Abstract
Consider the hypothetical case of a 75-year-old patient admitted to the intensive care unit (ICU) for acute hypoxic respiratory failure due to pneumonia and systolic heart failure. Although she suffers from a potentially treatable infection, her advanced age and chronic illness increase her risk of experiencing a poor outcome. Her family feels conflicted about whether the use of mechanical ventilation would be acceptable given what they understand about her values and preferences. In the ICU setting, clinicians, patients, and surrogate decision-makers frequently face challenges of prognostic uncertainty as well as uncertainty regarding patients’ goals and values. Time-limited trials (TLTs) of life-sustaining treatments in the ICU have been proposed as one strategy to help facilitate goal-concordant care in the midst of a complex and high-stakes decision-making environment. TLTs represent an agreement between clinicians and patients or surrogate decision-makers to employ a therapy for an agreed-upon time period, with a plan for subsequent reassessment of the patient’s progress according to previously-established criteria for improvement or decline. Herein, we review the concept of TLTs in intensive care, and explore their potential benefits, barriers, and challenges. Research demonstrates that, in practice, TLTs are conducted infrequently and often incompletely, and are challenged by system-level factors that diminish their effectiveness. The promise of TLTs in intensive care warrants continued research efforts, including implementation studies to improve adoption and fidelity, observational research to determine optimal timeframes for TLTs, and interventional trials to determine if TLTs ultimately improve the delivery of goal-concordant care in the ICU.
Keywords: Time-Limited Trials, End of Life Care, Goal-Concordant Care
Goal-Concordant Care in the Intensive Care Unit
For seriously ill patients admitted to an intensive care unit (ICU), the delivery of “goal-concordant care” is a fundamental priority. Goal-concordant care entails directing medical interventions towards patient-identified goals while respecting patients’ limitations on unacceptable interventions.1,2 Yet, delivering goal-concordant care for patients admitted to the ICU can be challenging. First, marked prognostic uncertainty early in the course of critical illness undermines the ability of ICU clinicians to accurately determine which goals (e.g., survival, functional recovery, maintenance of independence) are realistic for an individual patient based on the clinical context.3-5 Second, ICU clinicians do not typically have a longstanding relationship with their patients prior to ICU admission. Without a prior relationship, it can be challenging to establish trust,6,7 and it may take time to learn a patient’s values and preferences.8,9 Third, patients, their surrogate decision-makers, and their families are typically facing an unfamiliar, life-altering juncture at the time of ICU admission with little understanding of the medical interventions available to them in the ICU. Thus, patients and their surrogate decision makers may require support and time to understand the current situation, evaluate the interventions and range of potential outcomes, and establish goals by reconciling current realities with the patients’ underlying values and priorities.10 Lastly, surrogate decision makers may also face the perceived responsibility to pursue any possible intervention for the chance of a loved one’s recovery, the desire to not feel responsible for a love one’s death, and the pressure to maintain unity within the family unit in the midst of emotionally tumultuous circumstances.11
These challenges are exacerbated by system-level features of the ICU that generally promote invasive interventions and lack support for the complex, time-intensive process of providing goal-concordant care.12 The ICU environment is influenced by what has been previously labeled as “the technological imperative”13— the implicit notion that an intervention is “the correct moral treatment decision just because it exists as a technical option.”3 Implicit in this mindset are the biases that if a problem exists, action must be taken to fix it, and that, if a technological solution is possible, it must be attempted. This mindset fails to consider whether the use of a technological intervention (e.g. mechanical ventilation as a solution to the problem of hypoxic respiratory failure) helps to achieve a patient’s goal.13 The notion of the technological imperative and the intervention-permissive ICU environment contribute to a latent, system-level property of the ICU we recognize as “clinical momentum.”14 Clinical momentum is a phenomenon whereby individual signs, symptoms, and diagnoses are directly linked with their corresponding interventions, and one intervention often begets another. This process leads to an accumulation of invasive interventions over time and may bypass important opportunities to pause and consider how a trajectory of intervention aligns with a patient’s overarching goals of care.14
Time-limited trials (TLTs) of life-sustaining treatments in the ICU have been proposed as one strategy to help facilitate goal-concordant care in the midst of the complex and high-stakes decision-making environment of the ICU.3,8,15-17 Herein, we will review the concept of TLTs of intensive care, and explore the potential benefits, barriers, and challenges for TLTs, illustrated through the case of a hypothetical patient in the ICU.
A 75-year-old woman with chronic systolic heart failure develops acute hypoxic respiratory failure due to pneumonia and pulmonary edema. At the time of initial medical evaluation, she is encephalopathic and unable to participate in decision making about her care. The ICU team is concerned that her respiratory status will continue to deteriorate and she will soon require mechanical ventilation. Her family shares that she has never explicitly discussed her end-of-life wishes, but describes her as “a fighter, with a strong will to live”, but for whom long-term quality of life and independence is very important. She has previously commented that she would never want to be kept alive on machines or live in a nursing home. They want her to get better, but they also feel conflicted as to whether the use of mechanical ventilation is appropriate given what they understand about her values and preferences.
The Concept of a Time-Limited Trial in the ICU
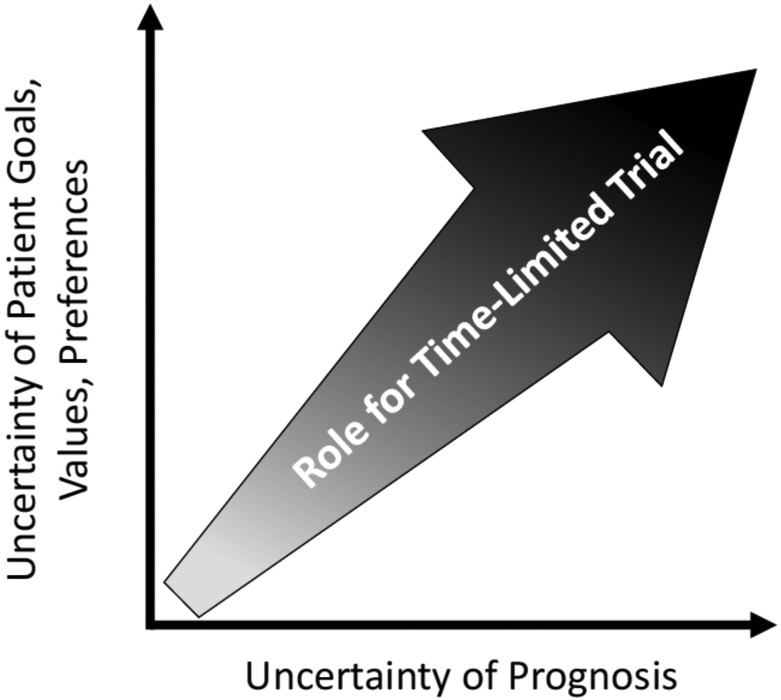
In one of the first formal descriptions of TLTs in the literature, Timothy Quill and Robert Holloway define TLT as “an agreement between clinicians and a patient/family to use certain medical therapies over a defined period to see if the patient improves or deteriorates according to agreed-on clinical outcomes.”8 TLTs, also known as “treatment trials,”11 have been described as a “third option” for patients, families, and clinicians who are facing high-stakes decisions about the pursuit of life-prolonging, intensive care or, alternatively, end-of-life care focused primarily on a patient’s comfort. This third option of a TLT alleviates the dichotomy of this decision and provides an opportunity to trial an intervention that can potentially restore the patient’s health and reevaluate its risks and benefits after the passage of time. TLTs are typically employed in the context of uncertainty,16 whether the uncertainty lies in prognosis, in the application of a patient’s goals, values, and preferences, or in both (Figure).
Figure.
Opportunities for time-limited trials of intensive care typically arise in the context of uncertainty around the patient’s prognosis (x-axis), uncertainty in how the patient’s goals, values, and treatment preferences ought to be applied in a given clinical context (y-axis), or both.
In a qualitative interview study of ICU physicians, investigators found unanimous recognition of the utility of TLTs. Participating physicians reported using TLTs for three distinct purposes: (1) to prepare for a shift toward comfort-focused end-of-life care, (2) to build consensus among clinicians, patients, surrogates, and families, and (3) to improve prognostic estimates with the passage of time.15 In addition to the broad arena of intensive care, TLTs have also been proposed as a mechanism to support goal-concordant care for patients after a stroke,18 for patients who face decisions about renal replacement therapy,3,19 for patients with chronic obstructive lung disease,20 for older patients with acute surgical conditions,17 and for critically ill patients with cancer.21,22
To begin a TLT, Quill and Holloway propose a five-step process (Table).8 First, ICU clinicians clearly define the patient’s clinical diagnosis and prognosis with and without the intervention or set of interventions in question. This involves consideration of not only factors related to the underlying disease, but also the patient’s insight into their condition and ability to participate in the decision-making process. Second, ICU clinicians elucidate the patient’s values and goals, including what treatments the patient would consider acceptable, and how the patient or their surrogate evaluates the level of uncertainty in prognosis that would justify or preclude any given treatment. Third, clinicians identify objective, measurable, and clearly defined markers for improvement or deterioration (e.g., improving creatinine and urine output in kidney failure, the ability to wean off vasoactive infusions, improving fraction of inspired oxygen in hypoxic respiratory failure, or improving mental status and interaction after stroke). Fourth, an appropriate, mutually determined time period for reevaluation is established. Quill and Holloway provide examples of “usual timeframes” of time-limited trials for specific conditions (e.g., 1 to 3 months of renal replacement therapy in a patient with limited cognitive status, or 3 to 7 days for a patient on mechanical ventilation for end-stage congestive heart failure). However, there are few empiric data to guide appropriate time frame selection and most experts acknowledge that TLT time frames must be individualized to account for the complexity of each patient’s circumstance.16 Finally, clinicians, patients and surrogates outline clear potential actions for follow up based on the patient’s clinical progress. These actions might include continuation of the intervention if there is evidence of benefit, extension of the proposed time-frame if the patient’s prognosis remains unclear, or discontinuation of the intervention in exchange for less invasive alternatives or comfort-focused interventions if the risks seem to outweigh the benefits. Potential actions at the end of the TLT should be described at the onset of the trial, and viewed not as binding contracts, but as a mutually useful tool to frame treatment decisions in the context of the patient’s changing condition. Neuman and colleagues describe this flexibility as a defining feature of TLTs, which should “explicitly recognize the possibility that an individual’s goals of care can change over time”, as part of “an uncertain process that will require iterative re-evaluation.”17
Table.
Potential Barriers and Facilitators to the Five Key Steps of a Time-Limited Trial
| Time-Limited Trial Key Step8 |
Potential Barriers | Potential Facilitators |
|---|---|---|
| Define clinical problem and prognosis | Diagnostic and Prognostic Uncertainty Multi-organ failure and/or multiple comorbid conditions15 Disagreement among clinicians8,15 |
Disease process with robust evidence of expected outcomes15 |
| Clarify patient’s goals and priorities | Disagreement among surrogates and family members8,15 Unknown or vague patient goals |
Concrete, measurable goals set at onset of TLT15 |
| Identify objective markers of improvement/deterioration | Unpredictable clinical course with new complications or diagnoses15 Specific criteria of effectiveness not discussed at onset of TLT11 |
Consistent, daily communication between clinicians, patients, surrogate decision makers16 |
| Suggest time frame for reevaluation | Lack of empiric data to suggest appropriate time frame8,16 Established time frame is too long or too short16 |
Time frame individualized and based on a patient’s clinical condition, the unique needs of the patient and their surrogates and family, and the specific interventions being trialed8,16 |
Despite widespread endorsement of TLTs, few empiric studies have described the use of TLTs in clinical practice.23 Schenker and colleagues audio-recorded and qualitatively analyzed 72 end-of-life care discussions between patients, families, and physicians in the ICU setting (including medical, surgical, and neurological specialties) to evaluate the use of TLTs.11 They found that TLTs are utilized infrequently and were presented as an option in only 13% of the recorded discussions. When implemented, TLTs were often missing essential key components, such as establishing the duration for the TLT, identifying the specific milestones for assessing clinical improvement or decline, or clarifying the potential next steps for action after TLT completion. Schenker’s investigation suggests that, despite the widespread recognition of the potential for TLTs to promote goal-concordant care, the adoption and fidelity of TLTs in clinical practice is low.
Back to the patient case: Given the potential reversibility of her acute condition, the patient’s family and physicians discuss the possibility of a time-limited trial of mechanical ventilation. To clearly guide the family through this process, the physician utilizes the 5 steps outlined by Quill and Holloway: They identify the patient’s major problem as respiratory failure secondary to heart failure and pneumonia. Her acute condition is a result of both a reversible condition (infection) and a chronic but acutely decompensated condition (heart failure), and her prognosis is guarded in light of her advanced age and chronic heart disease. After discussion, the family determines that the patient would want a chance at survival through limited, but not indefinite, use of mechanical ventilation, with the ultimate hope of living independently again. The physician proposes a time period of four days over which to monitor the patient’s progress. At the end of 3 days, if the patient’s oxygenation is not improving, they will discuss options for extubation to comfort-focused end-of-life care. If she markedly worsens during this time frame, they will not provide cardiopulmonary resuscitation and they will individually evaluate the value and burdens of any additional life-sustaining treatments. If her oxygenation is improving, they will discuss a plan for continued aggressive management of her infection and heart failure.
Benefits of Time-Limited Trials in the ICU
TLTs have the potential to promote goal-concordant ICU care, by providing a time period for the complex process of decision making to unfold for clinicians, patients, surrogate decision-makers, and their families. For clinicians, this passage of time may lessen the prognostic uncertainty and improve their ability to counsel patients and families about the range of expected outcomes and their respective likelihoods. TLTs may also help address the complex emotional needs faced by surrogate decision-makers and families when making difficult care decisions in the ICU.24 TLTs include the provision of a built-in time buffer for surrogate decision-makers and families to cognitively and emotionally process the condition of their loved one, to spend time with the patient, to reflect and communicate among themselves, and to coordinate logistics such as out-of-town travel.11,15 TLTs may allow surrogate decision-makers and families to feel that they allowed for any reasonable chance of recovery for their loved one —that “everything was done”—and by basing decisions to continue or withdraw interventions upon pre-agreed outcomes, relieve the burden of feeling that they are responsible for their family-member’s death.3,11,25
TLTs also provide an opportunity for clinicians, patients, surrogate decision-makers, and families to develop a trusting relationship in the ICU, which is known to influence the experience and delivery of end-of-life care in the ICU.6,7 This time period can be used as an iterative dialogue process that has the potential to decrease conflict between clinicians, patients, and families.8,16 In addition, the passage of time during a TLT may help mitigate the impact of decision-making biases that influence patients, families, and clinicians alike and are exacerbated by acute, stressful situations.26 The TLT, when conducted successfully, can become a shared communication platform and framework that facilitates negotiation and, therefore, relationship building between physicians, patients, surrogate decision-makers, and families.23
Concluding our case: While intubated, the patient was treated with empiric antibiotics and diuresis. Her oxygenation gradually improved, and she was extubated to nasal cannula on day three of her hospital stay. She was eventually discharged to a subacute rehab facility, and finally to home after four weeks of rehabilitation. After discharge, she reflected on these experiences with her family, primary care physician, and outpatient cardiologist. She determined that, while she was grateful for her recovery, if her breathing were to fail again in the future, she would elect to avoid intubation. She recognized that her heart condition was getting worse and that someday soon she would die; she decided to prioritize spending time with her family in the time preceding her death.
Challenges and Barriers to Time-Limited Trials in the ICU
Despite the potential benefits of TLTs, there are important challenges and barriers that limit their use and effectiveness (Table). According to ICU physicians, the most important barriers to effective TLTs are: division among family members, disagreement or inconsistency between members of the larger healthcare team (e.g., between consulting and ICU teams), and the utilization of arbitrary or unclear timelines or clinical end-points.15 ICU physicians also described how communication breakdowns caused by frequent handoffs and rotating call schedules, “impacted their ability to know whether a trial had been set, if the goals or variables of the trial had been determined, and whether there is a willingness to complete them.”15 Additionally, patients may develop new complications that challenge the boundaries or expectations of an established TLT (e.g., a patient with respiratory failure now develops shock and renal failure). Physicians also report greater difficulty in predicting timelines and establishing expectations for patients with multiple medical problems (e.g., multi-organ failure due to septic shock), compared to patients with single-organ failure (e.g., ischemic-anoxic brain injury), for whom the passage of time may lead to a clearer prognosis. Finally, despite statements of legal and ethical equivalence between treatment withdrawal and withholding, many physicians perceive a difference between the active processes of withdrawing life-sustaining treatments that have already been initiated as compared to withholding the same treatment.27-32 Surrogate decision-makers may also experience the act of treatment withdrawal as a burdensome decision, thus challenging the effectiveness of a TLT to improve and support this complex decision-making process.25,33 For these reasons, TLTs must be viewed not as one-time interventions to be followed algorithmically, but as an adaptable process requiring consistent communication and re-evaluation.17
ICU physicians readily acknowledge the difficulty in establishing an appropriate timeframe for a TLT,15 and there are very few published studies to help guide this decision. In a prospective clinical trial of an ICU triage process that included a TLT of ICU care for patients with cancer who required mechanical ventilation, investigators found that the pattern of organ failure progression was significantly different between survivors and nonsurvivors after six days of an ICU stay.22 Their findings suggest that six days may be an appropriate time period to consider for a TLT of ICU care in patients with cancer who develop acute respiratory failure. Another study using a decision-analysis microsimulation model demonstrated that, for patients with advanced malignancy due to solid tumors and high severity of acute critical illness, an ICU trial of one to four days may be sufficient to determine which patients are likely to achieve a survival benefit from ICU care.21 In this same study, the estimated duration of a TLT of ICU care for patients with hematologic malignancies or lower severity of acute critical illness was approximately two weeks.21 Thus, in the absence of clear and generalizable evidence, the time frame selected for a TLT must be individualized and based on a patient’s clinical condition, the unique needs of the patient and their surrogates and family, and the specific interventions being trialed.8,16
A Call for Continued Study of Time-Limited Trials in the ICU
Time-limited trials of intensive care, applied thoughtfully and in the appropriate context, can help achieve goal-concordant care for patients and support clinicians and surrogate decision makers during this complex, challenging process. Future work to improve the adoption and fidelity of TLTs in the ICU should include implementation studies of the five-step, consensus-based TLT process,8 to ensure that TLTs are considered when appropriate and are conducted in a clear, organized, and consistent fashion. To guide clinicians’ recommendations for the appropriate time frame of a TLT, further empiric research is necessary to clarify how patient and disease-specific prognostic estimates are affected by the passage of time. Additionally, qualitative studies that include patients, families, surrogate decision-makers, and the entire interprofessional ICU clinician team are necessary to improve our understanding of how TLTs impact experiences and outcomes for patients, families, and ICU clinicians. Finally, despite the promise and widespread support for TLTs, observational and interventional studies are necessary to measure the true impact of TLTs on the achievement of goal-concordant care in the ICU.
Acknowledgments
Sources of Support:
JMK: supported, in part, through NIH/NHLBI K23 HL146890. EMV: supported though NIH T32 HL7749-25. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The remaining authors report no relevant sources of support or conflicts of interest.
References
- 1.Sanders JJ, Curtis JR, Tulsky JA: Achieving Goal-Concordant Care: A Conceptual Model and Approach to Measuring Serious Illness Communication and Its Impact. J Palliat Med. 21:S17–S27, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Turnbull AE, Hartog CS: Goal-concordant care in the ICU: a conceptual framework for future research. Intensive Care Med. 43:1847–1849, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scherer JS, Holley JL: The Role of Time-Limited Trials in Dialysis Decision Making in Critically Ill Patients. Clin J Am Soc Nephrol. 11:344–53, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Detsky ME, Harhay MO, Bayard DF, et al. : Discriminative Accuracy of Physician and Nurse Predictions for Survival and Functional Outcomes 6 Months After an ICU Admission. JAMA. 317:2187–2195, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sinuff T, Adhikari NK, Cook DJ, et al. : Mortality predictions in the intensive care unit: comparing physicians with scoring systems. Crit Care Med. 34:878–85, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Hutchison PJ, McLaughlin K, Corbridge T, et al. : Dimensions and Role-Specific Mediators of Surrogate Trust in the ICU. Crit Care Med. 44:2208–2214, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnson SK, Bautista CA, Hong SY, et al. : An empirical study of surrogates’ preferred level of control over value-laden life support decisions in intensive care units. Am J Respir Crit Care Med. 183:915–21, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Quill TE, Holloway R: Time-limited trials near the end of life. JAMA. 306:1483–4, 2011 [DOI] [PubMed] [Google Scholar]
- 9.Scheunemann LP, Arnold RM, White DB: The facilitated values history: helping surrogates make authentic decisions for incapacitated patients with advanced illness. Am J Respir Crit Care Med. 186:480–6, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seaman JB, Arnold RM, Scheunemann LP, et al. : An Integrated Framework for Effective and Efficient Communication with Families in the Adult Intensive Care Unit. Ann Am Thorac Soc. 14:1015–1020, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schenker Y, Tiver GA, Hong SY, et al. : Discussion of treatment trials in intensive care. J Crit Care. 28:862–9, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lynn J, Arkes HR, Stevens M, et al. : Rethinking fundamental assumptions: SUPPORT’s implications for future reform. Study to Understand Prognoses and Preferences and Risks of Treatment. J Am Geriatr Soc. 48:S214–21, 2000 [DOI] [PubMed] [Google Scholar]
- 13.Hofmann B: Is there a technological imperative in health care? Int J Technol Assess Health Care. 18:675–89, 2002 [PubMed] [Google Scholar]
- 14.Kruser JM, Cox CE, Schwarze ML: Clinical Momentum in the Intensive Care Unit. A Latent Contributor to Unwanted Care. Ann Am Thorac Soc 14:426–431, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bruce CR, Liang C, Blumenthal-Barby JS, et al. : Barriers and Facilitators to Initiating and Completing Time-Limited Trials in Critical Care. Crit Care Med. 43:2535–43, 2015 [DOI] [PubMed] [Google Scholar]
- 16.Vink EE, Azoulay E, Caplan A, et al. : Time-limited trial of intensive care treatment: an overview of current literature. Intensive Care Med. 44:1369–1377, 2018 [DOI] [PubMed] [Google Scholar]
- 17.Neuman MD, Allen S, Schwarze ML, et al. : Using time-limited trials to improve surgical care for frail older adults. Ann Surg. 261:639–41, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Creutzfeldt CJ, Holloway RG: Treatment decisions after severe stroke: uncertainty and biases. Stroke. 43:3405–8, 2012 [DOI] [PubMed] [Google Scholar]
- 19.Lee GQ, Harrigan PR, Dong W, et al. : Comparison of population and 454 “deep” sequence analysis for HIV type 1 tropism versus the original trofile assay in non-B subtypes. AIDS Res Hum Retroviruses. 29:979–84, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fraenkel L, Street RL Jr., Towle V, et al. : A pilot randomized controlled trial of a decision support tool to improve the quality of communication and decision-making in individuals with atrial fibrillation. J Am Geriatr Soc. 60:1434–41, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shrime MG, Ferket BS, Scott DJ, et al. : Time-Limited Trials of Intensive Care for Critically Ill Patients With Cancer: How Long Is Long Enough? JAMA Oncol 2:76–83, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lecuyer L, Chevret S, Thiery G, et al. : The ICU trial: a new admission policy for cancer patients requiring mechanical ventilation. Crit Care Med. 35:808–14, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Aslakson R: Time-Limited Trials in the ICU: Seeing the Forest Beyond the Bark and Trees. Crit Care Med. 43:2676–8, 2015 [DOI] [PubMed] [Google Scholar]
- 24.Schenker Y, White DB, Crowley-Matoka M, et al. : “It hurts to know… and it helps”: exploring how surrogates in the ICU cope with prognostic information. J Palliat Med. 16:243–9, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schenker Y, Crowley-Matoka M, Dohan D, et al. : I don’t want to be the one saying ‘we should just let him die’: intrapersonal tensions experienced by surrogate decision makers in the ICU. J Gen Intern Med. 27:1657–65, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wilson ME, Hopkins RO, Brown SM: Long-Term Functional Outcome Data Should Not in General Be Used to Guide End-of-Life Decision-Making in the ICU. Crit Care Med. 47:264–267, 2019 [DOI] [PubMed] [Google Scholar]
- 27.Levin PD, Sprung CL: Withdrawing and withholding life-sustaining therapies are not the same. Crit Care. 9:230–2, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Phua J, Joynt GM, Nishimura M, et al. : Withholding and withdrawal of life-sustaining treatments in intensive care units in Asia. JAMA Intern Med. 175:363–71, 2015 [DOI] [PubMed] [Google Scholar]
- 29.Emmerich N, Gordijn B: Beyond the Equivalence Thesis: how to think about the ethics of withdrawing and withholding life-saving medical treatment. Theor Med Bioeth. 40:21–41, 2019 [DOI] [PubMed] [Google Scholar]
- 30.Luce JM: Withholding and withdrawing life support from the critically ill: how does it work in clinical practice? Respir Care. 36:417–26, 1991 [PubMed] [Google Scholar]
- 31.Luce JM, Alpers A: Legal aspects of withholding and withdrawing life support from critically ill patients in the United States and providing palliative care to them. Am J Respir Crit Care Med. 162:2029–32, 2000 [DOI] [PubMed] [Google Scholar]
- 32.Luce JM, White DB: The pressure to withhold or withdraw life-sustaining therapy from critically ill patients in the United States. Am J Respir Crit Care Med. 175:1104–8, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wiegand D: In their own time: the family experience during the process of withdrawal of life-sustaining therapy. J Palliat Med. 11:1115–21, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]