Abstract
Objective sleep quality can be measured by electroencephalography (EEG), a non-invasive technique to quantify electrical activity generated by the brain. With EEG, sleep depth is measured by appearance and an increase in slow wave activity (scalp-SWA). EEG slow waves (scalp-SW) are the manifestation of underlying synchronous membrane potential transitions between silent (DOWN) and active (UP) states. This bistable periodic rhythm is defined as slow oscillation (SO). During its “silent state” cortical neurons are hyperpolarized and appear inactive, while during its “active state” cortical neurons are depolarized, fire spikes and exhibit continuous synaptic activity, excitatory and inhibitory. In adults, data from high-density EEG revealed that scalp-SW propagate across the cortical mantle in complex patterns. However, scalp-SW propagation undergoes modifications across development. We present novel data from children, indicating that scalp-SW originate centro-parietally, and emerge more frontally by adolescence. Based on the concept that SO and SW could actively modify neuronal connectivity, we discuss whether they fulfill a key purpose in brain development by actively conveying modifications of the maturing brain.
Keywords: travelling waves, slow waves, slow oscillation, propagation, spatio-temporal, brain connectivity, function of sleep, high-density EEG, neurodevelopment marker, sleep regulation, brain maturation, myelination, white matter, consciousness, local sleep
Slow oscillations (SO) during sleep and their neuronal basis
Electroencephalography (EEG) is a non-invasive technique to measure the electrical activity of the brain. In sleep research, EEG assessments can reliably determine the objective quality and depth of sleep. When sleep deepens, the primary characteristics in the scalp-EEG are slow waves (scalp-SW) in the delta frequency (0.5–4.5 Hz) band, often quantified as slow wave activity (SWA in μV2) [4] (see box). SW and spindles dominate the EEG activity during Non-Rapid Eye Movement sleep (NREM) and particularly slow-wave sleep. In contrast, Rapid Eye Movement (REM) sleep and waking are dominated by low-amplitude, high frequency activity [5, 6].
Slow waves, Slow Oscillations, Delta Waves, Slow Wave Activity.
There is growing inconsistency regarding the terminology of brain activity during deep sleep. Recently, terms have been used interchangeably, which was possibly driven by the growing diversity of assessment methodology, analytical approach and species investigated.
Originally, the following terms were introduced:
Slow Oscillation (SO) (not oscillations) or Slow Rhythm: cycles of cellular activity-<1 Hz as a periodic process (Hz), consisting of an alternation of active and silent states, as measured with intracellular, depth electrodes or intracranial EEG from sleeping cats and humans [2].
Slow Wave (SW): individual negative positive wave in the scalp EEG or local field potential (LFP) deflection lasting several hundred milliseconds. (Incongruously, in human literature individual slow waves composing slow oscillation were called slow oscillations). Repeated slow waves as measured with intracellular or intracranial EEG are basis of slow oscillation.
Delta Waves orSlow Wave Activity (SWA): wave activity in scalp EEG or intracranial EEG (μV2), in the delta frequency 0.5–4.5 Hz, or subsets within this frequency. Sleep EEG power density is often quantified from spectral analysis (fast Fourier transform) in this frequency range in the human scalp EEG
These definitions have recently been challenged due to the evolution of our understanding the following three fundaments:
1). SW in the scalp EEG reflects SO cellular activity.
Specifically, the degree of synchronization among a multitude of cells determines the morphology of the scalp-SW. For example, highly synchronized SO activity among neuron populations is related to scalp-SW with high amplitude and steep slope. With low SO synchronization among neuron populations, scalp-SW demonstrate lower amplitude and flatter slope.
2). SW were discovered to travel across the cortical mantle.
Integration of the first point suggests that high synchronization of SO among neuron populations leads to near-simultaneous occurrence of scalp-SW across the cortex and also broader propagation of scalp-SW. Lower local synchronization is reflected in reduced amplitudes of SWs. In contrast, low long-distance synchronization is reflected in reduced simultaneous occurrence and shorter propagation distances (local, but not global SWs). Further, the simultaneous start of SWs from different scalp locations may appear as overlapping SWs with multiple peaks.
3). Recent data from sleep deprivation experiments provide key knowledge about the local occurrence of low-frequency EEG activity (SWs, theta waves) in the waking state.
These waves arise occasionally and appear as individual waves. They cannot be called SO, but they are rather SWs.
We propose nomenclature as outlined in the following table. We also propose for future studies to specify assessment method and/or behavioral state, i.e., scalp-SW, intracellular-SO, extracellular-SW, waking-SW, REM-SWA, etc.
| Proposed term | Assessment method | Morphology / physiology | Frequency | Unit |
|---|---|---|---|---|
| Slow oscillation (SO) | Intracellular, extracellular unit firing, cortical LFP and cortical EEG | De- and hyperpolarized membrane potential states, cycles of spiking activity, positive and negative field deflections. Each state lasts more than 100 ms (typically above 200 ms). | < 1 Hz | Hz |
| Slow wave (SW) | Intracellular, extracellular unit firing, cortical LFP and scalp EEG | Same as above, but individual wave, number / density / morphology thereof | Single wave | |
| Slow wave activity (SWA) | Intracranial EEG, scalp EEG | Integrated signal, representing multiple overlapping SW | 0.5–4.5 Hz | μV2 |
How neuronal dynamics generate scalp-SW remains a core target for ultimately unraveling the dynamics of sleep. More than two decades ago, the simultaneous assessment of local field potentials (LFP, i.e., assessing extracellular electric potential) with intracellular recordings was investigated in anesthetized cats [7]. A rhythm of periodic bistability was discovered and named slow oscillation (SO, <1Hz) [2]. These experiments demonstrated the hyperpolarization (DOWN states / silent states) of cortical neurons during depth-positive/surface negative components of LFP. In contrast, during the opposite components of LFP (depth-negative/surface positive), cortical neurons were depolarized, revealed rich synaptic activities and fired spikes [7] (UP states / active states). An identical relationship between LFP and intracellular activities were observed during natural slow wave sleep [8–10]; however, during wake and REM sleep, cortical neurons are in persistent active states showing continuous excitatory and inhibitory synaptic activity (Fig. 2) [8, 11–13]. Beside difference in frequency, other changes in neuronal activities recorded during SO or delta oscillations are currently unknown.
Figure 2.
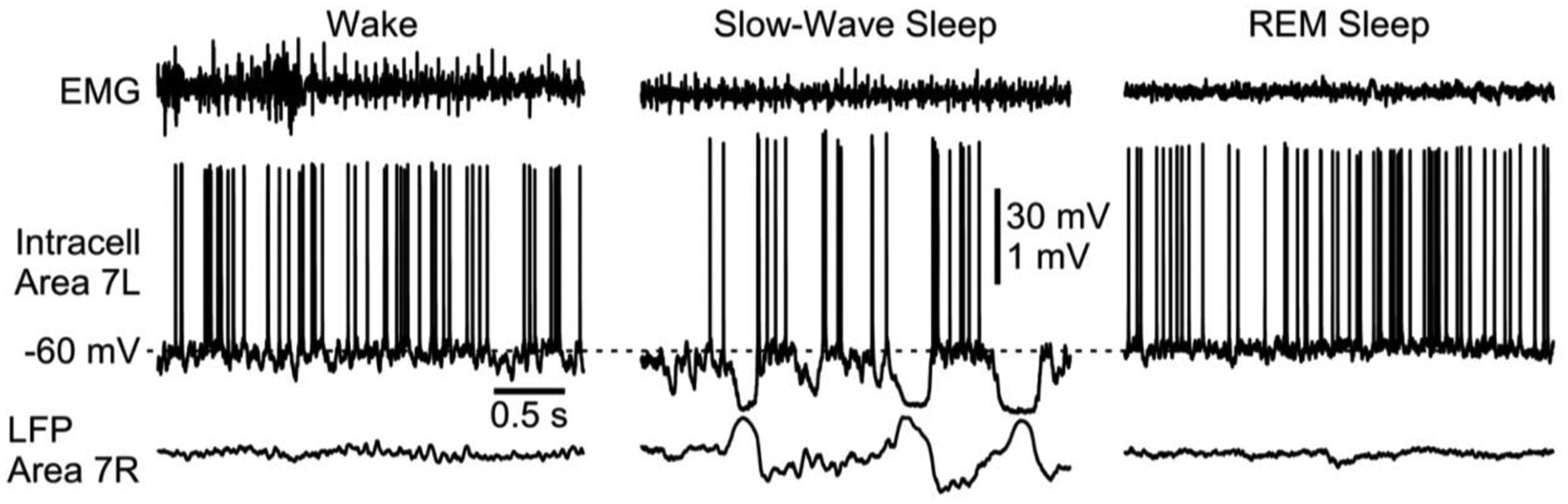
Neuronal and muscle activities in neocortex during wake, slow-wave sleep and rapid-eye movement (REM) sleep. Three segments of simultaneously recorded neck muscle electromyogram (EMG), intracellular activities from area 7 (left hemisphere) cortical neuron, and area 7 (right hemisphere) and extracellular activities from local field potential (LFP) in a cat (modified from [1]).
Origin and regulation of SO
The SO can emerge in isolated neocortical slabs [14, 15], neocortical slices [16, 17] and cortical cell cultures [18, 19], overall signifying that the SO originates in the neocortex. However, functional disconnection of cortex from the thalamus temporarily disrupts cortical-SWA [15, 20], and the SO rhythm is likewise absent in the thalamus of decorticated animals [21]. Further it is interesting that activity in higher-order thalamic nuclei precedes cortical active state onset [22, 23]. Together these observations indicate that cortical-SWA is controlled by subcortical structures.
Typical duration of the silent states of the SO in cats [24] and mice [25] is 100 −200 ms. The onset of these silent states in a subset of neurons is mediated by active inhibition [26]. Thereby, a subset of fast spiking, parvalbumin positive interneurons fires prior, or at the beginning of silent states [27]. Somatostatin interneurons are also active prior to the onset of silent states [28, 29]. The generation of small depth negative components prior to a large depth-positive wave [25] suggests that subsets of cortical neurons can be synchronously activated at the onset of cortical silent state.
Synchronized SO activation and termination
Silent states start almost simultaneously across large cortical territories [23, 30] implying a central coordination of inhibitory control. One likely source of this coordination is the thalamus, as (a) thalamic inactivation disrupts synchrony of active state onsets [26], and (b) occasional firing of thalamocortical cells at the beginning of silent states directly activates parvalbumin interneurons that inhibit cortical activity [27]. Another possible source is the claustrum, which might control synchronous and widespread onset cortical silent states [31].
This inhibitory drive shifts the balance of excitation and inhibition reported for the active states [12, 32] towards inhibition. Thus, some neurons become more polarized, their firing threshold is harder to reach, no action potential is generated and as a chain reaction, the cortical network goes to the silent state. Additional intracortical mechanisms of active state termination may depend on synaptic properties. For instance, long-lasting active states produce short-term synaptic depression in excitatory synapses, consequentially the synaptic drive is unable to bring target cells to the firing threshold so the network goes in to the silent state [1, 14]. This mechanism does not require an external regulator and can be responsible for triggering a local SO.
Differences between humans and animals
The six layers of the neocortex are each characteristically composed of specific neurons that connect with different cortical areas and the thalamus. Animal investigations reveal that cortical active states can start in any layer, but overall most commonly begin in layer 5. Layer 5 cells are the largest of the neocortex and exhibit the most extensive intracortical connectivity, and firing of layer 5 cells involves other cortical layers in ferrets [17], cats [10] and rats [33]. Yet, a divergent picture arises from intracranial recordings in humans, as tested in epileptic patients: current source density and neuronal firing analyses suggest that active states often start from layer 3 [34, 35]. There are several possible explanations for these differences between species: (a) Although data from healthy humans are lacking, the human epileptic brain may consist of reorganized connectivity and relatedly, the possible alteration of spatio-temporal involvement of cells entering the active state; (b) Reconstruction of pyramidal cells from resected cortical tissue suggests that in patients, the layer 5 pyramidal cells are typically smaller compared to pyramidal cells from layers 2–3 in the same patients [36], thus transmembrane currents from layer 2–3 neurons would be stronger; and (c) Layer 5 human pyramidal cells are considerably larger than layer 5 rat pyramidal cells, and due to their size, exhibit larger electrical compartmentalization that causes decreased sensing of dendritic activity at the level of soma [37] providing the possibility for distal dendrites to create strong excitatory currents and LFP signal without major impact at the level of neuronal soma.
Local and global waves
Silent states can be generated locally, if mediated by short-term synaptic depression due to local mechanisms, or globally, with the involvement of the subcortical drive of cortical interneurons. Wave detection within the frequency 0.1–4 Hz in high spatial resolution EEG recordings (high density EEG, 256 channels) from healthy human adults suggests that the majority of detected scalp-SW affect ~20% of all electrodes [38]. Observations at younger ages support this regional restriction, such that a typical scalp-SW in healthy children involves on average ~15% of the electrodes in high-density EEG (128 electrodes), which would be considered a local scalp-SW [39]. In line with this observation, intracranial LFP and multi-unit recordings from epileptic patients demonstrate that the majority (85%) of all cortical-SW are local [40], indicating that the global cortical-SW or scalp-SW only appear in a smaller fraction of occurrences. Additionally, data from intracellular recordings of anesthetized cats (suprasylvian gyrus, recorded at 12–15 mm distance, but receiving similar thalamic inputs) reveal that 80% of silent states coincide in time. In multi-site intracellular recordings (performed within 0.5 mm) almost all silent states occur simultaneously [10]. Considering these results in the context of a role of long-range projections of LP thalamic nucleus, we can conclude that the vast majority of sleep SO and SW are local also in animals [15, 24, 41].
Results from intracellular recordings in sleeping cats indicated the absence of neuronal firing during silent states, because neurons were hyperpolarized [8–10, 13]. However, LFP and multi-unit recordings from the same electrodes show a strong reduction, but no complete absence of spikes during cortical-SW in humans [34, 35, 40], rats [42] and mice [43]. This suggests that in local cortical constellations, not all cells enter into silent states simultaneously. Furthermore, although it is commonly assumed that the cortical SO rhythm is absent during wake, sleep deprivation can trigger singular incidents of local cortical SW during wakefulness in rats [44]. During prolonged waking periods, it is likely that local cortical SO occurs as a result of synaptic short-term depression. Yet, it is unclear why the synaptic depression does not induce singular waking-SW during the normal (not extended) waking state. Several studies suggest that increased network activity reduces overall synaptic dynamics, as observed in cortical slices from ferrets and in vivo recordings in cats [45, 46]. A possible explanation is that high cholinergic activity during waking reduces synaptic efficacy and leads to synaptic stabilization, as examined in mice, rats [47], and cats [48].
The onset of active states is triggered when the network is silent, and this condition experiences self-maintenance. Active states typically start in layer 5 cells and rapidly propagate to other cortical layers. Once initiated in one cortical location, the active states propagate across the cortical mantle. Studies in human [40] and mice [23, 49] demonstrated that the individual active states can generally start at any location, but most often they begin in the frontal cortex, confirming the wave propagation activity across the cortical mantle as observed with scalp-SW in adult humans [38].
Sleep SO alters effective cortical connectivity
Because scalp-SW propagate across EEG channels, they are sometimes referred to as “traveling waves” [38]. SW propagation patterns in humans are complex with signal dynamics involving convergence, divergence, and circulation [50]. Similar to SWA that accumulates and dissipates with homeostatic sleep pressure [51, 52], traveling scalp-SW and cortical-SW also undergo across-night dynamics in human adults [40] and children [39]. Dynamics of scalp-SW and cortical-SW are likely influenced by sleep-wake history (sleep homeostasis) and circadian timing and have been linked to neuronal connectivity in human adults [53] and mice [22].
Findings from experimental perturbations using Transcranial Magnetic Stimulation (TMS) have started to shed light on the relationship of SW with cortical connectivity. TMS is an established tool for assessing the interaction between cortical connectivity and consciousness in humans, which evokes EEG responses in targeted brain regions. Because TMS can elicit scalp-SW and cortical-SW in healthy humans and clinically diagnosed patients, investigating the role of natural SW in relation to cortical connectivity and states of consciousness is an important area of ongoing investigation [54].
Connectivity is quantified in manifold ways, e.g. (a) Cortical complexity capturing the interplay of both functionally segregated local areas, as well as their global integration during perception and behavior [55]; (b) Effective connectivity – the ability of a set of neuronal groups to causally affect the firing of other neuronal groups within a system [56]; or (c) Stability of connectivity – the dynamic (spatio-temporal) dimension of functional connectivity (temporal coactivation between brain regions), reflecting the time-varying signal propagation [57]. Elicited scalp-SW led to the discovery that with sleep onset, there is a breakdown in effective connectivity [58]. Interestingly and in contrast, cortical complexity does not greatly change across the wake period [53, 59]. Simultaneous EEG and functional Magnetic Resonance Imaging recordings support this observation, such that effective connectivity differs between sleep and wakefulness [60]. Specifically, in NREM sleep N2, connectivity is instable suggestive of a redistribution of within and across-network information. In contrast, effective connectivity is stable in N3, portraying slow wave sleep as a relatively inactive condition with possibly more local integration [60]. Overall, insights are emerging from data on scalp-SW and cortical-SW dynamics indicating that during the transition to deeper sleep, a breakdown of neuronal connectivity occurs that gradually relates to dissipating consciousness.
Sleep-like events in the waking period
The idea of the clearly separable vigilance states of sleep and wakefulness has recently been challenged by data showing the occurrence of local SW in waking. As mentioned above, in freely behaving rats, after a long period in the awake state, cortical neurons can briefly go “offline” as in sleep – this is reflected locally in one cortical area but not in others [44]. Investigations with scalp EEG in humans support the concept that local sleep-like events can be detected (particularly extended) during waking periods: Local sleep-like events were found to intrude on wakefulness [61]. This investigation quantified the cortical size of local events in children’s waking EEG by means of the number of electrodes involved in theta events. Approximately 6% −15% scalp electrodes were involved in a local sleep-like event for detection windows of 20–100 ms [61]. This study further revealed that theta waves (6–8 Hz) become more widespread in the evening and are associated with slower reaction times – suggesting specifically that theta waves are markers of local sleep in humans. Thus, while local sleep represents neuronal off periods, the synchronization of off periods can enter the spatial domain: OFF periods can propagate across the cortical mantle and become visible as traveling scalp-SW in sleep, or traveling theta waves during extended waking. Although these attempts take place in the time domain of seconds (off-states during waking in rats 50–100 ms [44], and theta waves in children 20–100 ms [61]), the transition from wakefulness to sleep includes modifications in several dimensions: alertness, neuronal connectivity and behavioral performance [53].
Local aspects of sleep yield similar electrographic patterns in mammals, reptiles [62], birds [63], and fish [64] and further revolutionize current understanding of brain states. In other words, it is now recognized that variability exists across cortical regions during wakefulness, NREM sleep and REM sleep states. For example, SW – classically the most typical characteristic of NREM sleep – was recently discovered to also exist in REM sleep of mice and men [65–67]. Thus, local SW also appear in REM sleep [66, 67] and wakefulness.
Although local aspects of sleep have been primarily investigated as cortical manifestations of NREM sleep, research in birds indicates that muscle tone during REM sleep also appears to be regulated at a local level [68]. One investigation demonstrated that REM sleep-related reductions in skeletal muscle tone appear largely restricted to muscles involved in head posture maintenance. Relatedly, it was proposed that muscle atonia and REM sleep are mediated by the brainstem, while SO in NREM sleep is detected in the hyperpallium (primary visual area in ostriches) [68]. Additionally, the findings in birds further imply a prominent role of thalamic input layers in the initiation of propagating SO. Further, SO propagation varies across layers of avian hyperpallium (the primary visual area), such that SO first occur in, then propagate through and outward from thalamic input layers.
The evolution of SW and SO across species unravels from “bottom-up” a reconstruction of the neurophysiology of our primary behavioral states, including specifically the transitions manifested in local sleep-like events. Yet, it is largely unknown to what extent our living context influences local sleep-like events from “top-down”. While the sleep/wake routine of our society largely defines a 24h rhythm, astronauts witness short sunrises several times a day, as the International Space Station orbits earth every 90 min. Even though an “artificial” 24h routine is created, a majority of crew members suffer from low sleep quality. Thus, markers of sleep pressure were examined in astronauts who were on a 6-month space mission using high-density EEG [69]. Using theta frequency, local sleep-like events were detected in the wake period. Interestingly, these events occurred across more widespread cortical areas when humans were in space than on earth (4.06 ± 0.66% and 3.26 ± 0.66% increase of electrodes involved in a sleep like event [69]). Average “globality” varied between 15 to 25% of scalp electrodes. This research also specifically linked wave globalization to the slowing of behavioral reaction time, thereby suggesting a link between cortical synchronization of SO and behavioral function.
Function of propagating SO
SO are universal across species. SO expand from micro to macro scales, and were described as the “default activity” of the cortical network [15], based on their continuing expression in situations of physical or functional disconnection of the cortex (for a comprehensive multispecies review see [70]). It is not clear whether SO activity per se serves a function regarding the neuronal network or behavior; however, the following proposals have been made supporting such a concept.
One notion is that scalp-SW reflect neuronal activity and that their propagation dynamics mirror brain connectivity in developing [71] and adult humans [72]. Accordingly, the non-invasive assessment of scalp-SW provides insight into cortico-cortical interactions, excitability of the cortex and a link to behavioral states. Indeed, intracranial SW propagation has been associated with consciousness (in epilepsy patients) [73]. This linkage is complex and, for instance, includes the possibility that specific long or short-range SW dynamics relating to particular states of vigilance and behavior, as suggested from research in freely behaving rats [74]. Similarly, altered spatio-temporal scalp-SW dynamics negatively affect performance in vigilance tests (humans) [53, 75]. In this context, scalp-SW were suggested to represent a form of “neuronal tiredness”. The concept has thus emerged that scalp-SWA goes beyond the pure reflection of neuronal dynamics and in addition may directly alter the neuronal network and, as a consequence, behavioral activity.
Another consideration is that the propagation of SW modifies neuronal connections. In particular, SW have been linked to memory consolidation processes during sleep (for reviews see [76, 77]. Enhancing SW using electrical stimulation appears to improve hippocampal-dependent memory performance in both humans [78] and rats [79]. A recent study demonstrated that electrical field stimulation induced a transformation of SW dynamics in mice and therefore proposed this method for manipulating memory processes [80]. Mechanistic underpinnings of the SW-memory link may include high-frequency signal propagation from cortex to hippocampus during wakefulness, and low-frequency activity in the opposite direction during slow wave sleep. Recent findings corroborate such a state-dependent turnaround of cortical–hippocampal communication in humans [81]. The pattern of SW-and SO-propagation may determine the synaptic strengths between neurons, as addressed in a thalamocortical network model [82]. Synaptic plasticity, cortical-SW, and phasic hippocampal discharges possibly trigger some form of plasticity during SO that contribute to sleep-dependent memory consolidation [82]. Furthermore, relevant to this context (at least in humans) is the balance between the circadian and homeostatic drive for sleep [83], as well as wave-specificity, such that different observations exist for local or global SW. For instance, using scalp-SW trough traveling profiles in healthy human subjects, only global SW moved anteriorly to posteriorly, in comparison with local and frontal SW [84]. Additionally, global scalp-SW revealed also stronger coupling with fast spindles. Hence, experimental research is needed to uncover which types of scalp-SW are causally relevant for cognition and memory processes, specifically because the exact mechanisms of sleep-dependent synaptic modifications (plasticity) is a matter of debate [1, 85, 86].
A further viewpoint can be taken from an evolutionary perspective. The mammalian cortex is viewed as the zenith of neuronal evolution, yet how its laminar cytoarchitecture mediates complex cognition remains poorly understood. Comparisons of sleep-related neuronal activity with non-mammalian groups lacking laminar cytoarchitecture provide insight into neocortex functioning [63]. The SO also exists in birds, yet interestingly, it propagates in neuronal activity of complex three-dimensional trails [63]. This represents a contrast to the two-dimensional SO propagation observed in mammals with laminar organization of the neocortex. Accordingly, it was proposed that the non-laminar, nuclear neuronal cytoarchiteture in birds may have emerged from computational properties of this specific dimensional geometry. Examinations in Nile crocodiles [87] also suggest that the SO propagation may connect to the evolutionary elaboration of nuclear structures, which may also relate to the advancement of complex cognition.
Building on these concepts, a further discussion emerges suggesting that propagating SO and SW possibly serve an active role in the modification of processes of brain development and brain evolution. To test whether the maturation of synaptic dynamics is related to sleep and wake states, juvenile mice served as a model system for human adolescence development. In line with dynamics in adult animals, cortical spines in 1 month old mice were increased during waking and decreased during sleep [88]. However, only the developing mice revealed an overall net increase in spine density. In addition to this maturation in terms of spines and SO, maturation dynamics also happen on a topographical perspective. In humans, the distribution of scalp-SWA changes considerably during childhood. These changes in the sleep EEG mirror cortical anatomical processes by shifting from predominantly posterior to anterior regions [89]. Furthermore, propagation parameters of scalp-SW change during childhood, as we reported in two recent investigations using high-density sleep EEG in children aged 2–16 years [39, 71]. This study focused on scalp-SW propagation properties including distance proliferated, propagation speed, and cortical involvement (number of channels in which a SW is detected). We found that scalp-SW propagation undergoes age-specific changes that are associated with white matter microstructure (brain myelin) [71]. Specifically, across development, scalp-SW propagation distance increases, which is associated with myelination of the corpus callosum. Propagating speed and cortical involvement also relate to myelination of the superior longitudinal fascicle. Furthermore, across-night dynamics of scalp-SW propagation are specific to age, possibly reflecting heightened plasticity in neuronal networks specific to sensitive developmental periods [39]. In preschool children propagation distance decreases across the night, while this decrease is neither found at school-age nor at adolescence. Interestingly, even though cortical involvement of the propagating scalp-SW appears relatively stable across age, it undergoes a homeostatic decrease across sleep. Our novel data presented here indicate that scalp-SW originate most often in centro-parietal areas in younger children, whereas when youth are approaching adolescence, frontal origins are more frequently observed (Fig. 3).
Figure 3.
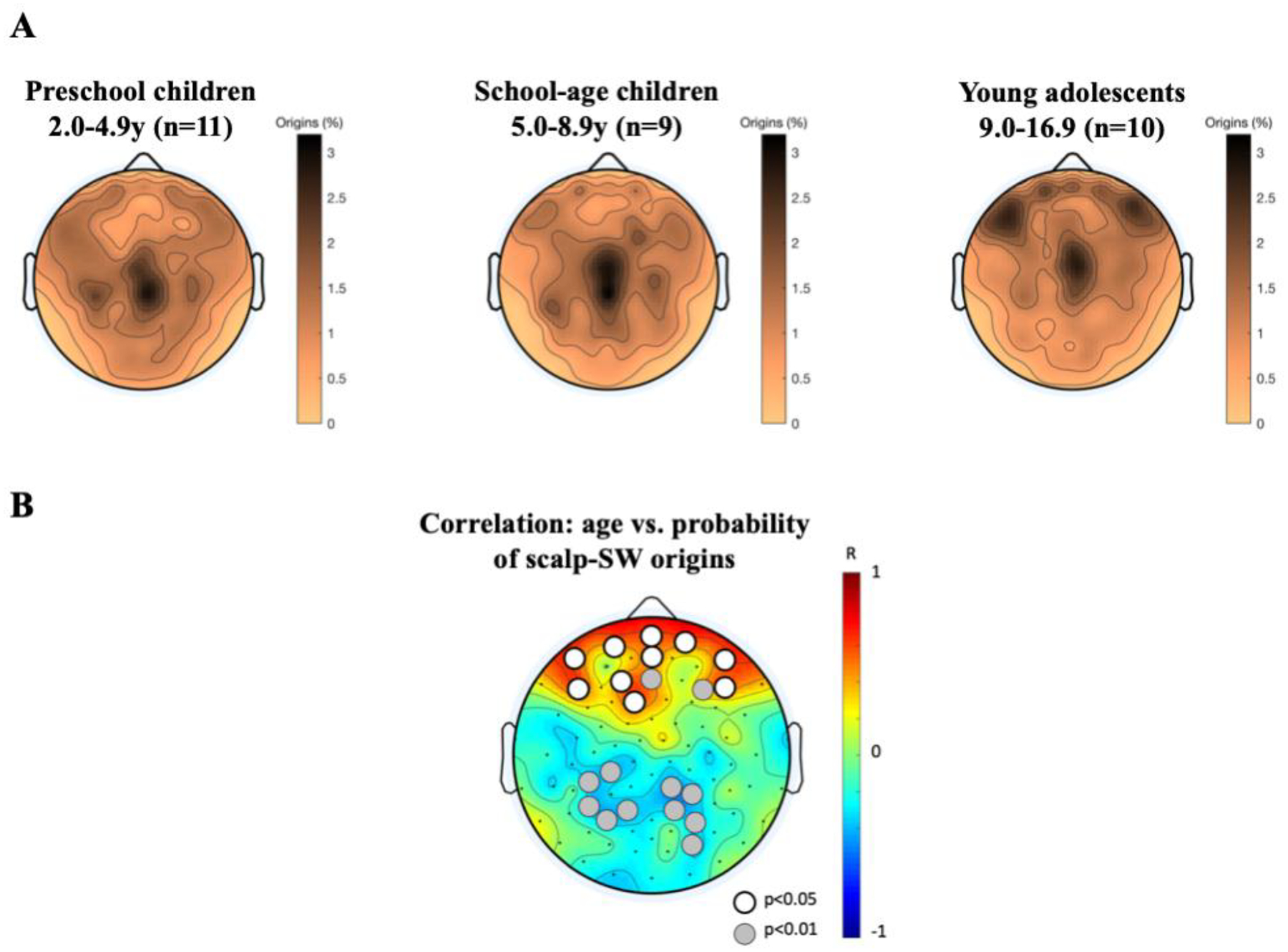
(A) Topographical distribution of probability of origins of scalp-SW in 30 healthy children. All-night high-density EEG at-home assessments were performed in 11 preschool children (2.0 – 4.9 years), 9 school-age children (5.0 – 8.9 years) and 10 adolescents (9.0 – 16.9 years). Dark colors refer to high probability of origins at the indicated electrode, light colors refer to low probability of scalp-SW origins. Data processing entailed band-pass filtering (0.5–40 Hz), rejection of artifact-containing channels and re-referencing to mastoids. A previously published algorithm was used for wave detection and computation of propagation delay, for details see [39, 71]. (B) Linear correlations of age with origin of scalp-SW. Pearson correlations were performed at each electrode. Red indicates significant positive correlation with age, while blue indicates negative correlation (p<0.05). The figure shows that with increasing age across childhood, scalp-SW are more likely to originate in frontal electrodes and less likely to originate in parietal channels.
Our findings address spatio-temporal scalp-SW dynamics and demonstrate the maturation from central towards frontal scalp-SW onset in developing humans. In human adults a frontal predominance of scalp-SW onset is typically observed [38] – a pattern that is highly reproducible across different recording nights, i.e., indicating high stability within participants. From a specific cortical focus location, scalp-SW travel across the cortex [38, 90] in mostly an anterior to-posterior direction, yet with complex pattern variation [50, 91]. The transformation from child to adult SW patterns thus highlights a connection between SW propagation and brain maturation and represents the potential of detecting deviations of developing cortical networks with sleep high-density EEG.
Data from developing rodents come from only a handful of studies, however, to our knowledge, findings in rodents generally align with regional scalp-SW dynamics reported in humans. Similar to humans [38, 40, 72], individual active states in mice most often start in the frontal cortex from which they propagate in anteroposterior/lateral direction over the cortex [23, 49]. Multielectrode arrays reduce the past limitation of poor spatial resolution signal from mice and rats and data from such studies indicate topographical differences in mice, such that faster DOWN-to-UP state transitions, higher firing rate during UP states, and more regular cycles are observed in the prefrontal cortex [49]. Triggering SW with stimulation in adult rats revealed likewise predominantly early cortical-SW propagation from frontal regions, an some isolated SW also originated from posterior areas [42]. Complementary knowledge from reports in mice indicate the subcortical control of cortical-SW: activity in centro-medial neurons in the thalamus precede the UP states in the cingulate cortex [22]. Future studies with nonhuman primates that use novel techniques of imaging [92, 93] or optogenetics [94] will allow to characterize primate SO thoroughly. Furthermore, they will provide direct testing of the functional role of SO and SW, as proposed in the context of propagating waves during waking [76].
It is possible that behavioral correlates of SO and SW maturation exist, for instance those involving the motor or cognitive domains. Accordingly, more frontalized scalp-SW onset may relate to advanced cognitive skills. We may further speculate that spurts of maturation in motor skills may be connected to increased probability of scalp-SW onset in the motor cortex, representative of critical developmental periods. Repeated longitudinal (within-subject) assessments across the time period of months to years when the specific skill maturation occurs are needed to capture such transitions. Furthermore, behavioral tasks known to specifically involve the cortical regions are needed (see [95]), and the involvement of white matter microstructure (myelin) in performance should be considered [71, 96, 97].
Conclusions
In summary, the recent trends in the field of spatio-temporal properties of SO and scalp-SW can be highlighted as follows:
Scalp-SW during sleep reflect the SO rhythm of hyperpolarization and silence of cortical neurons that occur synchronously across cortical regions. SO are controlled by subcortical structures involving the thalamus. While approximately 80% SO and scalp-SW are local, about 20% appear global.
Scalp-SW propagate across the cortical mantle in complex patterns. They originate mostly from the frontal cortex in adults, but from centro-parietal regions in young children.
Propagation patterns undergo across-night dynamics. Neuronal organization underlying SW and SO dynamics is connected to vigilance states and degrees of consciousness.
The function of isolated SW and SO remains unclear but they may (a) purely reflect neuronal activity; (b) modify neuronal connections, affect network connectivity, and maintain cognitive and memory processes; (c) be connected to the evolutionary elaboration of nuclear structures and complex cognition; and (d) actively convey modification processes of the developing brain.
Figure 1.
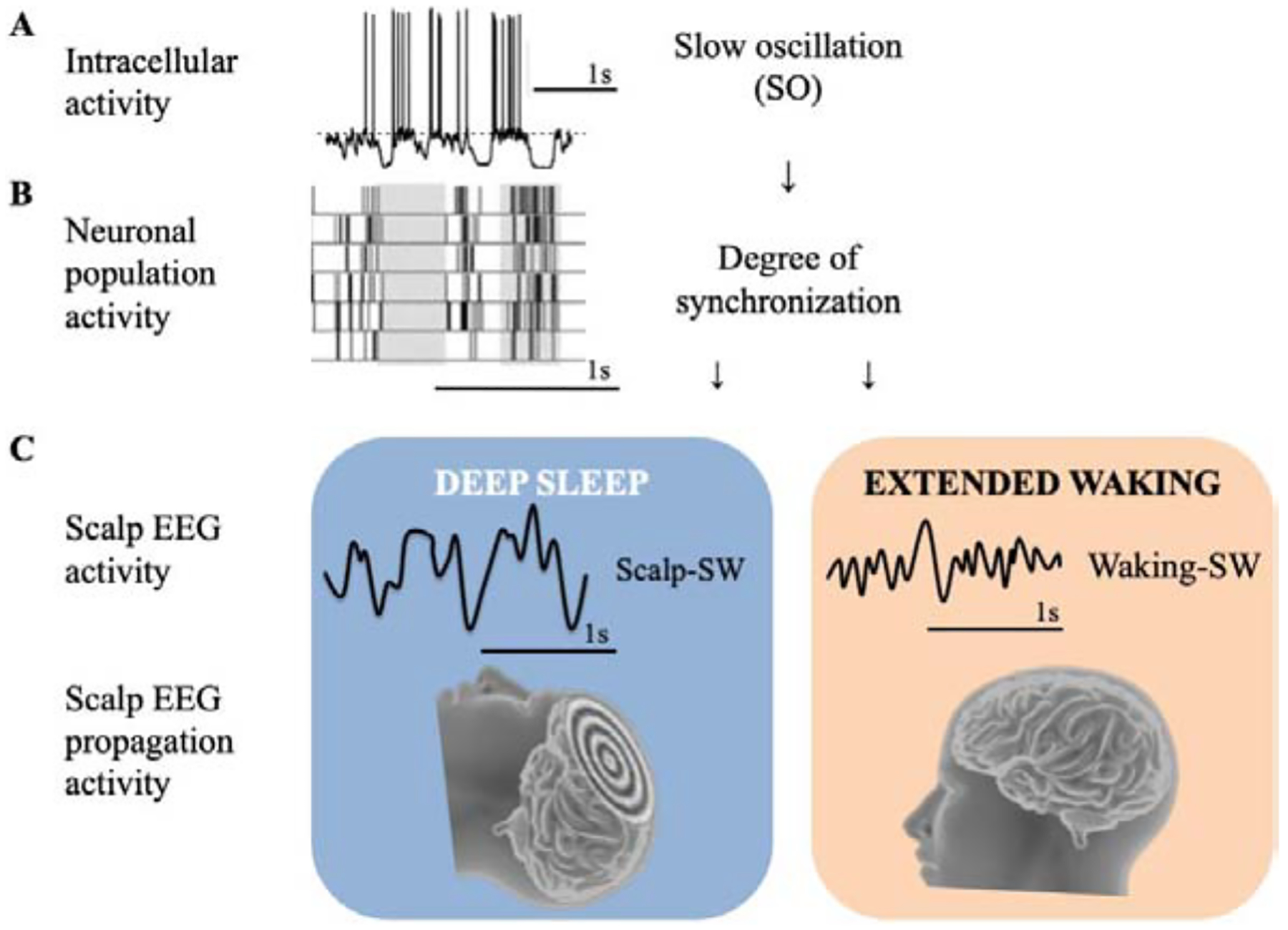
Schematic summary of the different levels of brain activity of sleep (A modified from [1], B modified from [3], C unpublished).
Acknowledgements
This work was supported by the University of Zurich (Clinical Research Priority Program Sleep and Health; Forschungskredit FK-18-047; Medical Faculty; to SK), the Swiss National Science Foundation (PBZHP3-138801, PBZHP3-147180; PCEFP1-181279 to SK; P0ZHP1-178697 to SFS; PP00A-114923 to RH), the National Institutes of Health (MH-086566 to MKL); Canadian Institutes of Health Research (MOP-136969, MOP-136967 to IT), the National Institutes of Health (NS104368 to IT) and National Sciences and Engineering Research Council of Canada (298475 to IT). We thank the anonymous reviewers for their constructive peer review of this work.
Abbreviations
- EEG
Electroencephalography
- LFP
Local Field Potential
- NREM
Non-Rapid Eye Movement
- REM
Rapid Eye Movement
- SO
Slow Oscillation(s)
- SW
Slow Wave(s)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Timofeev I, Chauvette S, Sleep slow oscillation and plasticity, Curr Opin Neurobiol 44 (2017) 116–126. [DOI] [PubMed] [Google Scholar]
- [2].Steriade M, Nunez A, Amzica F, A novel slow (< 1 Hz) oscillation of neocortical neurons in vivo: depolarizing and hyperpolarizing components, J Neurosci 13(8) (1993) 3252–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Vyazovskiy VV, Olcese U, Lazimy YM, Faraguna U, Esser SK, Williams JC, Cirelli C, Tononi G, Cortical firing and sleep homeostasis, Neuron 63(6) (2009) 865–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Borbély AA, Achermann P, Homeostasis of human sleep and models of sleep regulation, in: Kryger MH, Roth T, Dement WC (Eds.), Principles and Practice of Sleep Medicine W. B. Saunders, Philadelphia, 2000, pp. 377–390. [Google Scholar]
- [5].Iber C, Ancoli-Israel S, Chesson A, Quan S, Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications., American Academy of Sleep Medicine, Westchester, 2007. [Google Scholar]
- [6].Blake H, Gerard RW, Brain potentials during sleep, The American Journal of Physiology 119 (1937) 692–703. [Google Scholar]
- [7].Contreras D, Steriade M, Cellular basis of EEG slow rhythms: a study of dynamic corticothalamic relationships, J Neurosci 15(1 Pt 2) (1995) 604–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Steriade M, Timofeev I, Grenier F, Natural waking and sleep states: a view from inside neocortical neurons, J Neurophysiol 85(5) (2001) 1969–85. [DOI] [PubMed] [Google Scholar]
- [9].Timofeev I, Grenier F, Steriade M, Disfacilitation and active inhibition in the neocortex during the natural sleep-wake cycle: an intracellular study, Proc Natl Acad Sci U S A 98(4) (2001) 1924–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Chauvette S, Volgushev M, Timofeev I, Origin of active states in local neocortical networks during slow sleep oscillation, Cereb Cortex 20(11) (2010) 2660–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Mukovski M, Chauvette S, Timofeev I, Volgushev M, Detection of active and silent states in neocortical neurons from the field potential signal during slow-wave sleep, Cereb Cortex 17(2) (2007) 400–14. [DOI] [PubMed] [Google Scholar]
- [12].Rudolph M, Pospischil M, Timofeev I, Destexhe A, Inhibition determines membrane potential dynamics and controls action potential generation in awake and sleeping cat cortex, J Neurosci 27(20) (2007) 5280–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Timofeev I, Grenier F, Steriade M, Impact of intrinsic properties and synaptic factors on the activity of neocortical networks in vivo, J Physiol Paris 94(5–6) (2000) 343–55. [DOI] [PubMed] [Google Scholar]
- [14].Timofeev I, Grenier F, Bazhenov M, Sejnowski TJ, Steriade M, Origin of slow cortical oscillations in deafferented cortical slabs, Cereb Cortex 10(12) (2000) 1185–99. [DOI] [PubMed] [Google Scholar]
- [15].Lemieux M, Chen JY, Lonjers P, Bazhenov M, Timofeev I, The impact of cortical deafferentation on the neocortical slow oscillation, J Neurosci 34(16) (2014) 5689–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Sanchez-Vives MV, Mattia M, Compte A, Perez-Zabalza M, Winograd M, Descalzo VF, Reig R, Inhibitory modulation of cortical up states, J Neurophysiol 104(3) (2010) 1314–24. [DOI] [PubMed] [Google Scholar]
- [17].Sanchez-Vives MV, McCormick DA, Cellular and network mechanisms of rhythmic recurrent activity in neocortex, Nat Neurosci 3(10) (2000) 1027–34. [DOI] [PubMed] [Google Scholar]
- [18].Sun JJ, Kilb W, Luhmann HJ, Self-organization of repetitive spike patterns in developing neuronal networks in vitro, Eur J Neurosci 32(8) (2010) 1289–99. [DOI] [PubMed] [Google Scholar]
- [19].Hinard V, Mikhail C, Pradervand S, Curie T, Houtkooper RH, Auwerx J, Franken P, Tafti M, Key electrophysiological, molecular, and metabolic signatures of sleep and wakefulness revealed in primary cortical cultures, J Neurosci 32(36) (2012) 12506–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].David F, Schmiedt JT, Taylor HL, Orban G, Di Giovanni G, Uebele VN, Renger JJ, Lambert RC, Leresche N, Crunelli V, Essential thalamic contribution to slow waves of natural sleep, J Neurosci 33(50) (2013) 19599–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Timofeev I, Steriade M, Low-frequency rhythms in the thalamus of intact-cortex and decorticated cats, J Neurophysiol 76(6) (1996) 4152–68. [DOI] [PubMed] [Google Scholar]
- [22].Gent TC, Bandarabadi M, Herrera CG, Adamantidis AR, Thalamic dual control of sleep and wakefulness, Nat Neurosci 21(7) (2018) 974–984. [DOI] [PMC free article] [PubMed] [Google Scholar]; Gent, 2018: This study shows a thalamic contribution to cortical UP states in mice, by demonstrating phase-advanced spontaneous firing of centromedial thalamic neurons to global cortical UP states and NREM-wake transition. Optogenetic activation of centromedial thalamic neurons induced NREM-wake transitions, whereas burst activation mimicked UP states in the cingulate cortex and enhanced brain-wide synchrony of cortical slow waves during sleep, through a relay in the antedorsal thalamus. Authors suggest a dual control of sleep-wake states the firing pattern of centromedial thalamic neurons via the modulation of brain-wide cortical activity during sleep.
- [23].Sheroziya M, Timofeev I, Global intracellular slow-wave dynamics of the thalamocortical system, J Neurosci 34(26) (2014) 8875–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Chauvette S, Crochet S, Volgushev M, Timofeev I, Properties of slow oscillation during slow-wave sleep and anesthesia in cats, J Neurosci 31(42) (2011) 14998–5008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Bukhtiyarova O, Soltani S, Chauvette S, Timofeev I, Slow wave detection in sleeping mice: Comparison of traditional and machine learning methods, J Neurosci Methods 316 (2019) 35–45. [DOI] [PubMed] [Google Scholar]
- [26].Lemieux M, Chauvette S, Timofeev I, Neocortical inhibitory activities and long-range afferents contribute to the synchronous onset of silent states of the neocortical slow oscillation, J Neurophysiol 113(3) (2015) 768–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Zucca S, Pasquale V, Lagomarsino P de Leon Roig, S. Panzeri, T. Fellin, Thalamic Drive of Cortical Parvalbumin-Positive Interneurons during Down States in Anesthetized Mice, Curr Biol 29(9) (2019) 1481–1490 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Niethard N, Ngo HV, Ehrlich I, Born J, Cortical circuit activity underlying sleep slow oscillations and spindles, Proc Natl Acad Sci U S A 115(39) (2018) E9220–E9229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Funk CM, Peelman K, Bellesi M, Marshall W, Cirelli C, Tononi G, Role of Somatostatin-Positive Cortical Interneurons in the Generation of Sleep Slow Waves, J Neurosci 37(38) (2017) 9132–9148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Volgushev M, Chauvette S, Mukovski M, Timofeev I, Precise long-range synchronization of activity and silence in neocortical neurons during slow-wave oscillations [corrected], J Neurosci 26(21) (2006) 5665–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Narikiyo K, Mizuguchi R, Ajima A, Mitsui S, Shiozaki M, Hamanaka H, Johansen JP, Mori K, Yoshihara Y, The Claustrum Coordinates Cortical Slow-Wave Activity, PREPRINT, biorxiv 10.1101/286773 (TBD -preprint). [DOI] [PubMed] [Google Scholar]
- [32].Haider B, Duque A, Hasenstaub AR, McCormick DA, Neocortical network activity in vivo is generated through a dynamic balance of excitation and inhibition, J Neurosci 26(17) (2006) 4535–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Fiath R, Kerekes BP, Wittner L, Toth K, Beregszaszi P, Horvath D, Ulbert I, Laminar analysis of the slow wave activity in the somatosensory cortex of anesthetized rats, Eur J Neurosci 44(3) (2016) 1935–51. [DOI] [PubMed] [Google Scholar]
- [34].Cash SS, Halgren E, Dehghani N, Rossetti AO, Thesen T, Wang C, Devinsky O, Kuzniecky R, Doyle W, Madsen JR, Bromfield E, Eross L, Halasz P, Karmos G, Csercsa R, Wittner L, Ulbert I, The human K-complex represents an isolated cortical down-state, Science 324(5930) (2009) 1084–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Csercsa R, Dombovari B, Fabo D, Wittner L, Eross L, Entz L, Solyom A, Rasonyi G, Szucs A, Kelemen A, Jakus R, Juhos V, Grand L, Magony A, Halasz P, Freund TF, Magloczky Z, Cash SS, Papp L, Karmos G, Halgren E, Ulbert I, Laminar analysis of slow wave activity in humans, Brain 133(9) (2010) 2814–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Mohan H, Verhoog MB, Doreswamy KK, Eyal G, Aardse R, Lodder BN, Goriounova NA, Asamoah B, AB BB, Groot C, van der Sluis S, Testa-Silva G, Obermayer J, Boudewijns ZS, Narayanan RT, Baayen JC, Segev I, Mansvelder HD, de Kock CP, Dendritic and Axonal Architecture of Individual Pyramidal Neurons across Layers of Adult Human Neocortex, Cereb Cortex 25(12) (2015) 4839–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Beaulieu-Laroche L, Toloza EHS, van der Goes MS, Lafourcade M, Barnagian D, Williams ZM, Eskandar EN, Frosch MP, Cash SS, Harnett MT, Enhanced Dendritic Compartmentalization in Human Cortical Neurons, Cell 175(3) (2018) 643–651 e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Massimini M, Huber R, Ferrarelli F, Hill S, Tononi G, The sleep slow oscillation as a traveling wave, J Neurosci 24(31) (2004) 6862–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Schoch SF, Riedner BA, Deoni SC, Huber R, LeBourgeois MK, Kurth S, Across-night dynamics in traveling sleep slow waves throughout childhood, Sleep (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]; Schoch, 2018: Results of this study demonstrate that brain connectivity undergoes across-night dynamics specific to maturational periods. Using high-density EEG during sleep in healthy children, this study investigates age-related propagation of slow oscillations from preschool-age to young adolescence. Slow oscillation propagation distance decreased across a night of sleep, which was dependent on age and most prevalent in preschool children. Regardless of age, cortical involvement decreased across a night of sleep, while no across-night changes were observed in propagation speed.
- [40].Nir Y, Staba RJ, Andrillon T, Vyazovskiy VV, Cirelli C, Fried I, Tononi G, Regional slow waves and spindles in human sleep, Neuron 70(1) (2011) 153–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Avendano C, Rausell E, Reinoso-Suarez F, Thalamic projections to areas 5a and 5b of the parietal cortex in the cat: a retrograde horseradish peroxidase study, J Neurosci 5(6) (1985) 1446–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Vyazovskiy VV, Faraguna U, Cirelli C, Tononi G, Triggering slow waves during NREM sleep in the rat by intracortical electrical stimulation: effects of sleep/wake history and background activity, J Neurophysiol 101(4) (2009) 1921–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Fisher SP, Cui N, McKillop LE, Gemignani J, Bannerman DM, Oliver PL, Peirson SN, Vyazovskiy VV, Stereotypic wheel running decreases cortical activity in mice, Nat Commun 7 (2016) 13138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Vyazovskiy VV, Olcese U, Hanlon EC, Nir Y, Cirelli C, Tononi G, Local sleep in awake rats, Nature 472(7344) (2011) 443–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Reig R, Gallego R, Nowak LG, Sanchez-Vives MV, Impact of cortical network activity on short-term synaptic depression, Cereb Cortex 16(5) (2006) 688–95. [DOI] [PubMed] [Google Scholar]
- [46].Crochet S, Fuentealba P, Cisse Y, Timofeev I, Steriade M, Synaptic plasticity in local cortical network in vivo and its modulation by the level of neuronal activity, Cereb Cortex 16(5) (2006) 618–31. [DOI] [PubMed] [Google Scholar]
- [47].Gil Z, Connors BW, Amitai Y, Differential regulation of neocortical synapses by neuromodulators and activity, Neuron 19(3) (1997) 679–86. [DOI] [PubMed] [Google Scholar]
- [48].Chauvette S, Seigneur J, Timofeev I, Sleep oscillations in the thalamocortical system induce long-term neuronal plasticity, Neuron 75(6) (2012) 1105–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Ruiz-Mejias M, Ciria-Suarez L, Mattia M, Sanchez-Vives MV, Slow and fast rhythms generated in the cerebral cortex of the anesthetized mouse, J Neurophysiol 106(6) (2011) 2910–21. [DOI] [PubMed] [Google Scholar]
- [50].Hangya B, Tihanyi BT, Entz L, Fabo D, Eross L, Wittner L, Jakus R, Varga V, Freund TF, Ulbert I, Complex propagation patterns characterize human cortical activity during slow-wave sleep, J Neurosci 31(24) (2011) 8770–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Borbely AA, A two process model of sleep regulation, Hum Neurobiol 1(3) (1982) 195–204. [PubMed] [Google Scholar]
- [52].Cajochen C, Khalsa SB, Wyatt JK, Czeisler CA, Dijk DJ, EEG and ocular correlates of circadian melatonin phase and human performance decrements during sleep loss, Am J Physiol 277(3 Pt 2) (1999) R640–9. [DOI] [PubMed] [Google Scholar]
- [53].Gaggioni G, Ly JQM, Chellappa SL, Coppieters ‘t Wallant D, Rosanova M, Sarasso S, Luxen A, Salmon E, Middleton B, Massimini M, Schmidt C, Casali A, Phillips C, Vandewalle G, Human fronto-parietal response scattering subserves vigilance at night, Neuroimage 175 (2018) 354–364. [DOI] [PubMed] [Google Scholar]; Gaggioni, 2018: This study uses TMS-evoked EEG responses in waking to demonstrate that during night-time prolonged wakefulness the brain response propagation is decreased within the fronto-parietal cortex, which is associated with increased vigilance failure. This investigation thus indicates that worse/ lower alertness relate to lower effective brain connectivity.
- [54].Massimini M, Ferrarelli F, Esser SK, Riedner BA, Huber R, Murphy M, Peterson MJ, Tononi G, Triggering sleep slow waves by transcranial magnetic stimulation, Proc Natl Acad Sci U S A 104(20) (2007) 8496–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Tononi G, Sporns O, Edelman GM, A measure for brain complexity: relating functional segregation and integration in the nervous system, Proc Natl Acad Sci U S A 91(11) (1994) 5033–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Lee L, Harrison LM, Mechelli A, A report of the functional connectivity workshop, Dusseldorf 2002, Neuroimage 19(2 Pt 1) (2003) 457–65. [DOI] [PubMed] [Google Scholar]
- [57].Tagliazucchi E, van Someren EJW, The large-scale functional connectivity correlates of consciousness and arousal during the healthy and pathological human sleep cycle, Neuroimage 160 (2017) 55–72. [DOI] [PubMed] [Google Scholar]
- [58].Massimini M, Ferrarelli F, Huber R, Esser SK, Singh H, Tononi G, Breakdown of cortical effective connectivity during sleep, Science 309(5744) (2005) 2228–32. [DOI] [PubMed] [Google Scholar]
- [59].Abasolo D, Simons S, Morgado da Silva R, Tononi G, Vyazovskiy VV, Lempel-Ziv complexity of cortical activity during sleep and waking in rats, J Neurophysiol 113(7) (2015) 2742–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Kung YC, Li CW, Chen S, Chen SC, Lo CZ, Lane TJ, Biswal B, Wu CW, Lin CP, Instability of brain connectivity during nonrapid eye movement sleep reflects altered properties of information integration, Hum Brain Mapp 40(11) (2019) 3192–3202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Fattinger S, Kurth S, Ringli M, Jenni OG, Huber R, Theta waves in children’s waking electroencephalogram resemble local aspects of sleep during wakefulness, Sci Rep 7(1) (2017) 11187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Shein-Idelson M, Ondracek JM, Liaw HP, Reiter S, Laurent G, Slow waves, sharp waves, ripples, and REM in sleeping dragons, Science 352(6285) (2016) 590–5. [DOI] [PubMed] [Google Scholar]
- [63].Beckers GJ, van der Meij J, Lesku JA, Rattenborg NC, Plumes of neuronal activity propagate in three dimensions through the nuclear avian brain, BMC Biol 12 (2014) 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Leung LC, Wang GX, Madelaine R, Skariah G, Kawakami K, Deisseroth K, Urban AE, Mourrain P, Neural signatures of sleep in zebrafish, Nature 571(7764) (2019) 198–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Bernardi G, Betta M, Ricciardi E, Pietrini P, Tononi G, Siclari F, Regional Delta Waves In Human Rapid Eye Movement Sleep, J Neurosci 39(14) (2019) 2686–2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Funk CM, Honjoh S, Rodriguez AV, Cirelli C, Tononi G, Local Slow Waves in Superficial Layers of Primary Cortical Areas during REM Sleep, Curr Biol 26(3) (2016) 396–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Soltani S, Chauvette S, Bukhtiyarova O, Lina JM, Dube J, Seigneur J, Carrier J, Timofeev I, Sleep-Wake Cycle in Young and Older Mice, Front Syst Neurosci 13 (2019) 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Rattenborg NC, van der Meij J, Beckers GJL, Lesku JA, Local Aspects of Avian Non-REM and REM Sleep, Front Neurosci 13 (2019) 567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Petit G, Cebolla AM, Fattinger S, Petieau M, Summerer L, Cheron G, Huber R, Local sleep-like events during wakefulness and their relationship to decreased alertness in astronauts on ISS, NPJ Microgravity 5 (2019) 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Sanchez-Vives MV, Massimini M, Mattia M, Shaping the Default Activity Pattern of the Cortical Network, Neuron 94(5) (2017) 993–1001. [DOI] [PubMed] [Google Scholar]; Sanchez-Vives, 2017: This is a review presenting an updated version of how slow oscillations provide information about the underlying healthy or pathological brain network. The theory is discussed on how the “default activity” of slow oscillation is shaped, how it acts as attractor into UP and down states displaying bistable dynamics, and how its exiting this dynamics is necessary for the brain to recover its complexity levels of consciousness.
- [71].Kurth S, Riedner BA, Dean DC, O’Muircheartaigh J, Huber R, Jenni OG, Deoni SCL, LeBourgeois MK, Traveling Slow Oscillations During Sleep: A Marker of Brain Connectivity in Childhood, Sleep 40(9) (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]; Kurth, 2017: This study is the first to report maturational features of slow oscillation propagation in children using high-density EEG. Associations between slow oscillations and white matter myelin microstructure are examined by using multicomponent Driven Equilibrium Single Pulse Observation of T1 and T2-magnetic resonance imaging (mcDESPOT-MRI). Results show that primarily slow oscillation propagation distance increases across age. The myelination extent of the superior longitudinal fascicle, the corpus callosum and whole-brain appear relevant to slow oscillation propagation parameters.
- [72].Murphy M, Riedner BA, Huber R, Massimini M, Ferrarelli F, Tononi G, Source modeling sleep slow waves, Proc Natl Acad Sci U S A 106(5) (2009) 1608–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Schartner MM, Pigorini A, Gibbs SA, Arnulfo G, Sarasso S, Barnett L, Nobili L, Massimini M, Seth AK, Barrett AB, Global and local complexity of intracranial EEG decreases during NREM sleep, Neurosci Conscious 2017(1) (2017) niw022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Meisel C, Klaus A, Vyazovskiy VV, Plenz D, The Interplay between Long and Short-Range Temporal Correlations Shapes Cortex Dynamics across Vigilance States, J Neurosci 37(42) (2017) 10114–10124. [DOI] [PMC free article] [PubMed] [Google Scholar]; Meisel, 2017: This study demonstrates that cortical time scales measured at the individual neuronal level in rats change as a function of vigilance state and time awake. Data are relevant in that they provide mechanistic links among behavioral manifestations of sleep, wake and sleep deprivation and specific measurable changes in network dynamics relevant for information processing. Thereby, sleep was suggested to fulfill a reorganization of the cortex associated with ensuring information integration.
- [75].Fisher SP, Vyazovskiy VV, Local sleep taking care of high-maintenance cortical circuits under sleep restriction, Sleep 37(11) (2014) 1727–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Muller L, Chavane F, Reynolds J, Sejnowski TJ, Cortical travelling waves: mechanisms and computational principles, Nat Rev Neurosci 19(5) (2018) 255–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Molle M, Born J, Slow oscillations orchestrating fast oscillations and memory consolidation, Prog Brain Res 193 (2011) 93–110. [DOI] [PubMed] [Google Scholar]
- [78].Marshall L, Helgadottir H, Molle M, Born J, Boosting slow oscillations during sleep potentiates memory, Nature 444(7119) (2006) 610–3. [DOI] [PubMed] [Google Scholar]
- [79].Binder S, Berg K, Gasca F, Lafon B, Parra LC, Born J, Marshall L, Transcranial slow oscillation stimulation during sleep enhances memory consolidation in rats, Brain Stimul 7(4) (2014) 508–15. [DOI] [PubMed] [Google Scholar]
- [80].Greenberg A, Abadchi JK, Dickson CT, Mohajerani MH, New waves: Rhythmic electrical field stimulation systematically alters spontaneous slow dynamics across mouse neocortex, Neuroimage 174 (2018) 328–339. [DOI] [PubMed] [Google Scholar]; Greenberg, 2016: Using multi-site recordings in anesthetized rats, the authors found that sinusoidal electric field stimulation applied to the frontal cerebral cortex improved cortico hippocampal communication. Moderate-intensity field stimulation entrained slow wave activity in the hippocampus, as well as sharp-wave ripples and increased cortical spindles, whereas the reversed hippocampal-to-cortical communication at slow oscillation and gamma frequencies was enhanced.
- [81].Mitra A, Snyder AZ, Hacker CD, Pahwa M, Tagliazucchi E, Laufs H, Leuthardt EC, Raichle ME, Human cortical-hippocampal dialogue in wake and slow-wave sleep, Proc Natl Acad Sci U S A 113(44) (2016) E6868–E6876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Wei Y, Krishnan GP, Bazhenov M, Synaptic Mechanisms of Memory Consolidation during Sleep Slow Oscillations, J Neurosci 36(15) (2016) 4231–47. [DOI] [PMC free article] [PubMed] [Google Scholar]; Wei, 2016: Here, the authors propose how an interaction between hippocampal input and synaptic plasticity may underlie memory consolidation through preferential replay of cortical spike sequences in slow wave sleep. Effects of spike-timing-dependent synaptic plasticity on synaptic strength were tested in a thalamocortical network model. Spatio-temporal patterns of UP-state propagation appeared to determine a change of synaptic strength between neurons. An external input was applied to mimic hippocampal ripples, which, delivered to the cortical network – seemed to relate to input-specific change of synaptic weight, that persisted after removal of stimulation.
- [83].Ly JQM, Gaggioni G, Chellappa SL, Papachilleos S, Brzozowski A, Borsu C, Rosanova M, Sarasso S, Middleton B, Luxen A, Archer SN, Phillips C, Dijk DJ, Maquet P, Massimini M, Vandewalle G, Circadian regulation of human cortical excitability, Nat Commun 7 (2016) 11828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Malerba P, Whitehurst LN, Simons SB, Mednick SC, Spatio-temporal structure of sleep slow oscillations on the electrode manifold and its relation to spindles, Sleep 42(1) (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]; Malerba, 2019: This study suggests that slow oscillations that occur globally have traveling profiles and that primarily global slow oscillations are coupled with fast spindles.
- [85].Seibt J, Frank MG, Primed to Sleep: The Dynamics of Synaptic Plasticity Across Brain States, Front Syst Neurosci 13 (2019) 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Tononi G, Cirelli C, Sleep and the price of plasticity: from synaptic and cellular homeostasis to memory consolidation and integration, Neuron 81(1) (2014) 12–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Tisdale RK, Lesku JA, Beckers GJL, Rattenborg NC, Bird-like propagating brain activity in anesthetized Nile crocodiles, Sleep 41(8) (2018). [DOI] [PubMed] [Google Scholar]
- [88].Maret S, Faraguna U, Nelson AB, Cirelli C, Tononi G, Sleep and waking modulate spine turnover in the adolescent mouse cortex, Nat Neurosci 14(11) (2011) 1418–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Kurth S, Ringli M, Geiger A, LeBourgeois M, Jenni OG, Huber R, Mapping of cortical activity in the first two decades of life: a high-density sleep electroencephalogram study, J Neurosci 30(40) (2010) 13211–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Mohajerani MH, McVea DA, Fingas M, Murphy TH, Mirrored bilateral slow-wave cortical activity within local circuits revealed by fast bihemispheric voltage-sensitive dye imaging in anesthetized and awake mice, J Neurosci 30(10) (2010) 3745–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Mohajerani MH, Chan AW, Mohsenvand M, LeDue J, Liu R, McVea DA, Boyd JD, Wang YT, Reimers M, Murphy TH, Spontaneous cortical activity alternates between motifs defined by regional axonal projections, Nat Neurosci 16(10) (2013) 1426–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Khodagholy D, Gelinas JN, Thesen T, Doyle W, Devinsky O, Malliaras GG, Buzsaki G, NeuroGrid: recording action potentials from the surface of the brain, Nat Neurosci 18(2) (2015) 310–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Chemla S, Muller L, Reynaud A, Takerkart S, Destexhe A, Chavane F, Improving voltage-sensitive dye imaging: with a little help from computational approaches, Neurophotonics 4(3) (2017) 031215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Diester I, Kaufman MT, Mogri M, Pashaie R, Goo W, Yizhar O, Ramakrishnan C, Deisseroth K, Shenoy KV, An optogenetic toolbox designed for primates, Nat Neurosci 14(3) (2011) 387–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Kurth S, Ringli M, Lebourgeois MK, Geiger A, Buchmann A, Jenni OG, Huber R, Mapping the electrophysiological marker of sleep depth reveals skill maturation in children and adolescents, Neuroimage 63(2) (2012) 959–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Kurth S, Dean DC 3rd, Achermann P, O’Muircheartaigh J, Huber R, Deoni SC, LeBourgeois MK, Increased Sleep Depth in Developing Neural Networks: New Insights from Sleep Restriction in Children, Front Hum Neurosci 10 (2016) 456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].LeBourgeois MK, Dean DC, Deoni SCL, Kohler M, Kurth S, A simple sleep EEG marker in childhood predicts brain myelin 3.5 years later, Neuroimage 199 (2019) 342–350. [DOI] [PMC free article] [PubMed] [Google Scholar]


