Abstract
Objective
The aim of this study was to evaluate the efficacy and safety of tranexamic acid (TXA) in elderly patients with intertrochanteric fracture undergoing intramedullary fixation surgery.
Methods
We searched MEDLINE, the Cochrane Library and EMBASE for published randomized clinical trials relevant to use of TXA in elderly patients with intertrochanteric fracture treated with intramedullary fixation surgery. Meta-analysis was performed according to the guidelines of the Cochrane Reviewer’s Hand book.
Results
Five trials assessing 540 patients were included for meta-analysis. The pooled results showed that the mean total blood loss in TXA group was significant lower than that in the control group (mean difference − 172.83, 95% CI −241.43 to −104.23; p<0.00001, fixed-effect model). The intra- and postoperative transfusion rate for the TXA group was 34.4% (91/264) and for the control group was 49.27% (136/276), and the relative risk was 0.71 (95% CI 0.52 to 0.97; p<0.03, random-effect model) with substantial heterogeneity (I2=63%, p=0.03). The overall incidence of thrombotic events was 6.43% (17/264) in the intravenous TXA group, 7.63% (21/275) in the control group, with no significant difference (relative risk 0.84, 95% CI 0.46 to 1.54; p=0.57, fixed-effect model).
Conclusion
The present evidence shows that TXA can significantly reduce total and hidden blood loss, transfusion rate, and do not increase the risk of thrombotic events in elderly patients with intertrochanteric fracture undergoing intramedullary fixation surgery. However, the impact of TXA on thrombotic events needs to be researched in more high-quality, large-sample randomized clinical trials.
Level of Evidence
Level I Therapeutic Study
Keywords: Blood loss, Tranexamic acid, Transfusion, Intertrochanteric fractures, Randomized clinical trials, Meta-analysis
The incidence of intertrochanteric fractures (IFs) in elderly patients is increasing significantly as the population ages (1). Conservative treatment of IFs in elderly patients easily leads to pulmonary infection, pressure sores, urinary tract infections, and other complications, and the mortality and disability rate of conservative treatment are high. For these reasons, surgical treatment for these fractures is now recommended (2). Intramedullary fixation, such as by proximal femoral nail antirotation (PFNA), is widely used in the treatment of IFs among the elderly because of its minimally invasive procedure, reduced intra-operative blood loss, and reliable fixation (3, 4). However, reports in the literature show that significant hidden blood loss (HBL) after operation was observed, and that up to 28.3% of patients with stable intertrochanteric fractures (Evans grades I and II) required allogeneic blood transfusion (5). This patterns suggests that it is necessary to take blood protection measures for these patients.
Although the efficacy and safety of tranexamic acid (TXA) in reducing blood loss in major orthopedic operations have been confirmed by many clinical studies (6–8), the recommendation of TXA use in such minimally invasive surgery remains to be clarified, since HBL accounts for the vast majority of total blood loss. In recent years, some clinical studies focusing on this question have been published. However, most of these studies have small sample studies, with concomitant low test efficacy. In the present study, a meta-analysis of randomized controlled trials (RCTs) was conducted to evaluate the efficacy and safety of TXA in elderly patients with IFs treated by intramedullary fixation surgery.
Materials and Methods
Criteria for considering studies for this meta-analysis
All RCTs comparing a TXA group with a control group in elderly patients with IFs treated with intramedullary fixation surgery were eligible. Baseline characteristics of the experimental group and the control group were comparable. RCTs were excluded if they (1) were low-quality RCTs (see “Quality Assessment” below); (2) included patients younger than 60 years of age; (3) had incomplete reporting of primary outcomes or lost more than 20% participants prior to follow-up; (4) were repeated publication studies; (5) were published in a language other than English. We limited the literature search to only those studies of intertrochanteric fractures treated by intramedullary fixation aimed to evaluate a particular group of patients who have homogeneous features. The present study was registered at the International Prospective Register of Systematic Reviews (ID: CRD42019121457).
Outcome measures
The primary outcomes were total blood loss (TBL), transfusion rate, and transfusion volume. The secondary outcomes were intra- operative visible blood loss, HBL, postoperative hemoglobin values (Hb), the occurrence of thrombotic events including deep venous thrombosis, pulmonary embolism, cerebral infarction, and mortality within 3 months.
Search methods for identification of studies
Two reviewers independently searched the MEDLINE (1975–December 2018), Cochrane Library (Issue 12, 2018), and EMBASE (1975–December 2018). The keywords were “tranexamic acid,” “intertrochanteric fracture,” “hip fracture,” and “randomized controlled trial.” Furthermore the reviewers read and retrieved references from the literature as a supplementary method for finding studies that met the inclusion criteria.
Quality assessment
Methodological quality of included trials was evaluated from six perspectives: random sequence generation, allocation concealment, blindness, addressing of incomplete outcome data, selective reporting, and other bias risks, as recommended by the Cochrane Collaboration. This recommendation includes a description and a judgment for each entry in a “risk of bias” table, in which each entry addresses a specific feature of the study. The judgment for each entry involves answering a question, with answers “Yes” indicating low risk of bias, “No” indicating high risk of bias, and “Unclear” indicating either lack of information or uncertainty about the potential for bias (9). The quality of included trials was evaluated independently by two evaluators. Differences between the two evaluators were resolved by discussion.
Data synthesis and analysis
Two evaluators extracted data from the included studies, using a pre-designed data abstraction form. One extracted the research data, and the other checked the extracted data, with any controversy resolved through discussion. The outcome measures and general characteristics of the included studies were extracted, including study site, publication date, demographic characteristics, follow-up time, and treatment method. Data analysis used RevMan 5.0 software, with p<0.05 considered statistically significant. Relative risk (RR) and 95% confidence interval (CI) were used for dichotomous outcomes; mean difference (MD) and 95% CI were used for continuous outcomes (10). Chi-square tests were used to evaluate heterogeneity between groups, with the test level set to p<0.1 (11). I2 statistics were used to quantify heterogeneity. I2<25% indicated that there was no substantial heterogeneity between the results, 25%≤I2<50% indicated moderate heterogeneity, and I2≥50% indicated significant or substantial heterogeneity. When p>0.1, the summary effect size was analyzed by the fixed-effect model, but when p<0.1 and I2>50%, the random-effect model was used (12). Subgroup analysis was performed to explore the sources of heterogeneity, such as clinical heterogeneity and methodological heterogeneity.
The funnel diagram of meta-analysis, drawn using Revman 5.0 software, was used to check for publication bias. Fail-safe number (Nfs) was also used to evaluate publication bias. When the level of significance was set to 0.05, Nfs 0.05=(∑Z/1.645)2−K, in which Z is the Z value of an independent study, and K is the number of studies included (13). According to the standard recommended by Rosenthal, when Nfs 0.05 is greater than 5K+l0, the publication bias is considered to be effectively controlled (14).
Results
Description of studies
The paths of studies from initial search results to final inclusion or exclusion are shown in the flowchart (Figure 1). A total of 5 RCTs were included to final analysis (15–19). A total of 540 patients were enrolled in the five studies, including 264 patients in TXA groups and 276 patients in control groups. There was no significant difference in baseline between the two groups. The general characteristics of the included studies are shown in Table 1. Methodological quality assessment is shown in Table 2.
Figure 1.
Flow chart of searches (created using Preferred Reporting Items for Systematic Reviews and Meta-Analyses [PRISMA] 2009 Flow Diagram, version 2.1.3)
Table 1.
General characteristics of included studies
| Author and publication date | Number of patients enrolled TXA/Control | Mean age (years) TXA/Control | Fracture types | internal fixation methods | Anesthesia | Intervention methods | preventive measures for thrombotic events | Blood transfusion threshold | Lost to follow-up | |
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| TXA | Control | |||||||||
| Drakos 2016 | 200(100/10) | 81/80.7 | AO 31.A1–3 | Gamma nail | Epidural or general anesthesia | Local administration TXA 3g (500mg/5mL x6) | No TXA | Low-molecular- weight heparin for 30 days | Hb <8 g/dL or Hct <25% | No |
| Tengberg 2016 | 72(33/39) | 79.8/75.0 | AO 31A2.2–A3 | IMN | Epidural | 1g ivgtt TXA before surgery, 3 g post-operative 24-h infusion | placebo | Low-molecular- weight heparin | Hb <9.67 g/dL | No |
| Lei 2017 | 80(39/41) | 77.8/79.2 | AO 31.A 1–3 | PFNA | NR | TXA 1 g+NS 200 mL ivgtt | NS 200 mL ivgtt | NR | NR | 3 (3.7%) |
| Tian 2018 | 100(50/5) | 77.74/79.25 | AO 31.A 1–3 | PFNA | NR | TXA ( 10 mg/kg) ivgtt 15 minutes before operation and 3 hours after operation | No TXA | Low-molecular- weight heparin used before and operation | Hb <9g/dL | No |
| Luo 2019 | 100 (50/50) | 75.1/76.1 | AO 31.A 1–3 | PFNA | Spinal or general anesthesia | TXA (10mg/kg)+ NS 100 mL ivgtt 15 minutes before operation and 3 hours after operation | NS 100 mL ivgtt 15 minutes before operation | Low-molecular- weight heparin was administered every 24 hours postoperatively for 2 weeks | Hb <8g g/L | 10 (10%) |
PFNA: proximal femoral nail antirotation; Hct: hematocrit; Hb: hemoglobin; ivgtt: intravenously guttae; IMN: intramedullary nail
NS: normal saline; NR: not reported; TXA: tranexamic acid
Table 2.
Methodological quality assessment of included studies
| Study and publication date | Adequate sequence generation? | Allocation concealment? | Blinding? | Incomplete outcome data addressed? | Free of selective reporting? | Free of other bias? |
|---|---|---|---|---|---|---|
| Drakos 2016 | Yes | Yes | Yes | Yes | Yes | Unclear |
| Tengberg 2016 | Yes | Yes | Yes | Yes | Yes | Yes |
| Lei 2017 | Yes | Unclear | Unclear | Yes | Yes | Unclear |
| Tian 2018 | Yes | Unclear | Unclear | Yes | Yes | Yes |
| Luo 2019 | Yes | Yes | Yes | No | Yes | Unclear |
Meta-analysis results
TBL
Four included studies had reported TBL results (15, 17–19). The pooled results indicated that the TBL in the TXA group was significantly lower than in the control group (MD=–172.83, 95% CI −241.43 to −104.23; p<0.00001, Fixed-effect model; Figure 2). Nfs 0.05=35, that is say, to reverse the pooled results, 35 studies with negative results would be required, indicating that the pooled results were stable. Table 3 shows a summary of the findings.
Figure 2.
Forest plot of mean differences with confidence intervals for total blood loss
Table 3.
Summary of findings
| Outcomes | Studies | Participants | Statistical method | Effect estimate (95%CI) | p |
|---|---|---|---|---|---|
| TBL | 4 | 339 | Mean Difference (IV, Fixed, 95% CI) | −172.83 [−241.43, −104.23] | −0.00001 |
| Intra-operative visible blood loss | 4 | 339 | Mean Difference (IV, Fixed, 95% CI) | −33.46 [−52.40, −14.52] | 0.0005 |
| HBL | 3 | 267 | Mean Difference (IV, Fixed, 95% CI) | −144.20 [−210.74, −77.66] | −0.0001 |
| Intra- and postoperative transfusion rate | 5 | 540 | Risk Ratio (M-H,Random, 95% CI) | 0.71 [0.52, 0.97] | −0.03 |
| Intra- and postoperative transfusion rate | 5 | 540 | Risk Difference (M-H, Random, 95% CI) | −0.14 [−0.21, −0.07] | −0.0002 |
| Intra-and postoperative transfusion volume | 5 | 540 | Mean Difference (IV, Random, 95% CI) | −0.43 [−0.63, −0.23] | −0.0001 |
| Hb values on postoperative day 3 | 3 | 367 | Mean Difference (M-H, Fixed, 95% CI) | 0.32 [−0.09, 0.74] | =0.13 |
| Thrombotic events | 5 | 539 | Risk Ratio (M-H, Fixed, 95% CI) | 0.84[0.46, 1.54] | 0.57 |
| Thrombotic events | 5 | 539 | Risk Difference (M-H, Fixed, 95% CI) | −0.01[−0.06, 0.03] | 0.55 |
| Thrombotic events (subgroup analysis) | 4 | 339 | Risk Ratio (M-H,Random, 95% CI) | 0.76 [0.30, 1.90] | =0.56 |
| Mortality on postoperative day 30 | 3 | 239 | Risk Ratio (M-H, Random, 95% CI) | 1.69[0.20, 14.2] | 0.63 |
TBL: total blood loss; HBL: hidden blood loss; Hb: hemoglobin
Intra-operative visible blood loss
Four studies reported results of intra-operative visible blood loss (15, 17–19). The pooled results indicated that there was a significant difference between the two groups (MD=−33.46, CI −52.40 to −14.52; p=0.005, fixed-effect model). No heterogeneity was observed (p<0.51, I2=0%; Figure 3).
Figure 3.
Forest plot of mean differences with confidence intervals for intra-operative visible blood loss
HBL
Three studies reported HBL results (17–19). No significant heterogeneity was detected among the included studies (p<0.93, I2=0%). Therefore, a fixed-effect model was used to analyze the combined effect. This model found a significant difference between the two groups (MD=−144.20, 95% CI −210.74 to −77.66; p7<0.0001; Figure 4). The pooled results indicated that TXA significantly reduced postoperative HBL compared with the control group.
Figure 4.
Forest plot of mean differences with confidence intervals for hidden blood loss
Transfusion
Five studies reported intra- and postoperative transfusion rate (15–19). The intra- and postoperative transfusion incidence for the TXA group was 34.4% (91/264) and for the control group was 49.27% (136/276). The RR was 0.71 (95% CI 0.52 to 0.97; p=0.03, random-effect model) with substantial heterogeneity (I2=63%, p=0.03), showing that there was significant difference between the two groups (Figure 5). Compared with the control group, the TXA group reduced the RR of intra- and postoperative transfusion by 29% and the absolute risk by 14% (RD=−0.14, 95% CI −0.21 to −0.07; p<0.0002, fixed-effect model; Figure 6). Nfs 0.05=17, demonstrating that 17 studies with negative results would be required to reverse the pooled results. The use of TXA reduced the mean concentrated red blood cell (CRBC) infusion by 0.43 unit per patient (95% CI −0.63 to −0.23; p<0.0001; Figure 7).
Figure 5.
Forest plot of relative risk with confidence intervals for transfusion rate
Figure 6.
Forest plot of absolute risk with confidence intervals for transfusion rate
Figure 7.
Forest plot of mean differences with confidence intervals for concentrated red blood cell infusion
Hemoglobin values on postoperative day 3
Three studies reported Hb values on postoperative day three (16, 17, 19). The study by Drokos did not report standard deviations and so was not included to the combined analysis. The pooled results showed that there was no significant difference between the two groups (MD=0.32, 95% CI −0.09 to 0.74; p=0.13, fixed-effect model; Figure 8), with mild heterogeneity (p=0.24, I2=27%).
Figure 8.
Forest plot of mean differences with confidence intervals for Hb values on postoperative day 3
Thrombotic events
Five studies provided data on postoperative thrombotic events (15–19). The pooled results demonstrated that there was no significant difference between the two groups (RR=0.84, 95% CI 0.46 to 1.54; p=0.57, fixed-effect model), with no evidence of heterogeneity (p=0.59, I2=0%; Figure 9, 10). In subgroup analysis, the incidence of thrombotic events was 4.26% (7/164) in the intravenous TXA group, and 5.71% (10/175) in the control group (RR=0.76, 95% CI 0.30 to 1.90; p=0.56, fixed-effect model) with no heterogeneity, showing that intravenous TXA did not increase the risk of thrombotic events. However, Nfs 0.05<1,indicating an unstable meta-analysis result.
Figure 9.
Forest plot of relative risk with confidence intervals for thrombotic events
Figure 10.
Forest plot of absolute risk with confidence intervals for thrombotic events
Mortality on postoperative day 30
Three studies reported mortality on postoperative day thirty (15, 17, 19). The RR of mortality on postoperative day 30 was 1.69 (95% CI 0.20 to 14.2; p=0.63, random-effect model), with moderate heterogeneity (p=0.18, I2=44%), implying there was no significant difference between the compared groups (Figure 11).
Figure 11.
Forest plot of relative risk with confidence intervals for mortality on postoperative day 30
Publication bias
The funnel plot of transfusion volume and thrombotic events showed that the scattered points were roughly symmetrical, suggesting that publication bias among the included studies was mild (Figure 12, 13).
Figure 12.

Funnel plot of transfusion volume
Figure 13.

Funnel plot of thrombotic events
Discussion
In recent years, several systematic reviews and meta-analyses of TXA in hip fracture surgery have been published. Farrow et al. conducted a systematic review of the use of TXA in hip fracture surgery in 2016 including 7 RCTs (20). The authors believed that there was moderate-quality evidence demonstrating that TXA can reduce blood loss in hip fracture surgery, and that there was lower-quality evidence suggesting that TXA does not increase the risk of thrombotic events. Wang et al. published a meta-analysis in 2017 that included 4 RCTs on TXA topical use in IFs surgery (21). The results showed that topical use of TXA in IFs surgery could effectively reduce TBL and transfusion rate. Zhang et al. performed a meta-analysis (8 RCTs) on the use of TXA for hemostasis in hip fracture surgery, finding that intravenous use of TXA can effectively reduce TBL and transfusion rate (22). Zhu et al. reported a meta-analysis (7 RCTs) of TXA for internal fixation of IFs in 2018 (23). Their pooled results showed that TXA could effectively reduce TBL, but had no significant impact on blood transfusion rate or on the risk of thrombotic events. An updated meta-analysis including 11 RCTs indicated that intravenous TXA efficaciously reduces TBL and transfusion rate during hip fracture surgery without significantly increasing the risk of total thromboembolic events (24). However, all previous systematic reviews and meta-analyses included RCTs containing different ages groups, fracture types, and surgical methods, leading to significant clinical heterogeneity in their combined outcomes. In order to reduce clinical heterogeneity, we defined the inclusion criteria as closed reduction and intramedullary fixation surgery for IFs among the elderly. Five high-quality RCTs were therefore included in the meta- analysis. A funnel plot test showed no obvious publication bias in the present study. The combined results confirmed that TXA significantly reduced TBL, HBL, and transfusion rate, and did not increase the risk of thrombotic events in elderly patients with IFs undergoing intramedullary fixation surgery.
The combined effect of 4 RCTs showed that TXA could reduce TBL per capita by 172 mL (95% CI −241.43 to −104.23; p<0.00001) in elderly patients with IFs. This finding is clinically significant: Because IFs in elderly patients are often accompanied by anemia and many medical complications prior to operation, these patients’ tolerance of ischemia during operation is poor. TXA helps to maintain hemodynamic stability by reducing blood loss, and thus reduces the risk of cardio-cerebrovascular episodes induced by hemodynamic fluctuations. Because there are no major methodological flaws in each study, and the effects of each study are significant and consistent, we interpret the combined effect as strong evidence confirming the hemostatic effect of TXA when used in IFs surgery. In addition, Nfs 0.05=35, that is say, 35 studies with negative results would be required in order to reverse the pooled results, indicating that the pooled results are stable.
Patient statistics on postoperative blood transfusion and Hb level further verify the effect of TXA on reducing blood loss. The intra- and postoperative transfusion rates in the TXA group and the control group were 34.4% and 49.2% respectively. The combined effect RR was 0.71 (95% CI 0.52 to 0.97; p<0.03), indicating that TXA reduced the risk of transfusion by 29% compared with the control group. The calculated number of patients needed to be treat equals 6.75, meaning that one patient can be prevented from receiving transfusion therapy for every ~7 patients who use TXA. Although the average Hb level on operative day 3 was similar for the two groups (MD=0.32, 95% CI −0.09 to 0.74; p<0.13), patients in the control group received more units of CRBC infusion per capita than patients in the TXA group (MD=0.43, 95% CI −0.63 to −0.23; p<0.0001). These findings demonstrate that TXA has a definite hemostatic effect on the treatment of IFs among the elderly.
According to past research, the rate of asymptomatic deep venous thrombosis in major orthopedic surgery without anticoagulation is 30–80% (25, 26). Although the evidence suggests that TXA has a definite hemostatic effect, patients and doctors are concerned about the possible increase in risk of thrombotic events when intravenous antifibrinolytic therapy is used with TXA. In the present meta-analysis, we found that the incidence of thrombotic events was 4.26% (7/164) in the intravenous TXA group and 5.71% (10/175) in control group, with a RR of 0.76 (95% CI 0.30 to 1.90; p=0.56). The pooled results show that intravenous TXA in IFs surgery in the elderly does not significantly increase the risk of thrombotic events. Interestingly, the combined effects seem to favor TXA in the forest plot of thrombotic events. Although the difference was not statistically significant, this phenomenon was confirmed by a large-sample study. Roberts et al. reported that a stratified analysis of data from an international multicenter RCT (CRASH-2 trial) revealed a significant reduction in the risk of arterial thrombosis in patients treated with TXA (ratio 0.58, 95% CI 0.40 to 0.83; p=0.003), though not in the risk of venous thrombosis (ratio 0.83, 95% CI 0.59 to 1.17; p=0.295) (27). Franchini et al. published a meta-analysis regarding the safety of intravenous TXA in orthopedic surgery, in which a total of 73 RCTs including 4174 patients in the TXA group and 2779 in the control group were combined for analysis (28). The main safety measures were venous thromboembolism. The combined results showed that the incidence of venous thromboembolism was 2.1% in the TXA group and 2.0% in the control group, with a risk ratio of 1.067 (95% CI 0.760, to 1.496), again no significant difference. Current evidence suggests that intravenous administration of TXA is a safe drug therapy for reducing blood loss and transfusion requirements in major orthopedic surgery.
The major limitation of the present meta-analysis is the relatively small number of RCTs included. Although Zufferey et al.’s study contains 12 patients treated with intramedullary nail in whom tranexamic acid was used and 10 additional patients who were chosen as the placebo group, the RCT by Zufferey et al. (8) was not combined to meta-analysis. It was excluded because the authors did not separately report results for the 22 patients treated with intramedullary nail, and data could not be extracted for these patients. Although there is no statistical difference in the combined effects when it comes to thrombotic events, a possible real difference fail to be detected because of small sample size and insufficient statistical power. In addition, Nfs 0.05<1, indicating that the meta-analysis result for thrombotic events was unstable. Therefore, the evidence strength is considered moderate, and additional high-quality RCTs are needed to strengthen the findings.
Currently, there are few published RCTs assessing topical use of TXA in elderly patients with IFs undergoing intramedullary fixation. Whether topical use of TXA could equally effectively reduce surgical blood loss and transfusion deserves further research. Because the vast majority of studies on TXA excluded high-risk patients, such as those with previous venous embolism, cerebral infarction, myocardial infarction, and stenting, it is unclear whether TXA increased the risk of thrombotic events in patients with high risk of thrombosis. Previously published studies mainly focused on venous thrombosis in assessing the safety of TXA. However, other literature implies that TXA has a different impact on arteriovenous thrombosis. Future studies should report arterial and venous thrombotic events separately in assessing the safety of TXA.
In conclusion, the present evidence shows that TXA can significantly reduce TBL and HBL, as well as allogeneic blood transfusion rate, all without increasing the risk of thrombotic events in elderly patients with IFs undergoing intramedullary fixation surgery. However, the impact of TXA on thrombotic events needs to be researched in additional high-quality, large-sample RCTs.
| Section/topic | # | Checklist item | Reported on page # |
|---|---|---|---|
| Title | 1 | Efficacy and safety of tranexamic acid for reducing blood loss in elderly patients with intertrochanteric fracture treated with intramedullary fixation surgery: A meta-analysis of randomized controlled trials | 1 |
| ABSTRACT | |||
| Structured summary | 2 | Objectives: The aim of this study was to evaluate the efficacy and safety of tranexamic acid(TXA) in elderly patients with intertrochanteric fracture undergoing intramedullary fixation surgery. Methods: We searched MEDLINE, the Cochrane Library and EMBASE for published randomized clinical trials relevant to use of TXA in elderly patients with intertrochanteric fracture treated with intramedullary fixation surgery. Meta-analysis was performed according to the guidelines of the Cochrane Reviewer’s Hand book. Results: Five trials assessing 540 patients were included for meta-analysis. The pooled results showed that the mean total blood loss in TXA group was significant lower than that in the control group (mean difference −172.83, 95% CI −241.43 to −104.23; P < 0.00001, fixed-effect model). The intra- and postoperative transfusion rate for the TXA group was 34.4% (91/264) and for the control group was 49.2.4% (136/276), and the relative risk was 0.71 (95% CI 0.52 to 0.97; P < 0.03, random-effect model) with substantial heterogeneity (I2 = 63%, P = 0.03). The overall incidence of thrombotic events was 6.43% (17/264) in the intravenous TXA group, 7.63% (21/275) in the control group, with no significant difference (relative risk 0.84, 95% CI 0.46 to 1.54; P = 0.57, fixed-effect model) . Conclusion: The present evidence shows that TXA can significantly reduce total and hidden blood loss, transfusion rate,and do not increase the risk of thrombotic events in elderly patients with intertrochanteric fracture undergoing intramedullary fixation surgery. However, the impact of TXA on thrombotic events needs to be researched in more high-quality, large-sample randomized clinical trials. |
1 |
| INTRODUCTION | |||
| Rationale | 3 | Intramedullary fixation system, such as proximal femoral nail antirotation (PFNA), is widely used in the treatment of IFs in the elderly because of its minimally invasive procedure, less intra-operative blood loss and reliable fixation[3,4]. However, literature reported that significant hidden blood loss (HBL) after operation was observed and up to 41.6% of patients in this age-group required allogeneic blood transfusion[5], sugessting that it is necessary to take blood protection measures for these patients. Although the efficacy and safety of tranexamic acid (TXA) in reducing blood loss in major orthopaedic operations have been confirmed by many clinical studies[6–8],whether use of TXA in such minimally invasive surgery has not yet been clarified, of which hidden blood loss accounts for the vast majority of total blood loss. In recent years, some clinical studies focusing on this question have been published. However, most of them are small sample studies with low test efficacy. | 3 |
| Objectives | 4 | In this study, a meta-analysis of RCTs was conducted to evaluate the efficacy and safety of TXA in elderly patients with IF treated with intramedullary fixation surgery. | 3 |
| METHODS | |||
| Protocol and registration | 5 | A protocol was made before we carried out this meta-analysis. The study was registered at International Prospective Register of Systematic Reviews(ID:CRD42019121457). | 3 |
| Eligibility criteria | 6 | All RCTs comparing TXA group with a control group in elderly patients with IFs treated with intramedullary fixation surgery were eligible.Baseline characteristics of the experimental group and the control group were comparable. RCTs were excluded if they (1) were low-quality RCTs; (2) included patients younger than 60 years of age; (3) had incomplete reporting of primary outcomes or more than 20% participants lost to follow up; (4) were repeated publication study; (5) non-English written articles. | |
| Information sources | 7 | Two reviewers independently searched the MEDLINE (1975–12, 2018), Cochrane Library (Issue 12, 2018), EMBASE (1975–12, 2018), China Biology Medicine disc 1978–12, 2018), China National Knowledge Infrastructure (1999–12, 2018) and Wanfang Database (1980–12, 2018). | 5 |
| Search | 8 | (((((((((((((randomized controlled trial[Publication Type]) OR controlled clinical trial[Publication Type]) OR randomized[Title/Abstract]) OR placebo[Title/Abstract]) OR randomly[Title/Abstract]) OR trial[Title/Abstract]) OR groups[Title/Abstract])) AND humans)))))) AND ((((hip fracture[Title/Abstract]) OR intertrochanteric fracture[Title/Abstract])) AND tranexamic acid[Title/Abstract]) | |
| Study selection | 9 | Two reviewers independently searched the MEDLINE (1975–12, 2018), Cochrane Library (Issue 12, 2018), EMBASE (1975–12, 2018). Furthermore, the reviewers read and retrieved references from the literature as a supplementary method for finding studies that meets the inclusion criteria. | 4 |
| Data collection process | 10 | Two evaluators extract data from the included studies, using a pre-designed data abstraction forms. One extracted the research data, and the other checked the extracted data, where controversy was resolved through discussion. The outcome measures and general characteristics of the included studies were extracted, including the study site, publication date, demographic characteristics, follow-up time and treatment methods. | 5 |
| Data items | 11 | The primary outcomes were TBL, transfusion rate and transfusion volume. The secondary outcome was intra-operative visible blood loss, HBL, postoperative hemoglobin values (Hb), the occurrence of thrombotic events, including deep venous thrombosis, pulmonary embolism, cerebral infarction, and mortality within 3 months. | 4 |
| Risk of bias in individual studies | 12 | Methodological quality of included trials was evaluated from six aspects: random sequence generation, allocation concealment, blindness, incomplete outcome data addressed, selective reporting, and other bias risks, which was recommended by the Cochrane Collaboration. This comprises a description and a judgement for each entry in a “risk of bias” table, where each entry addresses a specific feature of the study. The judgement for each entry involves answering a question, with answers “Yes” indicating low risk of bias, “No” indicating high risk of bias, and “Unclear” indicating either lack of information or uncertainty over the potential for bias[9]. The quality of included trials was evaluated independently by two evaluators. If there is any difference, the decision will be made after discussion. | 5 |
| Summary measures | 13 | The efficacy outcomes were TBL, visible blood loss, HBL, transfusion rate and transfusion volume, postoperative hemoglobin values (Hb). The safety outcome was the occurrence of thrombotic events, including deep venous thrombosis, pulmonary embolism, cerebral infarction, and mortality within 3 months. | 4 |
| Synthesis of results | 14 | Data analysis using RevMan 5.0 software, P <0.05 is considered statistically significant difference. Relative risk (RR) and 95% confidence interval (CI) were used for dichotomous outcomes, mean difference (MD) and 95% CI [10] were used for continuous outcomes. Chi-square test was used to evaluate the heterogeneity between groups, and the test level was set to P < 0.1 [12]. I2 statistics was used to quantify the heterogeneity. I2 < 25% indicated that there was no substantial heterogeneity between the results, 25% < I2 < 50% indicated moderate heterogeneity, I2 > 50% indicated significant or substantial heterogeneity. When P > 0.1 the summary effect sizes was analyzed by the fixed-effect model, and when P < 0.1 and I 2 > 50% random-effect model was used [12]. Subgroup analysis was performed to explore the sources of heterogeneity, such as clinical heterogeneity and methodological heterogeneity. | 5 |
| Risk of bias across studies | 15 | The funnel diagram of meta-analysis was draw using Revman 5.0 software, from which the publication bias is checked. Fail-safe number ((Nfs) is also used to evaluate the publication bias. When the level of significance is set to 0.05, Nfs 0.05 = (∑Z/1.645) 2-K, in which Z is the Z value of independent study, K is the number of studies included. According to the standard recommended by Rosenthal [14], when Nfs 0.05 is greater than 5K + l, the publication bias is considered to be effectively controlled. | 6 |
| Additional analyses | 16 | Subgroup analysis was performed to explore the sources of heterogeneity, such as clinical heterogeneity and methodological heterogeneity. | 5 |
| RESULTS | |||
| Study selection | 17 | Studies from initial searches results to final inclusion or exclusion are showed in the flowchart (Fig. 1). A total of 5 RCTs were included to final analysis. | 6 |
| Study characteristics | 18 | There were 1166 patients enrolled in the 10 studies, including 582 patients in TXA group and 584 patients in the control group. There was no significant difference in baseline between the two groups. The general characteristics of the included studies are shown in Table 1. | 6 |
| Risk of bias within studies | 19 | Methodological quality assessment is shown in Table 2. | 6 |
| Results of individual studies | 20 | Summary data of Tengberg 2016 The TBL in in the TXA group was significantly lower than that in the the control group(MD=−570,95% CI −1071.04 to −70.56;P=0.003). There was no significant difference in the comparison of intra-operative blood loss and transfusion volume. The rater of patients that required blood transfusion ,thrombotic events and mortality on post-operative day 30 were not significant different between the two groups. More details of outcomes can be seen in Fig. (1).(2) |
|
Fig. (1).
Forest plot of outcomes of Tengberg 2016
Fig. (2).
Forest plot of outcomes of Tengberg 2016
Summary data of Drakos 2016
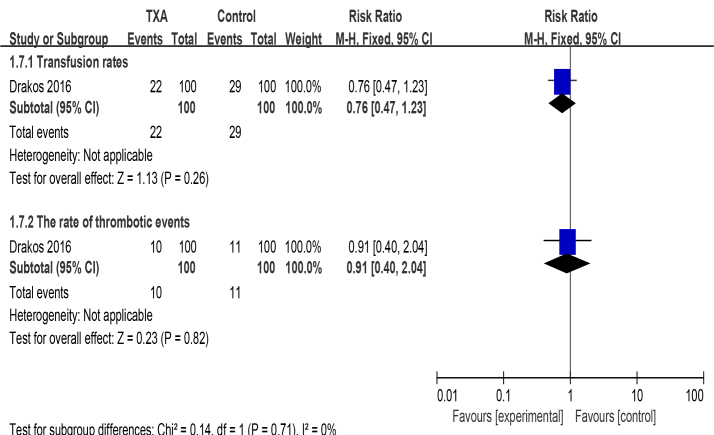
The number of patients that required blood transfusions was lower in the TXA group (22% vs 29%) than the control group(P=0.26). There was no significant difference in the incidence of thrombotic complications between the two groups (P >0.82). More details of outcomes can be seen in Fig. (3).(4)
Fig. (3).
Forest plot of outcomes of Drakos 2016
Fig. (4).
Forest plot of outcomes of Drakos 2016
Summary data of Lei 2017
The rater of patients that required blood transfusion was significant lower in the TXA group than the control group(RR 0.44, 95% CI 0.26 to 0.77;P=0.004). There was no significant difference in the incidence of thrombotic complications between the two groups(RR 1.62, 95% CI 0.29, 9.17;P=0.58). More details of outcomes can be seen in Fig. (5). (6)
Fig. (5).
Forest plot of outcomes of Lei 2017
Fig. (6).
Forest plot of outcomes of Lei 2017
Summary data of Tian 2018
The transfusion rate was not significantly different between the control groupand the TXA group (RR 0.71,0.59, 95% CI 0.50 to 1.00; P = 0.05). There was no significant difference in the incidence of thrombotic complications between the two groups(RR 1.50, 95% CI 0.26, 8.60;P=0.65).The average total blood loss for the contral group was significantly higher compared with the TXA group (MD −181.60, 95% CI −290.15 to −73.05; P =0.001). The average hidden blood loss for the control group was significantly higher compared with the TXA group (MD −130.60, 95% CI −231.37 to −29.83; P =0.01). More details of outcomes can be seen in Fig. (7). (8).
Fig. (7).
Forest plot of outcomes of Tian 2018
Fig. (8).
Forest plot of outcomes of Tian 2018
Summary data of Luo 2019
The transfusion rate was significantly different between the control group and the TXA group (RR 0.43, 95% CI 0.20, 0.94; P = 0.03). There was no significant difference in the incidence of thrombotic complications between the two groups(RR 0.26, 95% CI 0.03, 2.25;P=0.22).The average total blood loss for the control group was significantly higher compared with the TXA group (MD −181.70, 95% CI −332.12, to −31.28; P =0.02). The hidden bloo loss in the TXA group was significantly lower than in the contral group (MD −164.40, 95% CI −311.50 to −17.30; P =0.03). More details of outcomes can be seen in Fig. (9).(10).
Fig. (9).
Forest plot of outcomes of Luo 2018
Fig. (10).
Forest plot of outcomes of Luo 2018
| Synthesis of results | 21 | TBL Four included studies had reported the results of TBL[15,17~19,]. The pooled results indicated that the TBL in TXA group was significantly lower than that in the control group (MD= −172.83, 95% CI−241.43 to −104.23; P < 0.00001, Fixed-effect model Fig. 2). Nfs 0.05 = 35, that is say, to reverse the pooled results 35 studies with negative results were required, indicating that the pooled results are stable. Visible blood loss Four studies reported results of visible blood loss[15,17~19,]. The pooled results indicated that there was significant difference between the two groups (MD=−33.46% CI−52.40 to −14.52; P=0.005,fixed-effect model).No heterogeneity was observed (P < 0.51, I2 = 0%; Fig. 3) HBL Three studies reported HBL [17~19]. No significant heterogeneity was detected between the included studies(P<0.93, I2 =0%), therefore, a fixed-effect model was used to analyze the combined effect, finding that there was significant difference between the two groups (MD= −144.20, 95% CI−210.74 to −77.66; P < 0.0001, Figure 4). The pooled results indicate that TXA significantly reduced the postoperative HBL compared with the control group. Transfusion Five studies reported intra- and postoperative transfusion rate [15~19]. The intra- and postoperative transfusion rate for the TXA group was was 34.4% (91/264) and for the control group was 49.2.4% (136/276), and the relative risk was 0.71 (95% CI 0.52 to 0.97; P < 0.03, random-effect model) with substantial heterogeneity (I 2 = 63%, P = 0.03), showing that there was significant difference between the two groups (Fig. 5).Compared with the control group, the TXA group reduced the relative risk of intra- and postoperative transfusion by 29% and the absolute risk by 14% (RD = −0.14 ,95% CI − 0.21 to − 0.07; P < 0.0002, fixed-effect model, Fig. 6). Nfs 0.05 = 17, demonstrating that 17 studies with negative results were required to reverse the pooled results. With the use of TXA, concentrated red blood cell (CRBC) infusion was reduced by mean 0.43 unit per patient (95% CI −0.63 to −0.23 mL; P<0.0001, Fig. 7). Hb values on postoperative day 3 Three studies reported Hb values on postoperative day 3 [16, 17, 19]. The study by Drokos did not report standard deviations and was not included to the combined analysis. The pooled results showed that there was no significant difference between the two groups (MD = 0.32,95% CI−0.09 to 0.74; P = 0.13, fixed-effect model, Fig. 8), with mild heterogeneity(P = 0.24, I2 = 27%). Thrombotic events Five studies provided data of postoperative thrombotic events [15~19]. The pooled esults demonstrated that there was no significant difference between the two groups (RR = 0.84, 95%CI 0.46 to 1.54; P = 0.57, fixed-effect model), with no evidence of heterogeneity(P = 0.59, I2 = 0%, Fig. 9,10). In subgroup analysis, the incidence of thrombotic events was 4.26% (7/164) in the intravenous TXA group, and 5.71% (10/175) in the control group( RR = 0.76,95% CI 0.30 to 1.90; P = 0.56, fixed-effect model) with no heterogeneity, showing that intravenous TXA do not increase the risk of thrombotic events. However, Nfs0.05<1,indicating that the analysis result is unstable. Mortality on postoperative day 30 Three studies reported mortality on postoperative day 30 [15, 17, 19]. The relative risk of mortality on postoperative day 30 was 0.47(95%CI 0.20 to 1.10; P=0.08, fixed-effect model),with no heterogeneity (P=0.89, I2=0%), implying there was no significant difference between the compared groups (Fig. 11). |
6–9 |
| Risk of bias across studies | 22 | The funnel plot of transfusion volume and thrombotic events showed that the scattered points were roughly symmetrical, suggesting that publication bias in the included studies was mild (Fig. 12, 13). | 9 |
| Additional analysis | 23 | Give results of additional analyses, if done (e.g., sensitivity or subgroup analyses, meta-regression [see Item 16]). | |
| DISCUSSION | |||
| Summary of evidence | 24 | The combined effect of 4 RCTs showed that TXA could reduce the TBL per capita of 172 mL (95% CI−241.43 to −104.23; P < 0.00001) in elderly patients with IFs, which has important clinical significance. Because elderly patients with IFs are often accompanied by anemia and many medical complications before operation, their tolerance of ischemia during operation is poor. TXA helps to maintain hemodynamic stability by reducing blood loss, and thus reduces the risk of cardio-cerebrovascular diseases attack induced by hemodynamic fluctuations. We believe that the combined effect is a strong evidence confirming the hemostatic effect of TXA used in IFs surgery, because there are no major methodological flaws in each study, and the effects of each study are significant and consistent. In addition, Nfs 0.05 = 35, that is say, to reverse the pooled results 35 studies with negative results were required, indicating that the pooled results are stable. Postoperative blood transfusion and Hb level can further verify the effect of TXA on reducing blood loss. The intra- and postoperative transfusion rate in TXA group and control group were 34.4% and 49.2% respectively. The combined effect RR was 0.71 (95%CI 0.52, to 0.97; P < 0.03), indicating that TXA decreased the risk of transfusion by 29% compared with the control group. The calculated number of patients need to be treated (NNT) equals 6.75, meaning that the use of TXA in every 7 patients can prevent one patient from receiving transfusion therapy. Although the average Hb level on operative day 3 between the two groups was similar (MD = 0.32 ,95% CI -0.09 to 0.74; P < 0.13) patients in the control group received more unit of CRBC infusion per capita than patients in the TXA group(MD=0.43,95%CI −0.63 to −0.23;P<0.0001) These evidences demonstrate that TXA has a definite hemostatic effect in the treatment of IFs in the elderly. In the present meta-analysis, we found that the incidence of thrombotic events was 4.26% (7/164) in intravenous TXA group, and 5.71% (10/175) in control group, and RR was 0.76 (95% CI 0.30 to 1.90; P = 0.56). The pooled results show that intravenous TXA in IFs surgery in the elderly do not increase the risk of thrombotic events. |
10–11 |
| Limitations | 25 | The major limitation of the present meta-analysis is the small overall sample size in assessing thrombotic events. Although there is no statistical difference in the combined effects of thrombotic events, the real difference may not be detected because of small sample size and insufficient statistical test efficiency. In addition, Nfs0.05<1, indicating that the analysis result is unstable. Therefore, the evidence strength is moderate, and more high-quality RCTs are needed to strengthen the findings. | 12 |
| Conclusions | 26 | In conclusion, the present evidence shows that TXA can significantly reduce TBL and HBL, allogeneic blood transfusion rate, and do not increase the risk of thrombotic events in elderly patients with IFs undergoing intramedullary fixation surgery. However, the impact of TXA on thrombotic events needs to be researched in more high-quality, large-sample RCTs. | 13 |
| FUNDING | |||
| Funding | 27 | There was no any founding sources and other support role of founder provided for this meta-analysis. | |
From: Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group (2009). Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med 6(7): e1000097. doi:10.1371/journal.pmed1000097
For more information, visit: www.prisma-statement.org.
Footnotes
Ethics Committee Approval: N/A.
Informed Consent: N/A.
Author Contributions: Concept - X.L., H.H., X.T.; Design - X.L., H.H., X.T.; Supervision - X.L., H.H., X.T.; Resources - X.L., H.H., X.T.; Materials - X.L., H.H., X.T.; Data Collection and/or Processing - X.L., H.H., X.T.; Analysis and/or Interpretation - X.L., H.H., X.T.; Literature Search - X.L., H.H., X.T.; Writing Manuscript - X.L., H.H., X.T.; Critical Review - X.L., H.H., X.T.
Conflict of Interest: The authors have no conflicts of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.
Appendix 1. Supplementary data
Supplementary data to this article can be found online at 10.5152/j.aott.2020.01.88.
References
- 1.Niu E, Yang A, Harris AH, Bishop J. Which fixation device is preferred for surgical treatment of intertrochanteric hip fractures in the United States? A survey of orthopaedic surgeons. Clin Orthop Relat Res. 2015;473:3647–55. doi: 10.1007/s11999-015-4469-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Simunovic N, Devereaux PJ, Sprague S, et al. Effect of early surgery after hip fracture on mortality and complications: Systematic review and meta-analysis. CMAJ. 2010;82:1609–16. doi: 10.1503/cmaj.092220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu Y, Tao R, Liu F, et al. Mid-term outcomes after intramedullary fixation of peritrochanteric femoral fractures using the new proximal femoral nail antirotation (PFNA) Injury. 2010;41:810–7. doi: 10.1016/j.injury.2010.03.020. [DOI] [PubMed] [Google Scholar]
- 4.Tang P, Hu F, Shen J, Zhang L, Zhang L. Proximal femoral nail antirotation versus hemiarthroplasty: A study for the treatment of intertrochanteric fracture. Injury. 2012;43:876–81. doi: 10.1016/j.injury.2011.11.008. [DOI] [PubMed] [Google Scholar]
- 5.Cai L, Wang T, Di L, Hu W, Wang J. Comparison of intramedullary and extramedullary fixation of stable intertrochanteric fractures in the elderly: A prospective randomised controlled trial exploring hidden perioperative blood loss. BMC Musculoskelet Disord. 2016;17:475. doi: 10.1186/s12891-016-1333-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jiang X, Ma XL, Ma JX. Efficiency and safety of intravenous tranexamic acid in simultaneous bilateral total knee arthroplasty: A systematic review and meta-analysis. Orthop Surg. 2016;8:285–93. doi: 10.1111/os.12256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fillingham YA, Ramkumar DB, Jevsevar DS, et al. Tranexamic acid use in total joint arthroplasty: The clinical practice guidelines endorsed by the American Association of Hip and Knee Surgeons, American Society of Regional Anesthesia and Pain Medicine, American Academy of Orthopaedic Surgeons, Hip Society, and Knee Society. J Arthroplasty. 2018;33:3065–9. doi: 10.1016/j.arth.2018.08.002. [DOI] [PubMed] [Google Scholar]
- 8.Zufferey PJ, Miquet M, Quenet S, et al. Tranexamic acid in hip fracture surgery: A randomized controlled trial. Br J Anaesth. 2010;104:23–30. doi: 10.1093/bja/aep314. [DOI] [PubMed] [Google Scholar]
- 9.Higgins JPT, Green S. Cochrane handbook for systematic reviews for intervention. The Cochrane Collaboration. 2008. Version 5.0.0:180. Available from URL: http://www.cochrane-handbook.org. https://doi.org/10.1002/9780470712184.
- 10.Higgins JPT, Green S. Cochrane handbook for systematic reviews for intervention. The Cochrane Collaboration. Version 5.0.0. pp. 242–248. Available from: URL: http://www.cochrane-handbook.org.
- 11.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–58. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 12.Lau J, Ioannidis JP, Schmid CH. Quantitative synthesis in systematic reviews. Ann Intern Med. 1997;127:820–6. doi: 10.7326/0003-4819-127-9-199711010-00008. [DOI] [PubMed] [Google Scholar]
- 13.Rosenthal R. The “file drawer problem”and tolerance for null results. Psychol Bull. 1976;86:638–41. doi: 10.1037/0033-2909.86.3.638. [DOI] [Google Scholar]
- 14.Rosenthal R. Meta-analytic procedures for social research. J Clin Epidemiol. 1991;44:288–92. doi: 10.4135/9781412984997. [DOI] [Google Scholar]
- 15.Tengberg PT, Foss NB, Palm H, Kallemose T, Troelsen A. Tranexamic acid reduces blood loss in patients with extracapsular fractures of the hip: Results of a randomised controlled trial. Bone Joint J. 2016;98-B:747–53. doi: 10.1302/0301-620X.98B6.36645. [DOI] [PubMed] [Google Scholar]
- 16.Drakos A, Raoulis V, Karatzios K, et al. Efficacy of local administration of tranexamic acid for blood salvage in patients undergoing intertrochanteric fracture surgery. J Orthop Trauma. 2016;30:409–14. doi: 10.1097/BOT.0000000000000577. [DOI] [PubMed] [Google Scholar]
- 17.Lei J, Zhang B, Cong Y, et al. Tranexamic acid reduces hidden blood loss in the treatment of intertrochanteric fractures with PFNA: A single center randomized controlled trial. J Orthop Surg Res. 2017;12:124. doi: 10.1186/s13018-017-0625-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tian S, Shen Z, Liu Y, Zhang Y, Peng A. The effect of tranexamic acid on hidden bleeding in older intertrochanteric fracture patients treated with PFNA. Injury. 2018;49:680–4. doi: 10.1016/j.injury.2018.01.026. [DOI] [PubMed] [Google Scholar]
- 19.Luo X, He S, Lin Z, Li Z, Huang C, Li Q. Efficacy and safety of tranexamic acid for controlling bleeding during surgical treatment of intertrochanteric fragility fracture with proximal femoral nail anti- rotation: A randomized controlled trial. Indian J Orthop. 2019;53:263–9. doi: 10.4103/ortho.IJOrtho_401_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Farrow LS, Smith TO, Ashcroft GP, Myint PK. A systematic review of tranexamic acid in hip fracture surgery. Br J Clin Pharmacol. 2016;82:1458–70. doi: 10.1111/bcp.13079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang W, Yu J. Tranexamic acid reduces blood loss in intertrochanteric fractures: A meta-analysis from randomized controlled trials. Medicine (Baltimore) 2017;96:e9396. doi: 10.1097/MD.0000000000009396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang P, He J, Fang Y, Chen P, Liang Y, Wang J. Efficacy and safety of intravenous tranexamic acid administration in patients undergoing hip fracture surgery for hemostasis: A meta-analysis. Medicine (Baltimore) 2017;96:e6940. doi: 10.1097/MD.0000000000006940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhu Q, Yu C, Chen X, et al. Efficacy and safety of tranexamic acid for blood salvage in intertrochanteric fracture surgery: A meta-analysis. Clin Appl Thromb Hemost. 2018;24:1189–98. doi: 10.1177/1076029618783258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xiao C, Zhang S, Long N, Yu W, Jiang Y. Is intravenous tranexamic acid effective and safe during hip fracture surgery? An updated meta-analysis of randomized controlled trials. Arch Orthop Trauma Surg. 2019;139:893–902. doi: 10.1007/s00402-019-03118-6. [DOI] [PubMed] [Google Scholar]
- 25.Piovella F, Wang CJ, Lu H, et al. Deep-vein thrombosis rates after major orthopedic surgery in Asia. An epidemiological study based on postoperative screening with centrally adjudicated bilateral venography. J Thromb Haemost. 2005;3:2664–70. doi: 10.1111/j.1538-7836.2005.01621.x. [DOI] [PubMed] [Google Scholar]
- 26.Lee SY, Ro du H, Chung CY, et al. Incidence of deep vein thrombosis after major lower limb orthopedic surgery: Analysis of a nationwide claim registry. Yonsei Med J. 2015;56:139–45. doi: 10.3349/ymj.2015.56.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roberts I, Perel P, Prieto-Merino D, et al. Effect of tranexamic acid on mortality in patients with traumatic bleeding: Prespecified analysis of data from randomised controlled trial. BMJ. 2012;11:345, e5839. doi: 10.1136/bmj.e5839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Franchini M, Mengoli C, Marietta M, et al. Safety of intravenous tranexamic acid in patients undergoing majororthopaedic surgery: A meta-analysis of randomised controlled trials. Blood Transfus. 2018;16:36–43. doi: 10.2450//2017.0219-17. [DOI] [PMC free article] [PubMed] [Google Scholar]