Abstract
Objective
The aim of this study was to investigate the immunohistochemical stain profiling of adipocytic tumors.
Methods
From our archive files between the years of 2012–2018, excised, formalin-fixed and paraffin-embedded adipocytic tumors were retrospectively screened and 61 subjects were selected. The gender, age, tumor location and tumor diameter were evaluated. The cases were investigated in terms of p16, CD34, MDM2 expression and clinicopathological information.
Results
Of the 61 patients included in the study, we found that 2 had hibernoma, 4 had lipoblastoma, 14 had spindle cell lipoma (SCL), 10 had lipoma, 20 had atypical lipomatous tumor/well differentiated liposarcoma (ALT/WDL), and 11 had dedifferentiated liposarcoma (DDL). In terms of diameter, ALT/WDL and DDL were significantly different from the others (p=0.001, p=0.001, respectively). There was a significant difference between the groups according to the location (p=0.001). 35% (7/20) of ALT/WDLs were in the lower extremities (thighs) and 35% (7/20) were located in the retroperitoneal region. 70% of DDLs (7/11) were located in the retroperitoneum. When CD34 expression was evaluated among the groups, a significant difference was observed (p=0.001). CD34 was positive in 92.9% of SCL cases. p16 immunoreactivity was significantly different between the groups (p=0.001). p16 expression was observed in 50.5% of ALT / WDL cases and 79% of DDL cases.
Conclusion
p16 and CD34 expression are valuable in the differential diagnosis of lipomatous tumors when radiological and clinical considerations do not help to differential diagnosis of adipocytic tumors.
Level of Evidence
Level IV, Therapeutic Study
Keywords: Hibernoma, Lipoblastoma, Spindle Cell Lipoma, Lipoma, Atypical Lipomatous Tumor/Well-Differentiated Liposarcoma, Dedifferentiated Liposarcoma, p16, CD34, MDM2
A single-specialty medicine facility may not be sufficient to provide appropriate treatment and true diagnosis for every case in the domain of orthopedic oncology. Especially, in soft-tissue tumors, close cooperation among the radiologist, surgeon, pathologist, and oncologist is essential (1). The tumors of adipose tissue are the most general soft-tissue tumors in the adult age group (2). The benign neoplasms of adipose tissue usually comprise mature fat cells. Each cell contains a large lipid vacuole that replaces the nucleus and compresses it onto the cytoplasmic membrane (3).
The differential diagnosis of adipose tissue tumors is significant because of their clinical behavior. The differential diagnosis between lipoma, atypical lipomatous tumor/well-differentiated liposarcoma (ALT/ WDL), and dedifferentiated liposarcoma (DDL) is important because of their prognosis and treatment (4). Despite typical morphological features, spindle cell lipoma (SCL) defined by Enzinger first, can be confused with liposarcoma (5). The differential diagnosis of Hibernoma, which is usually observed in adults under 40 years of age with liposarcoma and lipoblastoma, can sometimes be fairly difficult (6). Lipoblastoma, which is a rare benign adipocytic tumor observed during childhood, shows histological similarities with myxoid liposarcoma or ALT/WDL (7).
There is a significant difference in morphological and biological activities between ALT/WDL, which has low metastatic potential, and DDL, which has high potential of distant metastasis. The most general subtype of liposarcoma is ALT/ WDL (40–45%); notably, ALT/WDL has no significant gender preference, and it generally has deep anatomical localization (8). The clinical presentation of most adipocytic tumors may be completely asymptomatic. In several cases, pain can be the primary symptom in the case of neurovascular compression due to the mass effect of lipomatous tumors (8). Although a wide variety of diagnostic tools are available for imaging, magnetic resonance imaging is the most generally used examination for the diagnosis and localization of adipocytic tumors (8).
However, at present, histological evaluation is the gold standard for the diagnosis of lipomatous tumors. However, the diagnosis of various types of liposarcomas is not always easy, even for an experienced pathologist, especially when the amount of the biopsy tissue to be examined is too less. Generally, ALT/WDL comprises mature adipocytic cells with hyperchromatic nuclei, and it does not contain any solid areas. However, atypical stromal cells are not always readily recognizable and, sometimes, require complete sampling of the tumor; this critical issue makes it impossible to diagnose only via core needle biopsy, thereby might leading to inadequate diagnosis of the tumor. Although performing a routine histopathological examination is generally adequate, yet the gold standard for differential diagnosis is the fluorescent in-situ hybridization (FISH) method for the amplification of MDM2 and CDK4 genes (4, 9). Although immunohistochemistry for MDM2 and CDK4 proteins has been reported to be useful in this regard, the FISH method provides greater sensitivity and specificity. Notably, p16, which inhibits cell-cycle progression by binding to CDK4 (cyclin-dependent kinase inhibitor 2A, i.e., CDKN2A) has been overexpressed in several neoplasia and proposed as a diagnostic marker for ALT/WDL (2, 4).
The hypothesis in this study is that CD34, p16, and MDM2 staining can be useful in the histological diagnosing the lipomatous tumor when radiological and clinical considerations do not help differentiate one adipocytic tumor from another.
Materials and Methods
From our archive files between the years 2006 and 2018, formalin-fixed and paraffin-embedded adipocytic tumors were screened retrospectively. All materials were obtained via excision surgeries performed by the department of orthopedic oncology. The patients who did not have paraffin blocks and had no or inadequate clinical information were excluded from the study. A total of 61 cases were selected, and the excised materials were stained immunohistochemically using 3 markers, namely, CD34, p16, and MDM2, for our study. Furthermore, the gender, age, tumor location, radiological data, and tumor diameter information of the cases were obtained using the archive files of the patients. We used macroscopic examination to define the size of adipocytic tumors. The cases were divided into two groups according to their diameter, and the diameter of 5 cm was selected as the limit value, as used in the American Joint Committee on Cancer Staging System for Soft Tissue Sarcomas. According to the protocols prescribed by the College Of American Pathologist, the cases were divided into the following five groups: lower extremities (cruris, inguinal, thigh, and gluteal), upper extremity (hand, arm, forearm, scapula, and shoulder), intraabdominal, retroperitoneal, and others (neck, back, thorax, and nape). All the diagnoses were based on morphology and were agreed upon by manuscript authors, who are soft-tissue specialists. The authors evaluated the immunoreactivity score together, and they all agreed.
Immunohistochemical staining was performed on 5-μm thick tissue sections of formalin-fixed, paraffin-embedded tumor samples. The p16 (anti-p16Ink4a, 1: 100, CINtec, Arizona, USA) mouse monoclonal antibody, MDM2 (1B10, Ventana, Arizona, USA) mouse monoclonal antibody, and CD34 (QBEnd/10, Ventana, Arizona, USA) antibody were used as primary antibodies. Subsequently, staining was performed on a Ventana Benchmark XT autostainer with the XT ultraView. All the slides were counter stained using haematoxylin. The presence of a brown precipitate indicated positive findings for the primary antibody. The immunostaining of the cells was evaluated, and the percentage of nuclear staining for MDM2 and p16 were scored. The CD34 nuclear or cytoplasmic staining was evaluated as positive or negative. We excluded the intensity of staining and non-specific staining of non-tumor areas. Only the percentages of specific tumor areas were evaluated and scored. For statistical analysis, Windows-compatible SPSS 20 version was used. The student t-test was performed to compare the continuous variables of the patients. The spearman test was performed for correlation. The significance level was set at p<0.05.
In this study, the investigation protocol was in accordance with the Helsinki committee requirement and was approved by the Institutional Ethical Committee, The Balıkesir University (Decision no: 2018/143).
Results
Of the 61 excised materials used in this study, 2 were diagnosed as hibernoma, 4 as lipoblastoma, 14 as SCL, 10 as lipoma, 20 as ALT/WDL, and 11 as DDL. The age, tumor diameter, and gender information of the cases are listed in Table 1.
Table 1.
Mean descriptive analysis of adipocytic tumors
| Sex (Male/Female) | Max. diameter (cm) | Age | |
|---|---|---|---|
| Hibernoma (n=2) | 1/1 | 12.5±9.1 | 49.5±27.5 |
| Lipoblastoma (n=4) | 1/3 | 5.5±2.5 | 2±0.8 |
| SCL (n=14) | 13/1 | 6.1±2.4 | 63.5±13.6 |
| ALT/WDL (n=20) | 11//9 | 16.5±7.1 | 64.8±11.7 |
| DDL (n=11) | 5/6 | 16.9±5.7 | 62.8±11.6 |
| Lipoma (n=10) | 6/4 | 5.6±4.6 | 56.1± 0.8 |
SCL: spindle cell lipoma; ALT/WDL: atypical lipomatous tumor/well differentiated liposarcoma; DDL: dedifferentiated liposarcoma
For 37 (60%) of the cases, the patients were male. Although no significant difference was observed in other adipocytic tumors in terms of gender, 92.8% of the SCL patients were male; the mean age was 57 (ranging from 1 to 83 years) years. The mean tumor diameter was 10.9 cm (ranging from 2 to 23 cm) (Table 2). For 85% of the ALT/WDL cases and 91% of the DDL cases, diameters were greater than 5 cm. For both ALT/WDL and DDL cases, the diameters were significantly larger than those for the others (p=0.001, p=0.001, respectively). Upon examining the cases according to their superficial and deep soft-tissue locations, SCLs (78.5%) and Lipomas (90%) were generally superficial, whereas ALT/WDLs (95%) and DDLs (91%) were deeply located (Table 3).
Table 2.
Diameter in adipocytic tumors
| ALT/WDL (n=20) | DDL (n=11) | Lipoma (n=10) | Hibernoma (n=2) | Lipoblastoma (n=4) | SCL (n=14) | |
|---|---|---|---|---|---|---|
| Diameter <5cm (n=15) | 3 (15%) | 1 (9%) | 7 (70%) | 0 (0%) | 1 (25%) | 3 (21%) |
| Diameter >5cm (n=46) | 17 (85%) | 10 (91%) | 3 (30%) | 2 (100%) | 3 (75%) | 11 (79%) |
Percentage of rows
SCL: spindle cell lipoma; ALT/WDL: atypical lipomatous tumor/well differentiated liposarcoma; DDL: dedifferentiated liposarcoma
Table 3.
Evaluation of superficial and deep presence of adipocytic tumors
| Superficial | Deep | |
|---|---|---|
| Hibernoma (n=2) | 1 (50%) | 1 (50%) |
| Lipoblastoma (n=4) | 3 (75%) | 1 (25%) |
| SCL (n=14) | 11 (78.5%) | 3 (21.5%) |
| ALT/WDL (n=20) | 1 (5%) | 19 (95%) |
| DDL (n=11) | 1 (9%) | 10 (91%) |
| Lipoma (n=10) | 9 (90%) | 1 (1%) |
SCL: spindle cell lipoma; ALT/WDL: atypical lipomatous tumor/well differentiated liposarcoma; DDL: dedifferentiated liposarcoma
A significant difference was observed between the groups in terms of the localization of adipocytic tumors (p=0.001). The subgroup analysis showed a significant difference between SCLs and ALT/WDL (p=0.002). The SCLs were observed to be significantly higher than ALT/WDL in other localizations (neck, back, thorax, and nape). However, DDL was observed to be significantly higher in retroperitoneal localization compared with SCLs (p=0.007). Moreover, finally comparing to ALT/WDL with DDL, lipoma was significantly higher, more generally, in the upper extremity (p=0.009), and other localizations (neck, back, thorax, and nape) (p=0.008). In addition, no differences were observed between ALT/WDL and DDL (p=0.604) in terms of lipoma and SCLs (p=0.293) according to the localization of adipocytic tumors (Table 4).
Table 4.
Localization of adipocytic tumors
| Lower extremity n=16 (%) | Upper extremity n=10 (%) | Other localizations (neck, back, thorax, and nape) n=17 (%) | Retroperitoneum n=14 (%) | Intraabdominal n=4 (%) | |
|---|---|---|---|---|---|
| Hibernoma (n=2) | 1 (6.2%) | 0 (0%) | 1 (5.8%) | 0 (0%) | 0 (0%) |
| Lipoblastoma (n=4) | 3 (18.6%) | 0 (0%) | 1 (5.8%) | 0 (0%) | 0 (0%) |
| SCL (n=14) | 1 (6.2%) | 3 (30%) | 10 (58.8%) | 0 (0%) | 0 (0%) |
| ALT/WDL (n=20) | 7 (44.2%) | 2 (20%) | 1 (5.8%) | 7 (50%) | 3 (75%) |
| DDL (n=11) | 2 (12.4%) | 1 (10%) | 0 (0%) | 7 (50%) | 1 (25%) |
| Lipoma (n=10) | 2 (12.4%) | 4 (40%) | 4 (23.8%) | 0 (0%) | 0 (0%) |
Percentage of columns
SCL: spindle cell lipoma; ALT/WDL: atypical lipomatous tumor/well differentiated liposarcoma; DDL: dedifferentiated liposarcoma
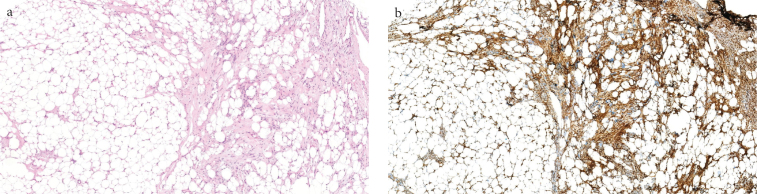
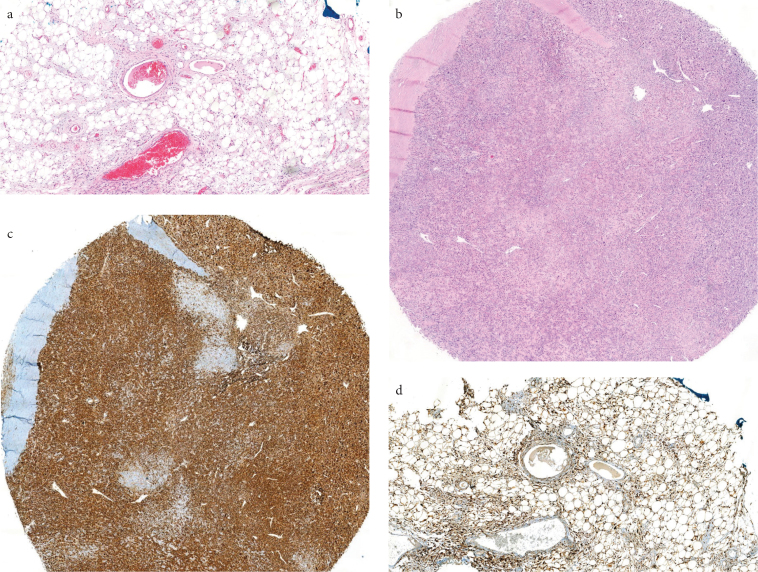
Table 5 presents the expression results of each antibody in adipocytic tumors. No significant difference was observed between the groups in terms of the MDM2 immunoreactivity (p=0.259). The CD34 expression was significantly different between the groups (p=0.001). In addition, CD34 was positive in 92.9% of the SCL cases (Figure 1, Table 5). However, all lipomas were evaluated as CD34 negative. The expression of p16 immunoreactivity was significantly different between the groups (Table 5) (p=0.001). The p16 expression was observed in 50.5% of the ALT/WDL cases and 79% of the DDL cases (Figure 2).
Table 5.
Mean expression percentage of p16, mdm2, and CD34 immunohistochemical expressions in adipocytic tumors
| mdm2 (%) | p16 (%) | CD34 (positive)* | CD34 (negative)* | |
|---|---|---|---|---|
| Hibernoma (n=2) | 0 | 2.5 | 1 (50%) | 1 (50%) |
| Lipoblastoma (n=4) | 0 | 20 | 3 (75%) | 1 (25%) |
| SCL (n=14) | 0 | 5 | 13 (92.9%) | 1 (7.1%) |
| ALT/WDL (n=20) | 1 | 50.5 | 9 (45%) | 11 (55%) |
| DDL (n=11) | 0.8 | 79 | 4 (36.3%) | 7 (63.7%) |
| Lipoma (n=10) | 0 | 23 | 0 (0%) | 10 (100%) |
SCL: spindle cell lipoma; ALT/WDL: atypical lipomatous tumor/well differentiated liposarcoma; DDL: dedifferentiated liposarcoma
Figure 1. a, b.
Histopathological view of SCL (H&E, ×40), (a). Spindle cells of the SCL tumor show wide cytoplasmic CD34 staining (CD34, ×40) (b)
Figure 2. a–d.
ALT/WDL (H&E, x40) (a), DDL (H&E, x40) (b), p16 staining DDL (x40) (c), p16 staining ALT/WDL (x40) (d)
Discussion
Liposarcomas are general soft-tissue tumors, and ALT/WDL and DDLs constitute an important part of liposarcomas (2, 10). Multiple genetic deffects such as the inactivation of tumor supressor genes and activation of oncogenes, play an important role for the development of soft-tissue tumors (11). MDM2 is an oncogene whose product links to p53 and inhibits the p53 function in the cell cycle in the following three ways: blockage of p53 interaction with DNA, inhibition of p53 transfer from cytoplasm to nucleus, and ubiquitination of p53. The action of MDM2 results in p53 inactivation without the mutation or deletion of p53, resulting in the inhibition of apoptosis. In addition, CDK4 is an oncogene whose product inhibits RB1. CDK4 protein phosphorylates RB1, which links to E2F and, thus, is inactive, thereby resulting in the loss of the G1-S checkpoint. MDM2 and CDK4 are the amplified genes in ALT–well-differentiated LPS/dedifferentiated LPS (12).
Previously conducted studies demonstrated that MDM2 and CDK4 are important tissue markers for the differentiation of ALT/WDL and DDLs. Thway et al. reported that CDK4, MDM2, and p16 immunohistochemical triad is an adjunct diagnostic tool that provides strong support for differentiating ALT/WDL and DDL from other adipocytic neoplasms (13). Contrarily, we observed that the MDM 2 expression was similar between both the adipositic tumors in our studied population.
However, Gonzales et al. recommended using p16 immunohistochemically for the differential diagnosis of ALT/WDLs by considering the false-positive rate of 14.3% in the laboratories where results and cost are prioritized (4). Compatible with this study, we observed that the p16 expression was important in the differential diagnosis of DDL from other adipocytic tumors (Table 5) (p=0.001).
While the brown fat tissue cells are similar to mature adipocytes, hibernoma can mimic lipoblasts and ALT/WDLs. In a study, 85% of hibernoma cases were stained positive with S-100, while none were stained with CD34 (6). In our study, no difference was observed between ALT/WDL and hibernoma cases in terms of p16 and CD34 expressions.
In a study that used p16 for the differential diagnosis of lipoma and ALT/WDL, none of the deep-located lipomas were p16 stained, whereas 83% of ALT/WDLs showed positive reactivity with p16 (14). In another study, 75% of ALT/WDL cases, 10% of lipoblastoma cases, and 31% of fat-necrosis cases demonstrated positive reactivity with p16; however, not all the lipoma cases were stained with p16 (7). Studies show that negative p16 staining may be helpful in the differential diagnosis of ALT/WDL in the light of clinical findings. However, this finding should be interpreted with caution because some liposarcomas are p16 negative, whereas benign conditions such as lipoblastoma or fat necrosis may be p16 positive.
Knosel et al. showed that the p16 expression had different patterns in different sarcomas (15). In the same study, a significant relationship was observed between a decreased p16 expression and shortened patient survival (15). Notably, ALT/WDL is a locally aggressive subtype of liposarcoma unless it is dedifferentiated, and the mechanism of dedifferentiation is unclear. However, the loss of p16 might be an early and critical event in progressing to dedifferentiation (14). In a study, Mai He et al. showed that ALT/WDL, DDL, and recurrent ALT/WDLs expressed the same density p16 (14). However, no correlation was observed between the hypermethylation of p16 promoter region and p16 protein expression (14).
Lipomas usually settle on the trunk, back, shoulder, neck, and proximal extremities (16). They are rare to be seen on hand, foot, face, lower leg, and retroperitoneum (16). Lipomas are usually seen in subcutaneous tissues, whereas liposarcomas are generally deeply localized (17, 18). For instance, SCL, which comprises mature adipocytes and spindle cells, is often localized in the subcutaneous superficial soft tissue of the neck, back, or shoulder (5, 19). It includes liposarcoma, neurofibroma, and nucal fibroma in the differential diagnosis (19). Immunohistochemically, the CD34 positivity in SCL is the most striking finding in our study. This positivity might be useful in differentiating SCL from a convetional lipoma, where radiological findings are inadequate to distinguish these two entities from each other.
A tumor of diameter 5 cm or greater and located in the subfascial deep soft-tissue area is likely to be a sarcoma (16). The most common locations for ALT/WDL are extremities and retroperitoneum (20). Despite the same histological features among all ALT/WDLs, the ones other than retroperitoneum generally follow a more benign course (21). Generally, ALT/WDLs and DDLs constitute diameter greater than 10 cm. Although few studies are based on this subject, our study found that ALT/WDLs and DDLs generally had diameter greater than 5 cm.
The major limitation of our study is that the number of some cases, such as hibernoma and lipoblastoma, is low and that several subtypes of liposarcoma, such as myxoid and pleomorphic liposarcomas, do not exist in this cohort. With higher number of cases of these lesions, p16, CD34, and MDM2 staining profile and differential diagnosis between lipomatous tumor entities can be certainly better understood. The MDM2 results revealed that this staining is not effective in differentiating lipomatous tumors one from another; however, this result might have been affected because of the limited number of cases in the cohort. Another limitation of this study is its retrospective design. In some of the cases, surgical excision without any prior biopsy was performed with the help of radiological diagnosis. Therefore, we could not to evaluate the concordance of the prior biopsy results with the histopathological results of the excised materials; the evaluation would have helped us understand the real necessity rate in order to examine some of the lesions with MDM2, p16, and CD34, in which there is an inconcordance between the prior biopsy results and histopathological results of the excised materials. Such concordance can be searched by performing a prospective study with higher number of cases. However, the strengths of this study is that it revealed significant p16 expression in DDL and ALT/WDL, CD34 positivity in SCL, and CD34 negativity in conventional lipoma cases; These data have can be helpful when radiological data are insufficient in state the definitive diagnosis and when tru-cut biopsy evaluation is inconclusive.
The histological diagnosis of adipocytic tumors is traditionally based on morphology. However, the use of p16, CDK4, and MDM2 in has gained popularity in the recent years, because of using different genetic backgrounds compared with other adipocytic neoplasms (2). In addition, p16 may be useful in the differential diagnosis of well-differentiated lipomatous neoplasms (4). In conclusion, in the differential diagnosis of lipomatous tumors, clinicopathological information such as tumor location, tumor diameter, gender, and age is important. In the diagnosis of soft-tissue tumors, clinical information significantly affects the diagnosis process followed by the pathologist. Using this information, we suggest that a panel of immunohistochemistry within p16 and CD34 is valuable in the differential diagnosis of lipomatous tumors when radiological and clinical considerations do not help to perform the differential diagnosis of adipocytic tumors.
MAIN POINTS.
The p16 and CD34 expressions are valuable in the differential diagnosis of lipomatous tumors.
Spindle cell lipoma is often localized in the subcutaneous superficial soft tissue of the neck, back, or shoulder and Dedifferentiated liposarcoma is often located in the retroperitoneum.
Atypical lipomatous tumor/well differentiated liposarcoma and Dedifferentiated liposarcoma constitute diameter greater than 10 cm.
Footnotes
Ethics Committee Approval: Ethics committee approval was received for this study from the ethics committee of Balıkesir University, School of Medicine (Decision No: 2018/143).
Informed Consent: N/A.
Author Contributions: Concept - G.K., E.A.; Design - E.A., G.K.; Supervision - H.Y.Y., G.K.; Resources - S.Y., E.A.; Materials - S.Y., E.A.; Data Collection and/or Processing - E.A., S.Y.; Analysis and/or Interpretation - E.A., S.Y.; Literature Search - E.A., S.Y.; Writing Manuscript - E.A., G.K.; Critical Review - G.K., H.Y.Y.
Conflict of Interest: The authors have no conflicts of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Yuceturk G, Sabah D, Kececi B, Kara AD, Yalcinkaya S. Prevalence of bone and soft tissue tumors. Acta Orthop Traumatol Turc. 2011;45:135–43. doi: 10.3944/AOTT.2011.2504. [DOI] [PubMed] [Google Scholar]
- 2.Thway K, Flora R, Shah C, Olmos D, Fisher C. Diagnostic utility of p16, CDK4, and MDM2 as an immunohistochemical panel in distinguishing well-differentiated and dedifferentiated liposarcomas from other adipocytic tumors. Am J Surg Pathol. 2012;36:462–9. doi: 10.1097/PAS.0b013e3182417330. [DOI] [PubMed] [Google Scholar]
- 3.Vinay Kumar Abul, Abbas JA. Robbins basic pathology. 9th Edition. Philadelphia: Elsevier Inc; 2014. pp. 791–5. [Google Scholar]
- 4.Gonzalez RS, Mcclain CM, Chamberlain BK, Coffin CM, Cates JM. Cyclin-dependent kinase inhibitor 2A (p16) distinguishes well-differentiated liposarcoma from lipoma. Histopathology. 2013;62:1109–11. doi: 10.1111/his.12112. [DOI] [PubMed] [Google Scholar]
- 5.Franz M. Enzınger DAH. Spindle cell lipoma. Cancer. 1975;36:1852–9. doi: 10.1002/1097-0142(197511)36:5<1852::aid-cncr2820360542>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 6.Furlong Ma, Fanburg-Smith JC, Miettinen M. The morphologic spectrum of hibernoma: A clinicopathologic study of 170 cases. Am J Surg Pathol. 2001;25:809–14. doi: 10.1097/00000478-200106000-00014. [DOI] [PubMed] [Google Scholar]
- 7.Cappellesso R, d’Amore ES, Dall’Igna P, et al. Immunohistochemical expression of p16 in lipoblastomas. Hum Pathol. 2016;47:64–9. doi: 10.1016/j.humpath.2015.08.019. [DOI] [PubMed] [Google Scholar]
- 8.Coran A, Ortolan P, Attar S, et al. Magnetic resonance imaging assessment of lipomatous soft-tissue tumors. In Vivo. 2017;31:387–95. doi: 10.21873/invivo.11071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sirvent N, Coindre JM, Maire G, Hostein I, Keslair F, Guillou L. Detection of MDM2-CDK4 amplification by fluorescence in situ hybridization in 200 paraffin-embedded tumor samples: Utility in diagnosing adipocytic lesions and comparison with immunohistochemistry and real-time PCR. Am J Surg Pathol. 2007;31:1476–89. doi: 10.1097/PAS.0b013e3180581fff. [DOI] [PubMed] [Google Scholar]
- 10.Dabak N, Cirakli A, Gulman B, Selcuk MB, Baris S. Distribution and evaluation of bone and soft tissue tumors in the middle Black Sea Region. Acta Orthop Traumatol Turc. 2014;48:17–24. doi: 10.3944/AOTT.2014.3013. [DOI] [PubMed] [Google Scholar]
- 11.Tuna B, Lebe B, Sis B, Yorukoglu K, Kargi A. Mdm2 gene expression in adipose-tissue tumors: Association with tumor progression in liposarcomas. Aegean Pathol J. 2004;1:15–22. [Google Scholar]
- 12.Binh MB, Garau XS, Guillou L, Aurias A, Coindre JM. Reproducibility of MDM2 and CDK4 staining in soft tissue tumors. Am J Clin Pathol. 2006;125:693–7. doi: 10.1309/VMBP67QUNN6Q3J0E. [DOI] [PubMed] [Google Scholar]
- 13.Thway K, Jones RL, Noujaim J, Zaidi S, Miah AB, Fisher C. Dedifferentiated liposarcoma: Updates on morphology, genetics, and therapeutic strategies. Adv Anat Pathol. 2016;23:30–40. doi: 10.1097/PAP.0000000000000101. [DOI] [PubMed] [Google Scholar]
- 14.He M, Aisner S, Benevenia J, Patterson F, Aviv H, Hameed M. p16 immunohistochemistry as an alternative marker to distinguish atypical lipomatous tumor. Appl Immunohistochem Mol Morphol. 2009;17:51–6. doi: 10.1097/PAI.0b013e3181719223. [DOI] [PubMed] [Google Scholar]
- 15.Knosel T, Altendorf-Hofmann A, Lindner L, et al. Loss of p16 ( INK4a ) is associated with reduced patient survival in soft tissue tumours, and indicates a senescence barrier. J Clin Pathol. 2014;16:1–7. doi: 10.1136/jclinpath-2013-202106. [DOI] [PubMed] [Google Scholar]
- 16.Rydholm A, Berg NO. Size, site and clinical incidence of lipoma: Factors in the differential diagnosis of lipoma and sarcoma. Acta Orthop Scand. 1983;54:929–34. doi: 10.3109/17453678308992936. [DOI] [PubMed] [Google Scholar]
- 17.Chhabra A, Soldatos T. Soft-tissue lesions: When can we exclude sarcoma? Am J Roentgenol. 2012;199:1345–57. doi: 10.2214/AJR.12.8719. [DOI] [PubMed] [Google Scholar]
- 18.Kransdorf MJ, Murphey MD. Radiologic evaluation of soft-tissue masses: A current perspective. AJR Am J Roentgenol. 2000;175:575–87. doi: 10.2214/ajr.175.3.1750575. [DOI] [PubMed] [Google Scholar]
- 19.Thompson LDR. Spindle-cell lipoma. Ear, Nose Throat J. 2009;88:7. doi: 10.1177/014556130908800704. [DOI] [PubMed] [Google Scholar]
- 20.Errani C, Cocchi S, Ali N, et al. Recurrence after marginal excision for atypical lipomatous tumors versus lipomas of the extremities. Orthopedics. 2016;39:e610–4. doi: 10.3928/01477447-20160610-02. [DOI] [PubMed] [Google Scholar]
- 21.Rozental TD, Khoury LD, Donthineni-Rao R, Lackman RD. Atypical lipomatous masses of the extremities. Clin Orthop Relat Res. 2002;398:203–11. doi: 10.1097/00003086-200205000-00029. [DOI] [PubMed] [Google Scholar]