Abstract
Purpose: Procalcitonin (PCT) is a peptide that is released in response to bacterial infections. The 2016 Infectious Diseases Society of America pneumonia guidelines recommend PCT monitoring to help guide antibiotic discontinuation. Utilization of PCT is well described in the literature; however, there is a paucity of literature regarding pharmacists’ involvement for using PCT in antibiotic interventions. The objective of this study was to investigate the effect of pharmacist-led intervention with PCT-guided antibiotic therapy in critically ill patients with pneumonia. Methods: This was a pre-post study conducted at a 1368-bed community teaching hospital in the United States. A prospective cohort with pharmacist intervention utilizing PCT-algorithm guidance was compared with a retrospective historical cohort with standard therapy. Adult patients admitted to the intensive care unit (ICU) with pneumonia were included. The primary endpoint was duration of antibiotic therapy. Secondary endpoints included 28-day mortality, ICU and hospital length of stay, reinitiation of antibiotic therapy, and the incidence of Clostridium difficile infection. Results: From August 2016 to July 2017, 113 patients were screened in the PCT group and 123 patients in the standard therapy group. Of these, 37 patients were included in the PCT group and 37 patients in the standard therapy group. Baseline characteristics were similar between the 2 groups. The antibiotic duration of therapy was 6.3 days in the PCT group versus 9.7 days in the standard therapy group (P < .001). There were no differences in secondary endpoints between the 2 groups. Conclusion: Clinical pharmacists’ intervention with PCT-guided antibiotic therapy led to a reduction in the duration of antibiotic therapy in critically ill patients with pneumonia without increasing complications.
Keywords: pharmacist, procalcitonin, pneumonia, antibiotic duration of therapy, critically ill
Background
Procalcitonin (PCT)-guided antibiotic therapy has been a topic of interest in the critically ill patients. It is a biomarker that is released in response to bacterial infections via a direct stimulation of cytokines, such as interleukin (IL)-1β, tumor necrosis factor-α, and IL-6, which has a prompt increase within 6 to 12 hours of infection with a daily decrease of around 50% when the infection is controlled.1 PCT has been shown to have better negative predictive value of infection and a better correlation with mortality as an outcome compared with other biomarkers such as leukocyte count and C-reactive protein that have low specificity, delayed response with late peak levels, and altered levels with steroid or other immunosuppressive therapies.2 Furthermore, upregulation of PCT is blocked during a viral infection by cytokine interferon-γ.1 Therefore, PCT can be used as a diagnostic and prognostic marker for bacterial infections, namely, pneumonia.
Studies investigating PCT-guided therapy have provided promising results in lower respiratory tract infections by reducing duration of antibiotics without compromising outcomes.3-5 PCT-guided therapy has also been studied in the intensive care unit (ICU) and provided similar results.6,7,8 Moreover, PCT-guided therapy has cost implications.9 Based on the cost analysis by Schuetz et al,9 PCT-guided therapy can have savings up to $1.6 billion for the whole United States insured population. Despite the clinical and economic advantages, PCT should be used as an adjunct to other clinical indicators along with a full assessment of the patient for diagnosing or discontinuing antibiotic therapy for pneumonia.
Excessive antibiotic use can lead to an increased emergence of resistance and adverse events (eg, nephrotoxicity, Clostridium difficile infection). The 2016 Infectious Diseases Society of America (IDSA) ventilator-associated pneumonia (VAP) and hospital-acquired pneumonia (HAP) guidelines recommend PCT plus clinical criteria to guide antibiotic discontinuation rather than clinical criteria alone.10 In 2017, the US Food and Drug Administration granted approval to use PCT to manage antibiotic treatment for lower respiratory tract infections and sepsis.11 However, in the United States, PCT levels are underutilized along with nonadherence to evidence-based PCT algorithms.12 Prior to the present study, at our institution, PCT-guided therapy was based on the provider’s discretion, as there were no protocols or order sets that encouraged their use. Clinical pharmacists are actively present and working in the ICUs from 0700 to 2100, play a key role in antibiotic stewardship, and assist providers in managing infectious processes such as pneumonia. The objective of this study was to investigate the impact of PCT-guided algorithm along with the assistance of clinical pharmacists on duration of antibiotic therapy in critically ill patients treated for pneumonia.
Methods
Study Design and Patients
This was a pre-post study conducted at a 1368-bed community teaching hospital in the United States and assessed clinical outcomes in critically ill patients treated for pneumonia. The study compared a prospective cohort (February 25-July 12, 2017) to a historical cohort (August 1, 2016-February 24, 2017). The included timeframe of both arms was after the publication of the 2016 IDSA VAP and HAP guidelines to reflect the most up-to-date antibiotic recommendations and duration of therapy.10 The study was approved by institutional review board (IRB no. 1001921-2). The requirement of informed consent was waived due to the observational nature of the study with minimal patient risk.
Patients were included if they were at least 18 years of age with the diagnosis of pneumonia and admitted to the medical ICU or mixed medical and surgical ICU. Pneumonia was the only included infection as preponderance of evidence regarding PCT was in patients being treated for pneumonia. Furthermore, the utilization of PCT was low in our institution and variable per the providers’ discretion; therefore, prior to widescale adoption of the PCT algorithm, we wanted to study it in patients being treated for pneumonia. The definitions of pneumonia were in accordance with the IDSA guidelines.10,13 Diagnosis of community-acquired pneumonia (CAP) was defined as the presence of clinical features, ie, cough, fever (temperature >100.4°F), sputum production, or pleuritic chest pain, and evidence of infiltrates on chest radiograph (or other imaging technique).13 VAP and HAP were defined as pneumonia that occurs with new lung infiltrate plus clinical features such as new-onset fever (temperature >100.4°F), purulent sputum, leukocytosis defined by white blood cells (WBC) >10 000/µL, and decline in oxygenation (O2 saturation <90%), where VAP occurs >48 hours after endotracheal intubation and HAP occurs ≥48 hours after hospital admission.10
Patients were excluded from either cohort if they were pregnant, immunocompromised (ie, HIV infection, presence of neoplasms, and active tuberculosis), diagnosed with cystic fibrosis, diagnosed with pancreatitis, scheduled for surgery or had undergone surgery in the past 14 days, diagnosed with septic shock (defined as fluid resuscitation not sufficient to maintain adequate perfusion, requiring vasopressors to maintain a mean arterial pressure of ≥65 mm Hg, and a serum lactate >2 mmol/L), and were diagnosed with a concomitant bacterial infection in addition to pneumonia at the time of antibiotic initiation. Patients in the historical cohort were also excluded if they had 2 or more consecutive PCT levels ordered within 24 to 48 hours following antibiotic initiation to prevent inclusion of patients who may have had influence of PCT algorithm in their infection management.
Procedures
Prior to the commencement of the study, all stakeholders were educated regarding the research project and protocol. Patients in both arms were screened for study inclusion through screening antibiotic orders initiated for pneumonia. Subsequently, these patients were reviewed for eligibility based on the study’s inclusion and exclusion criteria and included if all eligibility criteria were met. Patients in the prospective arm were screened until 37 consecutive patients were included. Patients in the historical cohort were retrospectively screened and randomly selected utilizing Microsoft Excel® randomization function until 37 patients were included. Data were collected through the institution’s electronic medical records (Cerner Millennium®).
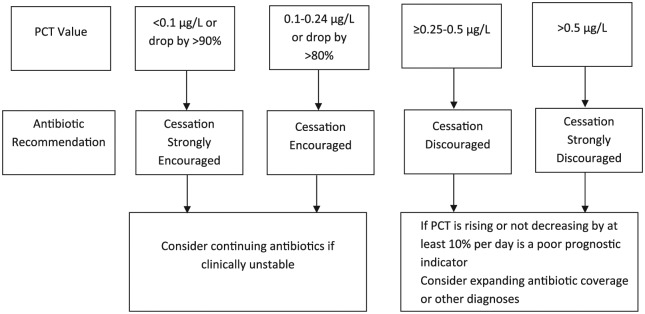
The historical cohort did not undergo any intervention, whereas, in the prospective cohort, clinical pharmacists contacted the prescribing providers and requested an order for a baseline PCT level within 24 hours of initiating antibiotics and a repeat 24- to 48-hour PCT level. Based on the 2 serial PCT levels, clinical pharmacists made antibiotic recommendations as listed in the PCT algorithm illustrated in Figure 1. The PCT algorithm was adopted from previous studies that included critically ill patients.14,15 When making these recommendations, pharmacists had a collaborative discussion with the attending physician and accounted for patients’ overall clinical status and objective data (eg, microbiology results, WBC, bands, fevers, imaging). Duration of antibiotics was in accordance with the current guideline recommendations and per the attending physician.10,13 The choice of antibiotics and final decision with respect to continuing or discontinuing antibiotics was also at the discretion of the attending physician.
Figure 1.
PCT algorithm.
Note. PCT = procalcitonin.
Data Collection
Baseline demographic data collection included age, sex, comorbid conditions (ie, coronary artery disease, congestive heart failure, cerebrovascular accident, chronic kidney disease, chronic obstructive pulmonary disease, and diabetes), signs and symptoms (ie, cough, sputum production, lowest oxygen saturation within 24 hours prior to starting antibiotics), corticosteroid therapy, and antibiotics before admission. Additional data included type of pneumonia (ie, CAP, HAP, VAP), maximum temperature in the last 24 hours prior to initiation of antibiotics, WBC at initiation of antibiotics, respiratory cultures and blood cultures during the duration of antibiotic therapy, types of micro-organism growth, sequential organ failure assessment (SOFA) score within 24 hours of starting antibiotics, Acute Physiologic Assessment and Chronic Health Evaluation III (APACHE III) score within 24 hours of antibiotics initiation, mechanical ventilation during antibiotic therapy, PCT level at baseline and a follow-up 24- to 48-hour level, and number of recommendations made to providers that were accepted or rejected. Clinical pharmacists assessed PCT levels and made recommendation to the attending physician whether to continue or discontinue antibiotics immediately after the receipt of the repeat PCT level. The rate of whether these recommendations were accepted or rejected was collected.
Outcomes
The primary endpoint was duration of antibiotic therapy defined by the number of days from initiation of antibiotic therapy to discontinuation. The duration of antibiotics was counted for the first course of antibiotic therapy (eg, if a patient was restarted on antibiotics later in their hospital stay, then it would not count toward the total duration of therapy). Patients discharged from the hospital on antibiotics had their outpatient prescription checked to ensure adequate documentation of duration of therapy. Secondary endpoints included 28-day all-cause mortality (patients discharged from the hospital prior to day 28 would be considered alive), ICU length of stay (LOS), hospital LOS, reinitiation of antibiotic therapy for the initial infection within 72 hours of antibiotic therapy discontinuation, and the incidence of C difficile infection at 28 days or prior to discharge.
Statistical Analysis
The statistical analysis was performed using SAS version 9.4, and 2-sided P < .05 was considered statistically significant. The Shapiro-Wilk test was used to test for normality. Descriptive statistics (median and interquartile range) were used to describe variables in each group. Mann-Whitney U test was used to compare continuous variables and Pearson χ2 test or Fisher exact test was used to compare categorical variables.
The sample size was calculated based on a 2-day decrease in duration of antibiotic therapy in the PCT group based on previous literature.7,8 With a power of 80% and α of 0.05, 37 patients in each group were needed to detect a 2-day difference in duration of antibiotic therapy (with a standard deviation of 3 days).
Results
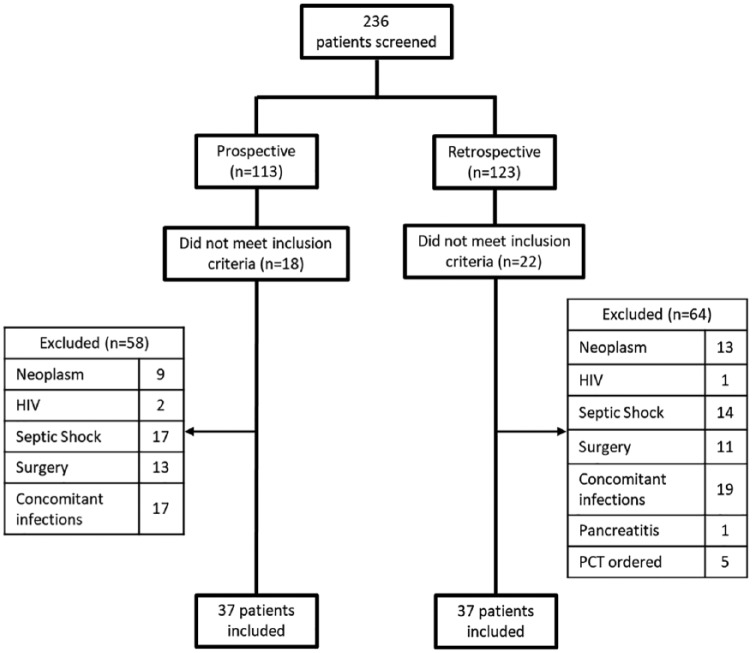
From August 2016 to July 2017, 236 patients were screened in 2 ICUs. Of these, 37 patients were included in the PCT group and 37 patients in the standard therapy group (Figure 2). Baseline characteristics such as age, sex, chronic comorbidities, type of pneumonia, APACHE III score, SOFA score, rates of mechanical ventilation, and positive cultures were similar between groups (Table 1). The majority of patients were diagnosed with CAP (83.8% in the PCT group vs 78.4% in the standard therapy group). Corticosteroids use was similar between the 2 groups (PCT group = 45.9% vs standard therapy group = 51.4%) and the indications were COPD exacerbation and CAP. Blood culture was positive in 1 patient in the PCT group versus 2 patients in the standard therapy group, which was deemed as contaminant and not treated. Fungal growth in the respiratory cultures was considered nonpathogenic in both groups, therefore not treated. The mean baseline PCT in the PCT group was 0.3 µg/L and subsequent 24- to 48-hour PCT level was 0.4 µg/L. Two patients in the historical cohort had single PCT ordered when initiating antibiotics without a repeat level; therefore, it would not have confounded the results in regard to discontinuation of antibiotics. In the prospective arm, 97% of the pharmacist recommendations were accepted.
Figure 2.
Patient inclusion.
Note. PCT = procalcitonin.
Table 1.
Baseline Characteristics of the Patients.
| Variables | Procalcitonin group (n = 37) |
Standard therapy group (n = 37) |
P |
|---|---|---|---|
| Age, y, median (IQR) | 66 (52-76) | 65 (54-79) | .98 |
| Sex, male, n (%) | 19 (51.4) | 22 (59.5) | .48 |
| APACHE III score, median (IQR) | 49 (38-64) | 47 (30-65) | .77 |
| SOFA score, median (IQR) | 3 (2-5) | 3 (0-4) | .09 |
| Chronic comorbidities, n (%) | |||
| CAD | 14 (37.8) | 8 (21.6) | .13 |
| CHF | 13 (35.1) | 9 (24.3) | .31 |
| CVA | 4 (10.8) | 5 (13.5) | .72 |
| CKD | 8 (21.6) | 3 (8.1) | .10 |
| COPD | 11 (29.7) | 13 (35.1) | .62 |
| DM | 20 (54.1) | 16 (43.2) | .35 |
| Patient presentation, n (%) | |||
| Cough | 20 (54.1) | 14 (37.8) | .16 |
| Sputum production | 11 (29.7) | 11 (29.7) | 1.00 |
| Diagnosis, n (%) | |||
| CAP | 31 (83.8) | 29 (78.4) | .55 |
| HAP | 4 (10.8) | 7 (18.9) | .33 |
| VAP | 2 (5.4) | 1 (2.7) | .56 |
| Mechanical ventilation, n (%) | 15 (40.5) | 12 (32.4) | .47 |
| Corticosteroids, n (%) | 17 (45.9) | 19 (51.4) | .64 |
| O2 Saturation, %, median (IQR) | 93 (89-97) | 93 (91-98) | .43 |
| Max temperature, °F, median (IQR) | 99 (98-100) | 99 (99-100) | .02 |
| WBC, 109 cells per L, median (IQR) | 14 (11-17) | 12 (9-17) | .59 |
| Antibiotics prior to admission, n (%) | 0 (0) | 0 (0) | 1.00 |
| Positive sputum culture, n (%) | 6 (16.2) | 6 (16.2) | 1.00 |
| Klebsiella pneumoniae (ESBL−) | 2 | 1 | |
| Escherichia coli (ESBL+) | 1 | 0 | |
| MRSA | 1 | 1 | |
| MSSA | 0 | 2 | |
| Pseudomonas aeruginosa | 1 | 2 | |
| Mucoid Pseudomonas aeruginosa | 0 | 1 | |
| Enterobacter cloacae complex | 0 | 1 | |
| Group B Streptococcus | 0 | 1 | |
| Acinetobacter baumannii | 1 | 0 | |
| Candida albicans | 2 | 1 | |
| Candida tropicalis | 1 | 0 | |
| Aspergillus fumigatus | 0 | 1 | |
| Positive bronchoscopy culture, n (%) | 7 (18.9) | 5 (13.5) | 0.53 |
| Pseudomonas aeruginosa | 2 | 2 | |
| Mucoid Pseudomonas aeruginosa | 0 | 1 | |
| MSSA | 2 | 2 | |
| Klebsiella oxytoca (ESBL−) | 1 | 0 | |
| Klebsiella pneumoniae (ESBL−) | 0 | 2 | |
| Acinetobacter baumannii | 1 | 0 | |
| Candida albicans | 2 | 0 | |
| Candida tropicalis | 1 | 0 | |
| Candida glabrata | 1 | 0 | |
| Positive blood culture, n (%) | 1 (2.7) | 2 (5.4) | .56 |
| Corynebacterium | 1 | 0 | |
| Coagulase-negative Staphylococcus | 0 | 2 | |
| Procalcitonin, µg/L, median (IQR) | |||
| Baseline | 0.34 (0.2-0.7) | — | — |
| 24 to 48 h | 0.38 (0.2-1.4) | — | — |
Note. Results are shown as median with IQR or as absolute number with the percentage of group. IQR = interquartile range; CAD = coronary artery disease; CHF = congestive heart failure; CVA = cerebrovascular accident; CKD = chronic kidney disease; COPD = chronic obstructive pulmonary disease; DM = diabetes mellitus; CAP = community-acquired pneumonia; HAP = hospital-acquired pneumonia; VAP = ventilator-acquired pneumonia; WBC = white blood cells; SOFA = sequential organ failure assessment; APACHE III = Acute Physiology and Chronic Health Evaluation III; ESBL = extended spectrum beta lactamases; MRSA = methicillin-resistant Staphylococcus aureus; MSSA = methicillin-sensitive Staphylococcus aureus.
Outcomes are shown in Table 2. The primary endpoint of antibiotic duration of therapy was shorter in the PCT group with 6.3 days versus 9.7 days in the standard therapy group (absolute difference = 3.4 days, P < .001). Secondary endpoints were similar between PCT group and standard therapy group, respectively: ICU LOS was 3.4 days versus 2.8 days, P = .88; hospital LOS was 8.2 days versus 10.9 days, P = .95; and all-cause 28-day mortality was 0% in both groups, P = 1.00. Antibiotic reinitiation at 72 hours and C difficile infection occurred in one patient in both groups.
Table 2.
Primary and Secondary Outcomes.
| Variables | Procalcitonin group (n = 37) |
Standard therapy group (n = 37) |
P |
|---|---|---|---|
| Duration of therapy, d, median (IQR) | 6.3 (4.4-8.6) | 9.7 (7.2-12) | <.001 |
| All-cause 28-d mortality, n (%) | 0 (0) | 0 (0) | 1.00 |
| ICU LOS, d, median (IQR) | 3.4 (1.4-6.8) | 2.8 (1.8-6.3) | .88 |
| Hospital LOS, d, median (IQR) | 8.2 (6.4-20.9) | 10.9 (6.5-15.3) | .95 |
| Antibiotics reinitiated at 72 h, n (%) | 1 (2.7) | 1 (2.7) | 1.00 |
| Clostridium difficile infection, n (%) | 1 (2.7) | 1 (2.7) | 1.00 |
Note. Results are shown as median with IQR or as absolute number with the percentage of group. IQR = interquartile range; ICU = intensive care unit; LOS = length of stay.
Discussion
This single-centered, pre-post study demonstrated that there was a significant reduction in antibiotic duration of therapy when utilizing a PCT-guided algorithm along with the intervention of clinical pharmacists in critically ill patients with pneumonia. Early discontinuation of therapy did not appear to compromise outcomes (ie, mortality and antibiotics reinitiation), similar to previously published literature.5,7,8,16 Although the secondary endpoints were similar between the 2 groups, our study was not powered to detect a difference in these endpoints.
The use of PCT-based algorithms to guide antibiotic therapy in critically ill patients has been extensively studied.7,8,15 The 2010 Procalcitonin to Reduce Patients’ Exposure to Antibiotics in Intensive Care Units (PRORATA) trial illustrated 2.7 additional antibiotic-free days. However, there was a higher mortality rate in the PCT group (not statistically significant).7 This led to safety concerns regarding utilization of PCT-guided algorithm to guide early antibiotic discontinuation. Subsequently, in 2014, the ProGuard study found no difference in antibiotic-free days when utilizing a lower PCT cut-off.15 To elucidate these concerns, the Stop Antibiotics on guidance of Procalcitonin Study (SAPS) trial was published in 2016, which was the largest study to date analyzing PCT algorithm in critically ill patients with sepsis.8 This study was powered to detect a difference in 28-day mortality and found a lower mortality in the PCT group (20% vs 25%; absolute difference = 5.4%, P = .012) and the difference remained at 1 year (35% vs 41%; absolute difference = 6.1%, P < .016). Furthermore, the PCT group had a significant reduction in antibiotic duration of therapy. These authors speculated that the difference may be due to early recognition of an alternative diagnosis to bacterial infection when the PCT value was low.8 A recent Cochrane review also found a mortality benefit in acute respiratory infections, and additional systematic reviews have illustrated mortality benefit when utilizing PCT-guided therapy to discontinue antibiotics.17-19 Thus, the implications of PCT utilization are limited to not only antibiotic exposure but also clinical outcome benefits. In our study, we found a decrease in antibiotic duration of therapy without improvement in clinical outcomes, which may be due to lack of power or short-term assessment of these endpoints.
Clinical pharmacists are an integral part of the multidisciplinary team at our institution. They participate in daily multidisciplinary rounds, perform order entry verification, participate in teaching and precepting, partake in quality improvement projects, and conduct research. This study evaluated the impact of clinical pharmacists utilizing PCT algorithm in antibiotic stewardship. Critical care pharmacists were actively involved in formulating the evidence based PCT algorithm, implementing its usage in critically ill patients, educating hospital staff the nuances of PCT levels and the algorithm, and reviewing and making recommendations to providers regarding antibiotic plan based on the algorithm plus full collaborative assessment of the patient. The average turnaround time for PCT at our institution was 1 to 2 hours and assessment of these levels was conducted immediately to avoid extension of antibiotic total duration of therapy. The acceptance rate of recommendations from clinical pharmacists was 97%, which is a higher adherence rate utilizing PCT algorithm than previous studies; however, this may also be due to direct intervention by clinical pharmacists.7,8 Several initiatives have emerged from this study. We began educating providers regarding the utilization of PCT with the notion of obtaining serial PCT levels to assess infection progress in the critically ill population. The utilization of PCT began increasing upon the inception of this study and we were in the process of adding PCT to all relevant institutional order sets. Furthermore, we intend to expand this study to include different infectious indications along with additional patient population in other ICU and medical floors and potentially conduct a multicenter study within our system.
There are some notable limitations to this study. This was a pre-post study that compared historical and prospective cohorts. There is a possibility for confounders when comparing cohorts of 2 separate time periods that may have not been controlled in the baseline characteristics. Nevertheless, the baseline characteristics that were analyzed in this study were similar. The possibility of selection bias and Hawthorne effect due to the study design cannot be ruled out. The patients enrolled in our study were less severe than previous studies (ie, exclusion of septic shock, low SOFA and APACHE III score); therefore, the generalizability to critically ill patients with higher severity may be limited. Our definition of immunocompromised status was limited to patients with HIV infection, presence of neoplasms, and active tuberculosis and did not account for other immunocompromised states. Finally, the retrospective chart review nature of this study heavily relied on documentation by the hospital staff.
Conclusion
In conclusion, this pre-post study shows that pharmacist-led PCT-guided antibiotic therapy led to a reduction in the duration of antibiotic therapy in critically ill patients with pneumonia. This reduction did not lead to increased complications such as mortality, reinitiation of antibiotic therapy, and C difficile infection. To our knowledge, this is the largest study evaluating critical care clinical pharmacists’ role in antibiotic stewardship utilizing PCT algorithm to treat critically ill patients with pneumonia. Future prospective studies are needed to validate clinical pharmacists’ role in antibiotic stewardship utilizing PCT in other patient populations.
Footnotes
Authors’ Note: Results of this research were presented at the Florida Residency Pharmacy Conference in May 19, 2017 (Tampa, Florida) and in the 47th Society of Critical Care Medicine Congress in February 27, 2018 (San Antonio, Texas). The research abstract was published in January 2018 Critical Care Medicine journal supplemental. Kristie Zappas is currently affiliated to Accelerate Diagnostics, Phoenix, Arizona.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Bibidh Subedi  https://orcid.org/0000-0002-4376-7696
https://orcid.org/0000-0002-4376-7696
References
- 1. Schuetz P, Amin DN, Greenwald JL. Role of procalcitonin in managing adult patients with respiratory tract infections. Chest. 2012;141(4):1063-1073. doi: 10.1378/chest.11-2430 [DOI] [PubMed] [Google Scholar]
- 2. Schuetz P, Christ-Crain M, Müller B. Biomarkers to improve diagnostic and prognostic accuracy in systemic infections. Curr Opin Crit Care. 2007;13(5):578-585. doi: 10.1097/MCC.0b013e3282c9ac2a [DOI] [PubMed] [Google Scholar]
- 3. Christ-Crain M, Jaccard-Stolz D, Bingisser R, et al. Effect of procalcitonin-guided treatment on antibiotic use and outcome in lower respiratory tract infections: cluster-randomised, single-blinded intervention trial. Lancet. 2004;363(9409):600-607. doi: 10.1016/S0140-6736(04)15591-8 [DOI] [PubMed] [Google Scholar]
- 4. Christ-Crain M, Stolz D, Bingisser R, et al. Procalcitonin guidance of antibiotic therapy in community-acquired pneumonia: a randomized trial. Am J Respir Crit Care Med. 2006;174(1):84-93. doi: 10.1164/rccm.200512-1922OC [DOI] [PubMed] [Google Scholar]
- 5. Schuetz P, Christ-Crain M, Thomann R, et al. Effect of procalcitonin-based guidelines vs standard guidelines on antibiotic use in lower respiratory tract infections: the ProHOSP randomized controlled trial. JAMA. 2009;302(10):1059-1066. doi: 10.1001/jama.2009.1297 [DOI] [PubMed] [Google Scholar]
- 6. Stolz D, Smyrnios N, Eggimann P, et al. Procalcitonin for reduced antibiotic exposure in ventilator-associated pneumonia: a randomised study. Eur Respir J. 2009;34(6):1364-1375. doi: 10.1183/09031936.00053209 [DOI] [PubMed] [Google Scholar]
- 7. Bouadma L, Luyt CE, Tubach F, et al. Use of procalcitonin to reduce patients’ exposure to antibiotics in intensive care units (PRORATA trial): a multicentre randomised controlled trial. Lancet. 2010;375(9713):463-474. doi: 10.1016/S0140-6736(09)61879-1 [DOI] [PubMed] [Google Scholar]
- 8. de Jong E, vanOers JA, Beishuizen A, et al. Efficacy and safety of procalcitonin guidance in reducing the duration of antibiotic treatment in critically ill patients: a randomised, controlled, open-label trial. Lancet Infect Dis. 2016;16(7):819-827. doi: 10.1016/S1473-3099(16)00053-0 [DOI] [PubMed] [Google Scholar]
- 9. Schuetz P, Balk R, Briel M, et al. Economic evaluation of procalcitonin-guided antibiotic therapy in acute respiratory infections: a US health system perspective. Clin Chem Lab Med. 2015;53(4):583-592. doi: 10.1515/cclm-2014-1015 [DOI] [PubMed] [Google Scholar]
- 10. Kalil AC, Metersky ML, Klompas M, et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis. 2016;63(5):e61-e111. doi: 10.1093/cid/ciw353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. FDA clears test to help manage antibiotic treatment for lower respiratory tract infections and sepsis. U.S. Food & Drug Administration; https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm543160.htm. Published February 23, 2017. Accessed February 23, 2017. [Google Scholar]
- 12. Chu DC, Mehta AB, Walkey AJ. Practice patterns and outcomes associated with procalcitonin use in critically ill patients with sepsis. Clin Infect Dis. 2017;64(11):1509-1515. doi: 10.1093/cid/cix179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44(suppl 2):S27-S72. doi: 10.1086/511159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bishop BM, Bon JJ, Trienski TL, Pasquale TR, Martin BR, File TM., Jr. Effect of introducing procalcitonin on antimicrobial therapy duration in patients with sepsis and/or pneumonia in the intensive care unit. Ann Pharmacother. 2014;48(5):577-583. doi: 10.1177/1060028014520957 [DOI] [PubMed] [Google Scholar]
- 15. Shehabi Y, Sterba M, Garrett PM, et al. Procalcitonin algorithm in critically ill adults with undifferentiated infection or suspected sepsis. A randomized controlled trial. Am J Respir Crit Care Med. 2014;190(10):1102-1110. doi: 10.1164/rccm.201408-1483OC [DOI] [PubMed] [Google Scholar]
- 16. Schuetz P, Briel M, Christ-Crain M, et al. Procalcitonin to guide initiation and duration of antibiotic treatment in acute respiratory infections: an individual patient data meta-analysis. Clin Infect Dis. 2012;55(5):651-662. doi: 10.1093/cid/cis464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schuetz P, Wirz Y, Sager R, et al. Procalcitonin to initiate or discontinue antibiotics in acute respiratory tract infections. Cochrane Database Syst Rev. 2017;10:CD007498. doi: 10.1002/14651858.CD007498.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lam SW, Bauer SR, Fowler R, Duggal A. Systematic review and meta-analysis of procalcitonin-guidance versus usual care for antimicrobial management in critically ill patients: focus on subgroups based on antibiotic initiation, cessation, or mixed strategies. Crit Care Med. 2018;46(5):684-690. doi: 10.1097/CCM.0000000000002953 [DOI] [PubMed] [Google Scholar]
- 19. Huang HB, Peng JM, Weng L, Wang CY, Jiang W, Du B. Procalcitonin-guided antibiotic therapy in intensive care unit patients: a systematic review and meta-analysis. Ann Intensive Care. 2017;7(1):114. doi: 10.1186/s13613-017-0338-6 [DOI] [PMC free article] [PubMed] [Google Scholar]