Abstract
Immune checkpoint inhibitors (ICIs) are now widely used to many malignant diseases, but some patients suffer from immune-related adverse events during or after ICI treatments. The monoclonal antibody infliximab is usually chosen as a salvage treatment to combat corticosteroid-resistant adverse events, but infliximab is not recommended as a response to hepatitis because of the potential risk of liver failure. An alternative treatment option has not been established. We treated a head and neck cancer patient (a 50-year-old Japanese male) who suffered from corticosteroid-resistant hepatitis during treatment with nivolumab, an anti-PD-1 ICI, and that was recovered by mycophenolate mofetil salvage therapy.
Keywords: immune-related adverse event, head and neck cancer, nivolumab, mycophenolate
INTRODUCTION
Immune checkpoint inhibitors (ICIs) have become the standard treatment options for many malignant diseases [1]. As treatments for head and neck cancer, the anti-programmed death-1 (PD-1) antibodies nivolumab and pembrolizumab provided prolonged overall survival in clinical trials [2, 3].
The safety profiles of ICIs differ from those of other antitumor drugs, and the adverse events that occur specifically in ICI treatments are categorized as immune-related adverse events (irAEs) [4]. Systemic corticosteroids are usually considered for treating irAEs, but an appropriate alternative option to corticosteroid-resistant liver toxicity has not been established [5].
Here we report the clinical sequence of a head and neck cancer patient who suffered from severe irAEs including liver toxicity during nivolumab treatment. The liver toxicity was resistant to corticosteroid, and the subsequent addition of mycophenolate mofetil (MMF) to the corticosteroid succeeded in improving the patient’s liver function.
CASE PRESENTATION
The patient was a 50-year-old Japanese male diagnosed with laryngeal cancer. The clinical stage was IVB (cT4N2cM0), and he underwent curative surgery, i.e. a total pharyngolaryngectomy and bilateral neck dissection. After the surgery, a CT scan revealed liver and pancreatic lesions which had been not observed before surgery, and the liver biopsy revealed squamous cell carcinoma that corresponded to the carcinoma of the larynx. The patient had a history of hepatitis B, and HB antigen was still positive (HBV-DNA was negative); entecavir prophylaxis was therefore started, and the combination of cetuximab, 5-fluorouracil, and cetuximab were administered with a partial response. After 9 months, recurrences in multiple lymph nodes were observed, and nivolumab 3 mg/kg every 2 weeks was started. The patient’s disease was stable while receiving nivolumab with no signs of disease progression. At the 13th cycle of nivolumab, the patient complained of fatigue, and his lab data showed that the levels of both cortisol and adrenocorticotropic hormone (ACTH) were low. Oral prednisolone (PSL) 5 mg/day was thus prescribed. The PSL prescription improved the patient’s fatigue, and the 14th cycle of nivolumab was administered with PSL continuation.
However, 2 weeks later the patient came to the hospital with complaints of diarrhea, systemic skin rash and fever, and he was hospitalized immediately. At admission, the patient’s performance status was ECOG 2, fatigue and diarrhea were grade 2, and systemic and severe grade 3 rashes were seen; bullous dermatitis in mucosa was not observed. Lab tests revealed that the patient’s cortisol and ACTH levels were still low and that hepatic transaminases were increased (AST 363 U/L and ALT 246 U/L); such increases had been not shown by the lab tests 2 weeks earlier. CT and abdominal echography did not show the progression of liver metastases or biliary obstruction.
Soon after the patient’s hospitalization, high-dose methylprednisolone (mPSL) 500 mg/day was intravenously administered, and his skin lesions and diarrhea immediately recovered. However, his hepatic transaminase levels had exacerbated during the mPSL therapy, and total bilirubin (T-Bil) and alkaline phosphatase (ALP) were also increased. Thus, after 3 days of mPSL pulse therapy, we added MMF 2000 mg/day to the intravenous PSL continuation, and a liver biopsy was planned. The biopsy on the sixth day of hospitalization revealed lymphocyte infiltration to Glisson’s capsule and piecemeal necrosis, which were consistent with hepatitis due to nivolumab treatment. On day 31 of hospitalization, the patient’s liver function test results had recovered to grade 0, and we began to gradually reduce the MMF and PSL. The patient was discharged with oral MMF 1500 mg/day and PSL 30 mg/day on day 68 of hospitalization. These drugs’ dosages were gradually reduced in the outpatient setting; the MMF was terminated on the 25th day from the discharge and the PSL was continued at 10 mg/day after that. The treatment sequence following nivolumab treatment is provided in Fig. 1.
Figure 1.
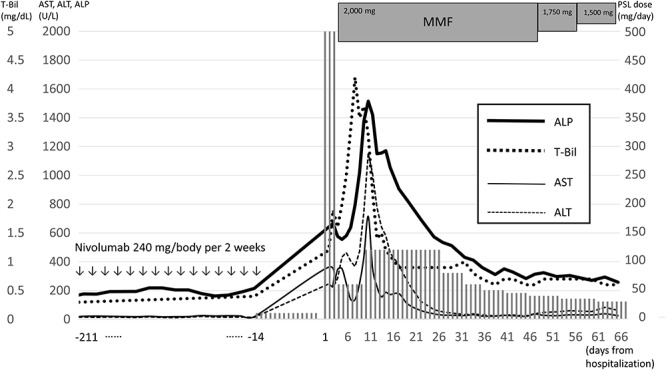
The treatment sequence of the patient, a 51-year-old male. The changes in the patient’s liver function test data following nivolumab treatment are also shown. ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; MMF, mycophenolate mofetil; PSL, prednisolone; T-Bil, total bilirubin.
The radiographic evaluation after the patient’s discharge showed tumor progression in multiple lymph node metastases, and palliative radiotherapy was performed; ICIs or other systemic chemotherapies were not administered, concerning about the recurrence of irAEs. The patient finally died of the laryngeal cancer progression at 16.3 months after the nivolumab induction, 9.7 months after the hospitalization due to irAEs.
DISCUSSION
During and after ICI treatments, irAEs can occur in organs all over the body, and the prevalence and severity of irAEs are different in injured organs [4], and the reported incidence of liver injury induced by ICIs is 3.5% [6].
In our present patient, multiple irAEs of skin lesions, endocrine abnormality and diarrhea were observed simultaneously. Most of these irAEs were rapidly improved by high-dose corticosteroid therapy, and only the patient’s liver dysfunction worsened despite the corticosteroid therapy.
MMF is a purine antagonist that inhibits the proliferation of lymphocytes and considered to be effective against autoimmune hepatitis that is resistant to corticosteroids [7]. Thus, MMF is considered to have the potential to be a treatment option for corticosteroid-resistant immune-related hepatitis induced by an ICI and shown as an option to corticosteroid-resistant hepatitis in the clinical guideline [5]. There are case reports of the successful treatment of hepatotoxicity due to ICI treatment by the addition of MMF to corticosteroid therapy [8, 9].
An additional concern in our patient’s case was that he was an HBV carrier and under a continued entecavir regimen. In our patient’s case, an HBV-DNA assay was conducted at the occurrence and multiple times during the treatment of the irAEs, but the HBV-DNA status was never positive regardless of the patient’s liver function disorders.
It cannot be denied that hepatitis B could be a risk factor for the liver injury that is induced by ICIs, but our patient’s hepatitis was not the reactivation of HBV. A small randomized trial of HBV carriers with idiopathic nephrotic syndrome indicated that MMF treatment did not cause HBV reactivation compared to corticosteroid [10].
We have reported a case of severe irAEs (particularly immune-related hepatitis that was resistant to corticosteroids) which was successfully treated with MMF. The provision of our patient’s treatment sequence herein may contribute to the optimal treatment of other patients with ICI-induced hepatitis that is resistant to corticosteroids and requires other salvage therapies, e.g. MMF.
DISCLOSURE STATEMENT
The authors have no conflicts of interests to declare.
ACKNOWLEDGEMENTS
We thank all the staff members of the Departments of Medical Oncology and Head and Neck Surgery at the Cancer Institute Hospital of the Japanese Foundation for Cancer Research for diagnosing and treating the patient.
FUNDING
None.
ETHICAL APPROVAL
No specific ethical approval was required.
CONSENT
Written informed consent was obtained from the patient for submission of this manuscript.
GUARANTOR
K.N. is the guarantor of this article.
REFERENCES
- 1. Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science 2018;359:1350–5. doi: 10.1126/science.aar4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ferris RL, Blumenschein G Jr, Fayette J, Guigay J, Colevas AD, Licitra L et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med 2016;375:1856–67. doi: 10.1056/NEJMoa1602252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Burtness B, Harrington KJ, Greil R, Soulières D, Tahara M, de Castro G Jr et al. KEYNOTE-048 investigators. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet 2019;394:1915–28. doi: 10.1016/S0140-6736(19)32591-7. [DOI] [PubMed] [Google Scholar]
- 4. Baxi S, Yang A, Gennareli RL, Khan N, Wang Z, Boyce L et al. Immune-related adverse events for anti-PD1- and anti-PD-L1 drugs: systematic review and meta-analysis. BMJ 2018;360:k793. doi: 10.1136/bmj.k793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brahmer JR, Lacchetti C, Schneider BJ, Atkins MB, Brassil KJ, Caterino JM et al. National Comprehensive Cancer Network. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol 2018;36:1714–68. doi: 10.1200/JCO.2017.77.6385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. De Martin E, Michot JM, Papouin B, Champiat S, Mateus C, Lambotte O et al. Characterization of liver injury induced by cancer immunotherapy using immune checkpoint inhibitors. J Hepatol 2018;68:1181–90. doi: 10.1016/j.jhep.2018.01.033. [DOI] [PubMed] [Google Scholar]
- 7. Heneghan MA, McFarlane IG. Current and novel immunosuppressive therapy for autoimmune hepatitis. Hepatology 2002;35:7–13. doi: 10.1053/jhep.2002.30991. [DOI] [PubMed] [Google Scholar]
- 8. Tanaka R, Fujisawa Y, Sae I, Maruyama H, Ito S, Hasegawa N et al. Severe hepatitis arising from ipilimumab administration, following melanoma treatment with nivolumab. Jpn J Clin Oncol 2017;47:175–8. doi: 10.1093/jjco/hyw167. [DOI] [PubMed] [Google Scholar]
- 9. Doherty GJ, Duckworth AM, Davies SE, Mells GF, Brais R, Harden SV et al. Severe steroid-resistant anti-PD1 T-cell checkpoint inhibitor-induced hepatotoxicity driven by biliary injury. ESMO Open 2017;2:e000268. doi: 10.1136/esmoopen-2017-000268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Li X, Tian J, Wu J, He Q, Han F, Li Q et al. A comparison of a standard-dose prednisone regimen and mycophenolate mofetil combined with a lower prednisone dose in Chinese adults with idiopathic syndrome who were carriers of hepatitis B surface antigen: a prospective cohort study. Clin Ther 2009;31:741–50. doi: 10.1016/j.clinthera.2009.04.011. [DOI] [PubMed] [Google Scholar]


