Abstract
Background
Whereas the independent effects of biomarkers, including 25-hydroxy vitamin D (25(OH)D), insulin-like growth factor 1, C-reactive protein, and interleukin 6 (IL-6), on gait speed in older adults have been evaluated, their joint effects on gait speed are not well understood.
Methods
Study subjects aged at least 65 at baseline (N = 970) were enrolled in the population-based Invecchiare in Chianti (InCHIANTI) study from 1998 to 2000 and were followed up at 3 and 6 years. All above biomarkers and gait speed data were measured at each of the three time points. Using a generalized estimating equation approach, we determined if slow gait speed (<0.8 m/s) was associated with the biomarkers. Further investigation was conducted for interactions between high IL-6 (≥.87 pg/mL) and other biomarkers focusing on low 25(OH)D (<20 ng/mL).
Results
After controlling for other biomarkers and potential confounders, IL-6 emerged as the only biomarker independently associated with gait speed. The association between high IL-6 and slow gait speed was enhanced by low 25(OH)D, with significant interaction between high IL-6 and low 25(OH)D (p = .038). The odds ratio of slow gait speed for low 25(OH)D and high IL-6 was 1.63 (95% confidence interval [CI]: 1.15, 2.32) compared with the reference groups with both biomarker levels at the other ends.
Conclusion
The association of low vitamin D with slow gait speed statistically interacts with high IL-6. Coexisting vitamin D insufficiency and inflammation may provide a better biomarker for identifying those at risk of developing impairments in gait speed than either factor alone.
Keywords: Gait Speed, IL-6, Vitamin D, Interaction
Vitamin D may play a role in older patients’ mobility (1,2) and slow gait speed, a major component of frailty. Systematic reviews have shown a positive association between vitamin D and walking speed in older adults (3). Potential mechanisms include aging-related cellular processes influenced by vitamin D such as mitochondrial dysfunction, epigenetic and DNA changes, and calcium and reactive oxygen signaling alterations (4). In a similar fashion, low insulin-like growth factor 1 (IGF-1), the hormone involved in muscle mass and strength, may also contribute to frailty (5) and incident disability (6).
Whereas vitamin D and IGF-1 are positively associated with mobility, inflammation has been shown to be inversely associated with muscle mass, strength, and function including gait speed (7). For example, short-term hospitalized geriatric patients with persistent inflammation (C-reactive protein [CRP] levels ≥10 mg/L) showed no improvement in handgrip strength 1 week following admission compared with those whose inflammation resolved (8). With aging, IGF-1 declines and inflammation increases (9), potentially contributing to a high prevalence of cardiovascular diseases and morbidity among older adults.
Vitamin D and inflammation are intricately associated, with inflammation contributing to diminished vitamin D levels, whereas vitamin D deficiency is associated with chronic inflammation (10,11). In our previous study from the National Health and Nutrition Examination Survey, vitamin D and CRP showed joint effects on slow gait speed in adults more than 50 years of age (12). In addition, in a study of older women, low IGF-1 levels were shown to have positive synergism with high interleukin 6 (IL-6) in association with subsequent disability and mortality (13).
Mobility disability (including slow gait speed) has multifactorial causes and targeting only one among multiple factors is unlikely to yield significant effects (14). Previous studies have examined the concurrent associations of multiple endocrine (15) or inflammatory (16) factors, either separately or in combination (17–19) with mobility disability, frailty, and mortality in various cohorts. Even though multiple factors were studied simultaneously, the earlier studies analyzed only additive effects of different factors on aging-related outcomes. Whereas one report assessed how the interaction between pairs of endocrine and inflammatory markers affects disability in older women (13), to date no published study has evaluated how the association between vitamin D and gait speed is affected by the presence of high IL-6, a marker of inflammation. To that end, the primary objective of this study was to assess the joint effects of high IL-6 and other biomarkers focusing on low vitamin D on the likelihood of slow gait speed in older adults over 6 years.
Methods
Participants and Data Collection
Study participants were enrolled in Invecchiare in Chianti, aging in the Chianti area (InCHIANTI), a prospective observational cohort study conducted in Greve in Chianti and Bagno a Ripoli (Tuscany, Italy). Details of the study were described elsewhere (20). Briefly, participants were recruited from 1998 to 2000 using multistage sampling methods. The study recruited 1,453 community-dwelling participants (640 men and 813 women) aged 20–102 years. Participants who had gait speed data available and aged at least 65 at baseline were included in the analysis. Interviews were conducted at home followed by blood sample collection within 3 weeks. Structured medical examination then followed within 2 weeks. Participants were revisited and the data were repeatedly collected 3 and 6 years after baseline.
Measurements
Gait speed, the dependent variable, was assessed using a 4-m walk at every visit. Participants were instructed to walk at their usual pace, with or without a cane or a walker. The cutoff for slow gait speed was less than 0.8 m/s (21). Blood samples were collected at baseline, and again at the 3- and 6-year follow-up visits.
25-Hydroxy Vitamin D
Baseline serum 25-hydroxy vitamin D (25(OH)D) was measured with radioimmunoassay (DiaSorin, Stillwater, MN). An enzyme immunoassay (OCTEIA 25-hydroxy vitamin D kit; Immunodiagnostic Systems, Inc., Fountain Hills, AZ) was used to measure serum 25(OH)D at 3-year and 6-year visits. Per recommendations by the Institute of Medicine, vitamin D insufficiency was considered to be less than 20 ng/mL (22). According to the report, sufficient level of 30 ng/mL is overestimated and should be revised (23).
Interleukin 6
Baseline serum IL-6 was measured in duplicate ultra-sensitive ELISA (CytoScreen Human IL-6; BioSource International Inc., Camarillo, CA). A regression equation was applied to derive IL-6 sandwich ELISA results. Plasma IL-6 at the follow-ups was measured by a solid-phase high-sensitivity quantitative sandwich ELISA (Quantikine HS Human IL-6 Immunoassay; R&D Systems Inc., Minneapolis, MN).
C-Reactive Protein
Immunonephelometric assay and monoclonal antibodies to CRP were used to measure high-sensitivity CRP levels in duplicate with the Dade Behring BN II Nephelometer (Dade Behring Inc., Deerfield, IL). Plasma was used for baseline and 3-year visit and serum was used for 6-year visit. The cutoff for high CRP was 3 mg/L (24).
Insulin-Like Growth Factor 1
Serum total IGF-1 levels were measured in duplicate with immunoradiometric assays (Diagnostic Systems Laboratories, Webster, TX). We used the median value of participants aged 65 years or older at baseline as the cutoff for high IL-6 and low IGF-1 (2.87 pg/mL and 112 ng/mL, respectively).
Statistical Analysis
We summarized baseline characteristics overall and by subgroup with or without slow gait speed. Between-group comparisons at baseline were conducted using an independent two-sample t test for continuous variables and a chi-squared test for categorical variables. Using generalized estimating equation approach with an unspecified correlation structure, that is, empirically estimated, we associated slow gait speed and the biomarkers individually and jointly, low 25(OH)D, high CRP, high IL-6, and low IGF-1 where the visit time was modeled by two dummy variables for the effects of 3-year and 6-year visits compared with baseline. An empirically estimated correlation matrix allowed us to use all the available time points and borrow information from other subjects for missing time points to help handle selection bias from attrition. Both unadjusted and adjusted models were fitted. In adjusted models, we included sex, age, site, any education completed, body mass index, physical activity, daily vitamin D intake (µg), and disease confounders as listed in Table 1. The disease diagnosis was determined by self-reported medical history and medication use.
Table 1.
Baseline Characteristics of Subjects Aged at Least 65 Years
| Slow gait speeda | ||||
|---|---|---|---|---|
| Overall (N = 970) | With (n = 217) | Without (n = 753) | p | |
| Gait speed (m/s) | 0.98 (0.29) | 0.58 (0.17) | 1.09 (0.19) | <.0001 |
| Slow gait speed | 217/970 (22.4) | n/a | n/a | n/a |
| Low 25(OH)Db | 587/930 (63.1) | 167 (81.5) | 420 (57.9) | <.0001 |
| High IL-6c | 487/959 (50.8) | 150 (70.8) | 337 (45.1) | <.0001 |
| Low IGF-1d | 476/940 (50.6) | 138 (65.4) | 338 (46.4) | <.0001 |
| High CRPe | 434/960 (45.2) | 115 (54.0) | 319 (42.7) | .004 |
| Age (years) | 74.6 (7.1) | 80.7 (7.5) | 72.8 (5.9) | <.0001 |
| Male gender | 430/970 (44.3) | 60 (27.7) | 370 (49.1) | <.0001 |
| Body mass index (kg/m2) | 27.5 (4.1) | 28.1 (4.8) | 27.3 (3.9) | .03 |
| Greve site | 450/970 (46.4) | 108 (49.8) | 342 (45.4) | .26 |
| No education | 277/970 (28.6) | 107 (49.3) | 170 (22.6) | <.0001 |
| Diabetes mellitus | 130/970 (13.4) | 41 (18.9) | 89 (11.8) | .02 |
| PAD | 172/970 (17.7) | 62 (28.6) | 110 (14.6) | <.0001 |
| Angina pectoris | 85/970 (8.8) | 33 (15.2) | 52 (6.9) | <.0001 |
| Heart failure | 238/970 (24.5) | 84 (38.7) | 154 (20.5) | <.0001 |
| Stroke or TIA | 71/970 (7.3) | 35 (16.1) | 36 (4.8) | <.0001 |
| COPD | 111/970 (11.4) | 27 (12.4) | 84 (11.2) | .87 |
| Physical activityf | 368/966 (38.1) | 23 (10.7) | 345 (46.0) | <.0001 |
| Daily vitamin D intake (µg) | 1.83 (0.85) | 1.67 (0.72) | 1.88 (0.88) | .0006 |
Note: Categorical variables summarized by frequencies (percentages) and tested by chi-squared test., continuous variables by means (standard deviations) and tested by two-sample t test. COPD = Chronic obstructive pulmonary disease; PAD = Peripheral arterial disease; TIA = Stroke or transient ischemic attack.
a<0.8 m/s;
b<20 ng/mL;
c≥2.87 pg/mL;
d<112 ng/mL;
e≥3 mg/L;
fLight exercise >4 h/wk or moderate exercise ≥1 h/wk in the last year.
High IL-6 was the only statistically significant biomarker after adjusting for other biomarkers and potential confounders. Furthermore, the interactions between high IL-6 and low 25(OH)D or low IGF-1 were investigated in the framework of generalized estimating equation adjusted for potential confounders. For sensitivity analysis, we relaxed the assumption of missing completely at random to missing at random and imputed data using fully conditional specification multiple imputation method. We set the number of burn-in iterations to 20 and generated 10 imputed data sets, where the regression coefficient estimates were pooled across data sets and converted to odds ratios. All statistical analyses were performed in SAS. A p value smaller than .05 was considered statistically significant.
Results
Of 1,155 participants aged 65 or older at baseline, 970 participants (84%) had gait speed data available. The sample size was 713 at 3-year visit and 600 at 6-year visit. Of them, 97 participants died between baseline and 3-year visit and another 65 died between 3-year and 6-year visits. Baseline characteristics of subjects are summarized in Table 1, overall and by subgroup with or without slow gait speed (<0.8 m/s). At baseline, a total of 217 participants (22%) had slow gait speed. Participants with slow gait speed were more likely to have low 25(OH)D (<20 ng/mL), low IGF-1 (<112 ng/mL), high CRP (≥3 mg/L), and high IL-6 (≥2.87 pg/mL) than participants without slow gait speed. The prevalence of vitamin D insufficiency at baseline in the entire sample was 63%. Compared with those without slow gait speed, participants with slow gait speed at baseline were older and less physically active and had a higher body mass index, lower daily vitamin D intake, and a higher prevalence of diseases including peripheral arterial disease, stroke or transient ischemic attack, diabetes, and congestive heart failure. In addition, slow gait speed at baseline was more prevalent among females and those with no education.
Low 25(OH)D, high CRP, high IL-6, and low IGF-1 levels were each associated with slow gait speed (p < .0001) without adjustment for other biomarkers (data not shown in the table). Table 2 presents the results from unadjusted and adjusted models including all four measures. In the unadjusted model, with the exception of CRP, each of the biomarkers was significantly (p < .0001) associated with slow gait speed. In the adjusted model, high IL-6 was the only biomarker that remained significant (p = .023). The risk of slow gait speed was higher at 3 years and 6 years than baseline. Prevalence of slow gait speed was 25.8% and 28.3% at 3-year and 6-year follow-up visits, respectively. In addition, there was no interaction between any biomarker and visit time and they were not included in the model.
Table 2.
Odds ratios (ORs) of slow gait speeda associated with low 25(OH)Db, high IL-6c, high CRPd, and low IGF-1e without and with adjustment for potential confoundersf
| Unadjusted | Adjusted | |||
|---|---|---|---|---|
| Variables of interest | OR (95% CI) | p | OR (95% CI) | p |
| Low 25(OH)D | 1.83 (1.50, 2.23) | <.0001 | 1.15 (0.87, 1.51) | .32 |
| High IL-6 | 1.69 (1.39, 2.04) | <.0001 | 1.35 (1.04, 1.74) | .023 |
| High CRP | 1.15 (0.93, 1.42) | .19 | 1.10 (0.82, 1.46) | .52 |
| Low IGF-1 | 1.68 (1.39, 2.03) | <.0001 | 1.25 (0.96, 1.62) | .10 |
| Follow-upg | ||||
| 3-year | 2.02 (1.67, 2.45) | <.0001 | 1.59 (1.20, 2.12) | .002 |
| 6-year | 1.82 (1.50, 2.22) | <.0001 | 1.30 (0.97, 1.74) | .08 |
Note: CI = Confidence interval; CRP = C-reactive protein; IGF-1 = Insulin-like growth factor 1; 25(OH)D = 25-hydroxy vitamin D; IL-6 = Interleukin 6.
a,b,c,d,eTime-dependent variables.
a<0.8 m/s;
b<20 ng/mL;
c≥2.87 pg/mL;
d≥3 mg/L;
e<112 ng/mL;
fIncluding demographic covariates, diseases, physical activity, daily vitamin D intake (µg), and body mass index;
gVisit time (reference = baseline visit).
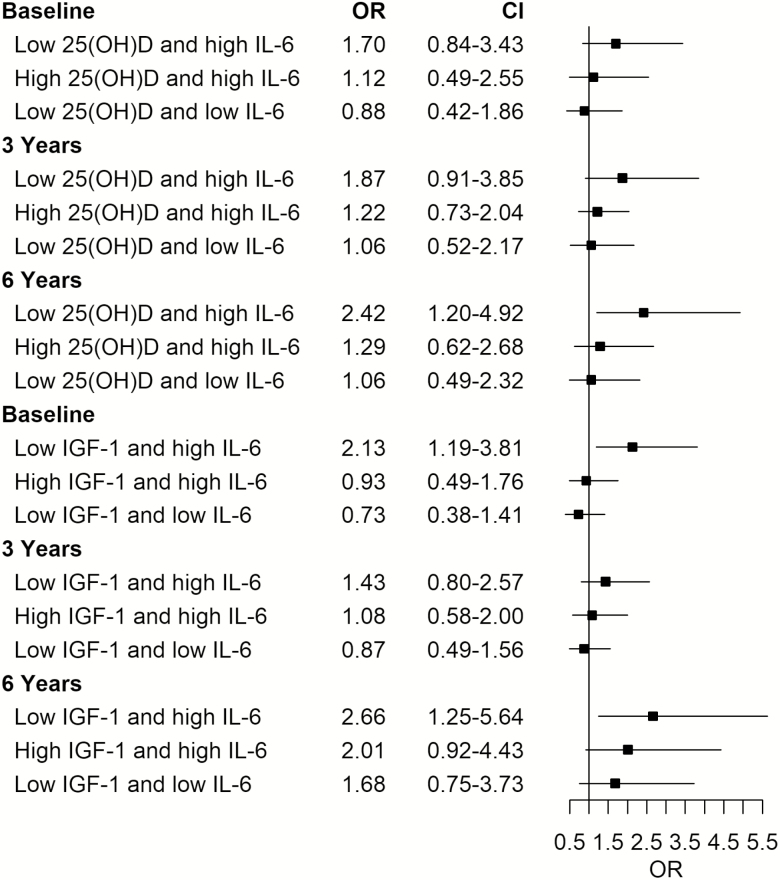
The interactions between IL-6 and vitamin D or IGF-1 were further investigated. Both interaction terms (low 25(OH)D × high IL-6 and low IGF-1 × high IL-6) were statistically significant (p = .038 and .022, respectively). Table 3 shows the joint effects of high IL-6 and low 25(OH)D or low IGF-1 with adjustment for visit time and potential confounders. Participants who had both low 25(OH)D and high IL-6 levels had the highest odds ratio (OR) of slow gait speed (OR [95% confidence interval] = 1.63 [1.15,2.32]) compared with the participants who had normal 25(OH)D and low IL-6 levels (reference group). The odds ratios associated with the groups of normal 25(OH)D and high IL-6 and low 25(OH)D and low IL-6 did not reach statistical significance. Similar findings were observed for low IGF-1 and high IL-6. Of note, none of the interactions between pairs of biomarkers and visit time was statistically significant and they were not included in the model. The results implied that the joint effects of 25(OH)D and IL-6 and IGF-1 and IL-6 did not significantly change with time as demonstrated in Figure 1. To evaluate the missing completely at random assumption, we applied the multiple imputation methods and found the joint effect results remained similar. We also performed analyses with gait speed as a continuous variable and the joint effects of low 25(OH)D and high IL-6 and low IGF-1 and high IL-6 remained significant. In addition, we performed sensitivity analyses using baseline gait speed as a covariate; although this resulted in one-third reduction of the sample size, the joint effect of low 25(OH)D and high IL-6 on slow gait speed remained significant. The joint effect of low IGF-1 and high IL-6, however, became insignificant likely due to a smaller sample size from the removal of the baseline data. Moreover, the interaction between low IGF-1 and high IL-6 was significant at baseline but not at 3 years and 6 years. Finally, we repeated analyses using the cutoff 30 ng/mL for low 25(OH)D and the findings were consistent.
Table 3.
Odds ratios (ORs) of slow gait speeda associated with the groups of low 25(OH)Db (yes vs. no) and high IL-6c (yes vs. no) in Model 1 and low IGF-1d (yes vs. no) and high IL-6 (yes vs. no) in Model 2 adjusted for potential confounderse
| Model 1: Joint effects of low 25(OH)D (yes vs. no) and high IL-6 (yes vs. no) | Model 2: Joint effects of low IGF-1 (yes vs. no) and high IL-6 (yes vs. no) | ||||
|---|---|---|---|---|---|
| Group | OR (95% CI) | p | Group | OR (95% CI) | p |
| Yes, Yes | 1.63 (1.15, 2.32) | .006 | Yes, Yes | 1.65 (1.19, 2.29) | .003 |
| No, Yes | 1.12 (0.80, 1.56) | .50 | No, Yes | 1.07 (0.75, 1.53) | .72 |
| Yes, No | 0.89 (0.60, 1.30) | .54 | Yes, No | 0.91 (0.65, 1.27) | .57 |
| No, No | Reference group | No, No | Reference group | ||
| Follow-upf | Follow-upf | ||||
| 3-year | 1.59 (1.20, 2.12) | .001 | 3-year | 1.51 (1.18, 1.93) | .001 |
| 6-year | 1.32 (0.99, 1.77) | .06 | 6-year | 1.28 (0.97, 1.69) | .08 |
Note: CI = Confidence interval; CRP = C-reactive protein; IGF-1 = Insulin-like growth factor 1; 25(OH)D = 25-hydroxy vitamin D; IL-6 = Interleukin 6.
a,b,c,dTime-dependent variables.
a<0.8 m/s;
b<20 ng/mL;
c≥2.87 pg/mL;
d<112 ng/mL;
eIncluding demographic covariates, diseases, physical activity, daily vitamin D intake (µg; model 1), and body mass index;
fVisit time (reference = baseline visit).
Figure 1.
Odds ratios of slow gait speeda associated with groups of low 25-hydroxy vitamin D (25(OH)D)b (yes vs. no) and high interleukin 6 (IL-6)c (yes vs. no) in Model 1 and low insulin-like growth factor 1 (IGF-1)d (yes vs. no) and high IL-6 (yes vs. no) in Model 2 at each time point; baseline, 3 years, and 6 years by cross-sectional logistic regression models with adjustment for potential confounderse. a<0.8 m/s, b<20 ng/mL, c≥2.87 pg/mL, d<112 ng/mL,encluding demographic covariates, diseases, physical activity, daily vitamin D intake (µg; model 1) and body mass index.
Discussion
Various studies have shown association of vitamin D, IGF-1, inflammatory markers (IL-6 and CRP) with mobility outcomes (1,3,5–7,25,26) but evidence on statistical interactions between these markers is scarce (13). Studying incident changes in older adults in the InCHIANTI cohort over three measurement time-points in 6 years of follow-up, we found that only IL-6 was independently associated with gait speed overall, accounting for the other three measures. We also found statistical interactions between low vitamin D and high IL-6 and between low IGF-1 and high IL-6 on slow gait speed. The effect of vitamin D insufficiency was insignificant unless paired with high IL-6 considering whether there is a presence of inflammation (high IL-6). This raises the concerns that inflammation may be a factor that needs consideration because it can affect how vitamin D works in a clinical setting.
Low vitamin D levels and inflammation frequently coexist. In a cross-sectional study in older adults from InCHIANTI, inverse association between 25(OH)D and IL-6 levels was recently reported (27), suggesting a potential anti-inflammatory role of vitamin D. Underlying mechanisms may involve downregulation of pro-inflammatory transcription factor kappa B activation by vitamin D (28).
The results support previous studies’ findings that showed independent association of vitamin D with physical performance including gait speed both from cross-sectional (25) and longitudinal studies (26) as well as the association of inflammatory markers including IL-6 with mobility (7,16). This study also showed consistent results with previous study reporting synergism of IGF-1 and IL-6 on mobility disability in white older females (13). In addition, inflammatory markers were reported to be associated with longitudinal changes in brain function in older adults (29). Such findings may indicate the linkage of inflammation with cognitive changes, which could affect mobility including gait speed.
Several studies have shown positive association of vitamin D with physical performance and strengths (3,25). Nutritional supplementation with protein and vitamin D in addition to physical activity improved muscle composition in mobility-limited older adults with vitamin D insufficiency (30). Recent meta-analysis showed reduction in injurious falls with calcium and vitamin D supplementation together with other interventions (31). However, conflicting results were also reported. Interventions to improve vitamin D status such as vitamin D supplementation did not improve mobility-related clinical outcomes such as gait speed (32), frailty (33), falls (34), bone turnover (35), or fractures (36). The Institute of Medicine reported minimal effects of vitamin D on falls (22,23). Such inconsistent results may be from studies that supplements were given to participants who were not truly deficient (37). Another explanation may also involve factors that interact with vitamin D, and hence, affect the final outcome. 25(OH)D was shown to inversely associate with IL-6 in the elderly individuals (27). Risk for type 2 diabetes of low 25(OH)D was suggested to be partially mediated by inflammation (38). Glycemic control can be affected by vitamin D insufficiency in the elderly individuals (39). This study raises the possibility that inflammation may influence how vitamin D and IGF-1 affect gait speed and thus intervention without consideration of coexisting inflammation might prove less satisfactory. As shown in Table 3, participants with vitamin D insufficiency or low IGF-1 without high IL-6 did not have an increasing odds ratio of slow gait speed compared with the reference group. Targeting subjects with vitamin D insufficiency or low IGF-1 and coexisting inflammation may better improve aging-related outcomes and yield better results in future clinical trials, at least from gait speed standpoint as shown in this study. Moreover, our findings provide a conceptual framework to advocate multicomponent strategies targeting both pathways that may offer joint benefits in terms of improved gait speed.
Although high CRP was individually associated with slow gait speed, the association between high CRP and slow gait speed was insignificant when other biomarkers were accounted for as shown in Table 2. This is likely from the overlapping effects with IL-6 and thus the effects were offset by IL-6 when both CRP and IL-6 were included.
Strengths of our study include the longitudinal nature of the study including a large sample of older adults. Because the population in the study is ambulatory and community-dwelling, the knowledge obtained would apply very well to help with healthy aging before older adults become more frail or even disabled and intervention might be too late to obtain meaningful results. In addition, not only gait speed but also the biomarkers were repeatedly measured, which allows us to establish associations with enhanced power through the use of a time course data. However, there are several limitations. First, there is a lack of racial diversity in this cohort. The study population was Caucasian from two small towns in the countryside of Italy. Therefore, generalization to multiracial populations requires careful consideration. Second, adjustment of confounders in vitamin D analyses was limited. There are no data on the amount of time spent outside although the subjects in this homogeneous cohort spend most of their time outside. Another limitation came from inconsistency of biomarker measurements at different visits. Measurement methods were different at baseline and subsequent visits for IL-6 and 25(OH)D and different types of samples (serum vs. plasma) were used for IL-6 and CRP. The measurement method and sample type, however, were consistent across samples at a particular visit. The batch effect from one visit to another was modeled via 3-year and 6-year visits versus baseline. Although we have found the differences between visits in the risk of slow gait speed, the driving force was a combination of time, measurement methods, and sample types. Furthermore, results of the study cannot make mechanistic claims in terms of potential causation of the markers studied. These interactions could arise if IL-6 is a downstream marker of vitamin D and IGF-1 and the power of the study is limited to further clarify this. Finally, it is also possible that the anti-aging protein, Klotho, which may play a role in vitamin D metabolism (40), may interact with IL-6 and/or IGF-1, impacting the likelihood of slow gait speed. We intend to explore the role of Klotho in future work.
Another aspect that deserves attention is the clinical implication of the finding of no statistical significance of low vitamin D in subjects with low IL-6, which raises the concerns that the cutoff level to determine vitamin D insufficiency might vary with inflammation status, for example, a higher cutoff for patients with inflammation reflected by high IL-6 in this study or vice versa. This will require further investigation.
Conclusion
Although our results need to be replicated in other data sets, the interaction of low vitamin D and high IL-6 with slow gait speed found in this study suggests that the coexistence of both factors in ambulatory older adults may indicate an augmented risk of having slow gait speed than having either individual factor alone.
Acknowledgment
This study was supported in part by Intramural Research Program of National Institute on Aging
Conflict of Interest
None.
References
- 1. Annweiler C, Schott AM, Berrut G, Fantino B, Beauchet O. Vitamin D-related changes in physical performance: a systematic review. J Nutr Health Aging. 2009;13:893–898. [DOI] [PubMed] [Google Scholar]
- 2. Murad MH, Elamin KB, Abu Elnour NO, et al. Clinical review: the effect of vitamin D on falls: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2011;96:2997–3006. doi: 10.1210/jc.2011-1193 [DOI] [PubMed] [Google Scholar]
- 3. Annweiler C, Henni S, Walrand S, Montero-Odasso M, Duque G, Duval GT. Vitamin D and walking speed in older adults: systematic review and meta-analysis. Maturitas. 2017;106:8–25. doi: 10.1016/j.maturitas.2017.07.012 [DOI] [PubMed] [Google Scholar]
- 4. Berridge MJ. Vitamin D deficiency accelerates ageing and age-related diseases: a novel hypothesis. J Physiol. 2017;595:6825–6836. doi: 10.1113/JP274887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Joseph C, Kenny AM, Taxel P, Lorenzo JA, Duque G, Kuchel GA. Role of endocrine-immune dysregulation in osteoporosis, sarcopenia, frailty and fracture risk. Mol Aspects Med. 2005;26:181–201. doi: 10.1016/j.mam.2005.01.004 [DOI] [PubMed] [Google Scholar]
- 6. Doi T, Shimada H, Makizako H, et al. Insulin-like growth factor-1 related to disability among older adults. J Gerontol A Biol Sci Med Sci. 2016;71:797–802. doi: 10.1093/gerona/glv167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Westbury LD, Fuggle NR, Syddall HE, et al. Relationships between markers of inflammation and muscle mass, strength and function: findings from the hertfordshire cohort study. Calcif Tissue Int. 2018;102:287–295. doi: 10.1007/s00223-017-0354-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Norheim KL, Bautmans I, Kjaer M. Handgrip strength shows no improvements in geriatric patients with persistent inflammation during hospitalization. Exp Gerontol. 2017;99:115–119. doi: 10.1016/j.exger.2017.10.006 [DOI] [PubMed] [Google Scholar]
- 9. Newman AB, Sanders JL, Kizer JR, et al. Trajectories of function and biomarkers with age: the CHS All Stars Study. Int J Epidemiol., 2016. 45(4):1135–1145. doi:10.1093/ije/dyw092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Reid D, Toole BJ, Knox S, et al. The relation between acute changes in the systemic inflammatory response and plasma 25-hydroxyvitamin D concentrations after elective knee arthroplasty. Am J Clin Nutr. 2011;93:1006–1011. doi: 10.3945/ajcn.110.008490 [DOI] [PubMed] [Google Scholar]
- 11. Jablonski KL, Chonchol M, Pierce GL, Walker AE, Seals DR. 25-Hydroxyvitamin D deficiency is associated with inflammation-linked vascular endothelial dysfunction in middle-aged and older adults. Hypertension. 2011;57:63–69. doi: 10.1161/HYPERTENSIONAHA.110.160929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kositsawat J, Barry LC, Kuchel GA. C-reactive protein, vitamin D deficiency, and slow gait speed. J Am Geriatr Soc. 2013;61:1574–1579. doi: 10.1111/jgs.12403 [DOI] [PubMed] [Google Scholar]
- 13. Cappola AR, Xue QL, Ferrucci L, Guralnik JM, Volpato S, Fried LP. Insulin-like growth factor I and interleukin-6 contribute synergistically to disability and mortality in older women. J Clin Endocrinol Metab., 2003. 88(5):2019–2025. doi: 10.1210/jc.2002-021694 [DOI] [PubMed] [Google Scholar]
- 14. Inouye SK, Studenski S, Tinetti ME, Kuchel GA. Geriatric syndromes: clinical, research, and policy implications of a core geriatric concept. J Am Geriatr Soc. 2007;55:780–791. doi: 10.1111/j.1532-5415.2007.01156.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Maggio M, Lauretani F, Ceda GP, et al. Relationship between low levels of anabolic hormones and 6-year mortality in older men: the aging in the Chianti Area (InCHIANTI) study. Arch Intern Med. 2007;167:2249–2254. doi: 10.1001/archinte.167.20.2249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cesari M, Penninx BW, Pahor M, et al. Inflammatory markers and physical performance in older persons: the InCHIANTI study. J Gerontol A Biol Sci Med Sci. 2004;59:242–248. doi: 10.1093/gerona/59.3.m242 [DOI] [PubMed] [Google Scholar]
- 17. Stenholm S, Maggio M, Lauretani F, et al. Anabolic and catabolic biomarkers as predictors of muscle strength decline: the InCHIANTI study. Rejuvenation Res. 2010;13:3–11. doi: 10.1089/rej.2009.0891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Baylis D, Bartlett DB, Syddall HE, et al. Immune-endocrine biomarkers as predictors of frailty and mortality: a 10-year longitudinal study in community-dwelling older people. Age (Dordr). 2013;35:963–971. doi: 10.1007/s11357-012-9396-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Meng Y, Wu H, Yang Y, et al. Relationship of anabolic and catabolic biomarkers with muscle strength and physical performance in older adults: a population-based cross-sectional study. BMC Musculoskelet Disord. 2015;16:202. doi: 10.1186/s12891-015-0654-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ferrucci L, Bandinelli S, Benvenuti E, et al. Subsystems contributing to the decline in ability to walk: bridging the gap between epidemiology and geriatric practice in the InCHIANTI study. J Am Geriatr Soc. 2000;48:1618–1625. [DOI] [PubMed] [Google Scholar]
- 21. Studenski S, Perera S, Patel K, et al. Gait speed and survival in older adults. JAMA. 2011;305:50–58. doi: 10.1001/jama.2010.1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ross AC, Manson JE, Abrams SA, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011;96:53–58. doi: 10.1210/jc.2010-2704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Aloia JF. Clinical Review: the 2011 report on dietary reference intake for vitamin D: where do we go from here? J Clin Endocrinol Metab. 2011;96:2987–2996. doi: 10.1210/jc.2011-0090 [DOI] [PubMed] [Google Scholar]
- 24. Alves FC, Sun J, Qureshi AR, et al. The higher mortality associated with low serum albumin is dependent on systemic inflammation in end-stage kidney disease. PLoS One. 2018;13:e0190410. doi: 10.1371/journal.pone.0190410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Houston DK, Cesari M, Ferrucci L, et al. Association between vitamin D status and physical performance: the InCHIANTI study. J Gerontol A Biol Sci Med Sci. 2007;62:440–446. doi: 10.1093/gerona/62.4.440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Houston DK, Tooze JA, Davis CC, et al. Serum 25-hydroxyvitamin D and physical function in older adults: the Cardiovascular Health Study All Stars. J Am Geriatr Soc. 2011;59:1793–1801. doi: 10.1111/j.1532-5415.2011.03601.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. De Vita F, Lauretani F, Bauer J, et al. Relationship between vitamin D and inflammatory markers in older individuals. Age (Dordr). 2014;36:9694. doi: 10.1007/s11357-014-9694-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Suzuki Y, Ichiyama T, Ohsaki A, Hasegawa S, Shiraishi M, Furukawa S. Anti-inflammatory effect of 1alpha,25-dihydroxyvitamin D(3) in human coronary arterial endothelial cells: implication for the treatment of Kawasaki disease. J Steroid Biochem Mol Biol. 2009;113:134–138. doi: 10.1016/j.jsbmb.2008.12.004 [DOI] [PubMed] [Google Scholar]
- 29. Warren KN, Beason-Held LL, Carlson O, et al. Elevated markers of inflammation are associated with longitudinal changes in brain function in older adults. J Gerontol A Biol Sci Med Sci. 2018;73:770–778. doi: 10.1093/gerona/glx199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Englund DA, Kirn DR, Koochek A, et al. Nutritional supplementation with physical activity improves muscle composition in mobility-limited older adults, the VIVE2 study: a randomized, double-blind, placebo-controlled trial. J Gerontol A Biol Sci Med Sci. 2017;73:95–101. doi: 10.1093/gerona/glx141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tricco AC, Thomas SM, Veroniki AA, et al. Comparisons of interventions for preventing falls in older adults: a systematic review and meta-analysis. JAMA. 2017;318:1687–1699. doi: 10.1001/jama.2017.15006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fielding RA, Travison TG, Kirn DR, et al. Effect of structured physical activity and nutritional supplementation on physical function in mobility-limited older adults: results from the VIVE2 randomized trial. J Nutr Health Aging. 2017;21:936–942. doi: 10.1007/s12603-017-0936-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bolzetta F, Stubbs B, Noale M, et al. Low-dose vitamin D supplementation and incident frailty in older people: an eight year longitudinal study. Exp Gerontol. 2018;101:1–6. doi: 10.1016/j.exger.2017.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Uusi-Rasi K, Patil R, Karinkanta S, et al. Exercise and vitamin D in fall prevention among older women: a randomized clinical trial. JAMA Intern Med, 2015. 175(5):703–711. doi: 10.1001/jamainternmed.2015.0225 [DOI] [PubMed] [Google Scholar]
- 35. Zittermann A, Ernst JB, Prokop S, et al. Vitamin D supplementation and bone turnover in advanced heart failure: the EVITA trial. Osteoporos Int. 2018;29:579–586. doi: 10.1007/s00198-017-4312-9 [DOI] [PubMed] [Google Scholar]
- 36. Zhao JG, Zeng XT, Wang J, Liu L. Association between calcium or Vitamin D supplementation and fracture incidence in community-dwelling older adults: a systematic review and meta-analysis. JAMA. 2017;318:2466–2482. doi: 10.1001/jama.2017.19344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Dhaliwal R, Aloia JF. Effect of vitamin D on falls and physical performance. Endocrinol Metab Clin North Am. 2017;46:919–933. doi: 10.1016/j.ecl.2017.07.004 [DOI] [PubMed] [Google Scholar]
- 38. Thorand B, Zierer A, Huth C, et al. Effect of serum 25-hydroxyvitamin D on risk for type 2 diabetes may be partially mediated by subclinical inflammation: results from the MONICA/KORA Augsburg study. Diabetes Care. 2011;34:2320–2322. doi: 10.2337/dc11-0775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kositsawat J, Kuchel GA, Tooze JA, et al. ; Health ABC Vitamin D insufficiency and abnormal hemoglobin a1c in black and white older persons. J Gerontol A Biol Sci Med Sci. 2015;70:525–531. doi: 10.1093/gerona/glu122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Torres PU, Prié D, Molina-Blétry V, Beck L, Silve C, Friedlander G. Klotho: an antiaging protein involved in mineral and vitamin D metabolism. Kidney Int. 2007;71:730–737. doi: 10.1038/sj.ki.5002163 [DOI] [PubMed] [Google Scholar]