Abstract
Background
There has been increasing effort to measure frailty in the U.S. Medicare data. The performance of claims-based frailty measures has not been compared.
Methods
This cross-sectional study included 3,097 community-dwelling fee-for-service Medicare beneficiaries (mean age 75.6 years) who participated in the 2008 Health and Retirement Study examination. Four claims-based frailty measures developed by Davidoff, Faurot, Segal, and Kim were compared against frailty phenotype, a deficit-accumulation frailty index (FI), and activities of daily living (ADL) dependence using Spearman correlation coefficients and C-statistics.
Results
Claims-based frailty measures were positively associated with frailty phenotype (prevalence in ≤10th vs >90th percentile: 8.0% vs 41.3% for Davidoff; 5.9% vs 53.1% for Faurot; 3.3% vs 48.0% for Segal; 2.9% vs 51.0% for Kim) and FI (mean in ≤10th vs >90th percentile: 0.17 vs 0.33 for Davidoff; 0.13 vs 0.37 for Faurot; 0.12 vs 0.31 for Segal; 0.10 vs 0.37 for Kim). The age and sex-adjusted C-statistics for frailty phenotype for Davidoff, Faurot, Segal, and Kim indices were 0.73, 0.74, 0.73, and 0.78, respectively, and partial correlation coefficients with FI were 0.18, 0.32, 0.26, and 0.55, respectively. The results for ADL dependence were similar (prevalence in ≤10th vs >90th percentile: 3.7% vs 50.5% for Davidoff; 2.3% vs 55.0% for Faurot; 3.0% vs 38.3% for Segal; 2.3% vs 50.8% for Kim). The age and sex-adjusted C-statistics for the indices were 0.79, 0.80, 0.74, and 0.81, respectively.
Conclusions
The choice of a claims-based frailty measure can influence the identification of older adults with frailty and disability in Medicare data.
Keywords: Frailty, Health services, Geriatric assessment, Medicare claims data
Administrative claims data provide longitudinal information on the use of medical treatments and clinical outcomes in a large representative population, including older adults with frailty (1). Since older adults with frailty are at increased risk for adverse health events (2, 3), practice guidelines (4–6) recommend that frailty should be considered in clinical decision making. However, there is little evidence from clinical trials to guide clinical decision making due to the exclusion of frail older adults. As a result, administrative claims datasets remain a potentially valuable data source to inform clinical and public health policy decisions for this population.
A major limitation of administrative claims data is the lack of detailed clinical information, such as frailty, which can lead to bias in evaluating the effectiveness of medical interventions (7–9). Because frailty is not routinely assessed by clinicians and diagnosis codes for frailty do not exist, researchers attempted to measure frailty using surrogates (ie, diagnosis codes and health service billing codes that occur more frequently in frail older adults) in administrative claims data, electronic health records data, and prescription claims data (see examples in Supplementary Table 1). For most claims-based frailty measures, predictors were selected from a large number of diagnosis codes and health service billing codes based on clinical knowledge (10–16), while others employed more rigorous data-driven approaches for variable selection and estimation (17–23). In the U.S. Medicare data, four claims-based frailty measures have been developed using data-driven approaches (17–23) (Table 1). There are differences in terms of the types of predictors and the reference standard outcome used for model development.
Table 1.
Medicare Claims-Based Measures of Frailty
| Index (Year) | Predictor Types | Predictor Assessment | Reference Standard | ||||
|---|---|---|---|---|---|---|---|
| Demographic | ICD Codes | CPT codes | HCPCS Codes | Other | |||
| Davidoff (2013) | • Sex | None | 16 variables • E&M visits • Procedures • Preventive services • Diagnostic studies |
7 variables • Transportation services • DMEs |
• Medicaid enrollment • Number of E&M visits |
Past 12 mo | Disability |
| Faurot (2015) | • Age • Sex • Race |
19 variables | None | 4 variables • Transportation services • DMEs |
None | Past 8 mo | Disability |
| Segal (2017) | • Age • Sex • Race |
16 variables | None | None | • Charlson Comorbidity Index • Admissions in the past 6 mo |
Past 6 mo | Frailty phenotype |
| Kim (2018) | None | 52 variables | 25 variables • E&M visits • Procedures • Preventive services • Diagnostic studies |
16 variables • Transportation services • DMEs • Vaccines • Injectable drugs |
None | Past 12 mo | Deficit- accumulation frailty index |
Note: CPT = Current Procedural Terminology; DME = durable medical equipment; E&M = evaluation and management; HCPCS = Healthcare Common Procedure Coding System; ICD = International Classification of Diseases.
This study aimed to evaluate four claims-based frailty measures against clinically validated frailty measures and prevalent disability in a representative Medicare population. Investigating the comparative performance of different approaches may inform choice of claims-based frailty measures for studies of medical interventions and health outcomes in older populations.
Methods
Health and Retirement Study (HRS)-Medicare Data
The HRS is a nationally representative survey of adults over age 50 years in the United States, sponsored by the National Institute on Aging (grant NIA U01AG009740) and conducted by the University of Michigan (24). The survey was conducted every 2 years to assess health status from respondents or their proxy. In over 80% of the participants, survey data were linked to Medicare fee-for-service data, which included inpatient, outpatient, skilled nursing facility, home health agency, carrier, and durable medical equipment files. This cross-sectional study included 3,097 participants who were ≥65 years old, were randomly selected for measurement of physical performance, had a 12-month continuous fee-for-service Medicare enrollment, and resided in the community in the 2008 HRS wave (Supplementary Figure 1). The HRS was approved by the Institutional Review Board at the University of Michigan, Ann Arbor, Michigan, and this study was approved by the Institutional Review Board at the Brigham and Women’s Hospital, Boston, Massachusetts.
Claims-Based Frailty Measures
Medicare data contain the International Classification of Diseases (ICD) diagnosis and procedure codes, Current Procedural Terminology (CPT) codes (codes for medical services and procedures), and Healthcare Common Procedure Coding System (HCPCS) codes (codes for supplies, equipment, and devices) that were generated from routine health care encounters. We estimated four claims-based frailty measures based on relevant ICD, CPT, and HCPCS codes in Medicare data (Table 1).
Davidoff index: This index uses sex, Medicaid enrollment, number of evaluation and management visits, 16 CPT code-derived variables, and 7 HCPCS code-derived variables in the past 12 months to predict disability that corresponded to the Eastern Cooperative Oncology Group scale 3 (“capable of only limited self-care, confined to bed or chair more than 50% of walking hours”) or 4 (“completely disabled, unable to carry out any self-care, and totally confined to bed or chair”) (17). Diagnosis codes or age were not used.
Faurot index: This index uses age, sex, race, 19 ICD diagnosis code-based variables and 4 HCPCS code-based variables in the past 8 months to predict activities of daily living (ADL) disability (18, 19). No CPT code-based variables were used.
Segal index: This index uses age, sex, race, 16 ICD diagnosis code-based variables, Charlson comorbidity index (25), and admissions in the past 6 months to predict the frailty phenotype (20, 21), which is the most commonly used definition of frailty (26). No CPT or HCPCS code-based variables were used.
Kim index: This index uses 52 ICD diagnosis code-based variables, 25 CPT code-based variables, and 16 HCPCS code-based variables in the past 12 months to predict the value of a deficit-accumulation frailty index (FI) (22, 23), which is the second most commonly used definition of frailty (26). Demographic variables were not used.
The estimated score represents the probability of disability for the Davidoff and Faurot indices and the probability of frailty phenotype for the Segal index, whereas the Kim index estimates the deficit-accumulation FI value (range: 0–1; higher values indicate greater frailty). Because the estimates of four claims-based frailty measures had different meanings, we classified participants into five categories according to the population distribution of each index: robust (≤10th percentile), well (25th percentile), vulnerable (26–75th percentile), frail (76–90th percentile), and very frail (>90th percentile).
Measurement of Frailty and Disability
We defined the frailty phenotype based on weight loss, exhaustion, low activity, slowness, and weakness according to the HRS-specific modification of the original definition (27). Participants were classified as frail if having ≥3 components, pre-frail if 1–2 components, robust if 0 components, or undetermined if there were insufficient information (missing data: n = 1,338 for gait speed, n = 1,005 for grip strength). In addition, we calculated a 43-item FI from the HRS examination according to the standard deficit-accumulation approach (Supplementary Table 3) (28). Participants were asked about needing help from another person in performing six ADLs (dressing, walking across a room, bathing, eating, transferring, and toileting).
Statistical Analysis
Population characteristics were summarized in mean and standard deviation (SD), median and interquartile range, or proportions. We estimated the Spearman correlation coefficient between each claims-based frailty measure and the FI and number of ADL difficulties from the HRS examination, and partial correlation coefficient to remove the effect of age and sex. For prevalent frailty phenotype and ADL dependence (any dependence or ≥2 dependencies), we estimated C-statistics of a logistic model that included age, sex, and each claims-based frailty measure, and tested improvement in C-statistics from the logistic model that included age and sex alone. Statistical analysis was performed using R software version 3.4 (R Foundation for Statistical Computing, Vienna, Austria).
Results
The 3,097 HRS participants included in the analysis had a mean age of 75.6 years (SD, 7.5), 2,958 (58.3%) women, 811 (16.0%) non-white races, 529 (14.9%) frailty phenotype, a median FI of 0.21 (interquartile range, 0.10–0.29), and 613 (12.1%) ADL dependence. The median (interquartile range) of claims-based frailty measures was 0.018 (0.008–0.042) for the Davidoff index, 0.034 (0.022–0.060) for the Faurot index, 0.062 (0.040–0.103) for the Segal index, and 0.147 (0.115–0.190) for the Kim index.
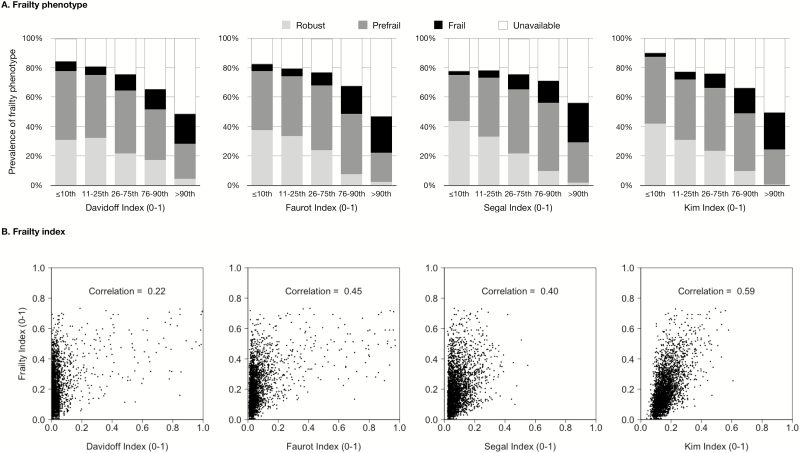
Frailty phenotype could not be determined in 1,523 participants (30.0%). Among those with available frailty phenotype data, more participants had frailty phenotype as claims-based frailty measures increased (Figure 1A): 8.0% (≤10th percentile) to 41.3% (>90th percentile) for the Davidoff index, 5.9% to 53.1% for the Faurot index, 3.3% to 48.0% for the Segal index, and 2.9% to 51.0% for the Kim index (Table 2). The C-statistics for frailty phenotype for four indices were 0.73, 0.74, 0.73, and 0.78, respectively. The improvement in C-statistics beyond age and sex (C-statistic: 0.68) was the largest for the Kim index.
Figure 1.
Claims-based measures of frailty versus clinically validated measures of frailty in the Health and Retirement Study. The 10th, 25th, 50th, 75th, and 90th percentile values of each claims-based frailty measure were 0.004, 0.008, 0.018, 0.042, and 0.074 for the Davidoff index; 0.015, 0.022, 0.034, 0.060, and 0.129 for the Faurot index; 0.030, 0.040, 0.062, 0.103, and 0.164 for the Segal index; and 0.099, 0.115, 0.147, 0.190, and 0.248 for the Kim index. Frailty phenotype was not available in 1,523 individuals due to missing gait speed or grip strength.
Table 2.
Comparison of Claims-Based Frailty Measures for Identifying Frailty and Prevalent Disability in the Health and Retirement Study
| Index | Frailty Category (Percentile Distribution) | Spearman Correlation (Partial Correlationa) | C-statistic (ΔC-statisticb) | ||||
|---|---|---|---|---|---|---|---|
| Robust (≤10th) | Well (11–25th) | Vulnerable (26–75th) | Frail (76–90th) | Very Frail (>90th) | |||
| Frailty phenotype,cn (%) | |||||||
| Davidoff index | 21 (8.0) | 26 (6.8) | 182 (14.5) | 48 (21.1) | 62 (41.3) | NA | 0.73 (0.04) |
| Faurot index | 15 (5.9) | 26 (6.9) | 133 (11.3) | 88 (28.1) | 77 (53.1) | NA | 0.74 (0.06) |
| Segal index | 8 (3.3) | 23 (6.2) | 156 (13.5) | 69 (21.0) | 83 (48.0) | NA | 0.73 (0.05) |
| Kim index | 8 (2.9) | 26 (7.2) | 148 (12.6) | 79 (25.8) | 78 (51.0) | NA | 0.78 (0.09) |
| Frailty index, mean (SD) | |||||||
| Davidoff index | 0.17 (0.10) | 0.15 (0.10) | 0.19 (0.12) | 0.21 (0.14) | 0.33 (0.17) | 0.22 (0.18) | NA |
| Faurot index | 0.13 (0.08) | 0.15 (0.10) | 0.17 (0.11) | 0.26 (0.13) | 0.37 (0.16) | 0.45 (0.32) | NA |
| Segal index | 0.12 (0.10) | 0.14 (0.10) | 0.19 (0.12) | 0.24 (0.14) | 0.31 (0.15) | 0.40 (0.26) | NA |
| Kim index | 0.10 (0.08) | 0.13 (0.09) | 0.18 (0.11) | 0.27 (0.12) | 0.37 (0.15) | 0.59 (0.55) | NA |
| Any ADL dependence, n (%) | |||||||
| Davidoff index | 19 (3.7) | 34 (4.5) | 233 (8.0) | 71 (18.1) | 256 (50.5) | NA | 0.79 (0.09) |
| Faurot index | 12 (2.3) | 26 (3.3) | 168 (6.7) | 128 (17.5) | 279 (55.0) | NA | 0.80 (0.10) |
| Segal index | 18 (3.0) | 36 (4.7) | 229 (9.4) | 136 (17.9) | 194 (38.3) | NA | 0.74 (0.04) |
| Kim index | 12 (2.3) | 83 (6.6) | 128 (6.3) | 132 (17.4) | 258 (50.8) | NA | 0.81 (0.11) |
| Two or more ADL dependencies, n (%) | |||||||
| Davidoff index | 3 (1.0) | 8 (1.7) | 48 (2.9) | 25 (7.2) | 70 (22.6) | NA | 0.79 (0.12) |
| Faurot index | 6 (1.9) | 17 (3.6) | 86 (5.6) | 57 (12.3) | 125 (40.3) | NA | 0.81 (0.14) |
| Segal index | 5 (1.6) | 6 (1.3) | 56 (3.6) | 38 (8.2) | 49 (15.9) | NA | 0.73 (0.06) |
| Kim index | NRd | 6 (1.3) | 34 (2.2) | 34 (7.3) | 80 (25.8) | NA | 0.84 (0.17) |
| Number of ADLs requiring help, mean (SD) | |||||||
| Davidoff index | 0.06 (0.38) | 0.08 (0.42) | 0.17 (0.70) | 0.51 (1.30) | 1.78 (2.19) | 0.30 (0.28) | NA |
| Faurot index | 0.04 (0.33) | 0.06 (0.36) | 0.14 (0.64) | 0.43 (1.16) | 1.87 (2.18) | 0.35 (0.28) | NA |
| Segal index | 0.07 (0.46) | 0.08 (0.45) | 0.23 (0.89) | 0.50 (1.30) | 1.24 (1.95) | 0.27 (0.16) | NA |
| Kim index | 0.06 (0.49) | 0.16 (0.73) | 0.13 (0.61) | 0.45 (1.23) | 1.66 (2.09) | 0.31 (0.27) | NA |
Notes: ADL = activity of daily living; NR = not reported; SD = standard deviation.
aPartial correlation coefficient was estimated after adjusting for age and sex;
bC-statistics for age and sex alone were 0.68 for frailty phenotype, 0.70 for any ADL dependence, and 0.69 for ≥2 ADL dependencies;
cFrailty phenotype was not available in 1,523 individuals due to missing gait speed or grip strength;
dThe cell size fewer than 3 was not reported.
All claims-based frailty measures were positively correlated with the FI (Figure 1B), with correlation coefficients ranging from 0.22 for the Davidoff index to 0.59 for the Kim index. The mean FI increased with claims-based frailty categories: 0.17 (≤10th percentile) to 0.33 (>90th percentile) for the Davidoff index, 0.13 to 0.37 for the Faurot index, 0.12 to 0.31 for the Segal index, and 0.10 to 0.37 for the Kim index (Table 2). After adjusting for age and sex, the correlation coefficients with FI attenuated more for the Faurot (0.45 to 0.32) and Segal (0.40 to 0.26) indices than for the Davidoff (0.22 to 0.18) and Kim indices (0.59 to 0.55).
Claims-based frailty measures were positively associated with prevalent any or ≥2 ADL dependencies and the number of ADLs requiring another person’s help (Table 2). The C-statistics for any ADL dependence were similar among the Davidoff (0.79), Faurot (0.80), and Kim (0.81) indices, which were higher than the Segal index (0.74) and age and sex (0.70). The C-statistics for ≥2 ADL dependencies were the highest with the Kim index (0.84), followed by the Faurot (0.81), Davidoff (0.79), and Segal indices (0.73), in comparison with age and sex (0.67). The partial correlation coefficients after adjusting for age and sex were similar among the Davidoff (0.28), Faurot (0.28), and Kim (0.27) indices, which were higher than the Segal index (0.16).
Discussion
Although the 4 claims-based frailty measures were derived to capture the level of vulnerability in administrative claims data in which clinical information on frailty was not available, we found that claims-based frailty measures varied widely in their discriminatory ability for frailty phenotype (C-statistics ranging 0.73–0.78) and correlation with FI (Spearman correlation coefficients ranging 0.22–0.59). Of the four measures, the Kim index outperformed the other indices in measuring the extent of frailty phenotype and FI that were not explained by age and sex. For ADL dependence, the Davidoff, Faurot, and Kim indices showed similar improvement in C-statistics for prevalent ADL dependence beyond age and sex, and similar partial correlation after age and sex adjustment. Our findings are useful in choosing a frailty measure for administrative database studies.
Different predictive performance of the four claims-based frailty measures may be attributed to the types (eg, demographic variables, ICD codes, CPT codes, and HCPCS codes) of data required to calculate each index. While the variables in each model were chosen using a variable-selection algorithm (eg, stepwise selection, penalized regression), the list of candidate and final predictors varied widely. As candidate predictors, the Davidoff index excluded ICD diagnosis codes, the Segal index excluded CPT or HCPCS codes, and the Kim index excluded demographic variables. The Faurot and Segal indices included demographic variables and mainly ICD diagnosis codes (the Faurot index also included four HCPCS code-based variables and the Segal index had the history of admissions). The Kim index included the largest number of ICD, CPT, and HCPCS codes. In administrative claims data that are generated for billing and payment of health services for clinical conditions, not considering diagnosis codes that are correlated with functional status (29, 30) (eg, Davidoff index) appears to limit the ability to measure frailty and disability. However, inclusion of demographic variables (eg, Faurot and Segal indices) reduced the chances for other variables based on ICD, CPT, and HCPCS codes to enter the final model. The consequence of this approach was a more parsimonious model at the expense of the discriminatory ability to explain the variation in frailty and disability beyond age and sex alone.
Our study offers useful insights for choosing a claims-based frailty measure for studies of health outcomes using administrative claims data. First, the estimates of the four claims-based frailty measures have different interpretations. The Kim index estimates a deficit-accumulation FI (a continuous value that represents the severity of frailty), while the other indices estimate the probability of a frailty state, defined as disability (a proxy of frailty) or the frailty phenotype. Second, the length of look-back period to measure predictors varies from 6 months for the Segal index and 8 months for the Faurot index to 12 months for the Davidoff and Kim indices. A longer look-back period may limit the amount of data available for outcome analysis. However, a shorter look-back period to measure predictors may lower discrimination, as individuals are likely to have fewer or even no health care encounters in shorter windows. Third, claims-based frailty measures that included demographic variables are unlikely to provide a large improvement in risk prediction and case-mix adjustment beyond demographic variables. Since demographic variables are recorded in administrative claims datasets, researchers are often interested in measuring health status that is not explained by these variables. Fourth, while the Kim index outperformed the other measures in measuring frailty phenotype, FI, and ≥2 ADL dependencies, the Davidoff, Faurot, and Kim indices showed comparable performance to measure any ADL dependence.
Our results should be interpreted with consideration of the following limitations. We compared the four claims-based frailty measures using an independent dataset of a representative Medicare population that was not used to develop these measures. Although frailty measures are often evaluated based on the ability to predict mortality or health care utilization, we compared claims-based frailty measures against the two most widely used clinical definitions of frailty. Nonetheless, frailty phenotype could not be determined in 30% of the population, and most of these participants had higher claims-based frailty scores. Therefore, the prevalence of frailty phenotype may have been underestimated in the high-risk categories of claims-based frailty measures. We acknowledge that the change in accuracy of diagnosis and billing codes, as well as coding practice (eg, ICD-9 to ICD-10 transition) may affect the performance of claims-based frailty measures. Inaccurate measurements of health conditions based on the billing codes may be responsible for C-statistics <0.80 for frailty phenotype and correlation with FI <0.60. Whether the performance can be enhanced by adding detailed clinical information from electronic health records to claims data or applying machine learning approach remains to be explored. Since our analysis was limited to four recently developed claims-based frailty measures, it is unknown how these measures compare to other similar measures (JEN FI with demographic variables achieved a C-statistic 0.81 for ≥2 ADL dependencies in the 2004 National Long-Term Care Survey (31)).
In conclusion, our comparative analysis of 4 recently developed claims-based frailty measures suggests that the choice of a claims-based frailty measure can influence the identification of older adults with frailty and disability. Claims-based frailty measures that included demographic variables showed limited ability to explain the variation in frailty and disability beyond age and sex. Future studies should examine the performance of claims-based frailty measures using more recent Medicare data and the usefulness of applying claims-based frailty measures in claims-based studies of medical interventions and health outcomes.
Funding
This study was funded by R01AG062713 from the National Institute on Aging (NIA) and the Paul B. Beeson Clinical Scientist Development Award in Aging (K08AG051187) to D.H.K. from NIA, American Federation for Aging Research, John A. Hartford Foundation, and Atlantic Philanthropies. This study is also supported by a career development grant K08AG055670 to E.P. from NIA. The funding sources had no role in the design, collection, analysis, or interpretation of the data, or the decision to submit the manuscript for publication.
Conflict of Interest
D.H.K. provides paid consultative services to Alosa Health, a nonprofit educational organization with no relationship to any drug or device manufacturers. E.P. is investigator of investigator-initiated grants to the Brigham and Women’s Hospital from Boehringer Ingelheim, unrelated to the topic of this study. S.S. is a consultant to WHISCON, LLC., Newton, Massachusetts, and to Aetion, Inc., New York, New York, a software manufacturer of which he also owns equity. He is the principal investigator of investigator-initiated grants to the Brigham and Women’s Hospital from Novartis, Basel, Switzerland; Genentech, San Francisco, California; and Boehringer Ingelheim, Ingelheim am Rhein, Germany, unrelated to the topic of this study. The other authors declare no competing interests.
Supplementary Material
Acknowledgments
D.H.K., S.S., and R.J.G. contributed to conception, design, and acquisition of data. D.H.K. and A.P. conducted statistical analysis. D.H.K. drafted the manuscript. D.H.K., E.P., A.P., H.L., S.S., and R.J.G. interpreted data, critically revised the manuscript for important intellectual content, and read and approved the final manuscript for submission.
References
- 1. Schneeweiss S, Avorn J. A review of uses of health care utilization databases for epidemiologic research on therapeutics. J Clin Epidemiol. 2005;58(4):323–337. doi:10.1016/j.jclinepi.2004.10.012 [DOI] [PubMed] [Google Scholar]
- 2. Rockwood K, Mitnitski A. Frailty defined by deficit accumulation and geriatric medicine defined by frailty. Clin Geriatr Med. 2011;27(1):17–26. doi:10.1016/j.cger.2010.08.008 [DOI] [PubMed] [Google Scholar]
- 3. Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–156. [DOI] [PubMed] [Google Scholar]
- 4. James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311(5):507–520. doi:10.1001/jama.2013.284427 [DOI] [PubMed] [Google Scholar]
- 5.American Geriatrics Society Expert Panel on Care of Older Adults with Diabetes M, Moreno G, Mangione CM, Kimbro L, Vaisberg E. Guidelines abstracted from the American Geriatrics Society Guidelines for Improving the Care of Older Adults with Diabetes Mellitus: 2013 update. J Am Geriatr Soc. 2013;61(11):2020–2026. doi:10.1111/jgs.12514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hurria A, Wildes T, Blair SL, et al. Senior adult oncology, version 2.2014: clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2014;12(1):82–126. [DOI] [PubMed] [Google Scholar]
- 7. Kim DH, Schneeweiss S. Measuring frailty using claims data for pharmacoepidemiologic studies of mortality in older adults: evidence and recommendations. Pharmacoepidemiol Drug Saf. 2014;23(9):891–901. doi:10.1002/pds.3674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dormuth CR, Patrick AR, Shrank WH, et al. Statin adherence and risk of accidents: a cautionary tale. Circulation. 2009;119(15):2051–2057. doi:10.1161/CIRCULATIONAHA.108.824151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Glynn RJ, Knight EL, Levin R, Avorn J. Paradoxical relations of drug treatment with mortality in older persons. Epidemiology. 2001;12(6):682–689. [DOI] [PubMed] [Google Scholar]
- 10. Abrams C, Lieberman R, Weiner JP.. Development and Evaluation of the Johns Hopkins University Risk Adjustment Models for Medicare+Choice Plan Payment. Baltimore, MD: Johns Hopkins University Press; 2003. [Google Scholar]
- 11. Sternberg SA, Bentur N, Abrams C, et al. Identifying frail older people using predictive modeling. Am J Manag Care. 2012;18(10):e392–397. [PubMed] [Google Scholar]
- 12. Chrischilles E, Schneider K, Wilwert J, et al. Beyond comorbidity: expanding the definition and measurement of complexity among older adults using administrative claims data. Med Care. 2014;52 (Suppl 3):S75–84. doi:10.1097/MLR.0000000000000026 [DOI] [PubMed] [Google Scholar]
- 13. JEN Associates. https://jen.com Accessed on May 25 2017.
- 14. Orkaby AR, Nussbaum L, Ho YL, et al. The burden of frailty among U.S. veterans and its association with mortality, 2002–2012. J Gerontol A Biol Sci Med Sci. 2018. doi:10.1093/gerona/gly232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Soong J, Poots AJ, Scott S, et al. Quantifying the prevalence of frailty in English hospitals. BMJ Open. 2015;5(10):e008456. doi:10.1136/bmjopen-2015–008456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Clegg A, Bates C, Young J, et al. Development and validation of an electronic frailty index using routine primary care electronic health record data. Age Ageing. 2016;45(3):353–360. doi:10.1093/ageing/afw039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Davidoff AJ, Zuckerman IH, Pandya N, et al. A novel approach to improve health status measurement in observational claims-based studies of cancer treatment and outcomes. J Geriatr Oncol. 2013;4(2):157–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Faurot KR, Jonsson Funk M, Pate V, et al. Using claims data to predict dependency in activities of daily living as a proxy for frailty. Pharmacoepidemiol Drug Saf. 2015;24(1):59–66. doi:10.1002/pds.3719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cuthbertson CC, Kucharska-Newton A, Faurot KR, et al. Controlling for frailty in pharmacoepidemiologic studies of older adults: validation of an existing Medicare claims-based algorithm. Epidemiology. 2018;29(4):556–561. doi:10.1097/EDE.0000000000000833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Segal JB, Chang HY, Du Y, Walston JD, Carlson MC, Varadhan R. Development of a claims-based frailty indicator anchored to a well-established frailty phenotype. Med Care. 2017;55(7):716–722. doi:10.1097/MLR.0000000000000729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Segal JB, Huang J, Roth DL, Varadhan R. External validation of the claims-based frailty index in the national health and aging trends study cohort. Am J Epidemiol. 2017;186(6):745–747. doi:10.1093/aje/kwx257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kim DH, Schneeweiss S, Glynn RJ, Lipsitz LA, Rockwood K, Avorn J. Measuring frailty in Medicare data: development and validation of a claims-based frailty index. J Gerontol A Biol Sci Med Sci. 2018;73(7):980–987. doi:10.1093/gerona/glx229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kim DH, Glynn RJ, Avorn J, et al. Validation of a claims-based frailty index against physical performance and adverse health outcomes in the health and retirement study. J Gerontol A Biol Sci Med Sci. 2018. doi:10.1093/gerona/gly197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sonnega A, Faul JD, Ofstedal MB, Langa KM, Phillips JW, Weir DR. Cohort profile: the Health and Retirement Study (HRS). Int J Epidemiol. 2014;43(2):576–585. doi:10.1093/ije/dyu067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J. Clin. Epidemiol. 1992;45(6):613–619. [DOI] [PubMed] [Google Scholar]
- 26. Buta BJ, Walston JD, Godino JG, et al. Frailty assessment instruments: systematic characterization of the uses and contexts of highly-cited instruments. Ageing Res Rev. 2016;26:53–61. doi:10.1016/j.arr.2015.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cigolle CT, Ofstedal MB, Tian Z, Blaum CS. Comparing models of frailty: the Health and Retirement Study. J Am Geriatr Soc. 2009;57(5):830–839. doi:JGS2225 [pii]10.1111/j.1532-5415.2009.02225.x [DOI] [PubMed] [Google Scholar]
- 28. Rockwood K, Mitnitski A. Frailty in relation to the accumulation of deficits. J Gerontol A Biol Sci Med Sci. 2007;62(7):722–727. doi:62/7/722 [pii] [DOI] [PubMed] [Google Scholar]
- 29. Wei MY, Kawachi I, Okereke OI, Mukamal KJ. Diverse cumulative impact of chronic diseases on physical health-related quality of life: implications for a measure of multimorbidity. Am J Epidemiol. 2016;184(5):357–365. doi:10.1093/aje/kwv456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wei MY, Kabeto MU, Langa KM, Mukamal KJ. Multimorbidity and physical and cognitive function: performance of a new multimorbidity-weighted index. J Gerontol A Biol Sci Med Sci. 2018;73(2):225–232. doi:10.1093/gerona/glx114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kinosian B, Wieland D, Gu X, Stallard E, Phibbs CS, Intrator O. Validation of the JEN frailty index in the National Long-Term Care Survey community population: identifying functionally impaired older adults from claims data. BMC Health Serv Res. 2018;18(1):908. doi:10.1186/s12913-018-3689-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.