Abstract
Objective: The objective of this review was to systematically review and synthesize evidence regarding benefits of using nonsteroidal anti-inflammatory drugs (NSAIDs) for the treatment of colorectal cancer (CRC). Data Sources: The data sources were MEDLINE, PubMed, NEJM, Google Scholar, and Google searches of references from relevant and eligible trials. Review Methods: We screened abstracts and full-text articles of identified references for eligibility and reviewed randomized controlled trials, cohort studies, and meta-analysis for evidence on benefits of using NSAIDs in CRC treatments. For all extracted data, completeness and relevance were checked. Results: The risk of any adenoma among frequent NSAID users was 26.8% vs 39.9% among placebo subjects who later used NSAIDs sporadically (adjusted relative risk = 0.62, 95% confidence interval [CI] = 0.39-0.98; P trend with NSAID use frequency = .03). Long-term use of aspirin reduces the risk of CRC. Aspirin also reduces the incidence of colon adenomas and mortality, especially when used for >10 years. Rofecoxib is associated with the reduction of CRC; however, it was associated with cardiovascular risk (with an overall unadjusted relative risk of 1.50 [95% CI = 0.76-2.94; P = .24]). Adenoma Prevention with Celecoxib trial shows that, for patients of all genotypes, the estimated cumulative incidence of one or more adenomas by year 3 was 59.8% for those randomized to placebo as compared with 43.3% for those randomized to low-dose (200 mg, twice daily) celecoxib (relative risk [RR] = 0.68; 95% CI = 0.59-0.79; P < .001) and 36.8% for those randomized to high-dose (400 mg, twice daily) celecoxib and 60.7% in placebo group (RR = 0.54; 95% CI = 0.46-0.64; P < .001). Conclusions: The use of COX-2 inhibitors both prior to and after diagnosis of CRC seemed to be mildly associated with the reduction in mortality of patients with CRC. Some literatures state that COX-2 inhibitors might play a synergistic role in adjuvant chemotherapy of FOLFOX regimen. Celecoxib was found to increase the radiosensitization of colon cancer cells.
Keywords: colorectal cancer, NSAID, COX-2 inhibitors
Introduction
Cancer is a disease characterized by the unchecked division and survival of abnormal cells.1 Colorectal cancer (CRC) refers to a slowly developing cancer that begins as a tumor or tissue growth on the inner lining of the rectum or colon.2
Colorectal cancer ranks third, only behind prostate cancer and lung cancer, for new cases in men (8% of all new cancer cases), and behind breast cancer and lung cancer for new cases in women (8% of all new cancer cases).2,3 Incidence and mortality rates have been declining for several decades because of historical changes in risk factors (eg, decreased smoking and red meat consumption and increased use of aspirin), the introduction and dissemination of screening tests, and improvements in treatment (mortality) in highly developed countries,3,4 whereas incidence and mortality rates are still rising rapidly in many low-income and middle-income countries.5 In 2017, there will be an estimated 95 520 new cases of colon cancer and 39 910 cases of rectal cancer diagnosed in the United States. Although the numbers for colon cancer are fairly equal in men (47 700) and women (47 820), a larger number of men (23 720) than women (16 190) will be diagnosed with rectal cancer. An estimated 27 150 men and 23 110 women will die from CRC in 2017.1
Despite increased awareness in the general population regarding CRC, it still remains a major cause of mortality and morbidity worldwide.6 Even though CRC is very well studied along with established diagnostic markers, most of CRC cancers present at late stages of disease and therefore have a poor prognosis.7 In addition, recurrence and metastasis of CRC also carry a very high mortality rate. Therefore, there is a need for improvement in the diagnosis of CRC as well as identification of newer therapeutic targets that can be specifically drugged to improve the management of these cancers.8
An important key survival molecule that is currently being investigated as a molecular marker and a potential therapeutic target is cyclooxygenase-2 (Cox-2) in various cancers. The main function of Cox is to synthesize prostaglandins (PGs) from arachidonic acid (AA). There are 2 isoforms of Cox: Cox-1 that is found to be expressed in normal cells and Cox-2 that is preferentially expressed in cancer cells and its expression is enhanced by pro-inflammatory cytokines and carcinogens.9 COX-1 and COX-2 catalyze the rate-limiting step in the metabolic conversion of AA to PGs. Prostaglandins are involved in a variety of pathologic processes. Specifically, prostaglandin E2 (PGE2) has been shown to mediate the pro-inflammatory and tumor-promoting effects of COX-2 in CRC.10 Cox-2 has been found to be overexpressed in a variety of cancers including breast, ovary, colorectal, thyroid, and lung.11 COX-2 overexpression is seen in precancerous and cancerous lesions in the colon and is associated with decreased colon cancer cell apoptosis and increased production of angiogenesis-promoting factors.12 Up to 50% of colorectal adenomas and 85% of sporadic colon carcinomas have elevated levels of COX-2, and COX-2 overexpression in CRC is associated with a worse survival. COX-2 appears to play a role in polyp formation, and COX-2 inhibition suppresses polyp growth, restores apoptosis, and decreases expression of proangiogenic factors. Inhibition of COX-2 also downregulates the phosphatidylinositol 3-kinase (PI3K) signaling pathway, which plays an important role in carcinogenesis and cancer cell resistance to apoptosis.13 Strong COX-2 expression was associated with high incidence and recurrence of CRC than weak or absent COX-2 expression.14
Aspirin and other nonsteroidal anti-inflammatory drugs (NSAIDs) have been tested in randomized clinical trials and have been successful in reducing the risk of CRC. Unfortunately, by inhibiting COX enzymes, they tend to inhibit physiologically important PGs that could ultimately lead to potentially fatal toxicities that exclude their long-term use for cancer chemoprevention. COX-2 inhibitors and NSAIDs are associated with an increased risk for cardiovascular events, gastrointestinal ulcerations, and bleeding. As for aspirin, even though it has a more favorable cardiovascular profile, hemorrhagic stroke and gastrointestinal bleeding are a concern particularly with prolonged use of the drug.10
Evidence for the anticancer effects of aspirin has emerged from in vitro and animal models, epidemiologic studies, and randomized data, with the most extensive evidence pertaining to CRC. Identifying biomarkers or clinical characteristics which predict benefit from aspirin use could lead to a more targeted intervention and protect some individuals from unnecessary treatment and possible side effects. A number of potential clinical, molecular, and genetic biomarkers have been evaluated including the following: genes mutated in CRC (PIK3CA and BRAF), molecules proposed to have a role in the mechanism through which aspirin exerts its anticancer effects (cyclo-oxygenase [COX] enzymes and human leukocyte antigen (HLA) class I expression), and key genetic polymorphisms that may influence the actions of aspirin.15
COX inhibitory activity of celecoxib and other NSAIDs and their promising chemopreventive activity have led investigators to study COX-independent mechanisms for the purpose of developing derivatives that lack COX inhibitory activity but retain or have improved anticancer activity.16
Although there were a lot of studies done, some of them were associated with side effects that hinder the use and others were associated with nonsignificant results. A study that compares aspirin and placebo stated that no differences between groups in the rate of recurrence (odds ratio [OR] 0.95; 95% confidence interval [CI] = 0.61-1.48), adverse effects, or secondary outcomes.17 Extended follow-up of women in the Women’s Health Initiative (WHI) Dietary Modification (WHI DM) trial did not confirm combined protective effects of aspirin and low-fat diet on CRC risk among the postmenopausal women. In one study, it was found that CRC incidence was not different in the DM from the comparison group among any type of NSAID users. None of the interactions with any category of NSAID use was statistically significant; however, there was most modest evidence for an interaction (P = .07) with aspirin use at baseline (hazard ratio [HR] = 0.81, 95% CI = 0.60-1.11 for users; HR = 1.12, 95% CI = 0.97-1.30 for nonusers). Strength and duration of aspirin use at baseline did not alter the associations.18 Therefore, it is important to review many studies to arrive at net effect of the using NSAIDs in the treatment of CRC.
Methods
Data Source and Search Strategy
A systemic literature search of MEDLINE, PubMed, NEJM, Google Scholar, and Google for identification of articles published between August 2006 and June 2017 was performed. We applied the same search strategy to the all databases using the appropriate controlled vocabulary with restriction on language (using only English language) and without restriction on publication status (published, in press, or in progress). The reference lists of the identified publications were reviewed for identification of additional studies. A total of 94 journal articles were downloaded of which 40 were excluded due to nonrelevance and a year of publication before 10 years.
Selection Criteria
Eligible studies had to meet the following inclusion criteria: (1) they are any type of observational study (case-control study, nested case-control study, randomized clinical trial, meta-analysis, and cohort study) investigating the effect of COX-2 inhibitors/NSAIDs administration on mortality, overall survival, and recurrence in patients with CRC; (2) those that reported the relative risk (RR), OR, or HR and its 95% CI for the association between COX-2 inhibitors/NSAIDs administration on mortality or recurrence in patients with CRC; and (3) if more than one article reported data from the same population, the most recent and complete articles were preferred.
Data Extraction and Quality Assessment
For each eligible study, the following information were extracted: first author’s surname, publication year, study region, study design, sampling subjects, sample size proportion of men, age at baseline, duration of follow-up, assessment of exposure and outcome, corresponding adjusted estimated size (RR, HR, or OR) and 95% CI, and covariates adjusted in the statistical analysis.
Search Terms
The following terms were used to searching, NSAIDs and CRC, aspirin and CRC, celecoxib and CRC, aspirin and CRC mortality, CRC prevention, role of NSAIDs in CRC.
Results and Discussion
Long-term NSAID use is associated with reduced recurrence and mortality from CRC.13,19-21 The risk of any adenoma among frequent NSAID users was 26.8% vs 39.9% among placebo subjects who later used NSAIDs sporadically (adjusted RR = 0.62, 95% CI = 0.39-0.98; P trend with NSAID use frequency = .03).20 The study done by Coghill et al on postmenopausal women showed that women who reported continued use (both baseline and year 3) experienced a significant reduction in CRC mortality (HR = 0.72; 95% CI = 0.54-0.95) compared with all noncontinuous users, including women who either initiated use after baseline or who discontinued their baseline use prior to year 3 (Table 1). Compared with continued NSAID users, women who consistently reported no NSAID use experienced 45% higher rates of CRC mortality (HR = 1.45; 95% CI = 1.08-1.85). Use for >10 years was associated with lower CRC mortality (HR = 0.64; 95% CI = 0.40-1.01) compared with no baseline use. Among the baseline NSAID users, each quartile increase in the duration of use was associated with a 14% reduction in the risk of CRC mortality (HR = 0.86; 95% CI = 0.75-1.00).19 The meta-analysis of 9 studies with 8521 subjects indicated that the RRs of any recurrence of adenoma in patients receiving NSAIDs compared with the placebo group were 0.68 (95% CI = 0.63-0.73, P = .001) for patients with a 1-year follow-up, 0.75 (95% CI = 0.68-0.83, P = .246) with 3 years, and 1.43 (95% CI = 1.14-1.79, P = .127) with follow-up of over 3 years. Using pooled risk ratios, NSAIDs were associated with a significant decrease in adenoma recurrence at 1 and 3 years, although this association was lost beyond 3 years of follow-up. For secondary prevention of advanced adenomas, the pooled risk ratios (compared with placebo) were 0.51 (95% CI = 0.43-0.60, P = .026) after 1 year, 0.61 (95% CI = 0.50-0.76, P = .887) at 3 years, and 1.39 (95% CI = 0.89-2.16, P = .829) after 3 years.22
Table 1.
Hazard Ratios for CRC Mortality and Baseline NSAID Use.19
| Categories | Any NSAID |
Aspirin |
Non-aspirin NSAID |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Total/events | HR | 95% CI | Categories | Total/events | HR | 95% CI | Categories | Total/events | HR | 95% CI | |
| Duration of use in years | |||||||||||
| None | 1 | Reference | None | 1 | Reference | None | 1 | Reference | |||
| <1 y | 12 605/43 | 1.12 | 0.80-1.56 | <1 y | 5163/16 | 0.92 | 0.54-1.57 | <6 mo | 5250/19 | 1.37 | 0.86-2.17 |
| 1 to 3 y | 15 757/57 | 1.06 | 0.78-1.44 | 1 to 3 y | 8738/39 | 1.27 | 0.89-1.81 | 0.5 to 2 | 5811/19 | 1.01 | 0.61-1.67 |
| 3 to 6 y | 13 834/34 | 0.82 | 0.57-1.17 | 3 to 9 y | 9760/27 | 0.87 | 0.58-1.29 | 2 to 5 | 7198/16 | 0.78 | 0.47-1.31 |
| 6+ y | 14 847/39 | 0.75 | 0.52-1.08 | 9+ y | 7944/19 | 0.59 | 0.34-1.00 | 5+ y | 7179/18 | 0.89 | 0.55-1.45 |
| P trend = .12 | |||||||||||
| Strength in milligrams | |||||||||||
| None | None | 1 | Reference | None | |||||||
| <200 mg | 10 466/31 | 0.84 | 0.56-1.25 | <81 mg | 421/1 | — | — | <200 mg | 3296/6 | 0.64 | 0.29-1.44 |
| 200-324.9 mg | 13 719/50 | 1.29 | 0.95-1.75 | 81 mg | 6563/23 | 0.97 | 0.62-1.53 | 200 mg | 10 070/37 | 1.34 | 0.95-1.90 |
| 325 mg | 20 180/66 | 0.95 | 0.71-1.26 | 325 mg | 20 173/66 | 0.96 | 0.72-1.26 | 201-500 mg | 7691/18 | 0.80 | 0.49-1.31 |
| >325 mg | 12 678/26 | 0.61 | 0.39-0.94 | >325 mg | 3134/6 | 0.52 | 0.22-1.27 | >500 mg | 438/11 | 0.78 | 0.40-1.51 |
| P trend = .20 | |||||||||||
Note. CRC = colorectal cancer; NSAID = nonsteroidal anti-inflammatory drugs; HR = hazard ratio; CI = confidence interval.
Nonsteroidal anti-inflammatory drugs are found to decrease postoperative anastomotic leakage. A study that was conducted on 1495 patients (411 randomized to NSAIDs and 1081 to placebo) warranted that the frequency of anastomotic leakage was slightly lower in the patients treated with NSAIDs: 11.4% (47/ 411) compared with 14.4% (156/1084) of the patients not receiving any NSAID. In the univariate logistic regression analysis, the OR for anastomotic leakage in relation to NSAID treatment was 0.77 (95% CI = 0.53-1.12). In the complete case multivariable analysis, this relationship was attenuated (OR 0.83; 95% CI = 0.63-1.05), and even more so after multiple imputation (OR 0.88; 95% CI = 0.65-1.20), indicating no increased risk of anastomotic leakage after NSAID treatment. There was no difference between nonselective and selective NSAIDs. Risk of leakage was (OR 0.91; 95% CI = 0.62-1.35) after treatment with nonselective agents and (OR 0.82; 95% CI = 0.63-1.06) for COX-2 selective agent. Ibuprofen was the most used NSAID, administered to 286 (69.6%) of patients treated with NSAIDs.23
Aspirin and CRC
Long-term use of aspirin reduces the risk of CRC. Aspirin also reduces the incidence of colon adenomas and mortality, especially when used for >10 years.14,19,24,25 This finding was in accordance with the study done by Cao et al. which suggests that the apparent benefit of aspirin use for gastrointestinal tract cancers and CRCs appears with longer duration (>10 years) of use of 0.5 to 1.5 standard aspirin tablets per week compared with people using higher dosages.26 A study done by Ranger also suggests similar finding. It suggests that allocation to aspirin for 5 years or longer reduced risk of right-sided cancer by 70% and also reduced risk of rectal cancer.27 A study done by Chan, Ogino and Fuchs states that statistically significant reduction in the number of cases of COX-2–positive cancer was not evident until aspirin had been used regularly for more than 10 years (multivariate RR = 0.59; 95% CI = 0.42-0.82; P for trend <.001).14 Aspirin has different effects according to the natural history of the polyp: a good chemopreventive effect observed only after long-term use (ie, 7-10 years),24,28 whereas antitumor effect observed within short period (at 1 year).24,29 In the Association Prevention of Colorectal Cancer and Aspirin (APACC), 272 patients with a history of colorectal adenomas were given 160 or 320 mg aspirin daily or placebo. After a follow-up of 4 years, the incidence of at least one adenoma was seen in 30% in the aspirin group vs 41% in the placebo (P = .08) (Table 2). Adenomas of >5 mm diameter were seen in 10% of patients on aspirin compared with 23% of patients in the placebo group (P = .01).30
Table 2.
Effects of Aspirin on Adenoma Recurrence Compared With Placebo in the Four Published Randomized Controlled Trials.
| Study (references) | No. of patients | Daily dose of aspirin (mg) MDTM | Exposure (py) recurrence (R) with placebo | Patients with at least one adenoma | Patients with at least one advanced adenoma | Mean ± SD no. of adenomas/patient |
|---|---|---|---|---|---|---|
| APACC 1 y | 238 | 160/300 1 y |
Asp 38/126 (30%) vs Pla 46/112 (41%) RR 0.73 (0.52 to 1.04) P = .04 |
Asp 8/126 (6%) vs Pla 13/112 (12%) RR 0.55 (0.24-1.27) NS |
Asp 0.45 + 0.15 vs Pla 0.86 ± 0.3 P = .01 |
|
| APACC 4 y | 185 | 160/300 3.4 y |
629 py 207 py |
Asp 65/128(51%) vs Pla 61/115 (53%) RR 0.96 (0.75-1.22) NS |
Asp 18/128 (14%) vs Pla 18/115 (16%) RR 0.90 (0.50-1.64) NS |
Asp 1 ± 1.5 vs Pla 1.5 ± 2.4 P = .07 |
| UKcap30 | 954 | 300 2.6 y |
2218 py 452R |
Asp 99/434 (23%) vs Pla 121/419 (29%) RR 0.79 (0.63-0.99) P = .04 |
Asp 41/434 (9%) vs Pla 63/419 (15%) RR 0.63 (0.43-0.91) P = .013 |
Asp 0.3 ± 0.7 vs Pla 0.5 ± 0.9 P = .015 |
| CAPS28 | 635 | 325 2.6 y |
1344 py 181 R |
Asp 43/259 (17%) vs Pla 70/258 (27%) RR 0.65 (0.46-0.91) P = .004 |
NS | Asp 0.3 ± 0.9vs Pla 0.5 ± 1 P = .003 |
| AFPPS29 | 1121 | 81/325 2.7 y |
2927 py 461 R |
140/366 (38%) (38%) Asp 81 mg vs 160/355 (45%) Asp 325 mg vs 171/363 (47%) Pla RR Asp 81 mg:0.81 (0.69-0.96 P = .04 RR Asp 325 mg:0.96 (0.81-1.13) |
28/366 (8%) Asp 81 mg vs 38/355 (13%) Pla RR Asp 81 mg:0.59 (0.38-0.92) P < .05 |
Not done |
Note. Asp = Aspirin; MDTM = mean duration of trial medication; py, patient years; APACC = Association Prevention of Colorectal Cancer and Aspirin; Pla = placebo; RR = relative risk; AFPPS = Aspirin/Folate Polyp Prevention Study.24
A double-blinded randomized placebo-controlled trial done in Asian patients shows that 63.2% (6/152) patients did not experience colorectal tumor recurrence in the aspirin group as compared with 54% (86/159) patients in the placebo group.29 Individuals who were randomized to 81 mg/d of aspirin treatment were less likely to have a recurrence as compared with those randomized to the placebo arm (P = .017), whereas those who were randomized to 325 mg/d aspirin (P = .86) and folate had no significant effect (P = .40).31 Factors such as smoking and alcohol consumption affects the effect of aspirin. Smoking displays strong effect modification on the main effect of aspirin (P for interaction = .004); that is, the OR for nonsmokers was 0.37 (95% CI = 0.21-0.68), and this value was significantly different from the OR for smokers (OR 3.44, 95% CI = 1.12-10.64) after adjustment for age, sex, and the number of tumors. For alcohol consumption, the OR was 0.72 (95% CI = 0.37-1.40) and 0.44 (95% CI = 0.21-0.95; P = .05) for drinkers and occasional drinkers, respectively.29,31 The retrospective observational study analysis that compares aspirin (100 mg/d) +capecitabine and capecitabine only showed that improved disease control rate, progression-free survival (PFS), and overall survival (OS) compared with those receiving the same treatment but who are not on chronic treatment with aspirin. The result showed that 12 (60%) partial responses were seen in patients on treatment with aspirin, compared with 3 (6%) partial responses in the remaining patients (P = .00007). Sixteen patients on aspirin (80%) obtained disease control vs 14 (30%) patients who were not on aspirin (P = .000377). Median PFS for patients receiving treatment with aspirin was 6.5 months vs 3.3 months for patients who were not on aspirin (HR = 0.48, 95% CI = 0.30-0.79, P = .0042). A significantly improved OS was also evident in aspirin users (median OS, respectively, 14.7 vs 8.7 months; HR = 0.43, 95% CI = 0.26-0.72, P = .0023). No significant differences were observed between aspirin users and patients in the control group in terms of toxicity.32 Among patients with mutated PIK3CA CRCs, regular use of aspirin after diagnosis was associated with superior CRC-specific survival (HR = 0.18; 95% CI = 0.06-0.61; P < .001 by the log-rank test) and OS (HR = 0.54; 95% CI = 0.31-0.94; P = .01 by the log-rank test).13
The study that used data from 2 ongoing prospective studies: the Nurses’ Health Study (NHS), a cohort study of 121 700 US female nurses aged 30 to 55 years at enrollment in 1976, and the Health Professionals Follow-up Study (HPFS), a cohort study of 51 529 US male health care professionals aged 40 to 75 years at enrollment in 1986 which was followed for 32 years, suggests that compared with nonregular use, regular aspirin use was associated with a lower risk for overall cancer (RR = 0.97; 95% CI = 0.94-0.99), which was primarily owing to a lower incidence of gastrointestinal tract cancers (RR = 0.85; 95% CI = 0.80-0.91), especially CRC (RR = 0.81; 95% CI = 0.75-0.88), with similar estimates in women and men. Regular aspirin use was associated with a nonsignificant reduced risk for gastro-esophageal cancer (RR = 0.85; 95% CI = 0.70-1.03). Regular use of aspirin was not associated with the risk for non-gastrointestinal tract cancers (RR = 0.99; 95% CI = 0.97-1.02). Specifically, we did not observe a significant association between aspirin and breast, advanced prostate, or lung cancer. The apparent benefit of aspirin on gastrointestinal tract cancers (including CRC) appeared to be dose dependent, emerging at 0.5 to 1.5 standard aspirin tablets per week or the equivalent of a daily dose of low-dose aspirin, and no departure from linearity was observed (P < .001 for trend). The estimates were similar after applying a 6- to 8-year lag. For CRC, specifically compared with no reported aspirin use, the multivariable RRs were 0.86 (95% CI = 0.76-0.97) for 0.5 to 1.5 standard aspirin tablets per week; 0.84 (95% CI = 0.75-0.93) for 2 to 5 tablets per week; 0.76 (95% CI = 0.68-0.86) for 6 to 14 tablets per week; and 0.61 (95% CI = 0.45-0.81) for at least 15 tablets per week (P < .001 for trend). During the first 5 years of use, we did not observe any significant reduction in the risk for cancer compared with nonregular users. Beyond 5 years, we observed a progressively greater reduction in the risk for gastrointestinal tract cancers and CRCs (P < .001 for trend).26
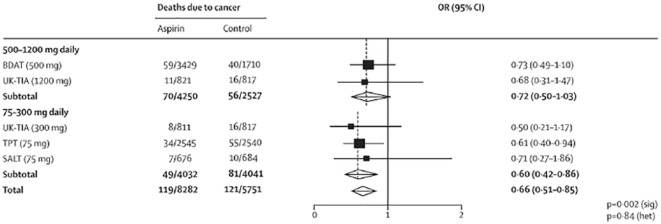
A study that was conducted on women of age 70 and older indicated that there was an indication of an inverse association between all metrics of aspirin use and cancer incidence, although trends were statistically nonsignificant. Compared with aspirin nonusers, the HRs of incident aspirin-sensitive cancer for women who reported using aspirin 6+ times per week, using aspirin for 10+ years, and using a regular to high dose of aspirin were 0.95 (95% CI = 0.80-1.13), 0.93 (95% CI = 0.74-1.17), and 0.87 (95% CI = 0.72-1.06), respectively. Using the combined aspirin usage measures, compared with nonusers, the HRs of incident aspirin-sensitive cancer for women who took 60 000 mg of aspirin per year and 280 000 mg of aspirin in their lifetime were 0.87 (95% CI = 0.70-1.09) and 0.95 (95% CI = 0.75-1.21), respectively. The inverse association was stronger for breast, pancreatic, or ovarian cancer incidence; results were attenuated for colon cancer incidence. Results were similar after stratification by history of heart disease, with no evidence for effect modification (P values ranged from 0.10 to 0.48 for the various aspirin metrics in the multivariable analysis). Using death from any type of cancer as an end point, results were consistent with HRs trending slightly lower than those for incident aspirin-sensitive cancers and a significant inverse association was observed between lifetime aspirin dose and cancer mortality (95 000 mg vs nonuser HR = 0.76; 95% CI = 0.61-0.95).25 A phase III randomized, parallel, 2 × 2 factorial design trial of celecoxib (active vs placebo) crossed with a selenium supplement (active vs placebo) for prevention of metachronous (recurrent) colorectal adenomas done in Arizona, Colorado, Texas, and New York showed adenoma recurrence rate of 49%, a marginal OR of 0.6 due to selenium (a 25% reduction in the recurrence rate), and a marginal OR for low-dose aspirin of 0.8 (a 10% reduction in the recurrence rate), and an interaction OR of 0.5 or less (a combined 33% reduction in the adenoma recurrence rate from selenium + low-dose aspirin).21 Meta-analysis of the effect of allocation to aspirin vs control on long-term risk of death due to CRC showed reductions in mortality on 75 mg daily, 300 mg daily, and 500 to 1200 mg daily (Figure 1). The reduction in risk of CRC in the aspirin vs control groups increased with scheduled treatment duration (P = .04 for mortality; P = .05 for incidence), In a pooled analysis of the 4 trials of aspirin vs control, aspirin at any dose, with a mean duration of scheduled treatment of 5.8 years (range 1.0-8.5), reduced long-term risk of colon cancer, but not rectal cancer.33
Figure 1.
Meta-analysis of effect of aspirin on long-term risk of death due to colorectal cancer in randomized trials of aspirin vs control.33
Note. OR = odds ratio; CI = confidence interval.
The Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial, which focused on the serum level of inflammatory markers and was done on men and women of age 55 to 74 years, showed that regular aspirin use was inversely associated with chemokine C-C motif ligand 15 ([CCL15] OR 0.5; 95% CI = 0.3-0.8; moderate use vs nonregular use), soluble vascular endothelial growth factor receptor 2 ([sVEGFR2] OR 0.7; 95% CI = 0.4-1.0), and soluble tumor necrosis factor receptor 1 ([sTNFR1] OR 0.6; 95% CI = 0.4-0.9) and positively associated with interleukin 4 ([IL-4] OR 1.6; 95% CI = 0.9-2.8), CCL13 (OR 1.3; 95% CI = 0.8-2.1), and CCL17 (OR 1.1; 95% CI = 0.7-1.9).34
Celecoxib and CRC
Celecoxib therapy was associated with decreased number of advanced adenomas (Table 3).35 Adenoma Prevention with Celecoxib (APC) trial shows that for patients of all genotypes, the estimated cumulative incidence of one or more adenomas by year 3 was 59.8% for those randomized to placebo as compared with 43.3% for those randomized to low-dose (200 mg, twice daily) celecoxib (RR = 0.68;95% CI = 0.59-0.79; P < .001) and 36.8% for those randomized to high-dose (400 mg, twice daily) celecoxib and 60.7% in placebo group (RR = 0.54; 95% CI = 0.46-0.64; P < .001). At year 5, among patients who used low-dose aspirin, there appeared to be a greater risk of recurrent adenoma among patients who were previously on low-dose celecoxib (RR = 1.45; 95% CI = 0.89-2.34) and high-dose celecoxib (RR = 1.53; 95% CI = 1.00-2.53).36 A randomized clinical trial that compares celecoxib placebo (CXB + PBO) with celecoxib and difluoromethylornithine (CXB + DFMO) showed that total polyp burden was decreased by an average of 40% (SE 6%) from baseline to 6 months in the CXB + DFMO group and decreased by 27% (SE 6%) in the CXB + PBO group (P = .13).37 According to the study done by Nadir et al, of the 557 subjects in the placebo group and the 840 subjects in the celecoxib group, the cumulative rate of adenomas detected through year 3 was 33.6% in the celecoxib group and 49.3% in the placebo group (RR = 0.64; 95% CI = 0.56-0.75; P < .001). The cumulative rate of advanced adenomas detected through year 3 was 5.3% in the celecoxib group and 10.4% in the placebo group (RR = 0.49; 95% CI = 0.33-0.73; P < .001). Adjudicated serious cardiovascular events occurred in 2.5% of subjects in the celecoxib group and 1.9% of those in the placebo group (RR = 1.30; 95% CI = 0.65-2.62).38 This result was consistent with the study done by Thompson et al which states that in the placebo and celecoxib arms of 356 participants each, adenoma detection was 47.5% and 49.7% (RR = 1.04, 95% CI = 0.90-1.21, P = .58), respectively, after median periods of 13.6 and 14.2 months on intervention. Among participants colonoscoped within 12 months of discontinuing intervention (n = 244), overall adenoma recurrence (RR = 0.69, 95% CI = 0.48-0.98, P = .04) and recurrence with advanced adenomas (RR = 0.23, 95% CI = 0.07-0.80, P = .02) were reduced with celecoxib. Reduction in adenoma recurrence was greatest in participants with previous advanced adenomas. Celecoxib increased risk of hypertension in participants with preexisting cardiovascular risk factors compared with placebo (HR = 2.19, 95% CI = 1.07-4.50, P = .03).21
Table 3.
Use of Celecoxib and Risk of Adenomas.35
| Variable | Placebo (N = 679) |
Celecoxib 200 mg twice daily (N = 685) |
Celecoxib 400 mg twice daily (N = 671) |
|---|---|---|---|
| Detection of any adenoma | |||
| All patients | |||
| Year 1 colonoscopy—no. with adenoma/total no. at risk (%) | 271/608 (44.6) | 186/613 (30.3) | 137/601 (22.8) |
| Year 3 colonoscopy—no. with adenoma/total no. at risk (%) | 83/286 (29.0) | 66/357 (18.5) | 76/400 (19) |
| Cumulative incidence of adenomas detected through year 3 (%) | 60.7 ± 2.1 | 43.2 ± 2.1 | 37.5 ± 2.1 |
| Risk ratio (95% CI) | — | 0.67 (0.59-.77) | 0.55 (0.48-0.64) |
| P value | — | <.001 | <.00 |
| Patients taking aspirin | |||
| Cumulative incidence of adenomas detected through year 3 (%) | 59.9 ± 3.7 | 45.7 ± 3.8 | 38.2 ± 3.8 |
| Risk ratio (95% CI) | — | 0.71 (0.57-0.90) | 0.55 (0.43-0.72) |
| P value | — | .004 | <.001 |
| Patients not taking aspirin | |||
| Cumulative incidence of adenomas detected through year 3 (%) | 60.9 ± 2.5 | 42.1 ± 2.5 | 37.2 ± 2.4 |
| Risk ratio (95% CI) | — | 0.65 (0.55-0.77) | 0.55 (0.46-0.65) |
| P value | — | <.001 | <.001 |
| Sensitivity analysis imputing adenoma for patients without an end point determination | |||
| Cumulative incidence of adenoma detected through year 3 (%) | 70.1 ± 1.8 | 57.5 ± 1.9 | 51.7 ± 1.0 |
| Risk ratio (95% CI) | — | 0.76 (0.69-0.85) | 0.66 (0.59-0.73) |
| P value | — | <0.001 | <0.001 |
| Analysis of patients who adhered to protocol | |||
| Cumulative incidence of adenoma detected through year 3 (%) | 60.7 ± 2.8 | 38.8 ± 2.8 | 35.4 ± 2.7 |
| Risk ratio (95% CI) | — | 0.59 (0.48-0.73) | 0.51 (0.41-0.64) |
| P value | — | <.001 | <.001 |
| Detection of advanced adenomas | |||
| All patients | |||
| Year 1 colonoscopy—no. with adenoma/total no. at risk (%) | 67/608 (11.0) | 26/613 (4.2) | 27/601 (2.8) |
| Year 3 colonoscopy—no. with adenoma/total no. at risk (%) | 32/459 (7.0) | 18/487 (3.7) | 18/503 (3.6) |
| Cumulative incidence of adenomas detected through year 3 (%) | 17.2 ± 1.6 | 7.8 ± 1.1 | 6.3 ± 1.0 |
| Risk ratio (95% CI) | — | 0.43 (0.31-0.61) | 0.34 (0.24-0.50) |
| P value | — | <.001 | <.001 |
Note. CI = confidence interval.
Celecoxib increases the radiosensitization of colon cancer cells.39-41 Pretreatment with 5 μM celecoxib for 4 hours followed by the irradiation (0-6Gy) and 0.25 μM BI-69A11 for 48 hours significantly augmented the radiation-induced cell death in both HCT116 (SER0.05 1.81) and SW480 (SER0.05 2.27) cell lines.39 Celecoxib enhanced the radiosensitivity of HeLa (a human cervical carcinoma cell line), A549 (a human lung carcinoma cell line), and HCT116 cells (a human CRC cell line). Among these cells, COX-2 expression was undetected in HCT116 cells. Treatment with celecoxib significantly increased BCCIP expression in COX-2–negative HCT116 cells. Celecoxib affects the functions of p53 and inhibits the recovery from the irradiation-induced injury by upregulating the expression of BCCIP and subsequently regulates the expressions of genes such as p21 and Cyclin B1 to enhance the radiosensitivity of HCT116 cells in a COX-2–independent manner.40 Doxorubicin and celecoxib induced concentration-dependent reductions in cell viability which reached a maximum of >90% cell death at 3 and 100 µM, respectively.41
One animal study showed that co-administration of celecoxib with DSS (distilled water), DMH, and DMH + DSS significantly reduced expression of PCNA (a marker of cell proliferation). Celecoxib significantly downregulated expression of protein Bcl-2 (an anti-apoptotic protein), whereas it upregulated expression of proteins Bad and Bax, the pro-apoptotic proteins, in mice. Also, the immunofluorescence and immunoblot studies of caspase 3, the critical component of intrinsic apoptotic pathway, expression was significantly elevated with the administration of celecoxib in these groups.42
The exploratory analysis performed using OS and COX-2 expression category stated that among patients censored for OS (9/17 patients), the median OS (80% CI) of patients in the low COX-2 expression group was 17.8 months (15.5 months, not reached [NR]), and the median OS of patients in the high COX-2 expression group was not reached at the time of the analysis (19.4 months, NR). The log-rank test P value for comparison of these 2 groups was .09. The HR and 80% CI for progression among patients in the high COX-2 expression group vs the (reference) low COX-2 expression group were 0.25 (0.08-0.75).43 The Prevention of Colorectal Sporadic Adenomatous Polyps (PreSAP) trial, which randomly assigned 1561 patients with previous history of adenomas to receive 400 mg of celecoxib or placebo, revealed that celecoxib was successful in decreasing the rate of adenomas (33.6% vs 49.6%, P < .001) and the rate of advanced adenomas (5.3% vs 10.4%, P < .001).10 The cohort study done in the Taiwan proposed that after weighting with inverse probability treatment weight (IPTW), patients using COX-2 inhibitors both prior to and after diagnosis had the best 5-year OS (P < .001) at a significant level. Patients who used COX-2 inhibitors both prior to and after diagnosis had a 10% decreased risk of all-cause mortality than patients in the nonuser group (adjusted HR with IPTW = 0.91; 95% CI = 0.86-0.96; P < .001). The subgroup analysis among different cancer sites, patients with colon cancer diagnosis who used COX-2 inhibitors both prior to and after diagnosis had a slightly decreased risk of mortality (adjusted HR with IPTW = 0.93; 95% CI = 0.87-1.00; P = .064). Among those with diagnosis of rectal cancer, patients who used COX-2 inhibitors both prior to and after diagnosis had significantly decreased (14%) risk of mortality (adjusted HR with IPTW = 0.86; 95% CI = 0.79-0.94; P = .001). In addition, there was significant reduction in mortality among patients using COX-2 inhibitors both prior to and after diagnosis who underwent operation (OP) followed by chemoradiation therapy (CRT; adjusted HR with IPTW = 0.57; 95% CI = 0.43-0.77; P < .001). In this study, patients who received FOLFOX regimen using COX-2 inhibitors both prior to and after diagnosis or only after diagnosis had reduced risk of mortality.44
Celecoxib was found with improving depression in the patient with CRC. A randomized, double-blind, placebo-controlled trial that randomly categorizes patients into either celecoxib 400 mg/d or placebo states that celecoxib was associated with improvement in mild to moderate depression. The results showed that, over 6 weeks, patients who received celecoxib showed significant improvement in scores of the Hamilton Depression Rating Scale (HDRS; P = .003). When comparing the mean difference (95% CI) between the 2 groups of therapy, the celecoxib group demonstrated greater reduction in HDRS score during the study period at weeks 4 (1.95, 95% CI = 0.27-3.63, P = .024) and 6 (2.60, 95% CI = 0.96-4.23, P = .003). This study indicated celecoxib as a potential monotherapy treatment strategy for mild to moderate depression in patients with CRC who underwent chemotherapy (CT). The risk of side effects that have been attributed to the administration of celecoxib, especially abdominal pain and diarrhea, was not significantly different between the intervention groups. No symptom of cardiovascular adverse event occurred in the study population, neither clinically nor by electrocardiographic investigation. No one experienced any serious adverse event which would lead to treatment discontinuation.45
Celecoxib increases the NK-92 cell-mediated lysis. The study done by Kim and colleagues suggests that in DELFIA cytotoxicity assay HCT-15 cells were more susceptible to NK cell-mediated lysis compared with HT-29 cells, which were resistant to NK-92 cell-mediated lysis. However, the susceptibility of both cells to NK-92 cell-mediated lysis was increased by treatment with celecoxib. When NKG2D receptor and DR5 were blocked simultaneously, the increased susceptibility of celecoxib-treated cells to NK-92 cell-mediated lysis was suppressed to the level of control cells in HCT-15 cells, but not in HT-29 cells, suggesting that some additional factors may be involved in celecoxib-mediated increase in susceptibility to NK-92 cell-mediated lysis in HT-29 cells. In soft agar colony forming assay, HCT-15 clonogenic cells were also more susceptible to NK cell-mediated lysis compared with HT-29 clonogenic cells. In the presence of celecoxib, the susceptibility of both HCT-15 and HT-29 clonogenic cells to NK-92 cell-mediated lysis was significantly increased.46
Doses of Aspirin and Celecoxib
Regarding the dose of aspirin, some literature advocate low dose. The Aspirin/Folate Polyp Prevention Study (AFPPS) involved 1121 patients randomly assigned to 325 mg aspirin/d or 81 mg aspirin/d or placebo. The incidence of one or more adenoma was 47%, 38%, and 45% in the placebo, 81 mg/d group, and 325 mg/d group, respectively (P = .04).10 Individuals who were randomized to 81 mg/d of aspirin treatment were less likely to have a recurrence compared with those randomized to the placebo arm (P = .017), whereas 325 mg/d aspirin (P = .86) and folate had no significant effect (P = .40). Finally, the mean follow-up time did not differ according to adenoma recurrence status (P = .32).31 Another study suggests that evidence for cancer prophylaxis is based on acetyl salicylic acid (ASA) doses of at least 75 mg/d.47
For celecoxib, high dose was associated with the better outcome. For patients of all genotypes, the estimated cumulative incidence of one or more adenomas by year 3 was 43.3% for those randomized to low-dose (200 mg, twice daily) celecoxib (RR = 0.68; 95% CI = 0.59-0.79; P < .001) and 36.8% for those randomized to high-dose (400 mg, twice daily) celecoxib (RR = 0.54; 95% CI = 0.46-0.64; P < .001; Table 4).36 Study that randomized 83 participants from 2 institutions to 3 arms (ie, high-dose celecoxib, low-dose celecoxib, and placebo) showed that 23 of 30 participants in the high-dose arm had a reduction in their colorectal polyp burden, an average reduction of 28% overall.37
Table 4.
Risk of Adenoma According to Celecoxib Treatment, Stratified by Aspirin Use and UGT1A6.36
| Placebo | Celecoxib 200 mg twice daily | Celecoxib 400 mg twice daily | |
|---|---|---|---|
| All genotypes | |||
| All patients, no. at risk | 493 | 497 | 494 |
| Cumulative incidence, 3 y, % ±SE | 59.8 ± 2.3 | 43.3 ± 2.3 | 36.8 ± 2.3 |
| RR (95% CI) | 1.0 | 0.66 (0.59-0.79) | 0.54 (0.46-0.64) |
| P value | — | <.001 | <.001 |
| Aspirin users, no. at risk | 162 | 160 | 152 |
| Cumulative incidence, 3 y, % ±SE | 61.0 ± 4.0 | 46.0 ± 4.1 | 38.7 ± 4.1 |
| RR (95% CI) | 1.0 | 0.69 (0.54-0.89) | 0.54 (0.41-0.73) |
| P value | — | .004 | <.001 |
| Non-aspirin users, no. at risk | 331 | 337 | 342 |
| Cumulative incidence, 3 y, % ±SE | 59.2 ± 2.8 | 42.0 ± 3.5 | 36+2.7 |
| RR (95% CI) | 1.0 | 0.67 (0.56-0.81) | 0.54 (0.44-0.66) |
| P value | — | <.001 | <.001 |
| Patients with wild-type genotypes | |||
| All wild-type genotypes, no. at risk | 212 | 213 | 221 |
| Cumulative incidence, 3 y, % ±SE | 62.3 ± 3.4 | 42+3.5 | 36.3 ± 3.4 |
| RR (95% CI) | 1.0 | 0.63 (0.50-0.78) | 0.51 (0.40-0.65) |
| P value | — | <.001 | <.001 |
| Aspirin users, no. at risk | 74 | 69 | 69 |
| Cumulative incidence, 3 y, % ±SE | 64.3 ± 3.4 | 44 ± 6.2 | 35.7 ± 6.1 |
| RR (95% CI) | 1.0 | 0.64 (0.44-0.92) | 0.51 (0.33-0.77) |
| P value | — | .013 | <.001 |
| Non-aspirin users no. at risk | 138 | 144 | 152 |
| Cumulative incidence, 3 y,% ±SE | 61.4 ± 4.2 | 41 ± 4.3 | 36.5 ± 4 |
| RR (95% CI) | 1.0 | 0.62 (0.47-0.82) | 0.51 (0.38-0.69) |
| P value | — | <.001 | <.001 |
| Patients with variant genotypes | |||
| All variant genotypes, no. at risk | 281 | 284 | 273 |
| Cumulative incidence, 3 y,% ±SE | 57.9 ± 3.1 | 44.2 ± 3.1 | 37.1 ± 3 |
| RR (95% CI) | 1.0 | 0.72 (0.59-0.88) | 0.57 (0.45-0.71) |
| P value | — | .001 | <.001 |
| Aspirin users, no. at risk | 88 | 91 | 83 |
| Cumulative incidence | 58.4 ± 5.4 | 47.4 ± 5.1 | 40.7 ± 5.6 |
| RR (95% CI) | 1.0 | 0.74 (0.53-1.04) | 0.57 (0.38-0.86) |
| P value | — | .084 | .005 |
| Non-aspirin users, no. at risk | 193 | 193 | 190 |
| Cumulative incidence | 57.6 ± 3.7 | 42.6 ± 3.7 | 35.6 ± 3.6 |
| RR (95% CI) | 1.0 | 0.71 (0.56-0.91) | 0.56 (0.44-0.73) |
| P value | — | .006 | <.001 |
Note. RR = relative risk; CI = confidence interval.
Other NSAIDs
Adenoma recurrence was less frequent for rofecoxib subjects than for those randomized to placebo (41% vs 55%; P < .0001; RR = 0.76; 95% CI = 0.69-0.83). Rofecoxib also conferred a reduction in risk of advanced adenomas (P < .01). The chemopreventive effect was more pronounced in the first year (RR = 0.65; 95% CI = 0.57-0.73) than in the subsequent 2 years (RR = 0.81; 95% CI = 0.71-0.93).48 A study that compared 1167 patients in the rofecoxib group and 1160 patients in the placebo group found that it is associated with the reduction in CRC; however, it was associated with cardiovascular risk (with an overall unadjusted RR of 1.50 [95% CI = 0.76-2.94; P = .24]).49
Dimethyl-celecoxib (DMC) increases apoptosis when combined with imatinib. Imatinib and its combination with DMC significantly increased caspase 3 enzyme activity. The most potent apoptotic effects in combination treatment were observed at a concentration of 3.5 µM imatinib and 12 µM DMC which was statistically significant compared with control. Treatment with imatinib (3.5 µM) and DMC (12 µM) for 24 hours increased apoptosis to 76% vs control (P < .001). The combined treatment showed more apoptosis induction than each of the drugs alone compared with control (50% for 7 µM imatinib and 38% for 24 µM DMC). According to real-time reverse transcription-polymerase chain reaction results, there was an approximately 2-fold and 1-fold increase in caspase-3 mRNA (messenger RNA) as a result of 7 µM imatinib (P = .05) and 24 µM DMC (P < .05) treatment compared with the vehicle-treated control group in HT-29 cells, respectively. However, the combined treatment with 3.5 µM imatinib and 12 µM DMC noticeably increased the level of caspase-3 mRNA in HT-29 cells (3-fold) as compared with the untreated control group (P < .001). These results indicate that imatinib increased caspase 3 mRNA expression more effectively when combined with DMC.50
The PLCO Cancer Screening Trial, which focused on the serum level of inflammatory markers and done on men and women of age 55 to 74 years, showed that regular ibuprofen use was inversely associated with chemokine C-X-C motif ligand 5 ([CXCL5] OR 0.3; 95% CI = 0.1-0.7; moderate use vs nonregular use), CCL24 (OR 0.7; 95% CI = 0.3-1.8), CCL13 (OR 0.5; 95% CI = 0.2-1.2), soluble interleukin 1 receptor II ([sIL-1RII] OR 0.5; 95% CI = 0.2-1.4), CCL17 (OR 0.8; 95% CI = 0.3-2.1), and serum amyloid P ([SAP] OR 0.5; 95% CI = 0.1-1.8) and positively associated with CXCL10 (OR 2.5; 95% CI = 0.9-7.0), CXCL9 (OR 2.0; 95% CI = 0.6-6.5), and CCL15 (OR 2.0; 95% CI = 0.8-5.1).34 The study that used the human colon cancer cell line, Caco-2, to assess the effects of ibuprofen salts on cell death in which celecoxib and the cancer killing drug doxorubicin were used as positive controls showed that doxorubicin and celecoxib induced concentration-dependent reductions in cell viability which reached a maximum of >90% cell death at 3 and 100 µM, respectively. Ibuprofen arginate had relatively weak effects on cell viability with no response seen at concentrations up to 100 µM reflecting its lower potency as a COX-2 inhibitor compared with celecoxib. However, at 1 mM, ibuprofen arginate, but not ibuprofen sodium, caused a small but statistically significant reduction in Caco-2 cell viability. l-arginine at concentrations calculated to be equivalent to those delivered in fully dissociated ibuprofen arginate increased survival at 15 to 150 mM (P < .001) with no effect seen at 0.5 mM(1-way analysis of variance followed by Dunnett test; P > .05).51 A meta-analysis that used 15 randomized controlled trials (12 234 patients) comparing 10 different strategies stated that compared with placebo, non-aspirin NSAIDs were ranked best for preventing advanced metachronous neoplasia (OR 0.37, 95% CI = 0.24-0.53; SUCRA = 0.98; high-quality evidence), followed by low-dose aspirin (OR 0.71, 95% CI = 0.41-1.23; SUCRA = 0.67; low-quality evidence). Low-dose aspirin, however, was ranked the safest among chemoprevention agents (OR 0.78, 95% CI = 0.43-1.38; SUCRA = 0.84), whereas non-aspirin NSAIDs (OR 1.23, 95% CI = 0.95-1.64; SUCRA = 0.26) were ranked low for safety. High-dose aspirin (≥300 mg/d) was comparable with low-dose aspirin (≤160 mg/d) in efficacy (OR 1.12, 95% CI = 0.59-2.10; SUCRA = 0.58) but had an inferior safety profile (SUCRA = 0.51).41
An animal control study was divided into 9 different groups having 5 animals in each group: Group 1: Control Group (animals were kept on normal diet and water), Group 2: Control Group + vehicle treated (1 mM EDTA saline subcutaneously) in a weekly injection and 0.5% carboxymethyl cellulose sodium salt orally daily, Group 3: DMH treated (DMH weekly at a dose of 30 mg/kg subcutaneously), Group 4: DMH + celecoxib 6 mg/kg was co-administered orally daily, Group 5: DMH + etoricoxib 0.6 mg/kg was co-administered orally daily, Group 6: DMH + diclofenac 8 mg/kg was co-administered orally daily, Group 7: celecoxib daily at a dose of 6 mg/kg orally, Group 8: etoricoxib 0.6 mg/kg orally daily, Group 9: diclofenac 8 mg/kg orally daily. This study showed that a number of aberrant crypt foci, a group of atypical tube-like glands in the lining of colon, were minimum in DMH + celecoxib, DMH + etoricoxib, and DMH + diclofenac groups, whereas they were maximum in DMH group. It also suggested that when co-administered with DMH, celecoxib, etoricoxib, and diclofenac were found to regress the COX-2 expression, which was otherwise overexpressed in DMH-treated group.52
Paeonol inhibits human CRC cell proliferation, induces cell apoptosis, and downregulates the expression of COX-2 in CRC cells in a dose-dependent manner. After treating the 3 human CRC cell lines with 30 mg/L paeonol, the viability of cells was assessed by MTT assay from 24 to 72 hours, and the proliferation of these cell lines was significantly inhibited, especially in the LoVo cells. The percentage of apoptotic cells treated with paeonol was significantly higher (increased from 15.4% to 38.6%) compared with that in the control group (P < .01), indicating that paeonol may inhibit the growth of CRC cells by inducing apoptosis. The expression of COX-2 in cells treated with 30 mg/L paeonol was observed to be lower than that in the control group. Treatment with 120 mg/L paeonol led to a further decrease, which indicates a dose-dependent decrease in COX-2 expression.53
Indomethacin was found with protective effect in CRC. Lönnroth and colleagues54 reported that the stem cell master regulator SOX2 was increased by NSAIDs (P < .01), as well as the tumor suppressor miR-630 (P < .01), whereas BMP7, a marker for poor prognosis in CRC, was downregulated by NSAIDs (indomethacin; P < .02).
Conclusions
Long-term use of aspirin is associated with reduction in adenoma recurrence, reduced mortality, and increased disease-free and OS. The benefit is significant in the patients with strong COX-2 expression. Globally, some data seem to suggest that aspirin use may improve the clinical outcome of patients with metastatic CRC receiving CT. Moreover, it has been suggested that a potential synergic activity may exist for concomitant aspirin use and capecitabine-based treatment. Factors such as smoking and alcohol drinking reduce the benefit of aspirin use in the CRC. Even though some literature recommends low-dose aspirin over high-dose aspirin, further study is needed to arrive at a clear conclusion regarding the most beneficial dose of aspirin.
The use of selective COX-2 inhibitors both prior to and after diagnosis of CRC seemed to be mildly associated with the reduction in mortality of patients with CRC. This survival benefit was also shown in patients diagnosed with rectal cancer or those undergoing OP followed by CRT within 180 days after diagnosis. Some literature state that COX-2 inhibitors might play a synergistic role in adjuvant CT of FOLFOX regimen. Celecoxib was found to increase the radiosensitization of colon cancer cells. High-dose celecoxib (400 mg twice a day) was associated with better outcome than low-dose celecoxib (200 mg twice a day).
The use of rofecoxib is associated with reducing the risk of CRC; however, it is associated with cardiovascular risk and it is not recommended to use it for the prevention of CRC. It is currently withdrawn from the market due to its side effects.
Among individuals with previous colorectal neoplasia, non-aspirin NSAIDs are the most effective agents for the prevention of advanced metachronous neoplasia, whereas low-dose aspirin has the most favorable risk:benefit profile. One study states that ibuprofen is associated with reducing inflammatory mediators. Further control study is needed in this area to come up with clear-cut point with the use of NSAIDs in the CRC.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. American Cancer Society (ACS). Colorectal Cancer Screening* (%), in adults 50 years and older. Color Cancer Facts Fig 2017-2019. 2017:1-40. [Google Scholar]
- 2. Marley AR, Nan H. Epidemiology of colorectal cancer. Int J Mol Epidemiol Genet. 2016;7(3):105-114. [PMC free article] [PubMed] [Google Scholar]
- 3. Siegel RL, Miller KD, Fedewa SA, et al. Colorectal Cancer Statistics. CA Cancer J Clin. 2017;67(3):177-193. [DOI] [PubMed] [Google Scholar]
- 4. Longo DL. Colorectal adenomas. N Engl J Med. 2016;374(11):1065-1075. [DOI] [PubMed] [Google Scholar]
- 5. Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut. 2017;66(4):683-691. [DOI] [PubMed] [Google Scholar]
- 6. Ross JS, Torres-Mora J, Wagle N, Jennings TA, Jones DM. Biomarker-based prediction of response to therapy for colorectal cancer: current perspective. Am J Clin Pathol. 2010;134:478-490. [DOI] [PubMed] [Google Scholar]
- 7. Worthley DL, Leggett BA. Colorectal cancer: molecular features and clinical opportunities. Clin Biochem Rev. 2010;31(2):31-38. [PMC free article] [PubMed] [Google Scholar]
- 8. Ahmed M, Hussain AR, Siraj AK, et al. Co-targeting of cyclooxygenase-2 and FoxM1 is a viable strategy in inducing anticancer effects in colorectal cancer cells. Mol Cancer. 2015;14:131. doi: 10.1186/s12943-015-0406-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhu YM, Azahri NSM, Yu DCW, Woll PJ. Effects of COX-2 inhibition on expression of vascular endothelial growth factor and interleukin-8 in lung cancer cells. BMC Cancer. 2008;10:218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Temraz S, Mukherji D, Shamseddine A. Potential targets for colorectal cancer prevention. Int J Mol Sci. 2013;14:17279-17303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Howe LR. Inflammation and breast cancer. Cyclooxygenase/prostaglandin signaling and breast cancer. Breast Cancer Res. 2007;9:210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Thun MJ, Jacobs EJ, Patrono C. The role of aspirin in cancer prevention. Nat Rev Clin Oncol. 2012;9(5):259-267. doi: 10.1038/nrclinonc.2011.199. [DOI] [PubMed] [Google Scholar]
- 13. Liao X, Lochhead P, Nishihara R, Morikawa T, Qian ZR. Aspirin use, tumor PIK3CA mutation, and colorectal-cancer survival. N Engl J Med. 2012;367(17):596-1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chan AT, Ogino S, Fuchs CS. Aspirin and the risk of colorectal cancer in relation to the expression of COX-2. N Engl J Med. 2007;356(21):2131-2142. [DOI] [PubMed] [Google Scholar]
- 15. Coyle C, Cafferty FH, Langley RE, Langley RE, Coyle C, Cafferty FH. Aspirin and colorectal cancer prevention and treatment: is it for everyone? Curr Colorectal Cancer Rep. 2016;12:27-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fajardo AM, Piazza GA. Chemoprevention in gastrointestinal physiology and disease. Anti-inflammatory approaches for colorectal cancer chemoprevention. Am J Physiol Gastrointest Liver Physiol. 2015;309:G59-G70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pommergaard H, Burcharth J, Rosenberg J, Raskov H. Aspirin, calcitriol, and calcium do not prevent adenoma recurrence in a randomized controlled trial. Gastroenterology. 2016;150(1):114-122.e4. doi: 10.1053/j.gastro.2015.09.010. [DOI] [PubMed] [Google Scholar]
- 18. Kato I, Lane D, Womack CR, et al. Interaction between nonsteroidal anti-inflammatory drugs and low-fat dietary intervention on colorectal cancer incidence; the Women’s Health Initiative (WHI) Dietary Modification Trial. J Am Coll Nutr. 2017;36(6):462-469. doi: 10.1080/07315724.2017.1321505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Coghill AE, Phipps AI, Bavry AA, et al. The association between NSAID use and colorectal cancer mortality: results from the Women’s Health Initiative. Cancer Epidemiol Biomarkers Prev. 2012;21(11):1966-1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Grau MV, Sandler RS, McKeown-Eyssen G, et al. Nonsteroidal anti-inflammatory drug use after 3 years of aspirin use and colorectal adenoma risk: observational follow-up of a randomized study. J Natl Cancer Inst. 2009;10(4):267-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Thompson P, Roe DJ, Fales L, et al. Design and baseline characteristics of participants in a phase III randomized trial of celecoxib and selenium for colorectal adenoma prevention. Cancer Prev Res (Phila). 2012;5(12):1381-1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wang Y, Zhang F, Wang J. The efficacy and safety of non-steroidal anti-inflammatory drugs in preventing the recurrence of colorectal adenoma: a meta-analysis and systematic review of randomized trials. Colorectal Dis. 2014;17:188-196. [DOI] [PubMed] [Google Scholar]
- 23. Hultberg DK, Angenete E, Lydrup M-L, Ruteg J, Matthiessen P, Ruteg M. Nonsteroidal anti-inflammatory drugs and the risk of anastomotic leakage after anterior resection for rectal. Eur J Surg Oncol. 2017;43:1908-1914. [DOI] [PubMed] [Google Scholar]
- 24. Benamouzig R, Uzzan B, Deyra J, et al. Prevention by daily soluble aspirin of colorectal adenoma recurrence: 4-year results of the APACC randomised trial. Gut. 2012;61:255-261. [DOI] [PubMed] [Google Scholar]
- 25. Vaughan LE, Prizment A, Blair CK, Thomas W, Anderson KE, Anderson KE. Aspirin use and the incidence of breast, colon, ovarian, and pancreatic cancers in elderly women in the Iowa Women’s Health Study. Cancer Causes Control. 2016;27(11):1395-1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cao Y, Nishihara R, Wu K, et al. Population-wide impact of long-term use of aspirin and the risk for cancer. JAMA Oncol. 2016;2(6):762-769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ranger GS. The role of aspirin in colorectal cancer chemoprevention. Crit Rev Oncol Hematol. 2016;104:87-90. doi: 10.1016/j.critrevonc.2016.05.011. [DOI] [PubMed] [Google Scholar]
- 28. Burn J, Gerdes A, Macrae F, et al. Long-term effect of aspirin on cancer risk in carriers of hereditary colorectal cancer: an analysis from the CAPP2 randomised controlled trial. Lancet. 2011;378(9809):2081-2087. doi: 10.1016/S0140-6736(11)61049-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ishikawa H, Mutoh M, Suzuki S, et al. The preventive effects of low-dose enteric-coated aspirin tablets on the development of colorectal tumours in Asian patients: a randomised trial. Gut. 2014;63:1755-1759. [DOI] [PubMed] [Google Scholar]
- 30. Benamouzig R, Uzzan B, Martin A, et al. Cyclooxygenase-2 expression and recurrence of colorectal adenomas: effect of aspirin chemoprevention. Gut. 2010;59:622-629. [DOI] [PubMed] [Google Scholar]
- 31. Barry EL, Sansbury LB, Grau MV, et al. Cyclooxygenase-2 polymorphisms, aspirin treatment, and risk for colorectal adenoma recurrence—data from a randomized clinical trial. Cancer Epidemiol Biomarkers Prev. 2009;18(10):2726-2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Giampieri R, Restivo A, Pusceddu V, et al. The role of aspirin as antitumoral agent for heavily pretreated patients with metastatic colorectal cancer receiving capecitabine monotherapy. Clin Colorectal Cancer. 2017;16(1):38-43.doi: 10.1016/j.clcc.2016.07.011. [DOI] [PubMed] [Google Scholar]
- 33. Rothwell PM, Wilson M, Elwin C, et al. Long-term effect of aspirin on colorectal cancer incidence and mortality: 20-year follow-up of five randomised trials. Lancet. 2010;376(9754):1741-1750. doi: 10.1016/S0140-6736(10)61543-7. [DOI] [PubMed] [Google Scholar]
- 34. Lang Kuhs KA, Hildesheim A, Trabert B, et al. Association between regular aspirin use and circulating markers of inflammation: a study within the Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial. Cancer Epidemiol Biomarkers Prev. 2015;24(5):825-832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bertagnolli MM, Eagle CJ, Zauber AG, et al. Celecoxib for the prevention of sporadic colorectal adenomas. N Engl J Med. 2006;355(9):873-884. [DOI] [PubMed] [Google Scholar]
- 36. Chan AT, Hsu M, Zauber AG, Hawk ET, Bertagnolli MM. The influence of UGT1A6 variants and aspirin use in a randomized trial of celecoxib for prevention of colorectal adenoma. Cancer Prev Res (Phila). 2012;5(1):61-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lynch PM, Burke CA, Phillips R, et al. An international randomised trial of celecoxib versus celecoxib plus difluoromethylornithine in patients with familial adenomatous polyposis. Gut. 2015;65(2):286-295. [DOI] [PubMed] [Google Scholar]
- 38. Nadir A, Eagle CJ, Dite P, et al. Celecoxib for the prevention of colorectal adenomatous polyps. N Engl J Med. 2006;355:885-895. [DOI] [PubMed] [Google Scholar]
- 39. Pal I, Dey KK, Chaurasia M, Parida S. Cooperative effect of BI-69A11 and celecoxib enhances radiosensitization by modulating DNA damage repair in colon carcinoma. Int Soc Oncol Biomarkers. 2016;37(5):6389-6402. [DOI] [PubMed] [Google Scholar]
- 40. Xu X, Hu W, Zhou J, Tu Y. Celecoxib enhances the radiosensitivity of HCT116 cells in a COX-2 independent manner by up-regulating BCCIP. Am J Transl Res. 2017;9(3):1088-1100. [PMC free article] [PubMed] [Google Scholar]
- 41. Dulai PS, Singh S, Marquez E, et al. Chemoprevention of colorectal cancer in individuals with previous colorectal neoplasia: systematic review and network meta-analysis. BMJ. 2016;355:i6188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Setia S, Nehru B, Sanyal SN. Celecoxib prevents colitis associated colon carcinogenesis: an upregulation of apoptosis. Pharmacol Rep. 2014;66:1083-1091. doi: 10.1016/j.pharep.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 43. Almhanna K, El-Rayes B, Sethi S, Dyson G, Heilbrun L. NIH public access. NIH Public Access. 2013;32(8):3559-3563. [PMC free article] [PubMed] [Google Scholar]
- 44. Huang W, Hsieh K, Huang R, Yang Y. ScienceDirect Role of cyclooxygenase-2 inhibitors in the survival outcome of colorectal cancer patients: a population-based cohort study. Kaohsiung J Med Sci. 2017;33(6):308-314. doi: 10.1016/j.kjms.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 45. Alamdarsaravi M, Ghajar A, Noorbala A, et al. Efficacy and safety of celecoxib monotherapy for mild to moderate depression in patients with colorectal cancer: a randomized double-blind, placebo controlled trial. Psychiatry Res. 2017;5:59-65.doi: 10.1016/j.psychres.2017.05.029. [DOI] [PubMed] [Google Scholar]
- 46. Kim S, Ha G, Bae J, et al. COX-2- and endoplasmic reticulum stress-independent induction of ULBP-1 and enhancement of sensitivity to NK cell-mediated cytotoxicity by celecoxib in colon cancer cells. Exp Cell Res. 2015;330(2):451-439. doi: 10.1016/j.yexcr.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 47. Lawrence JR, Baxter GJ, Paterson JR. Aspirin for cancer is no mere antiplatelet prototype. There is potential in its ancient roots. Med Hypotheses. 2016;94:74-76. doi: 10.1016/j.mehy.2016.07.001. [DOI] [PubMed] [Google Scholar]
- 48. Baron JA, Sandler RS, Bresalier RS, et al. A randomized trial of rofecoxib for the chemoprevention of colorectal adenomas. Gastroenterology. 2006;131(6):1674-1682. [DOI] [PubMed] [Google Scholar]
- 49. Kerr DJ, Dunn JA, Smith JL, et al. Rofecoxib and cardiovascular adverse events in adjuvant treatment of colorectal cancer. N Engl J Med. 2007;357(4):360-369. [DOI] [PubMed] [Google Scholar]
- 50. Atari-Hajipirloo S, Nikanfar S, Heydari A, Kheradmand F. Imatinib and its combination with 2,5-dimethyl-celecoxib induces apoptosis of human HT-29 colorectal cancer cells. Res Pharm Sci. 2017;12(1):67-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ahmetaj-Shala B, Tesfai A, Constantinou C, et al. Pharmacological assessment of ibuprofen arginate on platelet aggregation and colon cancer cell killing. Biochem Biophys Res Commun. 2017;484(4):762-766. doi: 10.1016/j.bbrc.2017.01.161. [DOI] [PubMed] [Google Scholar]
- 52. Ghanghas P, Jain S, Rana C, Sanyal SN. Chemopreventive action of non-steroidal anti-inflammatory drugs on the inflammatory pathways in colon cancer. Biomed Pharmacother. 2016;78:239-247. [DOI] [PubMed] [Google Scholar]
- 53. Li M, Tan S, Wang X. Paeonol exerts an anticancer effect on human colorectal cancer cells through inhibition of PGE2 synthesis and COX-2 expression. Oncol Rep. 2014;32:2845-2853. [DOI] [PubMed] [Google Scholar]
- 54. Lönnroth C, Andersson M, Asting AG. Preoperative low dose NSAID treatment influences the genes for stemness, growth, invasion and metastasis in colorectal cancer. Int J Oncol. 2014;45:2208-2220. [DOI] [PMC free article] [PubMed] [Google Scholar]