Abstract
Intranasal insulin is a safe and effective method for ameliorating memory deficits associated with pathological brain aging. However, the impact of different formulations and the duration of treatment on insulin’s efficacy and the cellular processes targeted by the treatment remain unclear. Here, we tested whether intranasal insulin aspart, a short-acting insulin formulation, could alleviate memory decline associated with aging and whether long-term treatment affected regulation of insulin receptors and other potential targets. Outcome variables included measures of spatial learning and memory, autoradiography and immunohistochemistry of the insulin receptor, and hippocampal microarray analyses. Aged Fischer 344 rats receiving long-term (3 months) intranasal insulin did not show significant memory enhancement on the Morris water maze task. Autoradiography results showed that long-term treatment reduced insulin binding in the thalamus but not the hippocampus. Results from hippocampal immunofluorescence revealed age-related decreases in insulin immunoreactivity that were partially offset by intranasal administration. Microarray analyses highlighted numerous insulin-sensitive genes, suggesting insulin aspart was able to enter the brain and alter hippocampal RNA expression patterns including those associated with tumor suppression. Our work provides insights into potential mechanisms of intranasal insulin and insulin resistance, and highlights the importance of treatment duration and the brain regions targeted.
Keywords: Antiaging, Cognitive decline, Animal model
Intranasal insulin (INI) has become a well-recognized method for addressing numerous neurodegenerative conditions. Several laboratories have provided evidence that it is a favorable and relatively noninvasive technique for selective delivery to the brain (1–5). Using methods developed by Frey and colleagues (Frey WH II, Method for administering insulin to the brain; Patent 6,313,093 B1, issued November 6, 2001), INI’s potential as a therapeutic for mild cognitive impairment- or Alzheimer’s disease (AD)-associated memory decline has been investigated in both clinical and preclinical studies. These trials have been encouraging, citing both the safety of INI, as well as its positive impact on memory function (6) and components of peripheral metabolism (7,8). Early work from Craft and colleagues (9) highlighted INI’s impact on memory, reporting that individuals with early AD or amnestic mild cognitive impairment receiving 20 international units (IU) of INI for 21 days retained more verbal information than controls. However, the cognitive benefits of INI are not limited to only AD or mild cognitive impairment patients. In a study of 38 healthy male participants (aged 18–34 years), 8-week INI administration correlated with improved immediate and delayed word recall, attention, and mood (6). This was corroborated by improved word-list recall in healthy male participants following INI using the rapid-acting insulin analogue aspart (2). Together, these studies highlight the important role of insulin in both declarative and spatial memory and suggest INI may target areas associated with these processes. Potential mechanisms suggested to underlie these actions include alterations in glucose metabolism (3), reductions in inflammation and glial cell activation (3,10,11), and a rapid and reliable increase of cerebral blood flow (12,13). This latter mechanism is particularly interesting as healthy young participants also respond favorably to INI. However, as the nature of clinical studies limits their ability to identify mechanistic processes, further analyses in animal models are required.
Following clinical studies, investigations of INI in animals have used models mimicking early-stage AD or mild cognitive impairment (14). Critical work from Banks and colleagues (15) reported transport of INI into the brain parenchyma, while relatively low, is nevertheless effective and long lasting, as no efflux mechanisms for the peptide appear to exist (16). These same investigators recently reported that ligand transport did not differ between AD-like (older senescence accelerated mouse-prone 8) or AD-predisposed (younger senescence accelerated mouse-prone 8) mice compared to healthy controls (17), suggesting INI is a viable method for elevating insulin in the brain regardless of AD status or severity.
With respect to aging, Apostolatos and colleagues (18) have shown improved spatial memory on the radial arm water maze following 4 weeks of daily INI with human recombinant insulin in aged (18-month-old) male C57Bl/6 mice. Studies of older (16–18-month-old) female mice showed that 7-day INI prevented anesthesia-induced reductions in spatial memory performance while reducing tau hyperphosphorylation (19). Fadool and colleagues (20) have provided evidence that short-term (5-day) INI human recombinant insulin increases novel object recognition and odor discrimination in 2-month-old male C57Bl/6 mice. However, this effect was not present when extended exposures (30–60 days) were used in 5-month-old animals (21), suggesting that duration may affect efficacy. We have investigated the ability of INI lispro and detemir to offset age-related cognitive decline in the Fischer 344 (F344) rat model of aging. In these studies, INI improved memory recall of the platform location on the Morris water maze (MWM) test in aged animals (22). Another study in the same animals using INI glulisine did not improve performance, but did facilitate mechanisms that could promote memory, including increasing cerebral blood flow and insulin receptor (IR) signaling in aged animals (23). Overall, the evidence appears to robustly support the hypothesis that insulin is involved in memory processes in both humans and animal models, and makes a strong case for INI as a clinically relevant therapy to ameliorate age- and/or AD-associated cognitive decline. However, the impact of insulin formulation and treatment duration on the efficacy of INI, as well as the cellular mechanisms targeted by this approach, remain unclear.
The premise of our work has been to address age-related reductions in insulin activity, represented by declining insulin concentrations and IR density (24–26), with a restorative increase in brain insulin levels using INI. As recently highlighted, the elements responsible for brain insulin insensitivity include diminished IR signaling through its canonical pathway (ie, insulin receptor substrate 1, glycogen synthase kinase-3 beta), a reduction of the ligand in the brain, and/or decreased insulin transport at the blood–brain barrier (reviewed in 27). As these mechanisms are likely connected, alterations in any of these processes could lead to reduced insulin signaling or function. Here, we present a series of experiments designed to test: (i) whether insulin aspart, a clinically relevant formulation that has shown enhanced brain penetration (2,28), could alleviate cognitive decline in F344 animals, and (ii) if repeated, daily INI across three consecutive months could cause changes in IR expression and modify insulin’s impact on hippocampal function. We also investigated the hypothesis that downregulation of IRs in the hippocampus or elsewhere following chronic INI is more pronounced in aged animals. The following outcome variables were obtained: spatial learning and memory and reversal learning, IR autoradiography and immunohistochemistry, and hippocampal microarray analysis. Results showed a small albeit nonsignificant amelioration of memory performance on the MWM reversal probe test in animals treated with INI aspart. The treatment significantly reduced 125I-insulin binding in the thalamus, but not in the hippocampus. Hippocampal immunofluorescence revealed a significant age-related decrease in IRs in stratum pyramidale and oriens. Hippocampal microarray analyses identified several pathways sensitive to INI, including novel genes associated with tumor suppression, neurogenesis, and synaptic stabilization.
Methods
Animal Models
The work strictly adhered to the regulations of our institutional licensing committee for the care and use of animals (Institutional Animal Care and Use Committee). 22 young (2-month-old) and 26 aged (18-month-old) male F344 rats were obtained from the National Institute on Aging colony. One young and one aged animal died within a week of arrival. Animals were housed in pairs except for the two animals that lost their cage mates. Animals were tail marked for identification, maintained on a 12-hour ON, 12-hour OFF light schedule, and fed Teklad global 18 per cent protein rodent diet ad libitum (2018; Harlan Laboratories, Madison, WI). As expected, young animals gained weight during the duration of the study. Aged animals neither gained nor lost weight. INI aspart did not affect animal weights (data not shown). Experimenters were blinded to the treatment groups and codes were revealed only after statistical analyses were performed. All brain tissues used in this study were harvested from non-fasted animals.
INI Delivery
Intranasal delivery of insulin aspart to the remaining 46 animals began 1 week after arrival and followed our previously published protocol (22,23). Animals were transiently held supine in a DecapiCone (Braintree Scientific, Braintree, MA) whereas two 5 µL doses of either sterile saline or insulin aspart (NovoLog), given 1 minute apart, were delivered to the right naris using a P10 pipette. INI continued for 3 months (62–64 doses per animal: 5 days a week, once a day, for 12 weeks). Insulin aspart was made fresh weekly and diluted from a U-100 vial (Novo Nordisk Inc., Plainsboro, NJ) using sterile saline. We chose a concentration of 0.0715 IU/10 µL as it mimics the approximate dosage used in numerous clinical trials.
Spatial Behavior
On the 12th week of INI, animals (aged 5 or 21 months) underwent a spatial learning and memory test using the MWM. The testing pool measured 190 cm in diameter. A 15 cm escape platform was placed 1.5 cm below the water’s surface. Water was maintained between 25°C and 26°C and made opaque to hide the platform using black tempura paint. A semi-random drop location was used for each trial. Animals were allowed 60 seconds to find the platform, after which they were guided to its location by the investigator. A Videomex-V acquisition and Water Maze analysis software (v4.64; Columbus Instruments, Columbus, OH) was used to track and measure movement. Each animal remained on the platform for 30 seconds before returning to a heated holding chamber for approximately 2 minutes.
On the first day of MWM, a visual acuity test was performed with a white cup placed above the partially submerged platform for three consecutive trials. Three animals in the aged insulin aspart group failed to find the platform in all trials and were removed from the behavioral analysis. After a 2-day rest, animals were subjected to three training trials per day for 3 days (~2.5 minutes intertrial interval). Twenty-four hours after the last training day, a probe trial was initiated with the platform removed (up to 60 seconds of max swim time). The following day, the platform was placed in the opposite quadrant and animals were trained on its new location (reversal learning). After 72 hours, a reversal probe trial was initiated with the platform removed. Following behavioral testing, brains from 20 animals were used for autoradiography measures. Brains from the remaining 26 animals were hemisected; left tissues (hemispheres) were used for IR immunohistochemistry and right tissues (whole hippocampi) were used for microarray analyses.
We present behavioral data on path length measures and number of platform crossings on the 24-hour memory recall (probe), as well as time in goal quadrant in the first 30 seconds of the 72-hour reversal probe, from 43 animals (young saline n = 11, young aspart n = 10, aged saline n = 13, aged aspart n = 9). Swim speed was averaged from the three trials on the third training day. As previously presented (22,23) aged animals swam more slowly than young (F(1,39) = 24.9, p < 0.0001; data not shown) and INI did not have an impact on swim speed (F(1,39) = 0.4, p > 0.05).
125I-Insulin Receptor Autoradiography
Whole brains, including olfactory bulbs, were extracted from randomly selected animals (n = 5 per group) following anesthesia (5 per cent isoflurane). Brains were placed on finely crushed dry ice, covered by the ice, and submerged in chilled 2-methylbutane. Tissues (16 µm sections) were mounted on slides and prepared for 125I-insulin receptor autoradiography using the assay described by Kar and colleagues (29). Briefly, slides were incubated for 18 hours in 10 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid buffer (pH 8.0) containing 0.5 per cent bovine serum albumin, 0.025 per cent bacitracin, 0.0125 per cent N-ethylmaleimide and 100 kIU aprotinin (Sigma-Aldrich, Saint Louis, MO), and 25 pM 125I-insulin (2,000 Ci/mmol; PerkinElmer, Waltham, MA) at 4°C, then washed twice (5 minutes each, 4°C) with 10 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid buffer (pH 8.0). Slides were then washed once with a 10-fold dilution wash buffer and again with deionized water (10 seconds each, 4°C), air dried, and stored in a vacuum-sealed desiccator. The next day, slides were incubated on tritium-sensitive film (Amersham Hyperfilm MP; GE Healthcare, Chicago, IL) and stored in x-ray cassettes for 2 months. Films were then processed in a Kodak D-19 Developer for 5 minutes, run through a 30 seconds indicator stop bath, and exposed to Kodak rapid fixer for 5 minutes. Images were captured using a Northern Lights desktop illuminator (Model B95; Imaging Research, Ontario, Canada) and a Sony XC-77 CCD camera via Scion LG-3 frame grabber. ImageJ v1.59 (National Institutes of Health, Bethesda, MD) was used for quantitative image analysis. Data are reported as uncalibrated optical density (n = 17–18 animals). Two animals from the aged insulin aspart group were removed from analysis: one for poor tissue quality, one for failing the visual acuity test. For binding measures in the olfactory bulb (internal plexiform layer), data from one aged saline animal was removed due to poor tissue quality.
Immunohistochemistry
The immunohistochemistry groups were split as follows: young saline n = 6, young aspart n = 5, aged saline n = 8, aged aspart n = 7. Animals were anesthetized with Fatal-Plus (390 mg/mL pentobarbital) and perfused with oxygenated saline (~10 minutes), after which brains were harvested and hemisected. The left hemisphere was placed in 4 per cent paraformaldehyde for 48 hours, then transferred to 30 per cent sucrose for approximately 24 hours. Tissues were placed in an antifreeze solution at 20°C until sectioning. Tissue slices were cut on a cryostat (35 µm) and probed for insulin receptor alpha subunit using a standard immunohistochemistry protocol (1o antibody: Abcam 5500, 1:200; fluorescein isothiocyanate-conjugated 2o antibody: Abcam 150077, 1:200; Abcam, Cambridge, MA). Slices were placed on subbed glass slides, covered with 4′,6-diamidino-2-phenylindole (DAPI)-supplemented mounting medium (P36966; Invitrogen, Carlsbad, CA), and cover slipped. A Nikon fluorescent microscope using a spectral analysis camera and Nuance software (Nuance Communications, Burlington, MA) together with ImageJ was used to quantify percent area of a region of interest in the CA1 cell body region. Images were thresholded to identify individual cell bodies. Percent area positively labeled was determined from the particle size algorithm. Percent areas are reported in hippocampal subsections (strata pyramidale, radiatum, and oriens) obtained from four to eight animals per group. The same size region of interest was used for each subsection across slices. To control for cell density across age and hippocampal subfields, data were normalized to the immunopositive area for DAPI signal in each section. Data presented are derived from the average of two independent scorers.
Hippocampal RNA Extraction and Microarray
Right hippocampi from 26 animals (young saline n = 6, young aspart n = 5, aged saline n = 8, aged aspart n = 7) were isolated over ice and placed in a −80°C freezer until processed for RNA extraction. To extract RNA, hippocampi were thawed on ice and homogenized in RiboZol Extraction Reagent (97064-948; VWR, Radnor, PA). RNA was precipitated with chloroform and isopropanol, then resuspended in a 75 per cent ethanol solution. Following extraction, RNA integrity numbers were obtained for each sample using standard protocols (University of Kentucky Genomics Core, Lexington, KY). Mean values for each group were as follows: young saline = 7.32 ± 0.14, young aspart = 7.34 ± 0.15, aged saline = 7.33 ± 0.12, aged aspart = 7.29 ± 0.13. No significant difference in integrity numbers was noted between the groups (two-way analysis of variance [ANOVA]; p > 0.5). Samples were stored in a −80°C freezer until thawed for microarray analysis (Affymetrix rat Clariom S Assay; Thermo Fisher Scientific, Waltham, MA). Gene signal intensities were calculated using the Robust Multiarray Average algorithm at the transcript level and data were associated with vendor-provided annotation information.
Statistical Analysis
Spatial memory results are based on a total of 43 animals. Statistical outliers (>2 SDs from the mean) were excluded from analysis. Immunofluorescent data were filtered using an interquartile range approach. Drug and aging effects on endpoint measures were determined using two-way ANOVAs with Bonferroni post hoc tests. Significance for all comparisons was set at p < 0.05, except for microarray data, where a significance level of p < 0.03 was chosen.
Results
Spatial Learning and Memory
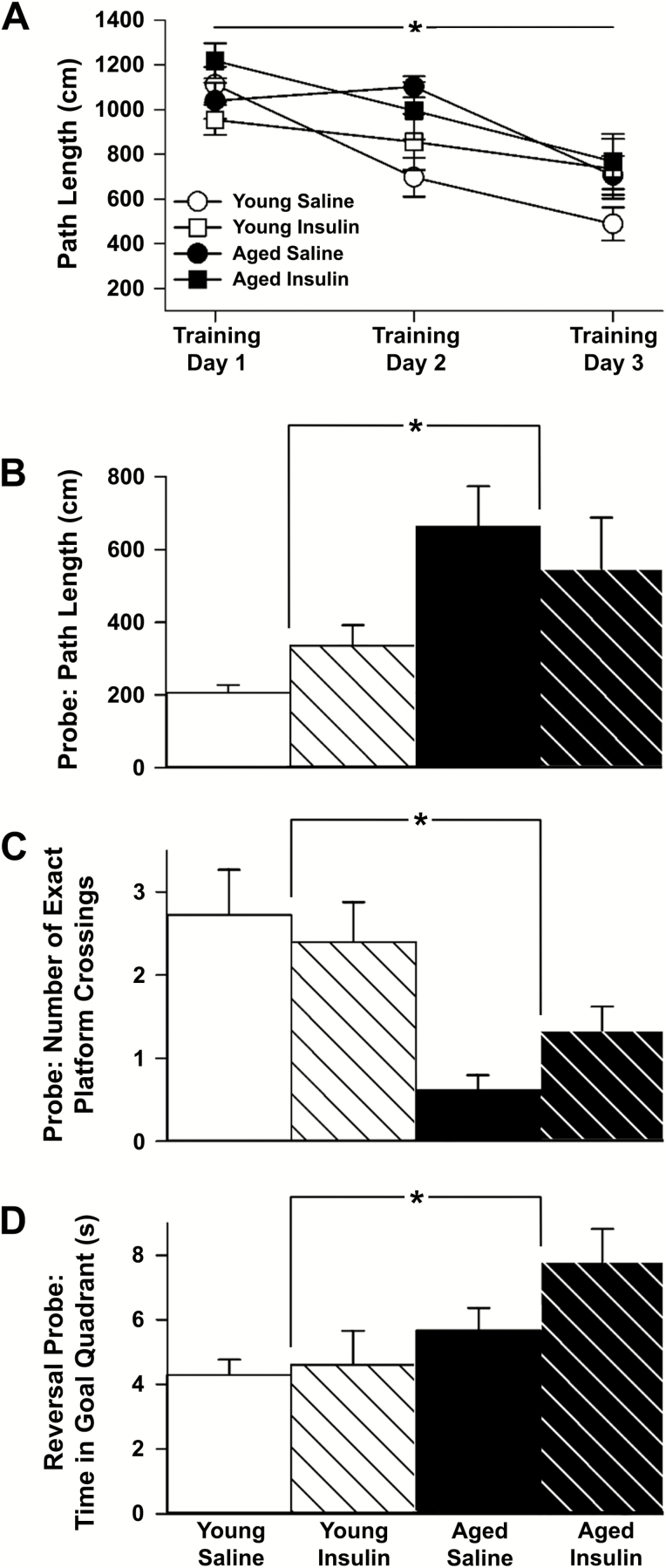
Young and aged animals were able to learn the spatial task and escape onto the platform with decreasing path lengths across the 3 days of training (young F(2,38) = 11.3, p = 0.0001; aged F(2,40) = 17.5, p < 0.0001; Figure 1A). INI did not alter learning rates in either age group (p > 0.05). These results align with our prior work showing that aged animals are capable of learning the task (22,23,30). Insulin aspart, much like detemir, lispro, or glulisine (22,23), does not show a measurable influence on the learning component of the task. The memory component was investigated with a 24-hour probe and 72-hour reversal probe following learning for a new platform location. Path length to goal during the 24-hour probe revealed significant memory effects from aging (F(1,39) = 12.5, p = 0.0011), but not from INI (p > 0.05, Figure 1B). Similarly, the number of exact platform crossings highlighted a significant aging difference (F(1,39) = 16.3, p = 0.0002) without an observed INI difference (p > 0.05, Figure 1C). We then trained the animals on a new platform location and waited 72 hours before probing again. As expected, analysis of this more demanding task revealed significant aging differences on time in goal quadrant during the first 30 seconds of the probe trial (F(1,39) = 7.9, p = 0.0076; Figure 1D), but also provided some evidence for a nonsignificant amelioration in memory performance (p = 0.14) in INI-treated animals. Although the effect did not reach significance, aged animals showed an approximately 30 per cent increase in time spent in the correct quadrant whereas young animals showed no such change. This is likely the reason for a lack of a main effect of INI on ANOVA testing. These results do not appear to depend on swim speed (F(1,39) = 0.4, p > 0.05, see Methods section).
Figure 1.
Spatial learning and memory. (A) Path length to goal measures from 43 animals (young saline n = 11, young aspart n = 10, aged saline n = 13, aged aspart n = 9) across 3 days of training showed improved learning over time, though no distinct drug effect was noted. (B) Memory recall on the probe task (24 h) shows young animals (n = 21) identifying the platform location more readily than aged animals (n = 22). (C) During the full 60 s probe task, young animals crossed the exact platform location more often. (D) The 72-h reversal probe showed that the young animals spent significantly more time in the new goal quadrant compared to the aged. Data represent means ± SEM. Asterisks (*) indicate significance at p < 0.05.
Overall, this INI regimen does not appear to have a greater impact on learning and memory performance in aged animals compared to shorter exposures previously used (22,23). Although this could suggest that longer exposures are less protective, it could also reflect a short-lived impact of INI that may have been missed using the current protocol. Given that chronic peripheral hyperinsulinemia or insulin resistance can reduce insulin transport into the central nervous system (31–33), we next tested whether 3-month INI could alter central nervous system IR expression similar to that seen at the blood–brain barrier (reviewed in 34) or in the periphery.
Quantitative Autoradiography
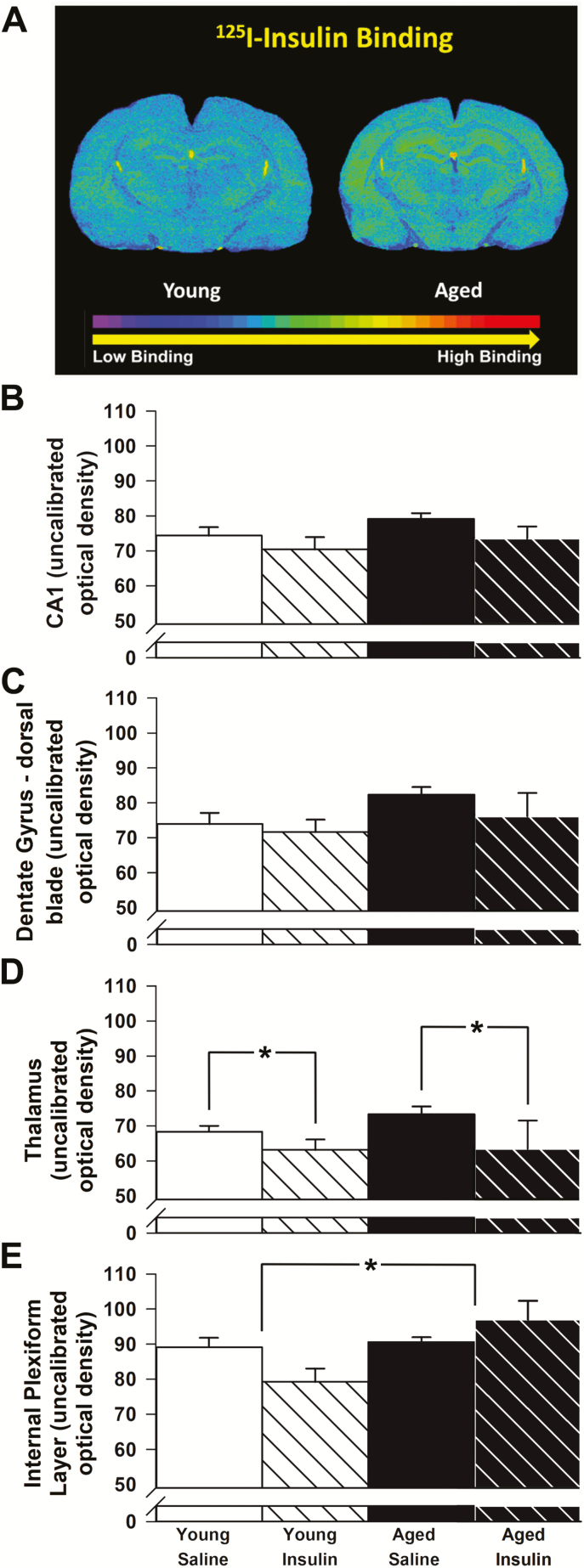
We harvested brains from randomly selected animals (n = 5 per group) to characterize IR binding using autoradiography (Figure 2). Although no significant differences with age or insulin treatment were found in field CA1 of the hippocampus (p > 0.05, Figure 2B), a nonsignificant aging effect was noted in the dorsal blade of the dentate gyrus (F(1,14) = 3.1, p = 0.10; Figure 2C). Binding of 125I-insulin in the thalamus (ventral posteromedial and ventral posterolateral thalamic nuclei) decreased significantly with long-term INI (F(1,14) = 4.7, p = 0.047; Figure 2D), but no aging effects were observed. A significant main effect of age, evidenced by greater binding in the outer plexiform layer (F(1,13) = 7.9, p = 0.014; Figure 2E) together with a significant interaction term in response to INI (F(1,13) = 5.7, p = 0.032; Figure 2E), was noted in the olfactory bulb. These results are somewhat surprising given previous work highlighting decreased cortical IR numbers (26) and overall IR mRNA levels with age (35), but are well aligned with several studies that did not find significant reductions in IR binding, except in the olfactory bulb of the aged rat (25,36).
Figure 2.
125I-Insulin receptor binding. (A) Representative images of 125I-insulin receptor binding on a young and aged control brain section. (B) No significant differences with age or insulin treatment were found in field CA1 of the hippocampus (n = 5 per group). (C) Although greater binding of insulin to the dorsal blade of the dentate gyrus was seen with age, this increase was not significant (p = 0.10). (D) Binding in the thalamus decreased significantly with long-term INI. (E) Binding in the internal plexiform layer of the olfactory bulb increased significantly with age. A significant interaction term was also noted, with intranasal insulin (INI) decreasing 125I-insulin binding in young while increasing it in aged. Data represent means ± SEM. Asterisks (*) indicate significance at p < 0.05.
Immunohistochemistry
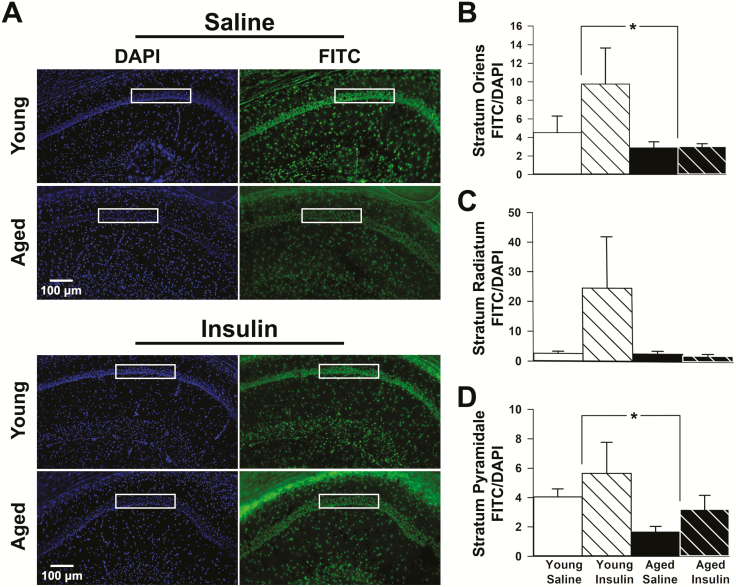
Aligned with prior work showing decreases in IR mRNA with aging (35), we show a significant reduction in immunolabeled area for IR in field CA1 of the hippocampus in aged animals compared to young (Figure 3). DAPI signal (percentage of area covered) did not change with age or treatment (Supplementary Figure 1). Although no significant effects of INI were noted, a significant age-dependent reduction in fluorescein isothiocyanate /DAPI was seen in stratum oriens (F(1,18) = 4.5, p = 0.047; Figure 3B) and stratum pyramidale (F(1,19) = 6.4, p = 0.021; Figure 3D). No significant difference was noted in stratum radiatum (Figure 3C). Similar quantification in the dorsal blade of the dentate gyrus did not show a significant main effect of age or INI (p > 0.05; Supplementary Figure 2).
Figure 3.
Insulin receptor (IR) immunofluorescence. (A) DAPI fluorescence (left) was used to normalize all FITC fluorescence (right) for each hippocampal section quantified. Immunopositive signals representing the presence of the IR were quantified within each region of interest (ROI; white boxes). Equally sized ROIs were used to quantify immunopositive areas across strata oriens (B), radiatum (C), and pyramidale (D) subfields. Strata oriens and pyramidale both showed a significant decrease in IR fluorescence with age (young n = 11, aged n = 15). Data represent means ± SEM. Asterisks (*) indicate significance at p < 0.05.
Microarray Analyses
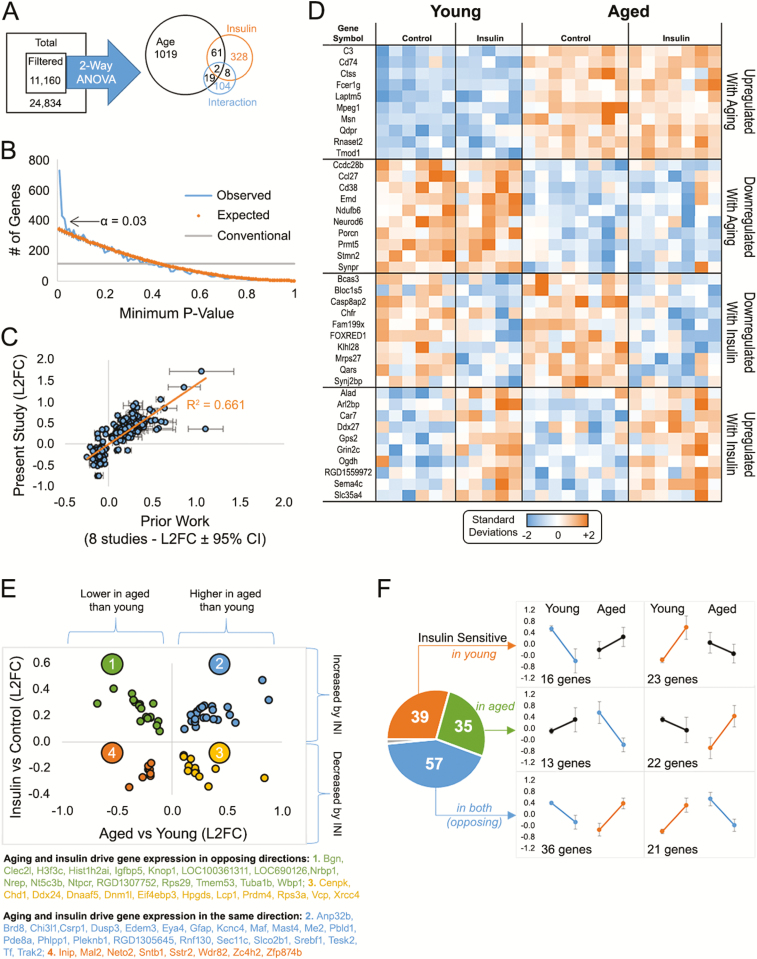
Microarray data are presented in Figure 4. Of the initial 11,160 filtered genes, significant main effects of age, insulin, and the interaction term identified 1541 genes (two-way ANOVA). We chose a significance level of 0.03 (false discovery rate = 0.16) based on the p value frequency distribution which indicated a large increase in significant genes below that value. As shown in Figure 4A, a greater number of genes were modified by aging (~1100) than by INI (~400).
Figure 4.
Microarray analyses. (A) Total gene set filtered to remove low-intensity signals yielded 11,160 genes. Two-way analysis of variance (ANOVA) analysis from 26 animals (young saline n = 6, young aspart n = 5, aged saline n = 8, aged aspart n = 7) identified ~1,500 genes that were significant by main effects of age, insulin, and/or the interaction (B) p value frequency histogram shows the increase in the number of significant genes with α < 0.03. The conventional line (gray) delineates a cutoff for significance near 112 as the first percentile (p < 0.01) of the 11,160 filtered genes. The orange line represents the p values obtain when testing for significance across a set of 11,160 randomly generated numbers through a two-way analysis of variance (ANOVA). The blue line highlights the p values obtain from our data set. (C) We validated ~1,000 age-sensitive genes across our prior studies and found a significant correlation with prior work. (D) Heatmap of significant genes (top 10) separated across participants by aging or drug effects. Each column represents one animal and each row represents one gene. Color-coded signal intensity values (standardized: orange represents increase, blue represents decrease) are shown. (E) Genes significant by both main effects (63) fall into four categories: 2 in the same direction (quadrants 4 and 2), and 2 in the opposite direction (quadrants 1 and 3). (F) Genes within the significant interaction space (~130) are divided as those modified in young animals (top), in aged animals (middle), or in both (bottom). This result suggests insulin sensitivity in the brain may not differ across aging (39 genes changed in young, and 35 changed in aged). Data represent means ± SEM.
Transcriptional targets of age and insulin are presented in Supplementary Table 1 (GO Accession # GSE130098). A large proportion of genes upregulated by age in the hippocampus combined into functional annotation clusters using the Database for Annotation, Visualization and Integrated Discovery (DAVID) hierarchical clustering analysis, including those involved in neutrophil activation, myelination, inflammation, and cell migration. We validated these changes by testing for alignment with eight transcriptional profiles of aging published in prior work ((37–39), reviewed in 40). As seen in Figure 4C, aging-significant genes identified here showed a strong correlation with those highlighted by prior profiles (192 genes; p = 1.09E-46; R2 = 0.661). The heatmap representation of the top 10 genes changed with aging or with insulin is shown in Figure 4D. Genes at the intersection of both main effects (age- and insulin-sensitive) are displayed graphically as a function of log2 fold change (Figure 4E). The nearly 140 genes representing the interaction term of the two-way ANOVA are presented in Figure 4F.
A DAVID heuristic categorization analysis of nonredundant genes upregulated by INI in the hippocampus identified main biological processes that included anti-inflammation (Synj2bp), synaptic stabilization (Pak1, Stx1a), and tumor suppression/antiproliferative function (Cdh11) genes. RNA signatures downregulated by INI were associated with cancer development (Erbb2, Myt1) and glial and neuronal growth (Atl1, Fgf9, Numb, Acap2), perhaps providing a path for stabilizing established synaptic connections. Alternatively, the strong presence of Erbb2 in astrocytes and Myt1 in oligodendrocytic precursor cells (41) suggest nonneuronal cell types may also be targets of INI. Analysis of genes that responded to aging and insulin in opposite directions, and are therefore likely reparative, corresponded to reduced inflammation, DNA repair, cell growth, and translation stability processes (Figure 4E). Genes increased with age and decreased by INI included two helicases (Chd1, Ddx24), a ligase (Xrcc4), and a programed cell death gene (Dnml1). Those decreased by age and increased by insulin included anti-inflammatory (Igfbp5), cellular repair (Nrep, H3f3c), vascular function (Nrep), tumor suppression (Nrbp1, Wbp1), and neuronal growth (Tuba1b, Nrbp1) genes. Surprisingly, very few, if any, genes targeted by chronic INI fell within the canonical insulin signaling pathway. It is also interesting that INI altered gene expression similarly in both young and aged animals ((35) and (39) genes, respectively), suggesting that the aged hippocampus may remain sensitive to insulin provided the ligand is present. Overall, the profiling analysis presented here reveals that INI likely entered the hippocampus and significantly altered expression of important genes associated with tumor suppression, neurogenesis, and synaptic stabilization.
Discussion
This study was undertaken to determine whether INI using a higher-penetrance insulin analogue could prevent aspects of brain aging. Aspart provided an observable, albeit small and nonsignificant, behavioral enhancement during a particularly challenging memory task in the aged animals. A significant reduction in IR autoradiography was seen in the thalamus in response to INI, but not in the hippocampus, although a modest increase in hippocampal IR immunofluorescence in stratum pyramidale was noted (nonsignificant). Compared to a prior study from our group using fewer insulin exposures (~10), but conducted in young and aged F344 rats with similar doses and INI techniques (22), this much longer study (~63 exposures) did not provide greater improvement on memory recall of spatial information in the aged animals. Importantly, however, we detected significant changes in gene signatures in the hippocampus of INI-treated animals including those associated with tumor suppression, synaptic stabilization, and anti-inflammation pathways, all of which are considered potential therapeutic targets for the amelioration of cognitive decline associated with aging and/or AD.
Why Insulin Aspart?
The fast-acting insulin analogue aspart includes molecular modifications that increase absorption rates and peak plasma concentrations to almost twice that of human insulin (reviewed in 42). Despite these pharmacokinetic differences, profiles of IR affinity, dissociation and tyrosine kinase activation rates, insulin-like growth factor 1 binding, metabolic potency, and ligand degradation rates are comparable between insulin aspart and human insulin (42,43). In the context of INI, insulin aspart’s inability to form hexamers may increase its absorption in the brain. In fact, recent work indicated insulin aspart was absorbed more rapidly than longer-acting formulations following nasal delivery in rats (28). In addition, INI aspart significantly improved word-list recall in healthy male participants (aged 18–35 years) compared to human insulin (2).
It is reasonable to assume that increasing the availability and absorption rate of insulin could enhance delivery to the brain. Although no statistical comparisons between our prior studies (22,23) and the current results were performed, insulin aspart does not appear superior at offsetting memory decline in aged animals compared to insulin detemir (longer-acting) or lispro (rapid-acting) (22). Given similar receptor affinities of the different insulin formulations, and assuming equivalent distribution in the brain, it is possible that the inconsistencies in memory performance in aged INI animals were due to different exposure frequencies. The greater number of exposures (~63 doses, one per day) did not yield larger improvements in memory recall compared to the fewer exposures (~10 doses) used in earlier studies (22). Interestingly, we previously reported that repeated doses of insulin glulisine (~18, one per day), another rapid-acting insulin, also did not improve memory recall in aged animals, although it did increase hippocampal IR signaling and cerebral blood flow (23).
Others investigating long-term (30–60 days), repeated dosing of INI in C57BL6/J mice showed that longer exposures are not as beneficial on olfactory or object recognition memory compared to acute (21). The authors speculated that longer exposures likely initiate a state of brain insulin resistance whereby continued IR signaling is not maintained. Indeed, current evidence indicates variability in results across laboratories and conditions may be due to the length of exposure (21), as long-term INI could potentially upregulate insulin degradation and cause a pharmacokinetic tolerance in the brain. Whether a larger number of insulin exposures (>10) weakens its impact or if the formulation used is responsible for this inconsistency remains to be determined. Regardless, the evidence of region-specific downregulation of IR presented here suggests that insulin resistance and/or decreases in signaling may not be a generalizable condition.
Difference Between 125I-Insulin Receptor Autoradiography and Immunofluorescence Results
Prior reports on IR binding in the brain of rodents indicated higher receptor density in the olfactory bulb and choroid plexus compared to other structures (25,44). In addition, a reduction in IR binding has been noted in the olfactory bulb in studies of aging (25,36), whereas others have shown reduced binding in the cortices of elderly non-demented participants (>65-years-old) compared to younger adults (24). Contrary to these studies, we do not show reduced IR binding with age in the internal plexiform layer of the olfactory bulb, and instead report greater binding in this area (Figure 2E). Given that insulin levels in the olfactory bulb fluctuate with feeding state (45), it is possible this might have influenced IR autoradiography. It is also interesting to speculate that the exposure to an enriched environment (eg, MWM and repeated daily handling) in this study might have increased IR mRNA levels, thereby offsetting the impact of aging on IR density.
As hypothesized, we show that chronic INI exposure does decrease binding, albeit only in the ventral posteromedial and ventral posterolateral thalamic nuclei of the thalamus. Given prior evidence that hippocampal–anterior thalamic pathways between the hippocampal formation and the thalamus contribute to episodic memory function (46), our results may reflect a potential mechanism by which INI alters thalamic IR density and possibly regulates learning and memory processes. Further, a significant interaction term (two-way ANOVA) showing an increase in aged and a decrease in young animals following INI was noted in the internal layer of the olfactory bulb. It appears distinct brain regions may respond differently to this particular dosing regimen, as areas associated with spatial memory processes (ie, the hippocampus) were less affected than others. With respect to aging, our results align well with a prior study showing no difference in insulin binding in the cortex or hippocampal formation (25). Further, data presented here should not reflect insulin-like growth factor 1 receptor binding, as an insulin dose well below the binding affinity for insulin-like growth factor 1 receptor (47) was used. Thus, our results are novel and given the paucity of binding studies in normal aging, continual investigations of insulin binding in aging and/or AD appear warranted.
In addition, the strong hippocampal immunofluorescence for the insulin receptor alpha subunit not only reflects the presence of IRs in neurons, but also shows substantial expression in stratum radiatum astrocytes and what are likely oligodendrocytes near the heavily myelinated fimbria above stratum oriens (Figure 3). Although surprising given evidence of stronger immunopositivity in primary neurons compared to other hippocampal cell types (48), this result was corroborated using another insulin receptor alpha subunit antibody from a different company (data not shown), suggesting that IRs in oligodendrocytes are perhaps relevant to observations of neuropathy in DM. Immunofluorescence in the primary neuronal cell layer was mostly somatic, as no dendritic elements appeared to be immunoresponsive. This was unexpected given previous evidence of synapse-centric insulin effects on hippocampal neurons (reviewed in 49).
Overall, the results from hippocampal IR immunofluorescence do not align well with those from autoradiography. We show a reduction in immunopositive area in stratum oriens and stratum pyramidale with age that is not reflected in measures of 125I-insulin binding. This is surprising as both approaches report on plasma membrane proteins and should represent functional IRs. Our immunofluorescence protocol did require the use of a mild detergent during washing; thus, it is possible that the quantification reported here includes intracellularly labeled nascent proteins. Further, the area quantified for autoradiography encompassed most of the hippocampus while more defined strata were quantified in the immunofluorescence assays, which could have influenced our results. Nevertheless, these results still clearly emphasize the complexity and dynamic aspect of insulin’s actions in the brain.
Analysis of Hippocampal Genes Altered by Aging and INI
For the first time, our studies provide a comprehensive analysis of the hippocampal transcriptome in aging that is sensitive to INI aspart. These gene targets appear to be involved with processes other than those strictly aligned with learning and memory. A number of the genes identified, particularly those modified by aging, are similar to genes characterized in prior studies, including those associated with myelination, inflammation, and cell migration. With respect to genes upregulated by INI, many were also previously recognized in studies of cardiac and brain health, and are primarily involved in anti-inflammatory (reviewed in 50) and synaptic stabilization processes (reviewed in 49). Our results offer new evidence that insulin in the brain may have antiproliferative properties and could potentially act as a tumor suppressor. In fact, hippocampal RNA signatures downregulated by INI included Erbb2, one of the most well-documented cancer-related genes (51). Perhaps of greater interest were genes that responded to INI in the opposite direction from aging and are likely beneficial. Again, these centered on processes associated with reductions in inflammation, DNA and cellular repair, and tumor suppression.
Insulin-mediated processes are thought to be reduced in both healthy and pathological aging and are often considered reflective of brain insulin resistance. However, we show that the number of genes altered by INI in the young and the aged brain are nearly identical. Given consistent evidence of maintained insulin sensitivity with age from our group, this is perhaps not surprising. Together, these results suggest that aging may not affect insulin sensitivity as much as previously thought (27).
Conclusion
With the expected increase in life expectancy, the resultant growth of the aged population will increase; therefore, it is important to consider novel therapies and perhaps earlier interventions for successful brain aging. We report that long-term INI aspart was well-tolerated and present evidence of mild improvements in memory recall in aged animals following repeated daily dosing. We also demonstrate the feasibility of using INI to offset changes associated with brain aging and provide new insights into potential mechanisms and physiological components that may contribute to its therapeutic efficacy.
Funding
This work is supported by the National Institutes of Health (R01AG033649 to O.T., T32DK007778 to H.N.F., and T32AG057461 to A.O.G.).
Conflict of interest statement None reported.
Supplementary Material
Acknowledgments
The authors are grateful for the invaluable help provided by Drs. Lawrence D. Brewer and John C. Gant during Morris water maze behavioral characterization.
References
- 1. Lochhead JJ, Thorne RG. Intranasal delivery of biologics to the central nervous system. Adv Drug Deliv Rev. 2012;64:614–628. doi:10.1016/j.addr.2011.11.002 [DOI] [PubMed] [Google Scholar]
- 2. Benedict C, Hallschmid M, Schmitz K, et al. Intranasal insulin improves memory in humans: superiority of insulin aspart. Neuropsychopharmacology. 2007;32:239–243. doi:10.1038/sj.npp.1301193 [DOI] [PubMed] [Google Scholar]
- 3. Brabazon F, Wilson CM, Jaiswal S, Reed J, Frey WH, Byrnes KR. Intranasal insulin treatment of an experimental model of moderate traumatic brain injury. J Cereb Blood Flow Metab. 2017;37:3203–3218. doi:10.1177/0271678X16685106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Born J, Lange T, Kern W, McGregor GP, Bickel U, Fehm HL. Sniffing neuropeptides: a transnasal approach to the human brain. Nat Neurosci. 2002;5:514–516. doi:10.1038/nn849 [DOI] [PubMed] [Google Scholar]
- 5. Thorne RG, Emory CR, Ala TA, Frey WH II. Quantitative analysis of the olfactory pathway for drug delivery to the brain. Brain Res. 1995;692:278–282. doi:10.1016/0006-8993(95)00637-6 [DOI] [PubMed] [Google Scholar]
- 6. Benedict C, Hallschmid M, Hatke A, et al. Intranasal insulin improves memory in humans. Psychoneuroendocrinology. 2004;29:1326–1334. doi:10.1016/j.psyneuen.2004.04.003 [DOI] [PubMed] [Google Scholar]
- 7. Dash S, Xiao C, Morgantini C, Koulajian K, Lewis GF. Intranasal insulin suppresses endogenous glucose production in humans compared with placebo in the presence of similar venous insulin concentrations. Diabetes. 2015;64:766–774. doi:10.2337/db14-0685 [DOI] [PubMed] [Google Scholar]
- 8. Heni M, Wagner R, Kullmann S, et al. Central insulin administration improves whole-body insulin sensitivity via hypothalamus and parasympathetic outputs in men. Diabetes. 2014;63:4083–4088. doi:10.2337/db14-0477 [DOI] [PubMed] [Google Scholar]
- 9. Reger MA, Watson GS, Green PS, et al. Intranasal insulin administration dose-dependently modulates verbal memory and plasma amyloid-beta in memory-impaired older adults. J Alzheimers Dis. 2008;13:323–331. doi:10.3233/JAD-2008-13309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rajasekar N, Nath C, Hanif K, Shukla R. Intranasal insulin administration ameliorates streptozotocin (ICV)-induced insulin receptor dysfunction, neuroinflammation, amyloidogenesis, and memory impairment in rats. Mol Neurobiol. 2017;54:6507–6522. doi:10.1007/s12035-016-0169-8 [DOI] [PubMed] [Google Scholar]
- 11. Adzovic L, Lynn AE, D’Angelo HM, et al. Insulin improves memory and reduces chronic neuroinflammation in the hippocampus of young but not aged brains. J Neuroinflammation. 2015;12:63. doi:10.1186/s12974-015-0282-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schilling TM, Ferreira de Sá DS, Westerhausen R, et al. Intranasal insulin increases regional cerebral blood flow in the insular cortex in men independently of cortisol manipulation. Hum Brain Mapp. 2014;35:1944–1956. doi:10.1002/hbm.22304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Akintola AA, van Opstal AM, Westendorp RG, Postmus I, van der Grond J, van Heemst D. Effect of intranasally administered insulin on cerebral blood flow and perfusion; a randomized experiment in young and older adults. Aging (Albany NY). 2017;9:790–802. doi:10.18632/aging.101192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schiöth HB, Craft S, Brooks SJ, Frey WH II, Benedict C. Brain insulin signaling and Alzheimer’s disease: current evidence and future directions. Mol Neurobiol. 2012;46:4–10. doi:10.1007/s12035-011-8229-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Salameh TS, Bullock KM, Hujoel IA, et al. Central nervous system delivery of intranasal insulin: mechanisms of uptake and effects on cognition. J Alzheimers Dis. 2015;47:715–728. doi:10.3233/JAD-150307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cashion MF, Banks WA, Kastin AJ. Sequestration of centrally administered insulin by the brain: effects of starvation, aluminum, and TNF-alpha. Horm Behav. 1996;30:280–286. doi:10.1006/hbeh.1996.0034 [DOI] [PubMed] [Google Scholar]
- 17. Rhea EM, Humann SR, Nirkhe S, Farr SA, Morley JE, Banks WA. Intranasal insulin transport is preserved in aged SAMP8 mice and is altered by albumin and insulin receptor inhibition. J Alzheimers Dis. 2017;57:241–252. doi:10.3233/JAD-161095 [DOI] [PubMed] [Google Scholar]
- 18. Apostolatos A, Song S, Acosta S, et al. Insulin promotes neuronal survival via the alternatively spliced protein kinase CδII isoform. J Biol Chem. 2012;287:9299–9310. doi:10.1074/jbc.M111.313080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhang Y, Dai CL, Chen Y, Iqbal K, Liu F, Gong CX. Intranasal insulin prevents anesthesia-induced spatial learning and memory deficit in mice. Sci Rep. 2016;6:21186. doi:10.1038/srep21186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Marks DR, Tucker K, Cavallin MA, Mast TG, Fadool DA. Awake intranasal insulin delivery modifies protein complexes and alters memory, anxiety, and olfactory behaviors. J Neurosci. 2009;29:6734–6751. doi:10.1523/JNEUROSCI.1350-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bell GA, Fadool DA. Awake, long-term intranasal insulin treatment does not affect object memory, odor discrimination, or reversal learning in mice. Physiol Behav. 2017;174:104–113. doi:10.1016/j.physbeh.2017.02.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Maimaiti S, Anderson KL, DeMoll C, et al. Intranasal insulin improves age-related cognitive deficits and reverses electrophysiological correlates of brain aging. J Gerontol A Biol Sci Med Sci. 2016;71:30–39. doi:10.1093/gerona/glu314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Anderson KL, Frazier HN, Maimaiti S, et al. Impact of single or repeated dose intranasal zinc-free insulin in young and aged F344 rats on cognition, signaling, and brain metabolism. J Gerontol A Biol Sci Med Sci. 2017;72:189–197. doi:10.1093/gerona/glw065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Frolich L, Blum-Degen D, Bernstein HG, et al. Brain insulin and insulin receptors in aging and sporadic Alzheimer’s disease. J Neural Transm (Vienna). 1998;105:423–438. doi:10.1007/s007020050068 [DOI] [PubMed] [Google Scholar]
- 25. Tchilian EZ, Zhelezarov IE, Petkov VV, Hadjiivanova CI. 125I-insulin binding is decreased in olfactory bulbs of aged rats. Neuropeptides. 1990;17:193–196. doi:10.1016/0143-4179(90)90035-W [DOI] [PubMed] [Google Scholar]
- 26. Zaia A, Piantanelli L. Insulin receptors in the brain cortex of aging mice. Mech Ageing Dev. 2000;113:227–232. doi:10.1016/S0047-6374(99)00118-9 [DOI] [PubMed] [Google Scholar]
- 27. Frazier HN, Ghoweri AO, Anderson KL, Lin RL, Porter NM, Thibault O. Broadening the definition of brain insulin resistance in aging and Alzheimer’s disease. Exp Neurol. 2019;313:79–87. doi:10.1016/j.expneurol.2018.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pillion DJ, Fyrberg MD, Meezan E. Nasal absorption of mixtures of fast-acting and long-acting insulins. Int J Pharm. 2010;388:202–208. doi:10.1016/j.ijpharm.2010.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kar S, Chabot JG, Quirion R. Quantitative autoradiographic localization of [125I]insulin-like growth factor I, [125I]insulin-like growth factor II, and [125I]insulin receptor binding sites in developing and adult rat brain. J Comp Neurol. 1993;333:375–397. doi:10.1002/cne.903330306 [DOI] [PubMed] [Google Scholar]
- 30. Blalock EM, Phelps JT, Pancani T, et al. Effects of long-term pioglitazone treatment on peripheral and central markers of aging. PLoS One. 2010;5:e10405. doi:10.1371/journal.pone.0010405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Craft S. Insulin resistance syndrome and Alzheimer’s disease: age- and obesity-related effects on memory, amyloid, and inflammation. Neurobiol Aging. 2005;26(suppl 1):65–69. doi:10.1016/j.neurobiolaging.2005.08.021 [DOI] [PubMed] [Google Scholar]
- 32. Israel PA, Park CR, Schwartz MW, et al. Effect of diet-induced obesity and experimental hyperinsulinemia on insulin uptake into CSF of the rat. Brain Res Bull. 1993;30:571–575. doi:10.1016/0361-9230(93)90084-O [DOI] [PubMed] [Google Scholar]
- 33. Vogt MC, Brüning JC. CNS insulin signaling in the control of energy homeostasis and glucose metabolism—from embryo to old age. Trends Endocrinol Metab. 2013;24:76–84. doi:10.1016/j.tem.2012.11.004 [DOI] [PubMed] [Google Scholar]
- 34. Banks WA, Owen JB, Erickson MA. Insulin in the brain: there and back again. Pharmacol Ther. 2012;136:82–93. doi:10.1016/j.pharmthera.2012.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhao WQ, Chen H, Quon MJ, Alkon DL. Insulin and the insulin receptor in experimental models of learning and memory. Eur J Pharmacol. 2004;490:71–81. doi:10.1016/j.ejphar.2004.02.045 [DOI] [PubMed] [Google Scholar]
- 36. Doré S, Kar S, Rowe W, Quirion R. Distribution and levels of [125I]IGF-I, [125I]IGF-II and [125I]insulin receptor binding sites in the hippocampus of aged memory-unimpaired and -impaired rats. Neuroscience. 1997;80:1033–1040. doi:10.1016/S0306-4522(97)00154-1 [DOI] [PubMed] [Google Scholar]
- 37. Burger C, Lopez MC, Baker HV, Mandel RJ, Muzyczka N. Genome-wide analysis of aging and learning-related genes in the hippocampal dentate gyrus. Neurobiol Learn Mem. 2008;89:379–396. doi:10.1016/j.nlm.2007.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Loerch PM, Lu T, Dakin KA, et al. Evolution of the aging brain transcriptome and synaptic regulation. PLoS One. 2008;3:e3329. doi:10.1371/journal.pone.0003329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Verbitsky M, Yonan AL, Malleret G, Kandel ER, Gilliam TC, Pavlidis P. Altered hippocampal transcript profile accompanies an age-related spatial memory deficit in mice. Learn Mem. 2004;11:253–260. doi:10.1101/lm.68204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hargis KE, Blalock EM. Transcriptional signatures of brain aging and Alzheimer’s disease: what are our rodent models telling us? Behav Brain Res. 2017;322(Pt B):311–328. doi:10.1016/j.bbr.2016.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zhang Y, Chen K, Sloan SA, Bennett ML, et al. An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J Neurosci. 2014;34:11929–11947. doi:10.1523/JNEUROSCI.1860-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lindholm A, Jacobsen LV. Clinical pharmacokinetics and pharmacodynamics of insulin aspart. Clin Pharmacokinet. 2001;40:641–659. doi:10.2165/00003088-200140090-00002 [DOI] [PubMed] [Google Scholar]
- 43. Duckworth WC. Insulin degradation: mechanisms, products, and significance. Endocr Rev. 1988;9:319–345. doi:10.1210/edrv-9-3-319 [DOI] [PubMed] [Google Scholar]
- 44. Werther GA, Hogg A, Oldfield BJ, et al. Localization and characterization of insulin receptors in rat brain and pituitary gland using in vitro autoradiography and computerized densitometry. Endocrinology. 1987;121:1562–1570. doi:10.1210/endo-121-4-1562 [DOI] [PubMed] [Google Scholar]
- 45. Fadool DA, Tucker K, Phillips JJ, Simmen JA. Brain insulin receptor causes activity-dependent current suppression in the olfactory bulb through multiple phosphorylation of Kv1.3. J Neurophysiol. 2000;83:2332–2348. doi:10.1152/jn.2000.83.4.2332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Aggleton JP, O’Mara SM, Vann SD, Wright NF, Tsanov M, Erichsen JT. Hippocampal-anterior thalamic pathways for memory: uncovering a network of direct and indirect actions. Eur J Neurosci. 2010;31:2292–2307. doi:10.1111/j.1460-9568.2010.07251.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Belfiore A, Frasca F, Pandini G, Sciacca L, Vigneri R. Insulin receptor isoforms and insulin receptor/insulin-like growth factor receptor hybrids in physiology and disease. Endocr Rev. 2009;30:586–623. doi:10.1210/er.2008-0047 [DOI] [PubMed] [Google Scholar]
- 48. Garwood CJ, Ratcliffe LE, Morgan SV, et al. Insulin and IGF1 signalling pathways in human astrocytes in vitro and in vivo; characterisation, subcellular localisation and modulation of the receptors. Mol Brain. 2015;8:51. doi:10.1186/s13041-015-0138-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ferrario CR, Reagan LP. Insulin-mediated synaptic plasticity in the CNS: anatomical, functional and temporal contexts. Neuropharmacology. 2018;136(Pt B):182–191. doi:10.1016/j.neuropharm.2017.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lioutas VA, Novak V. Intranasal insulin neuroprotection in ischemic stroke. Neural Regen Res. 2016;11:400–401. doi:10.4103/1673-5374.179040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Moasser MM. The oncogene HER2: its signaling and transforming functions and its role in human cancer pathogenesis. Oncogene. 2007;26:6469–6487. doi:10.1038/sj.onc.1210477 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.