Abstract
Rationale:
In black women, triglycerides are paradoxically normal in the presence of insulin resistance. This relationship may be explained by race-related differences in central adiposity and stearoyl-CoA desaturase-1 (SCD-1) enzyme activity index.
Objective:
In a cross-sectional study, to compare fasting and postprandial triglyceride-rich lipoprotein particle (TRLP) concentrations and size in black compared to white pre- and post-menopausal women and determine the relationship between TRLP subfractions and whole-body insulin sensitivity, hepatic and visceral fat, and SCD-1 levels.
Methods and Results:
In 122 federally employed women without diabetes, 73 black (58 African American and 15 African immigrant) and 49 white; age 44±10 (mean±SD); BMI 30.0±5.6 kg/m2 we measured lipoprotein subfractions using nuclear magnetic resonance. Hepatic fat was measured by proton MRS, insulin sensitivity index (SI) calculated by minimal modeling from a frequently-sampled intravenous glucose test, RBC fatty acid profiles by gas chromatography were used to estimate SCD-1 indices. Hepatic fat, SI, and SCD-1 were similar in black women and lower than in whites, regardless of menopausal status. Fasting and postprandial large, medium and small TRLPs, but not very small TRLPs, were lower in black women. Fasting large, medium and very small TRLPs negatively correlated with SIand positively correlated with visceral and hepatic fat, and SCD-1 activity in both groups. In multivariate models, visceral fat and SCD-1 were associated with total fasting TRLP concentrations (adjR2= 0.39, P=0.001). Black women had smaller postprandial changes in large (P=0.005) and medium TRLPs (P=0.007).
Conclusions:
Lower visceral fat and SCD-1 activity may contribute to the paradoxical association of lower fasting and postprandial TRLP subfractions despite insulin resistance in black compared to white pre- and post-menopausal women. Similar concentrations of very small TRLPs are related to insulin resistance and could be important mediators of cardiometabolic disease risk in women.
ClinicalTrial.gov:
Subject Terms: Epidemiology, Lipids and Cholesterol, Obesity, Race and Ethnicity, Risk Factors
Keywords: Triglyceride, insulin action, insulin resistance, race, liver, postprandial, obesity, lipids and lipoprotein metabolism, African ancestry
Graphical Abstract
INTRODUCTION
Critical race differences exist in the relationship between hypertriglyceridemia, insulin resistance and cardiometabolic disease 1, 2. In contrast to individuals of white or Hispanic descent, African ancestry individuals have high rates of obesity, insulin resistance and diabetes without the expected marked rise in fasting triglyceride concentrations 3-6. This triglyceride paradox has significant global public health ramifications because it is observed across multiple populations of African ancestry and limits the widespread utilization of triglyceride-based screening tests for cardiometabolic disease in these groups 7-11. Uncoupling the mechanisms of race/ethnic variations in triglyceride metabolism could help to enlighten viable strategies for primary prevention of cardiometabolic risk by identifying susceptibility pathways and resiliency factors to explain why triglyceride metabolism appears to remain intact despite other features of insulin resistance 12.
An important approach to understanding this dissociative relationship and improving risk prediction paradigms among populations of African descent, is to critically assess whether there are race-related differences in the subfractions of triglyceride-rich lipoprotein particle (TRLPs) concentrations13. TRLPs are heterogenous and differ in their size, apolipoprotein composition, and function; very low-density lipoproteins (VLDLs) are synthesized in the liver and chylomicrons are secreted from the intestines. Intermediate density lipoproteins (IDL) and chylomicron remnants are also triglyceride-rich lipoproteins but studies examining the triglyceride paradox in black compared to whites have primarily focused on fasting triglyceride and VLDL concentrations. These studies consistently show lower VLDL concentrations in black compared to white individuals that is paradoxically associated with greater insulin resistance in black women 14-17. The dissociative relationship between low VLDL and insulin resistance has been attributed to higher post-heparin lipoprotein lipase (LPL)-mediated VLDL clearance and lower Apolipoprotein CIII (a key inhibitor of LPL activity) 17-19. However, race-related differences in post-heparin LPL activity accounted for only 20% of the observed variance in fasting triglyceride concentrations 18. These fasting VLDL analyses are critical for defining the effect size of race-related differences in triglycerides, but do not account for the specific contribution of IDLs or chylomicrons to the triglyceride pool. Identifying differences in all TRLP subfractions during fasting and postprandial state is important especially since the kinetics of clearance of VLDL and chylomicrons differs after a meal and with insulin resistance 20.
Fewer studies have assessed postprandial triglyceride metabolism with conflicting results. Some studies show that pre-menopausal black compared to white women had lower postprandial triglyceride excursions 17, 21, 22. However, postprandial triglyceride excursions were not different after a high-fat meal in lean or obese black compared to white women 23, and there is a paucity of data in post-menopausal women. Variations in postprandial triglyceride concentrations could be secondary to diet or age-related differences in the relationship of insulin resistance with chylomicrons 24, 25. Alternatively, race-related differences in chylomicrons could be secondary to the well-described metabolic phenotype in black individuals (high rates of insulin resistance but low levels of visceral and hepatic adiposity) 26-28. Recently, we showed that black compared to white pre-menopausal women with insulin resistance had greater postprandial hyperinsulinemia, lower hepatic fat and higher postprandial FFA 29. These findings posit that ambient hyperinsulinemia may be permissive to lower hepatic fat accumulation and higher circulating FFA levels. These differences in body composition are important determinants of intrahepatic TRLP secretion but it is not known if the greater insulin resistance in black women would promote TRLP secretion potentially via increased de novo lipogenesis and apolipoprotein B gene regulation 30. De novo lipogenesis can be reliably estimated with a red blood marker, stearoyl-CoA desaturase activity index (SCD-1) for evaluating these race-related differences in TRLP subfraction concentrations 31, 32.
In this study, we tested the hypothesis that the lower insulin resistance and hepatic fat stores in black compared to white women were associated with lower TRLP concentrations, for all subclasses, under fasting and postprandial physiological conditions. Our objectives were to compare by race/ethnicity and menopausal status, fasting and postprandial TRLP subfractions and determine the relationship between TRLP concentrations and whole-body insulin sensitivity, hepatic and visceral adiposity and SCD-1 activity indices.
METHODS
The anonymized data that support the findings of this study are available from the corresponding author upon reasonable request. All supporting methods are available within the article and its online supplementary files.
Study cohort.
Participants were federally employed women and contractors age 43.5±9.7 years (mean±SD, range 24-62y), 49% African-American, 12% African immigrant, and 40% white who were enrolled in the Federal Women’s Study (Clinicaltrial.gov Identifier: NCT01809288). The Federal Women’s study was designed to elucidate the genetic, biological and socio-environmental mechanisms, associated with interethnic differences in cardiometabolic disease risk in African, African-American and white women who are federally employed, have access to health insurance, and live in the DC metropolitan area 29, 31, 33. This study design in federally employed women enabled comparisons by race/ethnicity while minimizing bias associated with lack of access to health care and health insurance, and low socio-economic status. All participants self-identified as healthy and were recruited from newspaper advertisements, flyers or the National Institutes of Health (NIH) website from September 2013 to June 2018. Online Figure I illustrates the participant flow diagram: 139 were screened and 122 women enrolled. Black women of African ancestry were defined as African-American (parents and participant identified as African-American born in the United States) or African (parents and participant born in Africa). White women identified self and both parents as white. Participants self-identified as healthy, did not report a history of diabetes, were not taking medications that influenced glucose or lipid metabolism such as oral hormonal contraceptive use, lipid-lowering medication, and did not participate in habitual alcohol intake (< 2 drinks/ week). The study was approved by the NIDDK Institutional Review Board. All enrollees gave written informed consent prior to participation and were evaluated in the Metabolic Clinical Research Unit at the NIH Clinical Center (CC), Bethesda MD for all study visits.
Study protocol.
Participants completed 4 study visits within 4-6 weeks. At visit 1 (screening), participants underwent history, physical and laboratory examination to exclude the presence of anemia, diabetes, liver or kidney disease and nutritional supplements or medications known to affect triglyceride concentrations or insulin sensitivity (e.g. oral contraceptive pills). Menopausal status was defined as having irregular periods and/ or an FSH >21 U/L. After the screening visit, women returned to the NIH CC after a 10-12 hour overnight fast for each of the 3 visits: oral glucose tolerance test (OGTT), insulin-modified frequently sampled intravenous glucose tolerance test (IM-FSIGT), and the mixed meal tolerance test (MMTT). Each visit was 7-14 days apart and completed within a 4-6 week period. The OGTT, IM-FSIGT and MMTT were completed consecutively, except in 10 women (5 black and 5 white) who had the MMTT before the IM-FSIGT because of scheduling difficulties. At the OGTT visit, fasting plasma samples were collected for red blood cell (RBC) fatty acid profile analysis, lipid and lipoprotein analyses and a 75g oral glucose tolerance test was performed to determine glucose tolerance status. Glucose tolerance status was defined according to American Diabetes Association guidelines; prediabetes: fasting glucose 100-125 mg/dL, and/ or 2-hour glucose 140-200 mg/dL 34. During the IM-FSIGT visit, one intravenous catheter was placed in each arm near or in the antecubital veins. Baseline blood samples were obtained and dextrose (0.3g/kg) was administered intravenously. Insulin was given as a bolus at 20 minutes (0.03U•kg−1min−1) and plasma glucose and insulin concentrations were obtained at 32-time points between baseline and 180 minutes 35.
For the MMTT, the test meal consisted of a bagel with cream cheese, a cheese omelet and orange juice to meet 33% of the estimated daily energy needs (52% carbohydrate, 15% protein, 33% fat) which was calculated using the Mifflin St. Jeor equation plus activity factor of 1.5 and was designed to simulate a typical American breakfast to examine the effects of the current American diet on lipid levels 36. Plasma samples for postprandial lipoprotein analyses were collected at 0, 2, 3, 4, and 5h. Women completed the breakfast meal within 20 mins (13.8±4.9 mins). Due to technical and scheduling difficulties, postprandial lipoprotein analyses were unavailable in 5 black and 7 white women.
To assess dietary habits, 3-7-day food records were collected, reviewed for accuracy with the participant and analyzed by nutrition staff, using Nutrition Data Systems for Research software (versions 2013-2017, Minneapolis, MN), to assess daily energy and macronutrient intake. Habitual physical activity in counts per minute (cpm) was assessed with an accelerometer (Actigraph™, Pensacola, FL) worn during the awake hours for 5-7 days during the same period as the food record collection. Physical activity levels were defined as either min●dy-1 spent in sedentary (0-99cpm), light (100-2019cpm) and moderate-vigorous (≥2020cpm). The average wear time was 932±72 minutes/day and did not vary by race (P=0.7).
Biochemical analyses and calculations.
Lipoprotein analyses:
All plasma samples were processed and stored at −80C prior to analysis by nuclear magnetic resonance (NMR) spectroscopy. Lipoprotein particle size and subclass concentrations were assessed by LP4 NMR LipoProfile® deconvolution analysis of the lipid methyl group signal envelope using the NMR Profiler and Vantera Clinical Analyzer platforms (LabCorp, formerly LipoScience) 37, 38. Triglyceride rich lipoproteins were defined as particles that are within the size range 26-240nm. Subclassification of TRLPs (large, medium, small and very small) was made based on particle size (nm diameter) as indicated in Online Table I. The reason for referring to these particles as TRLPs instead of VLDL and/or chylomicrons is that NMR cannot distinguish between intestinally-derived apoB-48 chylomicron particles and liver-derived apoB-100 VLDL particles. For this analysis, large TRLPs include the concentration of both very large (90-240 nm) and large (50-89 nm) TRLP particles. In addition, there is overlap in the types of particles that are in the size range 24-36 nm and both chylomicron remnants and intermediate density lipoproteins (IDL) can be found in this spectral range 39-41. Therefore, for this analysis we used the categorizations small TRLPs (30-36nm) and very small TRLP subclass particles (24-29 nm) to distinguish the particles solely on the basis of size and not by their apolipoprotein B structure. In unpublished data (run in the same lab that performed our NMR sample analysis) there were comparable ratios of triglyceride and cholesterol lipid compositions in the small and very small TRLPs (Online Table I) that indicates the heterogeneity in the particles in this spectral range. Apolipoprotein A-1 and apolipoprotein B were measured by immunoassays on the Cobas 6000 analyzer (Roche Diagnostics, Indianapolis, IN).
Red blood cell (RBC) fatty acid profiles.
RBC fatty acid profiles were quantified using gas chromatography as previously described 42, 43. In brief, the fatty acid methyl esters were separated and quantified with an Autosystem XL gas chromatograph (Perkin Elmer, Sheldon, CT). Peaks of interest were identified by comparison with fatty acid standards (Nu-Check-Prep, Elysian, MN). Individual fatty acids were expressed as the molar percentage (mol %) proportion of total fatty acids. RBC phospholipid fatty acid profiles were used to estimate stearoyl Co-A desaturase activity (SCD-1), a surrogate marker of de novo lipogenesis. SCD-1 activity was calculated as the product: precursor ration of:
Glucose and insulin.
Glucose, insulin and C-peptide concentrations were measured in serum using the Roche Cobas 6000 analyzer (Roche Diagnostics, Indianapolis, IN). The insulin sensitivity index (SI) during the FSIGT was determined from the minimal model as previously described (version 6.02; MinMoD Millenium, Los Angeles, CA) 44. The acute insulin response to glucose (AIRg) was calculated as the area under the curve, above basal, for insulin concentration between 0 and 10min and the disposition index, a surrogate marker of β-cell function relative to insulin sensitivity, was calculated as the product of SI and AIRg. Due to technical difficulties with sample collection, results were unavailable for SI in 8 black and 7 white women. During the MMTT, insulin relative to glucose response was calculated as the ratio of insulin: glucose concentrations for the incremental area under the respective curves for the first 30 minutes (insulin: glucose AUC0-30) and over the 300 minutes of the test (insulin: glucose AUC0-300).
Imaging studies.
Whole body composition measurements were performed with a dual-energy X-ray absorptiometry scans (Hologic Discovery, Bedford, MA). Hepatic fat content and intra- and extramyocellular fat percent in the tibialis anterior were measured by single volume localized proton magnetic resonance spectroscopy (MRS) at 3T (MAGNETOM Verio; Siemens) 45, 46. Additionally, visceral fat measurement was obtained using T1 images at the level of L2-L3 46. Because of difficulties with data acquisition, we were unable to obtain visceral fat in 16 black and 10 white women, hepatic fat in 19 black and 9 white women, and intramyocellular fat in 20 black and 12 white women.
Statistical analyses.
Data are presented as mean±SD and median (interquartile range), except where otherwise indicated. Student’s t-test and were used to compare continuous parametric variables (age, BMI, blood pressure, total physical activity, total fat mass, glucose, cholesterol) by race and menopausal status. All other variables had non-normal distribution and were compared by race and menopausal status with the Mann-Whitney test. Analysis of variance (ANOVA were used to compare variables by ethnicity, and Bonferroni test used to account for 3 comparisons between African-American, African, and White women.. The chi-square test was used to compare categorical variables by race and menopausal status. Spearman correlations (r) were performed with Bonferroni correction to account for 4 comparisons between TRLP subfractions and each variable (visceral and hepatic fat, SCD and insulin sensitivity). The Shapiro-Wilk test was used to determine normality. The incremental area under the curve during the first 30 minutes of the MMTT (iAUC30) calculated using a cubic spline to determine the integral (STATA, v15.1). Mixed effects models were used to analyze the change in TRLP concentrations during the MMTT (within-subject factor of time; between-subject factor of race; time x race interaction). Multi-linear regression models were created to determine the relationship of fasting total TRLP concentrations with whole-body insulin sensitivity, visceral and hepatic fat, and SCD-1 activity index. Due to collinearity between variables, four models were generated to assess the independent contributions of whole-body SI (Model A), visceral fat (Model B), hepatic fat (Model C), and intramyocellular fat (Model D). Models were adjusted for potential confounders such as age, moderate-vigorous exercise, and RBC MUFA content. Age (rather than menopausal status) was used in the models because of its stronger relationship with triglyceride concentrations 13. Non-parametric data (TRLP concentrations, SI, hepatic fat, visceral fat, SCD-1) were natural log-transformed (ln) prior to regression analyses. Statistical analyses were performed with STATA, v15.1 (College Station, Texas) and P-values <0.05 were considered statistically significant.
RESULTS
Participant characteristics.
Of the 122 women studied, 70% were pre-menopausal and 30% were post-menopausal (Online Figure I). African immigrant and African American women had similar sociodemographic characteristics, alcohol intake, fasting lipid profile and metabolic characteristics (Online Table II and III), and therefore were combined into one group for primary lipoprotein analyses.
Table 1 illustrates the participant characteristics by race and menopausal status. Overall, compared to white women, black women had similar age, BMI and prevalence rates of obesity and prediabetes. Total fat mass was not different by race, but black compared to white women had lower visceral and hepatic fat, yet higher intramyocellular fat in the tibialis anterior region. When stratified by menopausal status, the pattern of similar prevalence rates of obesity and prediabetes, but lower visceral and hepatic fat content in black women was evident in pre-menopausal but not post-menopausal women (Table 1).
Table 1.
Participant characteristics by race and menopausal status. Variables with a normal distribution are expressed as mean±SD and compared with unpaired Student’s t tests. Variables with non-normal distribution are expressed as median (25th-75th percentile) and compared with Mann-Whitney Test. Categorical variables are expressed as n(%) and compared with chi-squared tests.
| Entire Cohort (n=122) |
Pre-menopausal (n=86) |
Post-menopausal (n=36) |
Pre vs. Post Black |
Pre vs. Post White |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Black (n=73) |
White (n=49) |
P- value |
Black (n=53) |
White (n=33) |
P- value |
Black (n=20) |
White (n=16) |
P- value |
P- value |
P- value |
|
| Age (years) | 43±9 | 45±10 | 0.22 | 39±7 | 38±7 | 0.87 | 53±4 | 57±4 | 0.001 | 0.0001 | 0.0001 |
| BMI (kg/m2) | 30.2±5.8 | 29.8±5.4 | 0.68 | 30.5±6.1 | 30.8±5.6 | 0.84 | 29.6±4.9 | 27.9±4.6 | 0.29 | 0.55 | 0.08 |
| Obesity (%) | 32 (44) | 26 (53) | 0.32 | 25(47) | 21(64) | 0.14 | 7(35) | 5(31) | 0.81 | 0.35 | 0.03 |
| Systolic BP (mmHg) | 116±11 | 115±13 | 0.67 | 116±11 | 114±11 | 0.32 | 116±11 | 118±17 | 0.64 | 0.86 | 0.30 |
| Diastolic BP (mmHg) | 71±9 | 70±9 | 0.46 | 70±9 | 69±8 | 0.64 | 73±9 | 71±11 | 0.67 | 0.19 | 0.58 |
| Total physical activity (min/day) | 289±70 | 320±74 | 0.02 | 289±76 | 324±74 | 0.04 | 289±51 | 314±74 | 0.27 | 0.96 | 0.67 |
| Moderate-vigorous activity (min/day) | 20 (8-31) |
28 (18-42) |
0.006 | 22 (12-31) |
28 (19-47) |
0.04 | 8 (6-28) |
28 (16-36) |
0.04 | 0.11 | 0.57 |
|
Body fat distribution | |||||||||||
| Total fat mass (%) | 37.3±6.6 | 38.9±6.6 | 0.20 | 37.0±6.9 | 39.4±6.9 | 0.13 | 38.1±5.7 | 37.9±6.1 | 0.89 | 0.53 | 0.45 |
| Visceral fat L2-3 (cm2)* | 67 (42-111) |
98 (68-143) |
0.009 | 58 (34-104) |
100 (77-132) |
0.01 | 89 (58-128) |
92 (68-149) |
0.54 | 0.04 | 0.95 |
| Subcutaneous fat L2-3 (cm2)* | 265 (197-407) |
297 (194-358) |
0.72 | 265 (177-407) |
300 (253-366) |
0.44 | 277 (218-375) |
240 (194-337) |
0.48 | 0.64 | 0.30 |
| Hepatic fat (%)† | 0.7 (0.4-1.5) |
1.0 (0.7-2.5) |
0.04 | 0.6 (0.5-1.3) |
1.3 (0.7-2.9) |
0.01 | 1.2 (0.4-3.7) |
0.9 (0.5-1.7) |
0.90 | 0.45 | 0.31 |
|
Intra-myocellular (%)‡ Tibialis Anterior |
8 (4-15) | 5 (3-7) | 0.01 | 8 (4-16) | 5 (3-6) | 0.01 | 6(5-13) | 6(4-8) | 0.49 | 0.94 | 0.12 |
|
Extramyocellular (%)‡ Tibialis Anterior |
24 (15-40) |
21 (11-42) |
0.23 | 24 (15-47) |
16 (10-33) |
0.08 | 22 (14-38) |
23 (13-47) |
0.58 | 0.47 | 0.24 |
|
Metabolic Characteristics | |||||||||||
| Prediabetes (%) | 23 (32) | 16 (33) | 0.89 | 10 (19) | 7 (21) | 0.79 | 13 (65) | 9 (56) | 0.59 | 0.0001 | 0.01 |
| Fasting glucose (mg/dL) | 91±7 | 93±7 | 0.34 | 90±7 | 92±7 | 0.23 | 95±7 | 94±7 | 0.79 | 0.02 | 0.38 |
| 2-hour glucose (mg/dL) | 125±24 | 130±28 | 0.28 | 122±24 | 126±27 | 0.44 | 134±22 | 139±31 | 0.57 | 0.05 | 0.14 |
| Fasting insulin (mcU/mL) | 7.7 (4.5-10.6) |
7.0 (4.8-10.2) |
0.50 | 7.2 (4.5-11.2) |
7.5 (5.3-10.5) |
0.76 | 7.9 (5.4-10.0) |
5.0 (3.5-7.5) |
0.07 | 0.70 | 0.08 |
| Acute Insulin Response (mU/Lmin−1)§ | 732 (477-1178) |
428 (276-703) |
0.0001 | 793 (551-1186) |
529 (333-712) |
0.001 | 596 (341-1178) |
318 (183-394) |
0.02 | 0.16 | 0.03 |
| Insulin sensitivity index, SI (mU/L)−1min−1x10−4§ | 2.3 (1.7-3.2) |
2.7 (1.8-4.6) |
0.06 | 2.3 (1.6-3.3) |
2.5 (1.6-4.2) |
0.34 | 2.4 (1.7-3.0) |
4.5 (2.4-6.0) |
0.04 | 0.94 | 0.12 |
| Disposition index§ | 1693 (1138-2777) |
1325 (972-1802) |
0.02 | 1874 (1270-2784) |
1460 (972-1874) |
0.02 | 1240 (930-1937) |
1234 (970-1556) |
0.47 | 0.07 | 0.27 |
n=96 (black pre-menopause=26, post-menopause=13; white pre-menopause=41, post-menopause=16)
n=94 (black pre-menopause=27, post-menopause=13; white pre-menopause=38, post-menopause=16)
n=86 (black pre-menopause=23, post-menopause=12; white pre-menopause=35, post-menopause=16)
n=107(black pre-menopause=30, post-menopause=12; white pre-menopause=47, post-menopause=18). BP: blood pressure.
Insulin response and sensitivity.
The acute insulin response to glucose was higher and SI tended to be lower in black compared to white pre- and post-menopausal women indicating greater hyperinsulinemia and lower insulin sensitivity in black women (Table 1). During the mixed meal test, black women had greater hyperinsulinemia compared to white women: insulin: glucose AUC0-30 (black: 2.9 (1.7-4.8) vs. white 1.4 (0.9-2.0), P=0.0001) and insulin: glucose AUC0-300 (black: 89.1 (48.5-118.8) vs. white 63.5 (37.4-92.0), P=0.02).
Diet and activity profiles.
Dietary energy and macronutrient profiles were reported, and RBC fatty acid profiles measured as an indicator of longer-term diet composition. By report of dietary macronutrient intake, African immigrant women had lower total energy intake, total and saturated fatty acid consumption and added sugars compared to African American and white women (Online Table III). However, there were no differences in RBC fatty acid profiles between African Immigrant and African American women (Online Table IV). When black women were compared to whites, black women had lower RBC MUFA concentrations (P=0.004, Online Figure IIA). Black women also had lower total (P=0.02) and moderate-vigorous physical activity levels (P=0.006, Table 1).
Marker of de novo lipogenesis.
SCD-116 and SCD-118 activity indices were 20 and 7% lower, respectively, in black compared to white women (Online Figure IIB and C).
Lipid and lipoprotein profile.
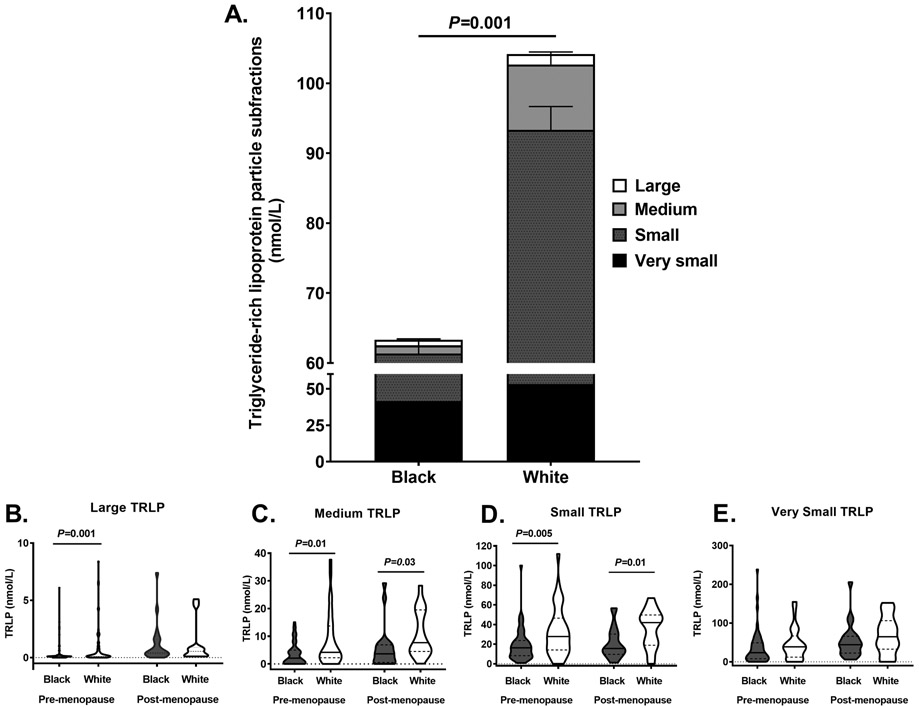
Consistent with prior findings, black compared to white women had lower triglyceride concentrations (P=0.0003) but there were no other differences in lipid, lipoprotein or apolipoprotein A1 or B profiles (Table 2). The majority of circulating fasting TRLPs subfractions were very small TRLPs (black:53±26 vs. white:47±27%, P=0.2) and small TRLPs (black:40±27 vs. white:42±27%, P=0.7) and this proportion did not differ by race. In contrast, black women tended to have a lower relative proportion of medium TRLPs (black:6±9 vs. white:9±9%, P=0.06) and had lower proportion of large TRLP subfractions (black:2±3 vs. white:3±4%, P=0.01). Figure 1 illustrates fasting TRLP concentrations by race. Total, large, medium, and small TRLPs were lower in black compared to white pre-menopausal women, with a similar pattern by race in post-menopausal women (Figure 1A-D). Very small TRLPs were not significantly different in black compared to white pre- or post-menopausal women (Figure 1E).
Table 2.
Fasting lipid and lipoprotein profile by race and menopausal status. Variables with a normal distribution are expressed as mean±SD and compared with unpaired Student’s t tests. Variables with non-normal distribution are expressed as median (25th-75th percentile) and compared with Mann-Whitney test.
| Entire Cohort (n=122) |
Pre-menopausal (n=86) |
Post-menopausal (n=36) |
Pre vs. Post Black |
Pre vs. Post White |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Black (n=73) |
White (n=49) |
P- value |
Black (n=53) |
White (n=33) |
P- value |
Black (n=20) |
White (n=16) |
P- value |
P- value |
P- value |
|
| Fasting Lipid Profile (mg/dL) | |||||||||||
| Total Cholesterol | 169±32 | 173±27 | 0.50 | 165±29 | 165±22 | 0.99 | 182±35 | 191±30 | 0.44 | 0.04 | 0.004 |
| Triglyceride | 57 (44-68) | 71 (58-98) | 0.0003 | 54 (41-67) | 69 (56-99) | 0.002 | 61 (54-77) | 74 (64-96) | 0.06 | 0.07 | 0.36 |
| HDL Cholesterol | 61±17 | 58±15 | 0.27 | 61±17 | 56±15 | 0.18 | 62±18 | 62±16 | 0.93 | 0.70 | 0.19 |
| LDL Cholesterol | 96±29 | 100±26 | 0.46 | 92±28 | 94±23 | 0.78 | 105±28 | 111±28 | 0.53 | 0.08 | 0.02 |
| Apolipoproteins (mg/dL) | |||||||||||
| Apo A1 | 131 (117-156) |
132 (123-144) |
0.88 | 131 (113-153) |
132 (119-142) |
0.79 | 136 (120-164) |
142 (128-150) |
0.59 | 0.23 | 0.08 |
| Apo B | 70 (60-89) |
75 (69-89) |
0.24 | 70 (60-87) |
74 (65-85) |
0.39 | 79 (65-94) |
84 (71-98) |
0.55 | 0.15 | 0.14 |
Figure 1. Fasting triglyceride-rich lipoprotein particle (TRLP) concentrations.
(A) Bar graph of TRLP concentrations after a 10-12 hour overnight fast; very small (black bar), small (grey bar with black dots), medium (light gray), large (white); Violin plots of large (B), medium (C), small (D), and very small (E) TRLP concentrations in black (dark gray, n=73) and white (white, n=49) pre- and post-menopausal women. The shape of the violin is the frequency distribution of the data, the solid black line represents the median and the dotted black line represents the interquartile range. Mann-Whitney tests were used to compare variables by race.
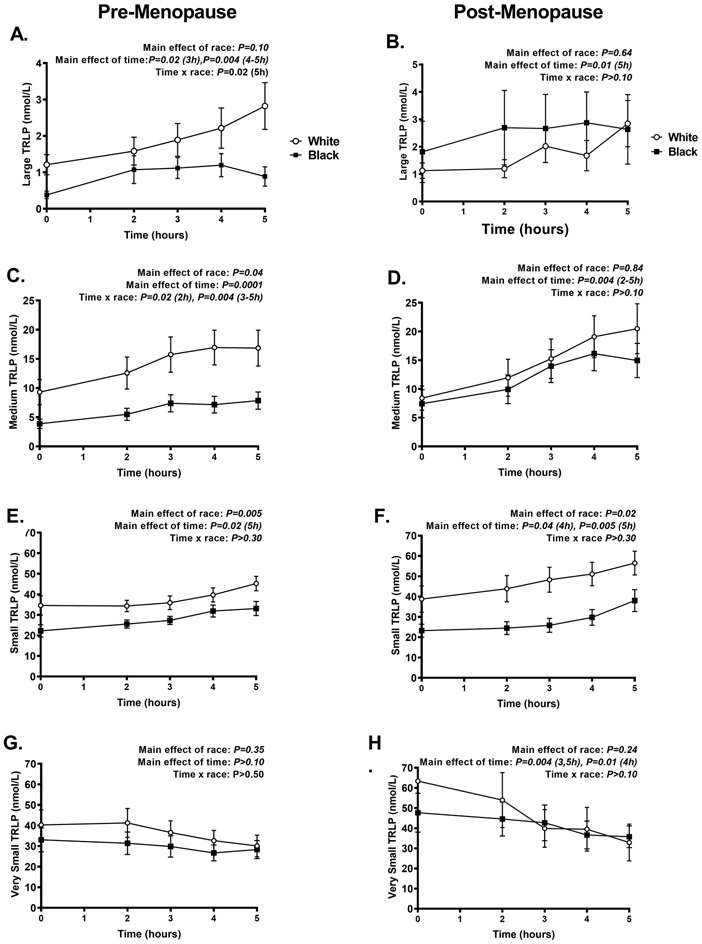
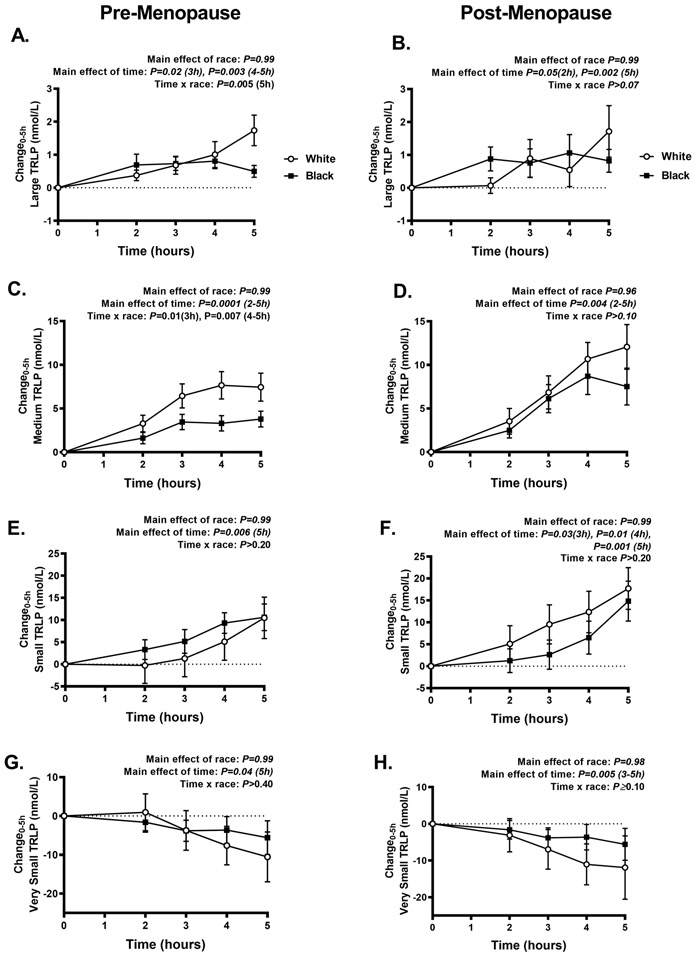
Figure 2 illustrates the TRLP concentrations of large (A-B), medium (C-D), small (E-F), and very small (G-H) during the MMTT in black compared to white pre- and post-menopausal women. Black compared to white pre-menopausal women had lower large, medium and tended to have lower small TRLP concentrations, but there were no differences in very small TRLPs (Figure 2A, C, E, G). The pattern of lower TRLP concentrations was similar in black compared to white post-menopausal women but was not statistically significant (Figure 2B, D, F, H). Of note, the lower large and medium TRLP concentrations in black pre-menopausal women were related to both lower TRLPs at baseline and smaller changes in TRLP concentrations during the MMTT (Figure 3).
Figure 2. Postprandial triglyceride-rich lipoprotein particle (TRLP) concentrations.
Concentrations of large (A-B), medium (C-D), small (E-F), and very small (G-H) TRLPs during the 5-hour mixed meal test in black (solid squares and lines, n=68) and white (white circles and lines, n=42) pre- and post-menopausal women. Data presented mean±SEM. Mixed effects models were used to analyze TRLP over time (within-subject factor of time; between-subject factor of race; time x race interaction).
Figure 3. The change in postprandial triglyceride-rich lipoprotein particle (TRLP) concentrations.
The change in large (A-B), medium (C-D), small (E-F), and very small (G-H) TRLP concentrations during the 5-hour mixed meal test in black (solid squares and lines, n=68) and white (white circles and lines, n=42) pre- and post-menopausal women. Data presented mean±SEM. Mixed effects models were used to analyze the change in TRLP over time (within-subject factor of time; between-subject factor of race; time x race interaction).
Correlations and determinants of TRLP subfractions.
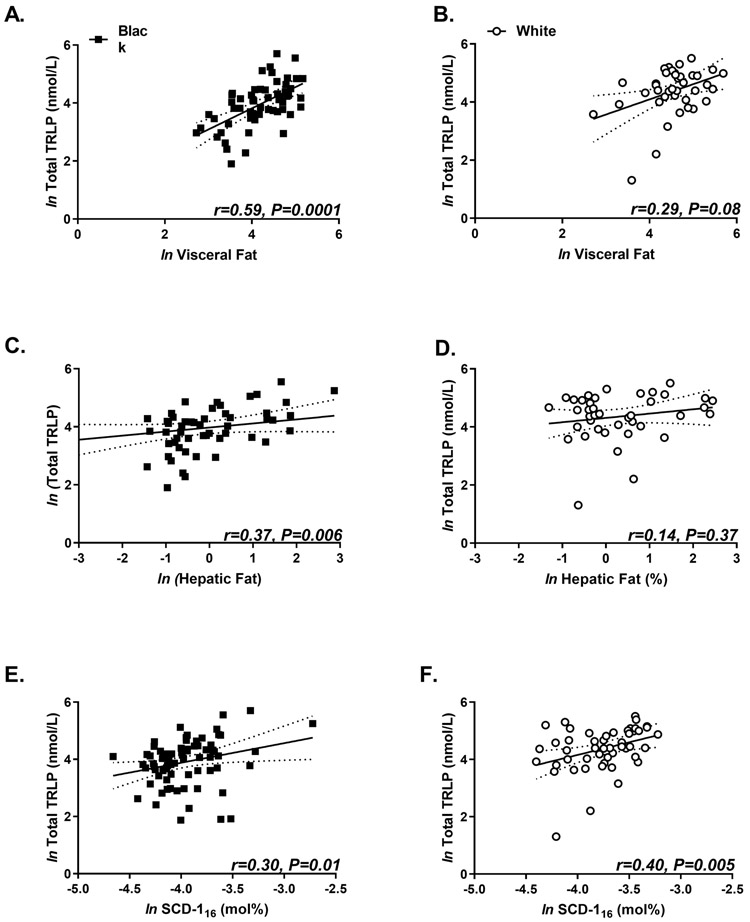
Fasting total TRLP concentrations positively correlated with visceral fat and SCD-116 activity in black and white women (Figure 4). However, total TRLP correlated with hepatic fat in black women only. Fasting TRLP subfractions (large, medium, very small, but not small) correlated with SI , hepatic and visceral fat, and SCD-116 activity although these relationships differed by race (Table 3). Table 4 depicts multiple regression models of the relationship between fasting total TRLP and whole-body insulin sensitivity (A), visceral fat (B), hepatic fat (C), and intramyocellular fat (D). Model B which included quantification of visceral fat content with SCD-116 activity accounted for ~40% of variance in TRLP. In contrast, SCD-116 activity with SI (Model A), hepatic (Model C) or intramyocellular fat (Model D) explained 31-33% of the variance in total fasting TRLP.
Figure 4. The relationship of fasting total triglyceride-rich lipoprotein particle (TRLP) concentrations with markers of insulin resistance and de novo lipogenesis.
Scatterplot of natural log (ln) of total TRLP and ln visceral fat (A-B), ln hepatic fat (C-D), and ln stearoyl-CoA desaturase activity (SCD-116) black (black squares) and white (white circles) women. Data presented as individual data points with corresponding regression line and 95% confidence interval in dotted line. Spearman correlations with Bonferroni corrections were used to determine correlation coefficients (r).
Table 3.
Correlates of fasting triglyceride-rich lipoprotein particles. Spearman correlations were used to determine correlation coefficients (r) with Bonferroni corrections to account for 4 comparisons between TRLP subfractions and each variable.
| Fasting TRLP Correlation coefficient (r) |
||||||||
|---|---|---|---|---|---|---|---|---|
| Large | Medium | Small | Very Small | |||||
| r | P-value | r | P-value | r | P-value | r | P-value | |
| All women | ||||||||
| Insulin sensitivity index (n=107) | −0.37 | 0.0007 | −0.19 | 0.472 | 0.01 | 1.000 | −0.201 | 0.379 |
| Visceral fat (n=96) | 0.56 | 0.0001 | 0.44 | 0.0001 | 0.16 | 1.000 | 0.42 | 0.0002 |
| Liver fat (n=94) | 0.50 | 0.0001 | 0.27 | 0.085 | 0.12 | 1.000 | 0.24 | 0.213 |
| SCD-116 index (n=122) | 0.33 | 0.002 | 0.40 | 0.0001 | 0.12 | 1.000 | 0.35 | 0.001 |
| SCD-118 index (n=122) | 0.23 | 0.117 | 0.34 | 0.002 | 0.05 | 1.000 | 0.31 | 0.005 |
| Black women | ||||||||
| Insulin sensitivity index (n=65) | −0.47 | 0.001 | −0.34 | 0.0495 | −0.13 | 1.000 | −0.38 | 0.016 |
| Visceral fat (n=57) | 0.57 | 0.0001 | 0.48 | 0.001 | 0.09 | 1.000 | 0.57 | 0.0001 |
| Liver fat (n=54) | 0.41 | 0.024 | 0.23 | 0.961 | −0.03 | 1.000 | 0.39 | 0.039 |
| SCD-116 index (n=73) | 0.19 | 1.000 | 0.32 | 0.052 | −0.02 | 1.000 | 0.30 | 0.088 |
| SCD-118 index (n=73) | 0.17 | 1.000 | 0.34 | 0.030 | −0.13 | 1.000 | 0.31 | 0.069 |
| White women | ||||||||
| Insulin sensitivity index (n=42) | −0.46 | 0.02 | −0.20 | 1.000 | −0.03 | 1.000 | 0.02 | 1.000 |
| Visceral fat (n=39) | 0.58 | 0.001 | 0.23 | 1.000 | 0.08 | 1.000 | 0.19 | 1.000 |
| Liver fat (n=40) | 0.60 | 0.0004 | 0.24 | 1.000 | 0.17 | 1.000 | 0.01 | 1.000 |
| SCD-116 index (n=49) | 0.41 | 0.033 | 0.32 | 0.252 | 0.14 | 1.000 | 0.34 | 0.157 |
| SCD-118 index (n=49) | 0.17 | 1.000 | 0.21 | 1.000 | 0.07 | 1.000 | 0.19 | 1.000 |
Table 4.
Determinants of fasting total triglyceride-rich lipoprotein particle (TRLP) concentrations. Multi-linear regression models were created to determine the relationship of fasting total TRLP concentrations with independent variables. All models were adjusted for age, moderate-vigorous activity, RBC monounsaturated fatty acid intake. ln: natural log, SCD-116: stearoyl-CoA desaturase-1 activity.
| Ln Total TRLP | |||
|---|---|---|---|
| Adjusted R2 | B (SE) | P value | |
| Model A, n=88 | 0.33 | ||
| Black | −0.41 (0.14) | 0.004 | |
| ln(Insulin Sensitivity Index) | −0.31 (0.11) | 0.006 | |
| ln(SCD-116 activity index) | 0.36 (0.27) | 0.19 | |
| Model B, n=96 | 0.39 | ||
| Black | −0.13 (0.15) | 0.383 | |
| ln(Visceral fat) | 0.44 (0.13) | 0.001 | |
| ln(SCD-116 activity index) | 0.41 (0.27) | 0.113 | |
| Model C, n=94 | 0.32 | ||
| Black | −0.25 (0.15) | 0.098 | |
| ln(Hepatic fat) | 0.05 (0.13) | 0.461 | |
| ln(SCD-116 activity index) | 0.59 (0.28) | 0.038 | |
| Model D, n=86 | 0.31 | ||
| Black | −0.38 (0.17) | 0.027 | |
| ln(Intramyocellular fat) | 0.13 (0.10) | 0.16 | |
| ln(SCD-116 activity index) | 0.64 (0.27) | 0.034 | |
Postprandial changes in total TRLP correlated with fasting insulin (r=0.22, P=0.02) and with age (r=0.39, P=0.0001), visceral (r=0.37, P=0.0002), and hepatic fat (r=0.37, P=0.0003), but not with SI (r=−0.07, P=0.50), monounsaturated fatty acids (r=0.14, P=0.15), or moderate-vigorous activity ((r=−0.05, P=0.61)). The change in medium vs. small and small vs. very small TRLPs were positively correlated in black women, but in white women only the change in small and very small TRLPs were correlated (Online Figure III).
DISCUSSION
The triglyceride paradox in black women is defined as low or normal triglyceride concentrations despite high rates of insulin resistance. This study helps to elucidate the underlying mechanisms of this paradox by demonstrating that race-related differences in TRLP subfraction concentrations is associated with differences in body composition (lower visceral and hepatic fat) and lower markers of de novo lipogenesis (SCD-1 activity). This novel association of TRLP concentrations with SCD-1 activity supports the hypothesis that hepatic secretion of TRLPs is an important contributor to lower TRLP concentrations in black compared to white women. Further, our findings also confirm that the triglyceride paradox is associated with 30-40% lower TRLP subclass concentrations in both pre- and post-menopausal women who were overweight/ obese during the fasting and post-prandial state. The lower TRLPs concentrations were evident in large, medium and small subfractions despite a trend for higher rates of insulin resistance, lower physical activity levels and modest differences in RBC MUFA concentrations in black women. These findings provide new information on the paradoxical association of individual TRLP subfractions with lower SI in 2 groups of women of African ancestry (African American and African immigrant) across a wide age range and in 2 physiologic states thereby strongly supporting an underlying genetic etiology for the interethnic variations in circulating triglyceride concentrations and particle distribution. Notably, by also demonstrating that very small TRLPs comprise a majority of circulating TRLPs that correlate with insulin resistance, we highlight the previously underrecognized contribution of these smaller particles to insulin resistance.
Demonstrating these race-related differences in TRLP subfractions concentrations in both the fasting and postprandial states is new and important because it suggests that black compared to white women have embedded resiliency mechanisms in the TRLP pathways i.e. a net reduction in TRLP production (assembly, secretion) and clearance despite insulin resistance 12. Our postprandial study design also offers insight into the cumulative effect of postprandial hyperinsulinemia and insulin resistance on changes in TRLP concentrations by race. Past metabolic studies were limited in their scope because greater LPL surrogate activity was associated with TRLP concentrations in the post-absorptive state without confirmatory kinetic studies 18, 19, 23, 47, 48. Although genetic analyses also linked variations in the LPL gene with lower triglyceride concentrations in individuals of African ancestry 15, 49, the association of changes in postprandial triglycerides with LPL mRNA expression and function were inconsistent and not observed in individuals with obesity 23. Moreover, previous postprandial analyses focused on changes in serum triglyceride concentrations which represent the sum of triglyceride concentrations within all TRLPs. In this study, we demonstrate that the trajectory of TRLP subclass concentrations are unique after a meal and differed by race. Black compared to white women with overweight and obesity had greater postprandial hyperinsulinemia and smaller postprandial TRLP excursions in large and medium TRLP concentrations (at 5 hours and 3-5 hours, respectively). These findings posit efficient chylomicron clearance may explain the lower postprandial levels in black compared to white women, although differences in the rate of chylomicron transfer or the release of triglycerides from the enterocyte-triglyceride pool could also play a role 50.
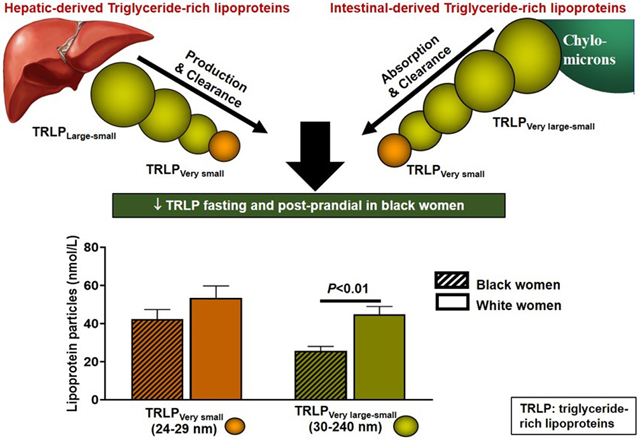
In contrast to the rise in large and medium TRLPs postprandially, we also showed that small and very small TRLPs remain unchanged or modestly decreased after a meal. By intentionally examining each TRLP subfraction by race and menopause in relation to insulin resistance, we uncovered the absence of racial differences in the concentrations of very small TRLPs. Very small TRLPs constituted approximately half (53% and 47%) of the total TRLP pool in black and white women respectively and positively correlated with insulin resistance, with no difference in this relationship by race. Since very small TRLPs represent remnant lipoproteins (IDLs and chylomicron remnants) that contain up to one third of non-fasting plasma cholesterol, 40 their presence and concentration may help to explain the overall high risk for cardiometabolic disease in black women, even when the fasting lipid panel is within the normal range. The relative importance of these very small particles (in 24-29nm range) as independent contributors to atherosclerotic cardiovascular disease is just coming into focus 51, 52. These very small TRLPs are highly atherogenic; they can directly enter the luminal wall without being oxidized and their intra-luminal hydrolysis is thought to incite a pro-inflammatory pro-atherogenic cascade 51. Therefore, the size and number of these very small TRLP, may be key mediators of cardiometabolic disease. This analysis shows the positive correlation of very small TRLPs with insulin resistance which supports their association with metabolic disease risk. The clinical relevance of elucidating very small TRLP metabolism and concentration is to illuminate their potential effect on cardiometabolic risk because their contribution would not be captured in clinical tests of fasting triglyceride analyses. In this study, in which 30% of participants had prediabetes, the large proportion of very small relative to total TRLP particles in both black and white women is intriguing and warrants further investigation into the role of these very small TRLPs in modifying cardiometabolic disease risk.
Another important study finding was the absence of race-related changes in postprandial TRLP concentrations by menopausal status. In contrast to the fasting state, in which TRLP concentrations were lower in black vs. white women, regardless of menopausal status, there were no racial differences in postprandial changes in TRLP. The similar TRLP excursions after a meal in post-menopausal women could have been related to a type 2 error owing to the smaller sample size of women in the post-menopause group, but could also indicate differences in metabolism by age or menopause. Notably, visceral and hepatic fat were not different by race in post-menopausal women and could contribute to the similar change in TRLP in black and white post-menopausal women. Kinetic studies designed to compare pre- and post-menopausal changes in postprandial TRLP metabolism are warranted.
The confirmation that lower visceral and hepatic fat was associated with lower concentrations of TRLP subfractions in this large group of women provides additional evidence to support tissue-specific insulin sensitivity in black compared to white pre-menopausal women31. The hepatic phenotype in black compared to white women, is characterized by greater hepatic, relative to whole-body insulin sensitivity, lower basal rates of gluconeogenesis and lower SCD-1 activity in black compared to white pre-menopausal women 31. The current TRLP analysis corroborates a similar hepatic phenotype, in a larger group of pre- and post-menopausal women, which is characterized by relatively preserved insulin sensitivity to triglyceride accumulation in black compared to white women. Together these studies from the Federal Women Study support pathways of resiliency to excess glucose and triglyceride production in 2 groups of women of African ancestry with insulin resistance who are at risk for cardiometabolic disease.
Importantly, because we did not undertake kinetic studies, we cannot definitively conclude preserved efficiency or resiliency in TRLP clearance pathways. Lower TRLP concentrations in black pre-menopausal women could also be secondary to decreased TRLP synthesis or some combination of both mechanisms. To address this question and acknowledge the important gate-keeper role of insulin in regulating intrahepatic TRLP assembly and secretion, we determined the association of TRLP subfractions with insulin sensitivity and factors related to hepatic TRLP secretion (visceral and hepatic fat) 47. TRLP subfractions correlated with insulin sensitivity index and visceral fat. In contrast, hepatic fat strongly correlated with total TRLP concentrations in black women only. To determine the individual contributions of hepatic and visceral adiposity, both visceral and hepatic fat were a priori determined as co-variates for the model analysis. The two models were created because visceral and hepatic fat are strong correlates and it is unknown whether FFAs must be deposited in the liver parenchyma and then released for TRLP secretion, or if visceral and hepatic fat accumulation are just corollaries of increased TRLP production 53. Of note, in black compared to white individuals, lower hepatic fat stores could limit the availability of free fatty acids (FFA) for re-esterification and contribute to lower fasting TRLP concentrations in the former group 28. Interestingly, in this study of women with obesity and insulin resistance but with relatively low hepatic fat content (2.1±2.85%), hepatic fat was not a significant independent determinant of the race-related differences in fasting TRLPs. Alternatively, race-related differences in insulin-mediated de novo lipogenesis needed for TRLP synthesis may exist 31. By using an independently RBC-derived marker of de novo lipogenesis, the SCD-116 index, we were able to assess the contribution of de novo lipogenesis in conjunction with and separate from hepatic fat accumulation by race and menopause. Lower visceral fat and SCD-116 activity in black than white women were important independent determinants of TRLP concentrations that accounted for almost 40% of race-related variations in TRLP in the multi-linear regression analyses. Although lower visceral fat has been associated with triglycerides 14, 16-18, 54, this is the first study to show that lower TRLP subfraction concentrations in black women is also associated with a marker of de novo lipogenesis. These data posit that race- related differences in both de novo lipogenesis and the size of the visceral fat compartment could be important mediators of lower fasting TRLP concentrations in black women.
Strengths and limitations.
This relatively large cross-sectional metabolic study compared TRLP concentrations during two physiological states to understand the interaction of socio-environmental factors to racial-related differences in triglyceride concentration. Using the Federal Women’s Study enabled comparisons in women who had similar access to health care and health insurance by race/ ethnicity29. We utilized validated and detailed measures of dietary intake –food records and RBC fatty acid profiles –to quantify short dietary exposure and fatty acid status over 3 months, respectively. Some study limitations are notable. This study included a relatively small sample of post-menopausal women with multiple comparisons to explore the relationship of the observed differences in TRLP by race/ethnicity and menopausal status. These comparisons increase the risk of observing false positive results. Larger studies, that account for multiple testing across the entire study, are needed to confirm our study observations. The use of frozen samples for lipoprotein analysesyields reproducible and reliable results however, the freeze-thaw cycle may underestimate the absolute concentrations of very large and large TRLPs 37. Since all samples were stored and analyzed in a similar manner, the results obtained remain a robust indicator of relative differences in TRLP concentrations between black and white women. In addition, the clearance of postprandial triglycerides may be an important indicator of insulin resistance and atherogenicity 55, but we only performed a 5-hour meal test which was insufficient for assessing the totality of chylomicron metabolism. The cross-sectional study design, without kinetic measurements, precluded quantitative assessments of TRLP production and clearance. A complete assessment of postprandial triglyceride clearance would require a 10-hour test with measurement of TRLP concentrations at frequent timepoints (e.g. 0, 3, 5, 7, and 10h). Additionally, the NMR assessment of lipoprotein particles is based on particle size and future studies to explore race/ ethnic variations in the metabolism of apoB-48 chylomicron remnants vs. apoB-100 intermediate density lipoproteins are needed. Finally, while we did not measure absolute rates of de novo lipogenesis; the SCD indices were a reliable indicator enzymatic activity between racial groups 32.
Conclusion.
In summary, pre- and post-menopausal black compared to white women had lower fasting and postprandial large, medium and small TRLP concentrations despite a trend for greater rates of insulin resistance. Lower visceral fat, and SCD-116 activity levels were important mediators of fasting TRLP concentrations and racial differences in these factors may contribute to the triglyceride paradox. The modest, albeit transient, racial differences in TRLP postprandial concentrations observed in pre-menopausal women, in the absence of race-related differences in very small TRLPs, warrants additional investigation to determine whether these differences, or the lack thereof, are significant mediators of higher cardiometabolic disease risk in black and white women.
Supplementary Material
NOVELTY AND SIGNIFICANCE.
What Is Known?
Globally, individuals of African descent have low to normal triglyceride levels but paradoxically high rates of insulin resistance, diabetes and heart disease.
These differences limit the use of common triglyceride-based tests to improve detection and early diabetes and heart disease in black populations.
What New Information Does This Article Contribute?
The paradoxical relationship of triglyceride levels with insulin resistance in black compared to white pre- and post-menopausal women is related to differences in the concentrations of triglyceride-rich lipoproteins (TRLP) subfractions.
In black compared to white women, large, medium and small TRLP concentrations were lower after an overnight fast and during a breakfast meal in both pre- and post-menopausal women.
The lower triglyceride subfractions (large, medium, and small) in black women were associated with less central fat stores and a lower marker of hepatic lipid synthesis (stearoyl-CoA desaturase 1 activity) in women with a similar diabetes risk.
Importantly, very small TRLP particles were similar in black and white women and correlated with insulin resistance, suggesting that these particles may be more useful for diabetes and heart disease risk assessment.
Triglyceride concentrations are paradoxically normal despite high rates of insulin resistance in individuals of African descent. This dissociative relationship hinders the widespread use of simple, cost-effective triglyceride-based screening tests for diabetes and heart disease in African ancestry populations. This study helped to clarify this paradoxical relationship to help improve cardiometabolic risk stratification regardless of race/ethnicity. We designed the Federal Women’s Study to compare triglyceride subfractions, insulin resistance, diet and activity profiles among pre- and post-menopausal women who had similar access to health care and health insurance. We showed that large, medium and small triglyceride-rich lipoproteins were lower in black compared to white women after an overnight fast and after a breakfast meal, despite similar diabetes risk factors. In black women, the lower triglyceride subfractions were related to lower visceral adiposity and lower markers of hepatic triglyceride synthesis (stearoyl Co-A desaturase activity). The presence of lower triglyceride subfractions after a meal also indicates efficient dietary triglyceride clearance, despite insulin resistance in black compared to white women. In contrast, the pro-atherogenic very small triglyceride-rich lipoproteins were not different by race/ethnicity, correlated with insulin resistance, and therefore may be useful in cardiometabolic risk stratification among black and white women.
ACKNOWLEDGEMENTS
We would like to thank the volunteers whose participation made this study possible. We sincerely thank James Otvos, PhD (LabCorp, Raleigh NC) for contributions to editing the manuscript and to his team for assisting with lipoprotein analyses, and Kong Chen, PhD and Robert Brychta, PhD for assisting with accelerometer data processing.
SOURCES OF FUNDING
The Division of Intramural Research supports STC, AES, RO, AG and LM (National Institute of Diabetes & Digestive & Kidney Diseases), ATR, MS (National Heart, Lung and Blood Institute), and AES (National Institute of Minority Health and Health Disparities). AC, SB, MLS are supported by the NIH Clinical Center. AHL is supported in part by the U. S. Department of Agriculture under agreement No. 58-1950-4-401.
Nonstandard Abbreviations and Acronyms:
- FFA
Free fatty acid
- FSH
Follicle stimulating hormone
- IM-FSIGT
Insulin-modified frequently sampled intravenous glucose tolerance test
- LPL
Lipoprotein lipase
- MMTT
Mixed meal tolerance test
- MUFA
Monounsaturated fatty acid
- NIH
National Institutes of Health
- OGTT
Oral glucose tolerance test
- RBC
Red blood cell
- SCD-1
Stearoyl-CoA desaturase-1 enzyme activity index
- TRLP
Triglyceride-rich lipoprotein particle
- VLDL
Very low-density lipoprotein
Footnotes
DISCLOSURES
I STC certify that neither I nor my co-authors have any conflicts of interest.
REFERENCES
- 1.Bentley AR, Rotimi CN. Interethnic differences in serum lipids and implications for cardiometabolic disease risk in african ancestry populations. Global heart. 2017;12:141–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yu SS, Castillo DC, Courville AB, Sumner AE. The triglyceride paradox in people of african descent. Metab Syndr Relat Disord. 2012;10:77–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sumner AE, Finley KB, Genovese DJ, Criqui MH, Boston RC. Fasting triglyceride and the triglyceride-hdl cholesterol ratio are not markers of insulin resistance in african americans. Archives of internal medicine. 2005;165:1395–1400 [DOI] [PubMed] [Google Scholar]
- 4.Sumner AE, Cowie CC. Ethnic differences in the ability of triglyceride levels to identify insulin resistance. Atherosclerosis. 2008;196:696–703 [DOI] [PubMed] [Google Scholar]
- 5.Sumner AE, Harman JL, Buxbaum SG, Miller BV 3rd, Tambay AV, Wyatt SB, Taylor HA, Rotimi CN, Sarpong DF. The triglyceride/high-density lipoprotein cholesterol ratio fails to predict insulin resistance in african-american women: An analysis of jackson heart study. Metab Syndr Relat Disord. 2010;8:511–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sumner AE. Ethnic differences in triglyceride levels and high-density lipoprotein lead to underdiagnosis of the metabolic syndrome in black children and adults. The Journal of pediatrics. 2009;155:S7 e7–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sumner AE, Zhou J, Doumatey A, Imoisili OE, Amoah A, Acheampong J, Oli J, Johnson T, Adebamowo C, Rotimi CN. Low hdl-cholesterol with normal triglyceride levels is the most common lipid pattern in west africans and african americans with metabolic syndrome: Implications for cardiovascular disease prevention. CVD Prev Control. 2010;5:75–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Foster T, Anania FA, Li D, Katz R, Budoff M. The prevalence and clinical correlates of nonalcoholic fatty liver disease (nafld) in african americans: The multiethnic study of atherosclerosis (mesa). Digestive diseases and sciences. 2013;58:2392–2398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu SS, Ramsey NL, Castillo DC, Ricks M, Sumner AE. Triglyceride-based screening tests fail to recognize cardiometabolic disease in african immigrant and african-american men. Metab Syndr Relat Disord. 2013;11:15–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ukegbu UJ, Castillo DC, Knight MG, Ricks M, Miller BV, 3rd, Onumah BM, Sumner AE. Metabolic syndrome does not detect metabolic risk in african men living in the u.S. Diabetes care. 2011;34:2297–2299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fezeu L, Balkau B, Kengne AP, Sobngwi E, Mbanya JC. Metabolic syndrome in a sub-saharan african setting: Central obesity may be the key determinant. Atherosclerosis. 2007;193:70–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bagby SP, Martin D, Chung ST, Rajapakse N. From the outside in: Biological mechanisms linking social and environmental exposures to chronic disease and to health disparities. American journal of public health. 2019;109:S56–S63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jackson KG, Abraham EC, Smith AM, Murray P, O’Malley B, Williams CM, Minihane AM. Impact of age and menopausal status on the postprandial triacylglycerol response in healthy women. Atherosclerosis. 2010;208:246–252 [DOI] [PubMed] [Google Scholar]
- 14.D’Adamo E, Northrup V, Weiss R, Santoro N, Pierpont B, Savoye M, O’Malley G, Caprio S. Ethnic differences in lipoprotein subclasses in obese adolescents: Importance of liver and intraabdominal fat accretion. The American journal of clinical nutrition. 2010;92:500–508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ellman N, Keswell D, Collins M, Tootla M, Goedecke JH. Ethnic differences in the association between lipid metabolism genes and lipid levels in black and white south african women. Atherosclerosis. 2015;240:311–317 [DOI] [PubMed] [Google Scholar]
- 16.Goedecke JH, Utzschneider K, Faulenbach MV, Rizzo M, Berneis K, Spinas GA, Dave JA, Levitt NS, Lambert EV, Olsson T, Kahn SE. Ethnic differences in serum lipoproteins and their determinants in south african women. Metabolism: clinical and experimental. 2010;59:1341–1350 [DOI] [PubMed] [Google Scholar]
- 17.Sumner A, Furtado J, Courville A, Ricks M, Younger Coleman N, Tulloch Reid M, Sacks F. Apoc-iii and visceral adipose tissue contribute to paradoxically normal triglyceride levels in insulin-resistant african-american women. Nutrition & metabolism. 2013;10:73–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Despres JP, Couillard C, Gagnon J, Bergeron J, Leon AS, Rao DC, Skinner JS, Wilmore JH, Bouchard C. Race, visceral adipose tissue, plasma lipids, and lipoprotein lipase activity in men and women: The health, risk factors, exercise training, and genetics (heritage) family study. Arterioscler Thromb Vasc Biol. 2000;20:1932–1938 [DOI] [PubMed] [Google Scholar]
- 19.Berk ES, Johnson JA, Lee M, Zhang K, Boozer CN, Pi-Sunyer FX, Fried SK, Albu JB. Higher post-absorptive skeletal muscle lpl activity in african american vs. Non-hispanic white pre-menopausal women. Obesity (Silver Spring). 2008;16:199–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Adiels M, Matikainen N, Westerbacka J, Soderlund S, Larsson T, Olofsson SO, Boren J, Taskinen MR. Postprandial accumulation of chylomicrons and chylomicron remnants is determined by the clearance capacity. Atherosclerosis. 2012;222:222–228 [DOI] [PubMed] [Google Scholar]
- 21.Punyadeera C, Crowther NJ, van der Merwe MT, Toman M, Immelman AR, Schlaphoff GP, Gray IP. Metabolic response to a mixed meal in obese and lean women from two south african populations. Obesity research. 2002;10:1207–1216 [DOI] [PubMed] [Google Scholar]
- 22.Punyadeera C, van der Merwe MT, Crowther NJ, Toman M, Schlaphoff GP, Gray IP. Ethnic differences in lipid metabolism in two groups of obese south african women. J Lipid Res. 2001;42:760–767 [PubMed] [Google Scholar]
- 23.Bower JF, Deshaies Y, Pfeifer M, Tanenberg RJ, Barakat HA. Ethnic differences in postprandial triglyceride response to a fatty meal and lipoprotein lipase in lean and obese african american and caucasian women. Metabolism: clinical and experimental. 2002;51:211–217 [DOI] [PubMed] [Google Scholar]
- 24.Masding MG, Stears AJ, Burdge GC, Wootton SA, Sandeman DD. The benefits of oestrogens on postprandial lipid metabolism are lost in post-menopausal women with type 2 diabetes. Diabetic medicine : a journal of the British Diabetic Association. 2006;23:768–774 [DOI] [PubMed] [Google Scholar]
- 25.Parks EJ, Krauss RM, Christiansen MP, Neese RA, Hellerstein MK. Effects of a low-fat, high-carbohydrate diet on vldl-triglyceride assembly, production, and clearance. J Clin Invest. 1999;104:1087–1096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sumner A, Micklesfield L, Ricks M, Tambay A, Avila N, Thomas F, Lambert E, Levitt N, Evans J, Rotimi C, Tulloch Reid M, Goedecke J. Waist circumference, bmi, and visceral adipose tissue in white women and women of african descent. Obesity. 2011;19:671–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kodama K, Tojjar D, Yamada S, Toda K, Patel C, Butte A. Ethnic differences in the relationship between insulin sensitivity and insulin response: A systematic review and meta-analysis. Diabetes care. 2013;36:1789–1796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guerrero R, Vega GL, Grundy SM, Browning JD. Ethnic differences in hepatic steatosis: An insulin resistance paradox? Hepatology. 2009;49:791–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chung ST, Galvan-De La Cruz M, Aldana PC, Mabundo LS, DuBose CW, Onuzuruike AU, Walter M, Gharib AM, Courville AB, Sherman AS, Sumner AE. Postprandial insulin response and clearance among black and white women: The federal women’s study. J Clin Endocrinol Metab. 2019;104:181–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jacome-Sosa MM, Parks EJ. Fatty acid sources and their fluxes as they contribute to plasma triglyceride concentrations and fatty liver in humans. Curr. Opin. Lipidology 2014;25:213–220 [DOI] [PubMed] [Google Scholar]
- 31.Chung ST, Courville AB, Onuzuruike AU, Galvan-De La Cruz M, Mabundo LS, DuBose CW, Kasturi K, Cai H, Gharib AM, Walter PJ, Garraffo HM, Chacko S, Haymond MW, Sumner AE. Gluconeogenesis and risk for fasting hyperglycemia in black and white women. JCI insight. 2018;3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee JJ, Lambert JE, Hovhannisyan Y, Ramos-Roman MA, Trombold JR, Wagner DA, Parks EJ. Palmitoleic acid is elevated in fatty liver disease and reflects hepatic lipogenesis. The American journal of clinical nutrition. 2015;101:34–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chung ST, Ha J, Onuzuruike AU, Kasturi K, Galvan-De La Cruz M, Bingham BA, Baker RL, Utumatwishima JN, Mabundo LS, Ricks M, Sherman AS, Sumner AE. Time to glucose peak during an oral glucose tolerance test identifies prediabetes risk. Clinical endocrinology. 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.American Diabetes A Classification and diagnosis of diabetes - standards of medical care. Diabetes care. 2017;40:S11–S24 [DOI] [PubMed] [Google Scholar]
- 35.Chow CC, Periwal V, Csako G, Ricks M, Courville AB, Miller BV, 3rd, Vega GL, Sumner AE. Higher acute insulin response to glucose may determine greater free fatty acid clearance in african-american women. J Clin Endocrinol Metab. 2011;96:2456–2463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mifflin MD, St Jeor ST, Hill LA, Scott BJ, Daugherty SA, Koh YO. A new predictive equation for resting energy expenditure in healthy individuals. The American journal of clinical nutrition. 1990;51:241–247 [DOI] [PubMed] [Google Scholar]
- 37.Jeyarajah EJ, Cromwell WC, Otvos JD. Lipoprotein particle analysis by nuclear magnetic resonance spectroscopy. Clin Lab Med. 2006;26:847–870 [DOI] [PubMed] [Google Scholar]
- 38.Matyus SP, Braun PJ, Wolak-Dinsmore J, Jeyarajah EJ, Shalaurova I, Xu Y, Warner SM, Clement TS, Connelly MA, Fischer TJ. Nmr measurement of ldl particle number using the vantera clinical analyzer. Clinical biochemistry. 2014;47:203–210 [DOI] [PubMed] [Google Scholar]
- 39.Rifai N, Warnick GR, Dominiczak MH. Handbook of lipoprotein testing. Washington, DC: AACC Press; 2000. [Google Scholar]
- 40.Balling M, Langsted A, Afzal S, Varbo A, Davey Smith G, Nordestgaard BG. A third of nonfasting plasma cholesterol is in remnant lipoproteins: Lipoprotein subclass profiling in 9293 individuals. Atherosclerosis. 2019;286:97–104 [DOI] [PubMed] [Google Scholar]
- 41.Kuchinskiene Z, Carlson LA. Composition, concentration, and size of low density lipoproteins and of subfractions of very low density lipoproteins from serum of normal men and women. J Lipid Res. 1982;23:762–769 [PubMed] [Google Scholar]
- 42.Matthan NR, Ip B, Resteghini N, Ausman LM, Lichtenstein AH. Long-term fatty acid stability in human serum cholesteryl ester, triglyceride, and phospholipid fractions. J Lipid Res. 2010;51:2826–2832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Erkkila AT, Matthan NR, Herrington DM, Lichtenstein AH. Higher plasma docosahexaenoic acid is associated with reduced progression of coronary atherosclerosis in women with cad. J Lipid Res. 2006;47:2814–2819 [DOI] [PubMed] [Google Scholar]
- 44.Sumner AE, Luercio MF, Frempong BA, Ricks M, Sen S, Kushner H, Tulloch-Reid MK. Validity of the reduced-sample insulin modified frequently-sampled intravenous glucose tolerance test using the nonlinear regression approach. Metabolism: clinical and experimental. 2009;58:220–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ouwerkerk R, Pettigrew RI, Gharib AM. Liver metabolite concentrations measured with 1h mr spectroscopy. Radiology. 2012;265:565–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Muniyappa R, Noureldin R, Ouwerkerk R, Liu EY, Madan R, Abel BS, Mullins K, Walter MF, Skarulis MC, Gharib AM. Myocardial fat accumulation is independent of measures of insulin sensitivity. J Clin Endocrinol Metab. 2015;100:3060–3068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jeppesen J, Hollenbeck CB, Zhou MY, Coulston AM, Jones C, Chen YD, Reaven GM. Relation between insulin resistance, hyperinsulinemia, postheparin plasma lipoprotein lipase activity, and postprandial lipemia. Arterioscler Thromb Vasc Biol. 1995;15:320–324 [DOI] [PubMed] [Google Scholar]
- 48.Sumner AE, Vega GL, Genovese DJ, Finley KB, Bergman RN, Boston RC. Normal triglyceride levels despite insulin resistance in african americans: Role of lipoprotein lipase. Metabolism: clinical and experimental. 2005;54:902–909 [DOI] [PubMed] [Google Scholar]
- 49.Bentley AR, Chen G, Shriner D, Doumatey AP, Zhou J, Huang H, Mullikin JC, Blakesley RW, Hansen NF, Bouffard GG, Cherukuri PF, Maskeri B, Young AC, Adeyemo A, Rotimi CN. Gene-based sequencing identifies lipid-influencing variants with ethnicity-specific effects in african americans. PLoS Genet. 2014;10:e1004190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chavez-Jauregui RN, Mattes RD, Parks EJ. Dynamics of fat absorption and effect of sham feeding on postprandial lipema. Gastroenterology. 2010;139:1538–1548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nordestgaard BG. Triglyceride-rich lipoproteins and atherosclerotic cardiovascular disease: New insights from epidemiology, genetics, and biology. Circulation research. 2016;118:547–563 [DOI] [PubMed] [Google Scholar]
- 52.Varbo A, Nordestgaard BG. Remnant cholesterol and triglyceride-rich lipoproteins in atherosclerosis progression and cardiovascular disease. Arterioscler Thromb Vasc Biol. 2016;36:2133–2135 [DOI] [PubMed] [Google Scholar]
- 53.Adeli K, Taghibiglou C, Van Iderstine SC, Lewis GF. Mechanisms of hepatic very low-density lipoprotein overproduction in insulin resistance. Trends Cardiovasc Med. 2001;11:170–176 [DOI] [PubMed] [Google Scholar]
- 54.Wang L, Sacks FM, Furtado JD, Ricks M, Courville AB, Sumner AE. Racial differences between african-american and white women in insulin resistance and visceral adiposity are associated with differences in apociii containing apoai and apob lipoproteins. Nutr Metab (Lond). 2014;11:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nakamura A, Monma Y, Kajitani S, Kozu K, Ikeda S, Noda K, Nakajima S, Endo H, Takahashi T, Nozaki E. Different postprandial lipid metabolism and insulin resistance between non-diabetic patients with and without coronary artery disease. Journal of cardiology. 2015;66:435–444 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.