changed the community composition of oral bacteria from that of mostly Methylobacterium komagatae and Enterobacteriaceae to Bacteroidales family S24-7, consisting of uncultivatable bacteria primarily found in laboratory animals (Lagkouvardos et al. 2016). These phenotypic characteristics combined with 16S rDNA expression and sequencing confirm a significant reduction and alteration of indigenous oral microbiota in our study.
Increased alpha diversity in the microbiota-depleted animals was seen in LIP and non-LIP models (Fig. 2B, Appendix Fig. 2). We hypothesize that depletion of the oral microbiota enabled recolonization of the oral cavity by microbes indigenous to the gut during coprophagy, as evidenced by the increased presence of gut-associated microbes, such as those from the order Clostridiales and S24-7. Another possible explanation for increased alpha diversity in Abx as compared with SPF animals is that Abx treatment decreases abundance of dominant taxa, such as Methylobacterium and Enterobacteriaceae, thereby enabling increased colonization of the oral cavity by a larger diversity of rare taxa, such as Ruminococcus gnavus and Roseburia (Gomez-Arango et al. 2017). Indeed, the oral microbiota harbors a high number of naturally Abx-resistant bacteria (Clemente et al. 2015).
The absence or depletion of gut microbiota causes osteopetrosis in the long bones of mice (Appendix Fig. 3, Veh group; Sjögren et al. 2012; Yan et al. 2016). Interestingly, there were no differences in bone mass between the Abx/ZOL and SPF/ZOL cohorts (Appendix Fig. 3, ZOL group), suggesting that the mechanism by which Abx-induced osteopetrosis occurs overlaps with that induced by ZOL. Moreover, the increased bone mass in germ-free mice was associated with a reduction in bone-related inflammatory cytokine expression and diminution of osteoclastogenesis (Sjögren et al. 2012). Together, this suggests that osteoclasts may play a central role in causing the osteopetrotic phenotype in Abx- and ZOL-treated mice.
Several studies have observed the sequela of Abx perturbation of the microbiota in the context of periodontal disease. Broad-spectrum Abx treatment consistently leads to significant reduction of oral bacterial load measured by relative (Tsukasaki et al. 2018) or absolute (Dutzan et al. 2018) quantification of 16S rDNA copies (Fig. 2A). In our study, LIP-mediated bone loss was reduced but not halted by microbial depletion, in agreement with previous reports (Abe and Hajishengallis 2013; Dutzan et al. 2018; Fig. 3). Ligature placement is proposed to induce periodontitis-like lesions in mice via inflammation and subsequent bone resorption that, in theory, occurs due to localized bacterial aggregation (Graves et al. 2008). However, our data suggest that the physical placement of the ligature may also elicit local inflammation and bone resorption, which could be due to the unique mechanosensing abilities of gingival cells, the importance of which was recently highlighted in periodontal bone loss (Dutzan et al. 2017). We observed increased osteonecrosis development in Abx/ZOL/LIP mice, suggesting that trauma or damage is another integral factor in MRONJ pathogenesis in addition to normal flora maintenance. Such an observation demonstrates that LIP-mediated bone loss is governed by entrapped bacteria on the ligature and physical and mechanical trauma that elicits osteoclastogenic responses. Further study is needed to define the role of the indigenous microbiota and physical/mechanical trauma in periodontal bone loss and MRONJ pathophysiology.
Previous studies have suggested that pathogenic bacteria colonize extraction sites in bisphosphonate-treated animals and humans and exacerbate ONJ lesion development (Hansen et al. 2006; Sedghizadeh et al. 2008; Kos, Brusco, et al. 2010; Katsarelis et al. 2015). In the present study, depletion of indigenous oral microbes increased the amount of osteonecrosis induced by BP following LIP and tooth extraction (Fig. 4). From these findings, we hypothesize that 1) “beneficial” bacteria may be present in the oral microbiota that help reduce osteonecrosis development or 2) Abx-resistant pathogenic bacteria may fill the niche left by Abx treatment and cause local immune responses and tissue destruction that ultimately leads to increased osteonecrosis development. Alternatively, low levels of immune stimulation by the microbiota may be important during osteonecrosis development, and Abx mice lacking these interactions may explain the increased osteonecrosis. While previous studies have identified a correlation between pathogenic bacteria and MRONJ lesions (Hansen et al. 2007; Kos, Kuebler, et al. 2010), our study identifies an important role for the indigenous microbes in MRONJ development. Collectively, these studies suggest that a delicate balance must be maintained between the clinical management of pathogenic bacteria and the preservation of the indigenous oral flora in the context of antiresorptive treatment and dental interventions such as a tooth extraction.
Here, we significantly reduced the oral and GI bacterial load and altered the remaining community composition of the microbiota (Fig. 2). Rodents are coprophagic and expose the oral cavity to intestinal microbes during their daily routine (Soave and Brand 1991), so it is technically challenging to reduce oral bacteria without also reducing gut bacteria. Thus, the effect of solely reducing oral-specific commensal bacteria on MRONJ pathophysiology remains unknown. Additionally, our model does not represent a germ-free condition. This limitation is mitigated by the fact that Abx treatment is a more clinically relevant strategy to alter the microbiota. Nevertheless, further study is necessary to integrate findings from this study to clinical practice.
In this study, we perturbed the microbiota by reducing the overall bacterial load and altering oral flora composition with Abx treatment, and we revealed that indigenous bacteria protect against osteonecrosis development in a mouse model of LIP but have no effect on osteonecrosis development in periodontal health. Our study has important implications because Abx regimens are often prescribed to patients with ONJ in a preventative and palliative manner (Ruggiero et al. 2014). However, further studies are needed to evaluate clinically relevant doses of Abx therapy as a strategy for reducing MRONJ development.
Author Contributions
D.W. Williams, contributed to conception, design, data acquisition, analysis, and interpretation, drafted and critically revised the manuscript; H.E. Vuong, contributed to design, data analysis and interpretation, critically revised the manuscript; S. Kim, contributed to data acquisition, critically revised the manuscript; A. Lenon, K. Ho, contributed to data acquisition and analysis, critically revised the manuscript; E.Y. Hsiao, E.C. Sung, contributed to data interpretation, critically revised the manuscript; R.H. Kim, contributed to conception, design, data interpretation, critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
Supplemental Material
Supplemental material, DS_10.1177_0022034520908594 for Indigenous Microbiota Protects against Inflammation-Induced Osteonecrosis by D.W. Williams, H.E. Vuong, S. Kim, A. Lenon, K. Ho, E.Y. Hsiao, E.C. Sung and R.H. Kim in Journal of Dental Research
Acknowledgments
We thank the University of California, Los Angeles (UCLA), Translational Procurement Core Laboratory for expedited and cooperative services. Additionally, we thank the UCLA Technology Center for Genomics and Bioinformatics for expedited sequencing service. We thank Teresa Wild and Niki Moutsopoulos for providing P. gingivalis and the microbiome quantification protocol.
Footnotes
A supplemental appendix to this article is available online.
This study was supported in part by the National Institutes of Health / National Institute of Dental and Craniofacial Research (grant DE025172 to D.W.W.; grant DE023348 to R.H.K.) and National Institutes of Health / National Institute of General Medical Sciences (grant GM106996 to H.E.V.), the ADA Foundation Dentsply Sirona Research Award (to D.W.W.), the Henry M. Thornton Fellowship (to D.W.W.), and UCLA School of Dentistry Dean’s Faculty Research Seed Grant (to R.H.K.). E.Y.H. is a New York Stem Cell Foundation–Robertson Investigator.
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
ORCID iD: D.W. Williams  https://orcid.org/0000-0002-7718-2098
https://orcid.org/0000-0002-7718-2098
References
- Abe T, Hajishengallis G. 2013. Optimization of the ligature-induced periodontitis model in mice. J Immunol Methods. 394(1–2):49–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aghaloo T, Hazboun R, Tetradis S. 2015. Pathophysiology of osteonecrosis of the jaws. Oral Maxillofac Surg Clin North Am. 27(4):489–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aghaloo TL, Kang B, Sung EC, Shoff M, Ronconi M, Gotcher JE, Bezouglaia O, Dry SM, Tetradis S. 2011. Periodontal disease and bisphosphonates induce osteonecrosis of the jaws in the rat. J Bone Miner Res. 26(8):1871–1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolyen E, Rideout JR, Dillon MR, Bokulich NA, Abnet CC, Al-Ghalith GA, Alexander H, Alm EJ, Arumugam M, Asnicar F, et al. 2019. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat Biotechnol. 37(8):852–857. Erratum in: Nat Biotechnol. 37(9):1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosshardt DD, Lang NP. 2005. The junctional epithelium: from health to disease. J Dent Res. 84(1):9–20. [DOI] [PubMed] [Google Scholar]
- Caporaso JG, Lauber CL, Walters WA, Berg-Lyons D, Huntley J, Fierer N, Owens SM, Betley J, Fraser L, Bauer M, et al. 2012. Ultra-high-throughput microbial community analysis on the illumina HiSeq and MiSeq platforms. ISME J. 6(8):1621–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang SY, Ko HJ, Kweon MN. 2014. Mucosal dendritic cells shape mucosal immunity. Exp Mol Med. 46(3):e84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JS, Sambrook PN. 2011. Antiresorptive therapies for osteoporosis: a clinical overview. Nat Rev Endocrinol. 8(2):81–91. [DOI] [PubMed] [Google Scholar]
- Clemente JC, Pehrsson EC, Blaser MJ, Sandhu K, Gao Z, Wang B, Magris M, Hidalgo G, Contreras M, Noya-Alarcon O, et al. 2015. The microbiome of uncontacted amerindians. Sci Adv. 1(3):e1500183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darveau RP. 2010. Periodontitis: a polymicrobial disruption of host homeostasis. Nat Rev Microbiol. 8(7):481–490. [DOI] [PubMed] [Google Scholar]
- Dutzan N, Abusleme L, Bridgeman H, Greenwell-Wild T, Zangerle-Murray T, Fife ME, Bouladoux N, Linley H, Brenchley L, Wemyss K, et al. 2017. On-going mechanical damage from mastication drives homeostatic Th17 cell responses at the oral barrier. Immunity. 46(1):133–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutzan N, Kajikawa T, Abusleme L, Greenwell-Wild T, Zuazo CE, Ikeuchi T, Brenchley L, Abe T, Hurabielle C, Martin D, et al. 2018. A dysbiotic microbiome triggers Th17 cells to mediate oral mucosal immunopathology in mice and humans. Sci Transl Med. 10(463):eaat0797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung TC, Vuong HE, Luna CDG, Pronovost GN, Aleksandrova AA, Riley NG, Vavilina A, McGinn J, Rendon T, Forrest LR, et al. 2019. Intestinal serotonin and fluoxetine exposure modulate bacterial colonization in the gut. Nat Microbiol. 4(12):2064–2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Arango LF, Barrett HL, McIntyre HD, Callaway LK, Morrison M, Dekker Nitert M. 2017. Antibiotic treatment at delivery shapes the initial oral microbiome in neonates. Sci Rep. 7:43481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon HA, Pesti L. 1971. The gnotobiotic animal as a tool in the study of host microbial relationships. Bacteriol Rev. 35(4):390–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves DT, Fine D, Teng YT, Van Dyke TE, Hajishengallis G. 2008. The use of rodent models to investigate host-bacteria interactions related to periodontal diseases. J Clin Periodontol. 35(2):89–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen T, Kunkel M, Springer E, Walter C, Weber A, Siegel E, Kirkpatrick CJ. 2007. Actinomycosis of the jaws—histopathological study of 45 patients shows significant involvement in bisphosphonate-associated osteonecrosis and infected osteoradionecrosis. Virchows Arch. 451(6):1009–1017. [DOI] [PubMed] [Google Scholar]
- Hansen T, Kunkel M, Weber A, James Kirkpatrick C. 2006. Osteonecrosis of the jaws in patients treated with bisphosphonates—histomorphologic analysis in comparison with infected osteoradionecrosis. J Oral Pathol Med. 35(3):155–160. [DOI] [PubMed] [Google Scholar]
- Katsarelis H, Shah NP, Dhariwal DK, Pazianas M. 2015. Infection and medication-related osteonecrosis of the jaw. J Dent Res. 94(4):534–539. [DOI] [PubMed] [Google Scholar]
- Kennedy EA, King KY, Baldridge MT. 2018. Mouse microbiota models: comparing germ-free mice and antibiotics treatment as tools for modifying gut bacteria. Front Physiol. 9:1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Williams DW, Lee C, Kim T, Arai A, Shi S, Li X, Shin KH, Kang MK, Park NH, et al. 2017. IL-36 induces bisphosphonate-related osteonecrosis of the jaw-like lesions in mice by inhibiting TGF-β-mediated collagen expression. J Bone Miner Res. 32(2):309–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim T, Kim S, Song M, Lee C, Yagita H, Williams DW, Sung EC, Hong C, Shin KH, Kang MK, et al. 2018. Removal of pre-existing periodontal inflammatory condition before tooth extraction ameliorates medication-related osteonecrosis of the jaw-like lesion in mice. Am J Pathol. 188(10):2318–2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kos M, Brusco D, Kuebler J, Engelke W. 2010. Clinical comparison of patients with osteonecrosis of the jaws, with and without a history of bisphosphonates administration. Int J Oral Maxillofac Surg. 39(11):1097–1102. [DOI] [PubMed] [Google Scholar]
- Kos M, Kuebler JF, Luczak K, Engelke W. 2010. Bisphosphonate-related osteonecrosis of the jaws: a review of 34 cases and evaluation of risk. J Craniomaxillofac Surg. 38(4):255–259. [DOI] [PubMed] [Google Scholar]
- Lagkouvardos I, Pukall R, Abt B, Foesel BU, Meier-Kolthoff JP, Kumar N, Bresciani A, Martinez I, Just S, Ziegler C, et al. 2016. The Mouse Intestinal Bacterial Collection (miBC) provides host-specific insight into cultured diversity and functional potential of the gut microbiota. Nat Microbiol. 1(10):16131. Erratum in: Nat Microbiol. 1(11):16219. [DOI] [PubMed] [Google Scholar]
- Marx RE. 2003. Pamidronate (Aredia) and zoledronate (Zometa) induced avascular necrosis of the jaws: a growing epidemic. J Oral Maxillofac Surg. 61(9):1115–1117. [DOI] [PubMed] [Google Scholar]
- Marx RE, Sawatari Y, Fortin M, Broumand V. 2005. Bisphosphonate-induced exposed bone (osteonecrosis/osteopetrosis) of the jaws: risk factors, recognition, prevention, and treatment. J Oral Maxillofac Surg. 63(11):1567–1575. [DOI] [PubMed] [Google Scholar]
- Reikvam DH, Erofeev A, Sandvik A, Grcic V, Jahnsen FL, Gaustad P, McCoy KD, Macpherson AJ, Meza-Zepeda LA, Johansen FE. 2011. Depletion of murine intestinal microbiota: effects on gut mucosa and epithelial gene expression. PloS One. 6(3):e17996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ripamonti CI, Maniezzo M, Campa T, Fagnoni E, Brunelli C, Saibene G, Bareggi C, Ascani L, Cislaghi E. 2009. Decreased occurrence of osteonecrosis of the jaw after implementation of dental preventive measures in solid tumour patients with bone metastases treated with bisphosphonates. The experience of the National Cancer Institute of Milan. Ann Oncol. 20(1):137–145. [DOI] [PubMed] [Google Scholar]
- Ruggiero SL, Dodson TB, Fantasia J, Goodday R, Aghaloo T, Mehrotra B, O’Ryan F; American Association of Oral and Maxillofacial Surgeons. 2014. American Association of Oral and Maxillofacial Surgeons position paper on medication-related osteonecrosis of the jaw—2014 update. J Oral Maxillofac Surg. 72(10):1938–1956. [DOI] [PubMed] [Google Scholar]
- Sedghizadeh PP, Kumar SK, Gorur A, Schaudinn C, Shuler CF, Costerton JW. 2008. Identification of microbial biofilms in osteonecrosis of the jaws secondary to bisphosphonate therapy. J Oral Maxillofac Surg. 66(4):767–775. [DOI] [PubMed] [Google Scholar]
- Shreiner AB, Kao JY, Young VB. 2015. The gut microbiome in health and in disease. Curr Opin Gastroenterol. 31(1):69–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjögren K, Engdahl C, Henning P, Lerner UH, Tremaroli V, Lagerquist MK, Bäckhed F, Ohlsson C. 2012. The gut microbiota regulates bone mass in mice. J Bone Miner Res. 27(6):1357–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soave O, Brand CD. 1991. Coprophagy in animals: a review. Cornell Vet. 81(4):357–364. [PubMed] [Google Scholar]
- Soundia A, Hadaya D, Esfandi N, Gkouveris I, Christensen R, Dry SM, Bezouglaia O, Pirih F, Nikitakis N, Aghaloo T, et al. 2018. Zoledronate impairs socket healing after extraction of teeth with experimental periodontitis. J Dent Res. 97(3):312–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong M, Jacobs JP, McHardy IH, Braun J. 2014. Sampling of intestinal microbiota and targeted amplification of bacterial 16S rRNA genes for microbial ecologic analysis. Curr Protoc Immunol. 107:7.41.1–7.41.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukasaki M, Komatsu N, Nagashima K, Nitta T, Pluemsakunthai W, Shukunami C, Iwakura Y, Nakashima T, Okamoto K, Takayanagi H. 2018. Host defense against oral microbiota by bone-damaging T cells. Nat Commun. 9(1):701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van ’t Hof RJ, Rose L, Bassonga E, Daroszewska A. 2017. Open source software for semi-automated histomorphometry of bone resorption and formation parameters. Bone. 99:69–79. [DOI] [PubMed] [Google Scholar]
- Williams DW, Lee C, Kim T, Yagita H, Wu H, Park S, Yang P, Liu H, Shi S, Shin KH, et al. 2014. Impaired bone resorption and woven bone formation are associated with development of osteonecrosis of the jaw-like lesions by bisphosphonate and anti-receptor activator of NF-κB ligand antibody in mice. Am J Pathol. 184(11):3084–3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan J, Herzog JW, Tsang K, Brennan CA, Bower MA, Garrett WS, Sartor BR, Aliprantis AO, Charles JF. 2016. Gut microbiota induce IGF-1 and promote bone formation and growth. Proc Natl Acad Sci U S A. 113(47):E7554–E7563. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, DS_10.1177_0022034520908594 for Indigenous Microbiota Protects against Inflammation-Induced Osteonecrosis by D.W. Williams, H.E. Vuong, S. Kim, A. Lenon, K. Ho, E.Y. Hsiao, E.C. Sung and R.H. Kim in Journal of Dental Research