Abstract
Sex cord tumour with annular tubules (SCTAT) is uncommon and distinctive type of sex cord-stromal tumours of the ovary which develops from sex cord cells. Most of SCTATs are strongly associated with Peutz–Jeghers syndrome (PJS) and have low malignancy potential; however, 20% of non-PJS-associated SCTATs have been reported to have high malignancy potential. Herein, we present a 13-year-old female who presented with severe abdominal pain localized in the right lower side, associated with nausea. Based on histopathological and immunohistochemical findings, the diagnosis was confirmed to be SCTAT. SCTAT of the ovary is extremely rare in the paediatric population as compared to the general population. Its occurrence among paediatrics as it was the case in the patient described in this paper may pose diagnostic challenges due to lack of clinical suspicion and therefore resulting in delay of diagnosis.
Keywords: ovarian tumours, sex cord tumours, annular tubules
INTRODUCTION
Sex cord tumour with annular tubules (SCTAT) is a rare tumour among the sex cord tumours with incidence of ˂1% and it is unique because of having peculiar intermediate phenotypical expression of pluripotent sex cord cell of the gonads [1]. Up to 1982, only 74 cases of this disease were reported [2]. These tumours are strongly associated with Peutz–Jeghers syndrome (PJS). SCTATs that are related to PJS are clinically benign, multifocal, bilateral, very small and calcified. On the other hand, those which are not associated with PJS are normally unilateral, unifocal and larger in size [3]. The PJS-related SCTATs occur in younger females with an average age of 27 years [4].
This paper describes a case of a 13-year-old female with sporadic SCTAT in the rare paediatric age group.
CASE REPORT
A 13-year-old female was admitted in the paediatric ward with severe abdominal pain localized in the right lower side, associated with nausea. On physical examination, there were no significant findings that could be recorded. Sertoli cell tumour, gonadoblastoma and granulosa cell tumour were considered for the differential clinical diagnoses. Abdominal pelvic ultrasound revealed a solid tumour involving the right ovary causing enlargement of the ovary. The adnexal mass measured 6.7 × 4.5 × 3.8 cm. The left ovary, fallopian tubes and uterus were normal. Ovarian serum tumour markers were not done due to financial constraints. Unilateral salpingo-oophorectomy was performed. The tumour was staged as Stage 1A by using the International Federation of Gynecology and Obstetrics (FIGO) staging system. This is because the tumour was confined to only one ovary with intact capsule, no ascites, no tumour on the external surfaces and without involving retroperitoneal or inguinal lymph nodes. The guidelines followed in treating the patient were based on the FIGO tumour stage as per institutional guidelines.
Microscopically, the tumour had ring-shaped tubules which were separated by stroma. The cells had small single nuclei and pale cytoplasm. Within the tubules, the cells were arranged in interconnecting configuration, and at the centre of each of these, there was a core of eosinophilic materials (Fig. 1). The nuclei of the cells were palisading both around the hyaline cores and at the periphery of the tubules. There was no necrosis and the capsule was free from the tumour. Both calretinin (Fig. 2) and inhibin (Fig. 3) were strongly positive, whereas cytokeratin AE1/AE3 (Fig. 4) was weakly positive. The diagnosis of SCTAT was confirmed. After 6 months, the patient’s mother was contacted and she reported no complaint regarding the condition of the patient.
Figure 1.

Ring-shaped tubules: palisading epithelial cells within tubules (H&E, ×40).
Figure 2.

Staining for calretinin: there is strong and diffuse staining of the tumour cells (IHC, ×40).
Figure 3.

Inhibin staining: the tumour cells are strongly and diffusely stained (IHC, ×40).
Figure 4.
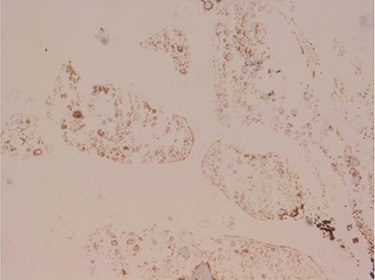
Staining of CK AE1/AE3: the tumour cells are weakly stained (IHC, ×40).
DISCUSSION
Occurrence of SCTATs has been reported to occur with the incidence ranging from 1 to 2.3% [5]. These tumours occur more in women of reproductive age than in the paediatric population. Qian et al. reported a cohort of 13 patients with SCTAT in China in 2015. In this series, the average age was 22.6 years [6]. Another series reported by Singh et al. in 1982 which comprised 74 patients with SCTAT in which the average age was 23.7 years [7].
It has been reported that majority of cases with SCTATs present with hyperestrinism, amenorrhea and postmenopausal bleeding [8]. In the literature, a few cases of malignant SCTATs have been reported. For instance, Lele et al. reported a 47-year-old female with malignant SCTAT which was bilateral [8]. Dart et al. reported one patient with metastasis from a series of three cases with SCTAT [9]. Recurrence and metastases tend to occur several months to years after removal of the primary tumour [8, 9]. Malignant SCTAT seems to spread mainly via the lymphatics and commonly involve the pelvic, para-aortic and supraclavicular lymph nodes [2, 8]. A recurrence rate of almost 50% has been reported in the series of Qian in which some of the patients had even repeated recurrences; the first recurrence was seen after 45.5 months [8].
Macroscopically, SCTATs have been reported to be solid, cystic, mixture of solid and cystic, yellow to tan, and some with haemorrhage and necrosis with size ranging up to 30 cm [4, 7]. Microscopically, SCTATs are typically characterized by circumscribed columnar epithelial nests composed of ring-shaped tubules, which are encircled by acidophilic hyalinized basement membrane-like material and some with calcification [9]. Mitotic counts, e.g. in the 13 cases of SCTAT reported having malignant characteristics, ranged from very few to 10 mitoses per 10 HPF as well as stromal and vascular space invasion by individual tumour cells and tumour cell clusters and one of which also had capsular infiltration [10].
A number of sex cord-stromal tumours including Sertoli–Leydig cell tumour, granulosa cell tumour and gonadoblastoma are the differential diagnoses of SCTATs. These tumours share some of the components that are found in SCTATs. Table 1 represents histological and immunohistochemical similarities and differences for such differential tumours and SCTAT.
Table 1.
Histopathological features and positive and negative immunohistochemical markers of SCTAT and its differential diagnoses
| Type of tumour | Histological features | Positive IHC markers | Negative markers |
|---|---|---|---|
| Sertoli–Leydig cell tumour | There is tubular arrangement of Sertoli cells with hyperchromatic nuclei with Reinke cystalloids which are large, bright, eosinophilic, intracytoplasmic and are positive for PAS and trichrome stains. There is also hepatoid differentiation as well as neuroendocrine differentiation. | Inhibin-A, AFP, chromogranin, calretinin | Negative for CK7, CAM 5.2, EMA, galectin-3 |
| Granulosa cell tumour | Immature tumour cells that are pleomorphic with brisk mitoses and follicles of varying size and shape typically punctuate the tumour. They contain Call–Exner bodies that help to distinguish GCTs from other SCSTs. | Inhibin-B, inhibin-A, calretinin, MIS, AMH, FRP | Galectin-3, CK7, EMA |
| Gonadoblastoma | Irregular clusters of immature Sertoli cells and germ cells surrounded by basement membranes with Sertoli cells encircling rounded hyaline nodules. Sertoli cells surround large germ cells and germ cells occupy centre of nests with peripheral ring of Sertoli cells. | Inhibin-A, P53, MIS, laminin, WT 1, cytokeratin, vimentin, PLAP | EMA, oestrogen |
| SCTAT | Composed of sex cord tumour cells aligned as ring-shaped tubules with antipodal arrangement of the nuclei, the presence of fibrous stroma and eosinophilic hyaline cores within the nests. They also lack germ cell. Nuclear atypia and mitotic figures are uncommon features. It can be distinguished from granulosa cell tumour by dominance of hyaline bodies in tubules, from gonadoblastoma by absence of germ cells and from Sertoli–Leydig cell tumour by complex tubules. | Inhibin-A, calretinin, WT 1, CK, vimentin | P53, AFP, EMA, PLAP, laminin, CD117 |
AFP, alpha-fetoprotein; CAM, cytokeratin antigen membrane; EMA, epithelial membrane antigen; PLAP, placenta-like alkaline phosphatase; WT, Wilms tumour; SCTAT, sex cord tumour with annular tubules; MIS, mullerian-inhibiting substance; AMH, anti-mullerian hormone; FRP, follicle regulating protein, GCT, granulosa cell tumour; SCST, sex cord-stromal tumour; IHC, immunohistochemistry, CK, cytokeratin.
SCTATs have been reported in literature to be treated by surgical removal of the involved organ [10]. Other treatment modalities include adjuvant chemotherapy and radiotherapy or combination of surgical treatment and chemotherapy and/or radiotherapy [8, 10]. The prognosis is usually better. Qian et al. [6] reported only one case of death among the 13 cases included in the study after 12 years of follow-up and the rest of the patients had either complete remission or partial remission. Bembde et al. [10] reported five cases of death out of 27 cases due to other causes rather than SCTAT. In summary, SCTATs are usually benign and they tend to occur most often in women of reproductive age.
ACKNOWLEDGEMENTS
None.
CONFLICT OF INTEREST STATEMENT
None declared.
FUNDING
No sources of funding.
ETHICAL APPROVAL
No ethical approval was needed.
INFORMED CONSENT
We confirm that we obtained a written informed consent from the parents (father) for publication of the case details and any accompanying images. A copy of this consent is available upon request by the Editor-in-Chief of this journal for review purposes.
GUARANTOR
Dr J.J.Y. is the guarantor of this paper.
REFERENCES
- 1. Scully RE. Sex cord tumor with annular tubules a distinctive ovarian tumor of the Peutz-Jeghers syndrome. Cancer 1970;5:1107–21. [DOI] [PubMed] [Google Scholar]
- 2. Purohit R, Alam S. Sex cord tumour of the ovary with annular tubules (SCTAT). Histopathology 1980;4:147–54. [DOI] [PubMed] [Google Scholar]
- 3. Chatziioannidou K, Botsikas D, Tille JC, Dubuisson J. Preservation of fertility in non-Peutz-Jegher syndrome-associated ovarian sex cord tumour with annular tubules. BMJ Case Rep 2015;23:20–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Erhan S, Gul A, Keser S, Acar S, Sensu S. Sex cord stromal tumor with annular tubules: a case report. Gynecol Obstet Case Rep 2016;2:1–3. [Google Scholar]
- 5. Kurman RJ, Carcangiu ML, Herrington S, Young RH. WHO classification of tumours of female reproductive organs. IARC 2014;5:12–21. [Google Scholar]
- 6. Qian Q, You Y, Yang J, Cao D, Zhu Z, Wu M, et al. Management and prognosis of patients with ovarian sex cord tumor with annular tubules: a retrospective study. BMC Cancer 2015;15:270–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Singh M, Mandal S, Majumdar K. Sex cord tumor with annular tubules: an incidental finding in an endometriotic cyst—the first known cooccurrence. Biomed Res Int 2014;1:79–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lele SM, Sawh RN, Zaharopoulos P, Adesokan A, Smith M, Linhart JM, et al. Malignant ovarian sex cord tumor with annular tubules in a patient with Peutz-Jeghers syndrome: a case report. Mod Pathol 2000;13:466–75. [DOI] [PubMed] [Google Scholar]
- 9. Dart K, Schwartzenfeld T, Brandes W, D’Errico A, Stender M. Metastatic ovarian sex-cord stromal tumor with annular tubules in a patient without Peutz-Jeghers syndrome. Ear Nose Throat J 2014;93:176–82. [PubMed] [Google Scholar]
- 10. Bembde AS, Manzoor I, Somani S, Mulay SS. Ovarian sex cord stromal tumor with annular tubules-a case report & review of literature. Int J Health Sci Res 2014;4:192–7. [Google Scholar]


