Abstract
Objective:
The 2010 Endovascular Aortic Repair-2 Trial (EVAR-2) reported that patients with comorbidity profiles rendering them unfit for open aneurysm repair who underwent Endovascular Aortic Repair (EVAR) did not experience a survival advantage compared those who did not undergo intervention. These patients experienced a 30-day mortality of 7.3%, while reports from similar cohorts report far lower mortality rates. The primary objective of our study was to compare the incidence of 30-day mortality in low-and high-risk patients undergoing EVAR in a contemporary dataset, utilizing patient risk stratification criteria from EVAR-2. Secondarily, we sought to identify risk factors associated with a disproportionate contribution to 30-day mortality risk.
Methods:
Data was obtained from the 2005–2013 American College of Surgeons – National Surgical Quality Improvement Program (ACS-NSQIP) participant user files (n = 24,813). Patients were included in the high-risk cohort with the presence of renal, respiratory, or cardiac preoperative criteria alone or in combination. Renal impairment criteria were defined as dialysis, or creatinine > 2.26 mg/dL. Respiratory impairment criteria included history of chronic obstructive pulmonary disease (COPD), or preoperative ventilator support. Cardiac impairment criteria included history of myocardial infarction, congestive heart failure, angina, or prior coronary intervention. Patient, procedural characteristics, and 30-day postoperative outcomes were compared using Pearson χ2 tests for categorical variables and Wilcoxon rank-sum tests for continuous variables.
Results:
Among 24,813 patients undergoing EVAR, 12,031 (48%) patients were characterized as high-risk (at least one impairment criteria) while 12,770 (52%) patients stratified as low-risk. Thirty-day mortality rate in the high-risk cohort was 1.9%, compared to the 7.3% reported by EVAR-2, and higher in the high-risk cohort compared to the low-risk cohort (1.9% vs. 0.9%, p<0.001). While the presence of each comorbidity increased the odds of 30-day mortality (respiratory OR: 1.62, 95% CI: 1.16–2.26, p=0.005; cardiac OR: 1.55, 95% CI: 1.14–2.10, p=0.005), the presence of renal criteria disproportionately increased the odds of mortality 3-fold (OR: 3.42, 95% CI: 2.31–5.09, p<0.001).
Conclusion:
Contemporary 30-day mortality following EVAR in high-risk patients is substantially lower than that reported in the EVAR-2 trial. While low-and high-risk stratification by current comorbidity criteria is appropriate, attention needs to be paid to disproportionate risk contribution from renal disease on mortality compared to cardiac and pulmonary comorbidities. Given the lower mortality risk than previously described, patients stratified as high-risk should be thoughtfully considered for definitive EVAR.
Introduction:
Endovascular aneurysm repair (EVAR), considered by some to be the standard of care for infrarenal abdominal aortic aneurysms (AAA), provides an alternative, often safer AAA treatment option for high-risk patients unfit for open repair.1–4 While randomized controlled trials have demonstrated improved perioperative safety of EVAR compared to open surgery, the utility of EVAR for high-risk patients categorized as unfit for open repair remains controversial.5–9 The 2010 randomized controlled trial EVAR-2, determined EVAR had no long-term survival advantage over no intervention in patients unfit for open repair.10 High 30-day mortality rates (7.3%) in the treatment arm contributed to the conclusions of this study. Following EVAR-2’s publication, several small studies using institutional data have reported significantly lower mortality rates (0%−2%) and no clinically significant differences in outcomes between high-and low-risk patients undergoing EVAR.5,6,11
One potential cause of deceptively high operative mortality rates seen in the EVAR-2 trial is the study period of 1999–2004, during the incipient stages of endovascular technology.12 Furthermore, patients enrolled in EVAR-2 had cardiac, renal, or respiratory comorbidities rendering them unfit for open repair, though it was unclear which comorbidity factors were most strongly correlated with the observed high mortality rates. The effect of each risk factor independently, and in combination, could not be discerned in the original study due to its design and lack of statistical power. Subsequent studies have detected small increases in mortality for patients with high-risk comorbidities, but lack statistical power to detect differences between patients assigned to low-and high-risk groups.11,13,14 Thus, more strongly powered datasets are needed to understand the impact of individual risk factors on high-risk patient outcomes following EVAR.
Using the original EVAR-2 inclusion criteria and American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP) data, contemporary 30-day outcomes following elective EVAR in patients who would have been stratified as unfit for open surgery by EVAR-2 criteria were examined. Given improvements in both EVAR technology and technical proficiency, we hypothesized 30-day mortality rates were lower in high-risk patients than those reported in the EVAR-2 trial with evaluation of a contemporary national dataset. We further sought to determine which preoperative risk factors disproportionately contribute to an increased risk of mortality after EVAR.
Methods:
The ACS-NSQIP is a validated multi-institutional clinical database that contains patient demographic and outcomes data obtained using an unbiased sampling system. The ACS-NSQIP and the hospitals participating in the ACS-NSQIP are the source of the data used herein; they have not verified and are not responsible for the statistical validity of the data analysis or the conclusions derived by the authors. As this is a retrospective study with no patient-identifiable data, patient informed consent was not obtained and IRB approval was waived.
Patients who underwent endovascular aneurysm repair (EVAR) using Current Procedure Terminology (CPT) diagnosis codes for EVAR (34800, 34801, 34802, 34803, 34804, 34805) were identified. To restrict our cohort to mimic the EVAR-2 trial, patients with ruptured aneurysm (ICD-9 code 441.3) were excluded, as were patients with systemic inflammatory response syndrome/sepsis/shock, ASA Class V, or on preoperative ventilation. Using criteria described above, a cohort of 24,813 patients was obtained. Inclusion criteria matched that of the EVAR-2 trial: 1) Renal: preoperative dialysis within 2 weeks or creatinine > 2.26 mg/dL (EVAR-2: creatinine > 2.26 mg/dL), 2) Pulmonary: history of chronic obstructive pulmonary disease (COPD) within 30 days (EVAR-2: FEV1 <1.0 L) or 3) Cardiac: history of myocardial infarction within 6 months, congestive heart failure (CHF) within 30 days, angina within 30 days, or any history of percutaneous or open cardiac intervention (EVAR-2: any history of MI, cardiac revascularization, angina, heart valve disease, arrhythmia, or uncontrolled CHF) (Table 1).
Table 1:
Comparison of EVAR-2 trial inclusion criteria to present study Comparison to EVAR-2 Trial Inclusion Criteria
| Comparison to EVAR-2 Trial Inclusion Criteria | ||
|---|---|---|
| EVAR-2 Trial | Present Study | |
| Cardiac Criteria | History of myocardial infarction | Myocardial Infarction within 6 months |
| History of cardiac revascularization | Any history of percutaneous or open cardiac intervention | |
| History of angina | Angina within 30-days | |
| Uncontrolled congestive heart failure | Congestive heart failure within 30-days | |
| History of heart valve disease | ||
| History of significant arrhythmia | ||
| Respiratory Criteria | FEV1 < 1.0 L | History of COPD within 30-days |
| Renal Criteria | Creatinine > 2.26 mg/dL | Creatinine > 2.26 mg/dL On dialysis |
Comparison of specific cardiac, renal, and respiratory inclusion criteria between EVAR-2 trial and this study
The primary outcome measure for this study was 30-day mortality compared between the historic EVAR-2 trial and the contemporary data set. Additionally, overall morbidity (defined as presence of any NSQIP outcome variable) and major complication (any complications except urinary tract infection, superficial surgical infections and deep vein thrombosis) rates were measured. Patient, procedural characteristics, and 30-day postoperative outcomes were compared using Pearson χ2 tests for categorical variables and Wilcoxon rank-sum tests for continuous variables. Post-hoc comparisons of stratified variables were performed with additional χ2 tests. To evaluate contributions of risk criteria to mortality while controlling for confounding variables, multivariate analysis was utilized in which a backward multivariable stepwise regression model with a probability of type I error set to 0.05 as the significance threshold for exclusion. All variables with missing values were excluded from analysis. The patient user files contain specific definitions of all variables addressed in this retrospective study. Statistical analyses were performed with Stata version 11.0 (StataCorp, College Station, TX).
Results:
After compiling the 2005–2013 NSQIP datasets, 24,813 patients undergoing EVAR were identified. Patient demographics are show in Table 2. Of all patients in the cohort, 81% were male with a median age of 74 (IQR 68–81), 30% were smokers, and 16% had diabetes. A subset of 12,031 (48%) patients were categorized high-risk with either a renal, respiratory, or cardiac comorbidity. Comparisons of patient comorbidities and characteristics between high-and low-risk cohorts are shown in Table 2. In the high-risk cohort, there was a higher prevalence of tobacco use (32% vs 29%, p<0.001) and diabetes (18% vs 14%, p<0.001). The high-risk cohort also had high frequencies of abnormal biochemical markers including albumin (11% vs 8%), hematocrit (35% vs 28%), and creatinine (41% vs. 33%). Bleeding disorders were more common in the high-risk group (14% vs 19%, p<0.001).
Table 2:
Cohort Demographics
| Variable | Total (n=24813) | Low Risk (n=12770) | High Risk (n=12043) | P-value |
|---|---|---|---|---|
| Male | 20155 (81%) | 10337 (81%) | 9818 (82%) | 0.139 |
| Median Age (IQR) | 74 (68–81) | 74 (68–80) | 75 (68–80) | 0.1793 |
| Current Smoker | 7525 (30%) | 3688 (29%) | 3787 (32%) | <0.001 |
| Non-independent Functional status | 930 (4%) | 373 (3%) | 557 (5%) | <0.001 |
| Preoperative transfusion (>1 units within 72 hours) | 110 (.44%) | 45 (.35%) | 65 (.54%) | 0.026 |
| DNR Status | 67 (.43%) | 15 (.23%) | 52 (.57%) | 0.001 |
| Alcohol | 682 (4%) | 307 (5%) | 375 (4%) | 0.084 |
| Diabetes | 3893 (16%) | 1726 (14%) | 2131 (18%) | <0.001 |
| Bleeding Disorder | 2823 (11%) | 1113 (9%) | 1710 (14%) | <0.001 |
| Steroid Use | 1010 (4%) | 359 (3%) | 651 (5%) | <.0.001 |
| Weight loss | 290 (1.17%) | 171 (1.42%) | 119 (0.93%) | <0.001 |
| Preoperative Albumin | <0.001 | |||
| Abnormal | 2323 (9%) | 998 (8%) | 1325 (11%) | |
| Normal | 9786 (39%) | 5014 (39%) | 4772 (40%) | |
| Normal | 9786 (39%) | 5014 (39%) | 4772 (40%) | |
| Missing | 12704 (51%) | 6770 (53%) | 5934 (49%) | |
| Preoperative Hematocrit | <0.001 | |||
| Abnormal | 7816 (32%) | 3607 (28%) | 4209 (35%) | |
| Normal | 16252 (61%) | 8758 (69%) | 7494 (62%) | |
| Missing | 745 (3%) | 417 (3%) | 328 (3%) | |
| Preoperative Creatinine | <0.001 | |||
| Abnormal | 9100 (37%) | 4166 (33%) | 4934 (41%) | |
| Normal | 15023 (61%) | 8230 (64%) | 6793 (56%) | |
| Missing | 690 (3%) | 386 (3%) | 304 (3%) | |
IQR: Interquartile Range, DNR: Do Not Resuscitate Status
Categoric data are shown as number (%) and continuous data as median (interquartile range)
Normal lab values are defined as the following: Albumin: 3.5–5.5 g/dL; Creatinine: 0.6–1.2 mg/dL in males, 0.5–1.1 mg/dL in females; Hematocrit: 38.8%−50% in males, 34.9%−44.5% in females.
Univariate comparisons for continuous variables made with Student’s t-test; comparisons for categorical variables made using Pearson’s chi-squared test or Fisher’s exact test, as appropriate.
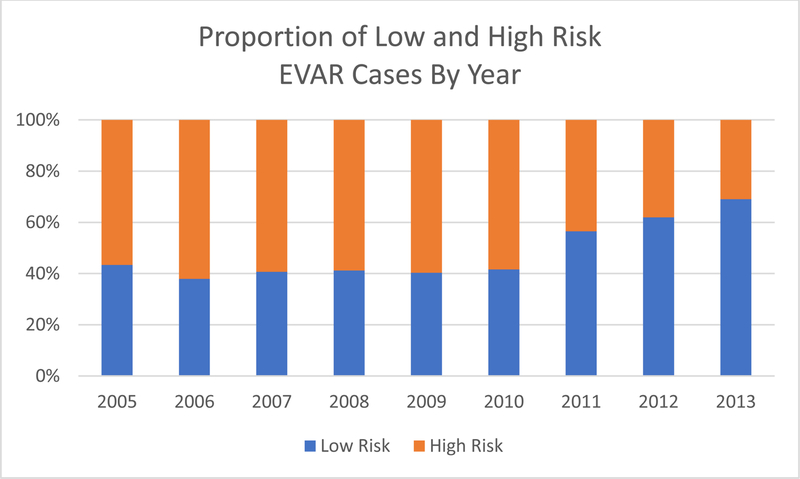
To determine the impact the EVAR-2 trial on current practice of treating high-risk patients with EVAR, we stratified our dataset by year and examined the proportion of high-risk patients treated with EVAR annually. Prior to the publication of the EVAR-2 Trial in 2010, nearly 60% of patients undergoing EVAR were stratified as high-risk as shown in Figure 1. In 2011, the proportion of high-risk patients intervened upon dropped to 43% and continued to fall, reaching 30% in 2013. Although the total number of EVAR cases increased per year after 2010, the number of high-risk cases decreased (Supplemental Figure 1). The EVAR-2 trial impacted surgical practice as seen by the disproportionate decrease in patients stratified as high-risk receiving endovascular intervention.
Figure 1:
Proportion of High-Risk cases drops after 2010
The contemporary NSQIP cohort of patients stratified as high-risk had a 30-day mortality rate of 1.9% following EVAR, significantly lower than the 7.3% reported by the EVAR-2 study (p<0.01). Subsequently, patient outcomes data between low-and high-risk groups were compared as displayed in Table 3. Overall, rates of 30-day mortality were low (1.4%), but increased from 0.9% in the low-risk group to 1.9% in the high-risk group. While no differences in operative mortality were detected (0.14% vs 0.10%, p=0.37), high-risk patients had increased overall morbidity (15% vs 12%, p<0.001), and risk of major complication (12% vs 9%, p<0.001), compared to patients stratified as low-risk. No differences between rates of deep vein thrombosis (DVT), myocardial infarction (MI), stroke, and pulmonary embolism (PE) were observed.
Table 3:
30-day Outcomes Stratified by Presence of Single Risk Factor
| Variable | Total (n=24813) | Low Risk (n=12770) | High Risk (n=12043) | P-value |
|---|---|---|---|---|
| 30-day Mortality | 336 (1.4%) | 113 (.9%) | 223 (1.9) | <0.001 |
| Operative Mortality | 30 (.12%) | 13 (.1%) | 17 (.14%) | 0.37 |
| Overall Morbidity | 3327 (13.4) | 1500 (11.7) | 1827 (15.2) | <0.001 |
| Major Complication Rate | 2648 (11) | 1208 (9) | 1440 (12) | <0.001 |
| Early return to OR | 1110 (4%) | 534 (4%) | 576 (4.79%) | 0.02 |
| Length of stay (hospital) (days) | 2 (1–3) | 2 (1–3) | 2 (1–4) | <0.001 |
| Operative time | 136 (104–181) | 132 (101–177) | 140 (106–185) | <0.001 |
| Superficial SSI | 385 (1.6%) | 156 (1.2%) | 229 (1.9%) | <0.001 |
| Deep SSI | 118 (.48%) | 53 (.41%) | 65 (.54%) | 0.151 |
| Organ Space SSI | 33 (.13%) | 17 (.13%) | 16 (.13%) | 1.00 |
| Wound Dehiscence | 70 (.28%) | 26 (.2%) | 44 (.37%) | 0.016 |
| Sepsis | 202 (.81%) | 79 (.62%) | 123 (1.02%) | <0.001 |
| Septic Shock | 157 (.63%) | 53 (.41%) | 104 (.86%) | <0.001 |
| Pneumonia | 329 (1.33%) | 115 (.9%) | 214 (1.78%) | <0.001 |
| Unplanned Reintubation | 376 (1.52%) | 116 (.91%) | 260 (2.16%) | <0.001 |
| Prolonged Ventilator Dependence | 304 (1.23%) | 99 (.77%) | 205 (1.70%) | <0.001 |
| PE | 54 (.22%) | 29 (.23%) | 25 (.21%) | 0.747 |
| Renal Failure | 207 (.83%) | 77 (.6%) | 130 (1.08%) | <0.001 |
| UTI | 431 (1.74%) | 196 (1.53%) | 235 (1.95%) | 0.011 |
| Stroke | 105 (.42%) | 51 (.4%) | 54 (.45%) | 0.546 |
| Cardiac Arrest | 124 (.5%) | 37 (.29%) | 87 (.72%) | <0.001 |
| MI | 228 (.92%) | 89 (.70%) | 139 (1.16 %) | <0.001 |
| Post-op Bleeding | 1753 (7.06%) | 856 (6.7%) | 897 (7.46%) | 0.02 |
| DVT | 134 (.54%) | 63 (.49%) | 71 (.59%) | 0.296 |
SSI: Surgical Site Infection, PE: Pulmonary Embolism, UTI: Urinary Tract Infection, MI: Mycoardial Infarction, DVT: Deep Vein Thrombosis. Early return to OR is defined by a return to the OR within 30 days of the primary procedure.
Categoric data are shown as number (%) and continuous data as median (interquartile range)
Univariate comparisons for continuous variables made with Student’s t-test; comparisons for categorical variables made using Pearson’s chi-squared test or Fisher’s exact test, as appropriate.
The incremental impact of each comorbidity criteria on 30-day postoperative outcomes after EVAR was assessed. Thirty-day mortality increases with an increasing number of respiratory, renal, or cardiac comorbidity criteria (p<0.001) (Table 3). Thirty-day mortality rates in patients with one comorbidity criterion was 1.6%, and up to 8.7% in patients meeting three criteria. The prevalence of other postoperative complications had concomitant increases with number of criteria, shown in higher rates of wound dehiscence, postoperative bleeding, and surgical site infection. Patients meeting all three comorbidity criteria had an overall morbidity rate of 38%, and a major complication rate of 36%. This is compared to 14% and 11%, respectively, in patients meeting one criteria.
Given a well-powered dataset, risk factors were analyzed, alone or in combination, to determine those associated with worse postoperative outcomes following EVAR (Table 4). Patients were stratified by respiratory, renal, or cardiac criteria in isolation and in combinations. While each risk factor additively increases risk of mortality, increases in mortality rates in patients on dialysis (5.2%), and those patients meeting both respiratory and renal criteria (8.3%) were substantial. No difference was found between the 30-day mortality in these patients and those meeting all three criteria (p=0.896). Similarly, major complication rates and overall morbidity were increased in high-risk, compared to low risk, patients irrespective of criteria, but no significant differences were observed in patients with renal criteria alone; renal and respiratory criteria in combination; or all three criteria.
Table 4:
Outcomes Stratified by Number of Risk Criteria
| Variable | Total (n=24813) | Low Risk (n=12770) | High Risk (n=12043) | P-value | ||
|---|---|---|---|---|---|---|
| 1 Risk Factor (n=9543) | 2 Risk Factors (n=2406) | 3 Risk Factors (n=94) | ||||
| 30-day Mortality | 336 (1.4%) | 113 (0.9%) | 148 (1.6%) | 67 (2.8%) | 8 (8.5%) | <0.001 |
| Operative Mortality | 30 (0.1%) | 13 (0.1%) | 13 (0.1%) | 4 (0.2%) | 0 (0%) | 0.778 |
| Overall Morbidity | 3327 (13.4%) | 1500 (11.8%) | 1363 (14.3%) | 429 (17.8%) | 35 (37.2%) | <0.001 |
| Major Complication Rate | 2648 (11.0%) | 1208 (9.5%) | 1061 (11.1%) | 346 (14.4%) | 33 (35.1%) | <0.001 |
| Early return to OR | 1110 (4.5%) | 534 (4.2%) | 444 (4.7%) | 120 (5.0%) | 12 (12.8%) | <0.001 |
| Length of stay (hospital) (days) | 2 (1–3) | 2 (1–3) | 2 (1–4) | 2 (1–5) | 5 (2–10) | 0.0001 |
| Operative time | 136 (104–181) | 132 (101–177) | 137 (105–182) | 148 (111–199) | 145 (121–198) | 0.0001 |
| Superficial SSI | 385 (1.6%) | 156 (1.2%) | 177 (1.9%) | 48 (2.0%) | 4 (4.3%) | <0.001 |
| Deep SSI | 118 (0.5%) | 53 (0.5%) | 48 (0.5%) | 13 (0.5%) | 4 (4.3%) | <0.001 |
| Organ Space SSI | 33 (0.1%) | 17 (0.1%) | 12 (0.1%) | 3 (0.1%) | 1 (1.1%) | 0.10 |
| Wound Dehiscence | 70 (0.3%) | 26 (0.2%) | 31 (0.3%) | 11 (0.5%) | 2 (2.1%) | <0.001 |
| Sepsis | 202 (0.8%) | 79 (0.6%) | 86 (0.9%) | 33 (1.4%) | 4 (4.3%) | <0.001 |
| Septic Shock | 157 (0.6%) | 53 (0.4%) | 62 (0.7%) | 36 (1.5%) | 6 (6.4%) | <0.001 |
| Pneumonia | 329 (1.3%) | 115 (0.9%) | 143 (1.5%) | 67 (2.8%) | 4 (4.3%) | <0.001 |
| Unplanned Reintubation | 376 (1.5%) | 116 (0.9%) | 170 (1.8%) | 82 (3.4%) | 8 (8.5%) | <0.001 |
| Prolonged Ventilator Dependence | 304 (1.2%) | 99 (0.8%) | 139 (1.5%) | 61 (2.5%) | 5 (5.3) | <0.001 |
| Pulmonary Embolism | 54 (0.2%) | 29 (0.2%) | 19 (0.2%) | 6 (0.3%) | 0 (0%) | 0.91 |
| Renal Failure | 207 (0.8%) | 77 (0.6%) | 81 (0.9%) | 37 (1.5%) | 12 (12.8%) | <0.001 |
| UTI | 431 (1.7%) | 196 (1.5%) | 169 (1.8%) | 63 (2.6%) | 3 (3.2%) | 0.002 |
| Stroke | 105 (0.4%) | 51 (0.4%) | 42 (0.4%) | 12 (0.5%) | 0 (0%) | 0.81 |
| Cardiac Arrest | 124 (0.5%) | 37 (0.3%) | 56 (0.6%) | 27 (1.1%) | 4 (4.3%) | <0.001 |
| MI | 228 (0.9%) | 89 (0.7%) | 96 (1.0%) | 43 (1.8%) | 0 (0%) | <0.001 |
| Post-op Bleeding | 1753 (7.0%) | 856 (6.7%) | 691 (7.2%) | 190 (7.9%) | 16 (17.0%) | <0.001 |
| DVT | 134 (0.5%) | 63 (0.5%) | 58 (0.6%) | 13 (0.5%) | 0 (0%) | 0.61 |
Categoric data are shown as number (%) and continuous data as median (interquartile range)
Univariate comparisons for continuous variables made with Student’s t-test; comparisons for categorical variables made using Pearson’s chi-squared test or Fisher’s exact test, as appropriate.
Finally, to identify which risk criteria are predictors of mortality while controlling for confounding variables, we performed a backward stepwise logistic regression (Table 5). Presence of renal criteria independently increased the odds of 30-day mortality by over 3-fold (OR: 3.42, 95% CI: 2.31–5.09, p<0.001). Respiratory criteria and cardiac criteria independently increased odds of 30-day mortality, albeit to a lesser extent (OR: 1.62, 95% CI:1.16–2.26, p=0.005; OR: 1.55, 95% CI: 1.14–2.10, p=0.005). Other preoperative variables increasing odds of 30-day mortality are dependent functional status (OR: 2.86, CI: 2.06–3.96, p<0.001), female sex (OR: 1.71, 95% CI: 1.35–2.18, p<0.001), steroid use (OR: 1.61, 95% CI: 1.06–2.43, p=0.024), and age (OR: 1.05, 95% CI:1.04–1.07, p<0.001). Notably, presence of a single of high-risk criterion did not increase the odds of 30-day mortality (OR: 1.01, 95% CI: 0.67–1.54, p=0.954). The presence of renal criteria constitutes either dialysis, or an elevated creatinine, although it is undetermined which criteria offers more predictive power of mortality. Renal insufficiency alone, and renal insufficiency on dialysis, independently increased the odds of mortality (OR: 3.36, 95% CI: 1.79–6.31, p<0.001); (OR: 2.22, 95% CI: 1.49–3.30, p<0.001) (Supplemental Table 1). Renal and respiratory criteria in combination synergistically increased odds of mortality by over 7-fold (OR: 7.18, 95% CI: 3.72–13.85, p<0.001). Meanwhile, combinations of cardiac/renal and cardiac/respiratory criteria had less than additive increased risks of 30-day mortality risk (OR: 2.04, 95% CI: 1.49–2.78, p<0.001; OR: 2.00, 95% CI: 1.50–2.78, p<0.001).
Table 5:
30-day Mortality and Morbidity by Risk Criteria
| Variable | Total (n=24813) | Low Risk (n=12770) | High Risk (n=12043) | P-value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 Risk Factor (n=9543) | 2 Risk Factors (n=2406) | 3 Risk Factors (n=94) | |||||||||
| Renal (n=327) | Cardiac (n=3,924) | Respiratory (n=5,292) | Cardiac/Renal (n=135) | Respiratory/Renal (n=137) | Cardiac/Respiratory (n=2129) | Cardiac/Renal/Respiratory | |||||
| No Dialysis (n=213) | Dialysis (n=114) | ||||||||||
| 30-day Mortality | 336 (1.4%) | 113 (.9%) | 6 (2.8%) | 6 (5.9%)* | 53 (1.4%) | 83 (1.6%)* | 5 (3.7%)* | 11 (8.3%)* | 51 (2.4%)* | 8 (8.7%)* | <0.001 |
| Operative Mortality | 30 (.12%) | 13 (.1%) | 1 (.47%) | 0 (0%) | 6 (.15%) | 6 (.11%) | 0 (0%)* | 2 (1.52%) | 6 (.09%) | 0 (0%) | 0.002 |
| Overall Morbidity | 3327 (13.4%) | 1500 (11.7%) | 60 (28.17%)* | 30 (29.41%)* | 463 (11.78%) | 810 (15.29%)* | 13 (22.96%)* | 49 (37.12%)* | 349 (16.38%)* | 35 (38.04%)* | <0.001 |
| Major Complication Rate | 2648 (11%) | 1208 (9%) | 52 (24.4%)* | 27 (26.5%)* | 334 (8.5%) | 648 (12.23%)* | 27 (20%)* | 46 (34.85%)* | 273 (12.81%)* | 33 (35.87%)* | <0.001 |
Asterisks () denote p<0.05 post-hoc 𝝌2-test compared to Low Risk cohort.
Categoric data are shown as number (%) and continuous data as median (interquartile range)
Univariate comparisons for continuous variables made with Student’s t-test; comparisons for categorical variables made using Pearson’s chi-squared test or Fisher’s exact test, as appropriate.
Discussion:
Thirty-day mortality rates in high-risk patients deemed unfit for open repair undergoing EVAR are nearly four times lower in a large contemporary cohort than reported by the EVAR-2 trial (1.9% vs 7.3%). Several reasons may account for the observed differences. First, our sample size was far greater than that of the EVAR-2 trial (n=404), making our study less susceptible to outliers and type I error. Second, while devices have not changed significantly, surgeon proficiency during EVAR is subject to a learning curve.15 Third, the proportion of EVAR to open cases continues to rise.16 Despite EVAR-2, practice patterns show that EVAR repairs are offered to high risk patients, and our study supports the safety of this practice. While methodologic differences exist between studies, the inclusion criteria from our retrospective study closely mirror those of the EVAR 2 study to allow for comparison of homogeneous cohorts. This allows for estimation of 30-day mortality of low-and high-risk patients in a contemporary cohort. We observed a strikingly lower mortality in our contemporary high-risk cohort compared to that observed by EVAR-2. As a secondary objective, we determined the incremental impact of each comorbidity on 30-day mortality after EVAR. Renal insufficiency had the greatest impact on 30-day mortality-risk, while effects of respiratory and cardiac comorbidities were moderate (OR: 1.62, 95% CI:1.16–2.26, p=0.005; OR: 1.55, 95% CI: 1.14–2.10, p=0.005, respectively). Mortality rates in high-risk patients are only slightly higher than mortality rates in a low-risk cohort (1.9% vs 0.9%, p<0.001). Indeed, most patients deemed high-risk by the presence of a single risk criteria have low 30-day mortality rates.
These findings confirm conclusions of previous reports suggesting that elevated creatinine and dialysis increase mortality risk disproportionate to respiratory and cardiac comorbidities. 5,13,14 Elevated creatinine alone, however, conferred a slightly increased risk of 30-day mortality compared to dialysis in our multivariate regression model (OR: 3.36 vs OR: 2.22). When examining the contribution of multiple comorbidities to mortality risk, renal and respiratory comorbidities in combination increased 30-day mortality risk by over 7-fold. Patients with only these two factors had similar adverse outcomes and mortality rates as patients with all three criteria, suggesting that cardiac comorbidities contribute little to 30-day outcomes. Surprisingly, the presence of a single risk factor, which can stratify patients as patients unfit for open repair, is not predictive of 30-day mortality.
Several studies have attempted to evaluate the effects of single risk factors that contribute to mortality after EVAR. 5,13,14,17 Given low mortality rates following EVAR, most previous studies have found small, statistically insignificant, differences between high-risk and low-risk patients. A retrospective study by Mehta et al. stratified patients by serum creatinine (<1.5 mg/dL, 1.5–2.0 mg/dL, and 2.1–3.5 mg/dL).13This study found that patients with serum creatinine values between 2.1 mg/dL and 3.5 mg/dL had a 7.4% mortality rate compared with 4.6% in patients with creatinine less than 1.5 mg/dL. As this was a relatively small case series (n = 200), it was underpowered to detect a statistically significant difference between groups. In addition, on multivariate analysis, a 2-fold increased risk of mortality was noted in patients with chronic renal insufficiency, but establishing the significance of this increase was again limited by lack of power. The authors concluded that EVAR could safely be performed in patients with renal insufficiency. Similar approaches have examined associations between COPD and 30-day mortality, but have found no significant differences.17 While our study confirms clinically small increases in patients with single risk factors, we demonstrate the statistically significant increased risk of mortality by the presence of renal criteria (high creatinine or dialysis) alone on mortality rates.
As single comorbidities offer little predictive power in determining mortality risk, combinations of comorbidities have been assessed to predict outcomes. Understanding the concerted effects of comorbidities may improve management by allowing more accurate risk stratification. Using a large retrospective cohort of 66,943 patients, Egorova et al. established a scoring system based on the risk conferred by preoperative variables on mortality based on results of a multivariate regression model.5 The following comorbidities were scored: renal failure with dialysis (score=7), renal failure without dialysis (score=3), lower extremity ischemia (score=5), age > 84 (score=3), 75–84 (score=2), 70–74 (score=1), heart failure (score=3), chronic liver disease (score=3), female gender (score=2), neurological disorders (score=2), COPD (score=2), surgeon inexperience (score=1), and hospital annual volume of EVAR < 7 (score=1). Concordant with our findings, patients with renal insufficiency on dialysis had the greatest risk of mortality and were given the highest risk score. However, heart failure had a higher score than COPD, which contrasts our finding that respiratory criteria increase mortality risk more than cardiac criteria. Such scoring systems can be used to understand the effect of multiple comorbidities, but assume additive effects of risk factors. Evaluation of the contemporary cohort when stratified by number of comorbidity criteria suggests that increasing numbers of criteria have synergistic, not additive, effects on mortality risk. Specifically, renal and respiratory criteria together confer over a 7-fold increased risk of mortality. However, addition of cardiac risk criteria added very little to mortality risk. Based on the scoring algorithm above, patients with cardiac and renal comorbidities would have a higher score than patients with renal and respiratory comorbidities.
Renal insufficiency and pulmonary disease act in concert to portend a poor prognosis in patients recovering from EVAR. The kidney and lung work together to maintain acid-base homeostasis in the blood. 18,19 It is conceivable that the function of this homeostatic mechanism is diminished in patients with concomitant disease. Both chronic hypercapnia of COPD and chronic renal insufficiency contribute to acidemia, and patients with both diseases lack adequate compensatory reserve.20,21 Several complications of AAA repair, such as ischemic colitis and abdominal compartment syndrome, can worsen existing acidosis through reperfusion and ischemic injury, respectively.22–26 These changes caused by aneurysm repair may thus perturb a precariously buffered pH state and cause an acute on chronic acidosis that leads to mortality. The effect of contrast dye during EVAR has not been reported to affect pH balance directly but can be nephrotoxic.27 Patients with only kidney disease may be able to compensate the acutely toxic effects of dye, whereas patients without a respiratory reserve may not. Further studies comparing mortality rates in these patients undergoing either EVAR or open repair will be particularly interesting and can address whether our observations are due to AAA repair in general or specifics of endovascular intervention.
Retrospective analysis of the NSQIP dataset has inherent limitations. These are apparent in the attempt to replicate cardiac risk factors of the EVAR-2 trial, with an inability to determine whether patients had valvular disease or a history or arrhythmia given the available data. Second, the NSQIP dataset follows outcomes only through 30-days, and extrapolation of long-term morbidity and mortality is inappropriate in this setting. Third, as a retrospective study of a nationally generated database, our data lacks the resolution that would be afforded by a prospective controlled trial. Finally, patients are not stratified on the extent of their aneurysmal disease, imposing a substantial indication bias.
Conclusion:
Contemporary 30-day mortality following EVAR in high-risk patients stratified as unfit for open AAA is substantially lower than that reported in the EVAR-2 trial. While low-and high-risk stratification by current comorbidity criteria is appropriate, attention needs to be paid to disproportionate risk contribution from renal disease on mortality compared to cardiac and pulmonary comorbidities. Given the lower mortality risk of contemporary endovascular repair of AAA than previously described, patients stratified as high-risk should be considered for definitive EVAR based on the patient’s preoperative comorbidity profile.
Supplementary Material
Table 6:
Predictors of 30-day Mortality: Backward Stepwise Elimination Multivariate Analysis
| Variable | OR | 95% CI | P-value |
|---|---|---|---|
| Single High-Risk Criteria | 1.01 | .67–1.54 | 0.95 |
| Renal Criteria | 3.42 | 2.31–5.09 | <0.001 |
| Respiratory Criteria | 1.62 | 1.16–2.26 | 0.005 |
| Cardiac Criteria | 1.55 | 1.14–2.10 | 0.005 |
| Cardiac and Renal Criteria | 2.04 | 1.49–2.78 | <.001 |
| Cardiac and Respiratory Criteria | 2.00 | 1.50–2.78 | <.001 |
| Renal and Respiratory Criteria | 7.18 | 3.72–13.85 | <.001 |
| Non-independent Functional Status | 2.86 | 2.06–3.96 | <0.001 |
| Female Sex | 1.71 | 1.35–2.18 | <0.001 |
| Steroid Use | 1.61 | 1.06–2.43 | 0.02 |
| Age | 1.05 | 1.04–1.07 | <0.001 |
CI: Confidence Interval; OR: Odds Ratio
References:
- 1.Paravastu SCV, Jayarajasingam R, Cottam R, Palfreyman SJ, Michaels JA, Thomas SM. Endovascular Repair of Abdominal Aortic Aneurysm. (Thomas SM, ed.). Chichester, UK: John Wiley & Sons, Ltd; 1996:1–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McHugh SM, Aherne T, Goetz T, et al. Endovascular versus open repair of ruptured abdominal aortic aneurysm. The Surgeon. 2015;14(5):1–4. [DOI] [PubMed] [Google Scholar]
- 3.Dangas G, O’Connor D, Firwana B, et al. Open Versus Endovascular Stent Graft Repair of Abdominal Aortic Aneurysms. JCIN. 2012;5(10):1071–1080. [DOI] [PubMed] [Google Scholar]
- 4.Morasch MD. EVAR for the Treatment of Ruptured AAA. Perspectives in Vascular Surgery and Endovascular Therapy. 2009;21(1):9–11. [DOI] [PubMed] [Google Scholar]
- 5.Egorova N, Giacovelli JK, Gelijns A, et al. Defining high-risk patients for endovascular aneurysm repair. J Vasc Surg. 2009;50(6):1271–1279.e1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Martino RR, Brooke BS, Robinson W, et al. Designation as “Unfit for Open Repair” Is Associated With Poor Outcomes After Endovascular Aortic Aneurysm Repair. Circ Cardiovasc Qual Outcomes. 2013;6(5):575–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prinssen M, Verhoeven E, Buth J. A randomized trial comparing conventional and endovascular repair of abdominal aortic aneurysms. N Engl J Med. [DOI] [PubMed] [Google Scholar]
- 8.Lederle FA, Freischlag JA, Kyriakides TC, et al. Long-term comparison of endovascular and open repair of abdominal aortic aneurysm. N Engl J Med. 2012;367(21):1988–1997. [DOI] [PubMed] [Google Scholar]
- 9.United Kingdom EVAR Trial Investigators. Endovascular versus open repair of abdominal aortic aneurysm. N Engl J Med. 2010;362(20):1863–1871. [DOI] [PubMed] [Google Scholar]
- 10.United Kingdom EVAR Trial Investigators, Greenhalgh RM, Brown LC, Powell JT, Thompson SG, Epstein D. Endovascular repair of aortic aneurysm in patients physically ineligible for open repair. N Engl J Med. 2010;362(20):1872–1880. [DOI] [PubMed] [Google Scholar]
- 11.Lim S, Halandras PM, Park T, et al. Outcomes of endovascular abdominal aortic aneurysm repair in high-risk patients. YMVA. 2015;61(4):862–868. [DOI] [PubMed] [Google Scholar]
- 12.Albuquerque FC, Tonnessen BH, Noll RE, Cires G, Kim JK, Sternbergh WC. Paradigm shifts in the treatment of abdominal aortic aneurysm: Trends in 721 patients between 1996 and 2008. YMVA. 2010;51(6):1348–1353. [DOI] [PubMed] [Google Scholar]
- 13.Mehta M, Veith FJ, Lipsitz EC, et al. Is elevated creatinine level a contraindication to endovascular aneurysm repair? J Vasc Surg. 2004;39(1):118–123. [DOI] [PubMed] [Google Scholar]
- 14.Guntani A, Okadome J, Kawakubo E, et al. Clinical Results of Endovascular Abdominal Aortic Aneurysm Repair in Patients with Renal Insufficiency without Hemodialysis. Annals of Vascular Diseases. 2012;5(2):1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Forbes TL, DeRose G, Lawlor DK, Harris KA. The Association Between a Surgeon’s Learning Curve With Endovascular Aortic Aneurysm Repair and Previous Institutional Experience. Vascular and Endovascular Surgery. 2016;41(1):14–18. [DOI] [PubMed] [Google Scholar]
- 16.Budtz-Lilly J, Venermo M, Debus S, et al. Assessment of International Outcomes of Intact Abdominal Aortic Aneurysm Repair over 9 Years. European Journal of Vascular & Endovascular Surgery. April 2017. [DOI] [PubMed] [Google Scholar]
- 17.Qureshi MA, Greenberg RK, Mastracci TM, Eagleton MJ, Hernandez AV. Patients with chronic obstructive pulmonary disease have shorter survival but superior endovascular outcomes after endovascular aneurysm repair. YMVA. 2012;56(4):911–919.e912. [DOI] [PubMed] [Google Scholar]
- 18.Turcios NL. Pulmonary Complications of Renal Disorders. Paediatric Respiratory Reviews. 2012;13(1):44–49. [DOI] [PubMed] [Google Scholar]
- 19.Pierson DJ. Respiratory considerations in the patient with renal failure. Respiratory Care. 2006;51(4):413–422. [PubMed] [Google Scholar]
- 20.Kraut JA, Kurtz I. Metabolic Acidosis of CKD: Diagnosis, Clinical Characteristics, and Treatment. American Journal of Kidney Diseases. 2005;45(6):978–993. [DOI] [PubMed] [Google Scholar]
- 21.Bruno CM, Valenti M, Bruno CM, Valenti M. Acid-Base Disorders in Patients with Chronic Obstructive Pulmonary Disease: A Pathophysiological Review. BioMed Research International. 2012;2012(7):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saggi BH, Ivatury R, Sugerman HJ. Abdominal compartment syndrome. 2001. [DOI] [PubMed] [Google Scholar]
- 23.Lee MJ, Daniels SL, Drake TM, Adam IJ. Risk factors for ischaemic colitis after surgery for abdominal aortic aneurysm: a systematic review and observational meta-analysis. Int J Colorectal Dis. 2016;31(7):1273–1281. [DOI] [PubMed] [Google Scholar]
- 24.Dariane C, Coscas R, Boulitrop C, et al. Acute Kidney Injury after Open Repair of Intact Abdominal Aortic Aneurysms. Annals of Vascular Surgery. November 2016. [DOI] [PubMed] [Google Scholar]
- 25.Katseni K, Chalkias A, Kotsis T, et al. The Effect of Perioperative Ischemia and Reperfusion on Multiorgan Dysfunction following Abdominal Aortic Aneurysm Repair. BioMed Research International. 2015;2015(3):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ersryd S, Djavani-Gidlund KA, Björck M. Editor’s Choice – Abdominal Compartment Syndrome After Surgery for Abdominal Aortic Aneurysm: A Nationwide Population Based Study. European Journal of Vascular & Endovascular Surgery. 2016;52(2):158–165. [DOI] [PubMed] [Google Scholar]
- 27.Wichmann JL, Katzberg RW, Litwin SE, et al. Contrast-Induced Nephropathy. Circulation. 2015;132(20):1931–1936. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.