Abstract
Chloroquine (CQ) and its analogue hydroxychloroquine (HCQ) have long been used worldwide as frontline drugs for the treatment and prophylaxis of human malaria. Since the first reported cases in Wuhan, China, in late December 2019, humans have been under threat from coronavirus disease 2019 (COVID-19) caused by the novel coronavirus SARS-CoV-2 (previously known as 2019-nCoV), subsequently declared a pandemic. While the world is searching for expedited approval for a vaccine, which may be only preventative and not a cure, physicians and country leaders are considering several concerted clinical trials suggesting that the age-old antimalarial drugs CQ/HCQ could be a potent therapeutic against COVID-19. Based on accumulating scientific reports, here we highlight the possible modes of action of CQ/HCQ that could justify its use against viral infections. Considering the global health crisis of the COVID-19 pandemic, the option of repurposing old drugs, e.g. CQ/HCQ, particularly HCQ, for the treatment of SARS-CoV-2 infection could be a good choice. CQ/HCQ has diverse modes of action, including alteration of the acidic environment inside lysosomes and late endosomes, preventing endocytosis, exosome release and phagolysosomal fusion, and inhibition of the host cytokine storm. One or more diverse mechanisms might work against viral infections and reduce mortality. As there is no cure for COVID-19, clinical testing of HCQ is urgently required to determine its potency against SARS-CoV-2, as this is the currently available treatment option. There remains a need to find other innovative drug candidates as possible candidates to enter clinical evaluation and testing.
Keywords: COVID-19, SARS-CoV-2, Chloroquine, Hydroxychloroquine, Pandemic
1. Introduction
Chloroquine (CQ) and its analogue hydroxychloroquine (HCQ) have long been used worldwide as frontline drugs for the treatment and prophylaxis of all types of human malaria. They have been used and tested against several pathophysiological conditions such us hepatic amoebiasis, lupus erythematosus, light-sensitive skin eruptions and rheumatoid arthritis [1], [2]. There are several in vitro and in vivo studies reporting the effect of CQ monotherapy or combination therapy on different cancers. Intraperitoneal administration of CQ at 60 mg/kg/day for 7 days delayed tumour growth in mice with epidermal growth factor receptor (EGFR)-over expressing glioblastoma xenografts [3]. In another study using a human intracranial glioblastoma xenograft mouse model, intratumoural injection of 5 μL of CQ (30 mM/day) for 17 days significantly reduced mitotic cells with apoptotic cell number increases through the p53 pathway of apoptotic induction [4], [5]. To support their anticancer potential, several clinical studies on CQ and HCQ as anticancer or antitumour drugs are currently underway (https://clinicaltrials.gov). CQ has been found to act as an antitumour as well as an anticancer agent [6], further indicating that CQ supplementation with conventional treatments of glioblastoma patients might improve the mid-term survival of these patients. Another study has found that CQ diminishes intratumoural hypoxia [7] making cancer cells more sensitive to radiotherapy and other oxygen-dependent therapies [8] and reducing metastasis. It is documented that autophagy, which occurs in cells under several conditions, promotes many cancers [9]. This degradative process initiates the formation of autophagosomes that retain degraded cell components, which then fuse with lysosomes to recycle these components. It is understood that autophagy is halted by CQ by disrupting the energy source of the autophagy pathway [10], [11]. Despite inhibition of the ‘autophagic flux’ by CQ and HCQ against cancer, another study has reported that these drugs affect the Toll-like receptor 9 (TLR9), p53 and CXCR4-CXCL12 pathways in cancer cells. In addition, in the tumour stroma, CQ affects the tumour vasculature, cancer-associated fibroblasts and the immune system [5].
CQ displays wide-ranging properties against bacterial, fungal, protozoal, parasitic and viral infections. Newman et al. showed that CQ boosts human macrophages to suppress the growth of yeast by limiting the availability of iron in macrophages [12]. CQ induces human mononuclear phagocytes to kill Cryptococcus neoformans, most commonly found in immunodeficient patients [12], [13], [14]. In addition to such immune modulation, CQ has shown promising effects in combination therapy against drug-resistant Candida albicans by inhibiting biofilm formation at low concentrations [15].
The coronavirus disease 2019 (COVID-19) pandemic caused by an outbreak of the novel coronavirus named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2; previously known as 2019-nCoV) [16], first reported in Wuhan, China, in late December 2019 [17], is a serious global threat to human health. As of 24 April 2020, approximately 2 620 000 people have been reported as confirmed cases of COVID-19, with over 181 000 deaths [18]. COVID-19 is an acute resolving disease that can be deadly if not immediately and properly managed. The severity of the disease is due to massive alveolar damage, with respiratory failure and subsequent death [17]. However, the pathology has not been clearly reported owing to barely accessible autopsy or biopsy reports of affected patients [17]. Countries worldwide are exhaustively trying to discover a cure or preventative measures against the virus. At present, all treatments are supportive in order to treat symptoms. Despite the fact that anti-inflammatory and antiviral treatments have been employed, no specific antiviral drugs have been confirmed to be effective.
Given that the development of a vaccine is time-dependent, physicians and scientists are trying to find quick but effective treatments for COVID-19. Currently available scientific reports of several clinical trials suggest that the age-old antimalarial drugs CQ and HCQ could be potent therapeutic agents against COVID-19. Accumulating scientific reports have proposed and highlighted the possible different modes of action of CQ/HCQ, particularly against viral infections.
2. History of chloroquine and development of its analogues
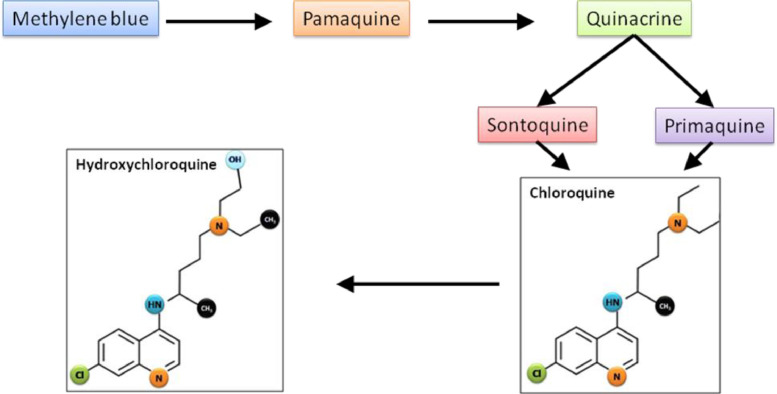
CQ (chloroquine phosphate), a 4-amino-quinoline, was synthesised in 1934 by Hans Andersag and co-workers at Bayer Laboratories and was introduced in 1945 to prevent and treat malaria. It is a weak base that exists in protonated and unprotonated forms. Before the synthesis of CQ, the natural compound quinine from cinchona tree bark was used as an antimalarial agent. In 1891, Paul Ehrlich's group discovered methylene blue, a dye that can selectively kill malaria parasites. Using chemistry and structure–activity relationships, by changing the basic methyl group resulted in a more effective antimalarial agent, pamaquine. Subsequent attachment of the basic side chain of pamaquine to several different heterocyclic ring systems resulted in synthesis of the acridine derivative quinacrine, which has an extra benzene ring. Further research resulted in the discovery of two CQ analogues, sontoquine and primaquine, which were improved and better antimalarial drugs [19]. Studies on these compounds then led to the discovery of ResochinⓇ. During World War II, investigations on ResochinⓇ led to the synthesis of CQ. Hydroxychloroquine (HCQ) sulfate, a derivative of CQ, was first synthesised in 1946 by introducing a hydroxyl group onto CQ and was demonstrated to be much less (~40%) toxic than CQ in animals [2], [20] (Fig. 1 ). It is also believed that HCQ has less blood–retinal barrier permeability and is less toxic to retinal cells than CQ [21], [22].
Fig. 1.
Development of chloroquine and its analogues.
3. Pharmacokinetic properties of hydroxychloroquine
The pharmacokinetic properties of HCQ are similar to those of CQ, but it is reported that HCQ is less active than CQ against resistant malaria parasites, leaving CQ as the only age-old drug against malaria until the emergence of CQ-resistant malaria parasites. The solubility of the drug and/or its analogues in water and its absorption occurs almost entirely in the digestive tract. The plasma level of the drug and its analogues has been found to reach a peak 4–12 h after the initial dose. The half-lives of CQ and HCQ are long, ranging from 40–50 days. CQ analogues have strong affinities to blood constituents and are highly protein-bound, particularly to thrombocytes and granulocytes, which reduces its plasma concentration. In addition, a major fraction of CQ analogues in plasma is bound to plasma proteins, primarily albumin [23], [24], [25].
4. Antiviral activities of chloroquine
Since the last decades, humans have been confronted with several emerging and re-emerging viruses such as dengue [26], [27], [28], Ebola [29], [30], avian influenza, severe acute respiratory syndrome (SARS) [31], [32], Middle East respiratory syndrome (MERS) [33], hepatitis C, chikungunya virus (CHIKV) [34], [35] and human immunodeficiency virus (HIV) [36], [37], [38], [39], etc. An in vitro study revealed that CQ is effective both at entry and post-entry stages of SARS-CoV-2 infection in Vero E6 cells [40].
Currently, there are no specific therapeutic strategies available for SARS-CoV-2 infection, which causes COVID-19. Physicians, researchers and scientists are involved in several clinical trials of CQ and/or HCQ at different stages (Table 1 ) in search for a treatment to curb the menace of the COVID-19 pandemic.
Table 1.
Ongoing trials with chloroquine (CQ) and hydroxychloroquine (HCQ) for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection/COVID-19 as of 19 April 2020.
| ClinicalTrials.gov IDa | Title | No. of participants | Phase |
|---|---|---|---|
| NCT04351724 | Austrian CoronaVirus Adaptive Clinical Trial (COVID-19) | 500 | 3 |
| NCT04321278 | Safety and efficacy of hydroxychloroquine associated with azithromycin in SARS-CoV2 virus (Coalition Covid-19 Brasil II) | 440 | 3 |
| NCT04303299 | Various combination of protease inhibitors, oseltamivir, favipiravir, and hydroxychloroquine for treatment of COVID-19: a randomized control trial | 320 | 3 |
| NCT04325893 | Hydroxychloroquine versus placebo in COVID-19 patients at risk for severe disease | 1300 | 3 |
| NCT04316377 | Norwegian coronavirus disease 2019 study | 202 | 4 |
| NCT04330144 | Hydroxychloroquine as post exposure prophylaxis for SARS-CoV-2 (HOPE Trial) | 2486 | 3 |
| NCT04342221 | Hydroxychloroquine for COVID-19 | 220 | 3 |
| NCT04340544 | Hydroxychloroquine for the treatment of mild COVID-19 disease | 2700 | 3 |
| NCT04346329 | Immune monitoring of prophylactic effect of hydroxychloroquine in healthcare providers highly exposed to COVID-19 | 86 | 3 |
| NCT04341727 | Hydroxychloroquine, hydroxychloroquine, azithromycin in the treatment of SARS CoV-2 infection | 500 | 3 |
| NCT04346667 | Post-exposure prophylaxis for asymptomatic SARS-CoV-2 COVID-19 patients with chloroquine compounds | 400 | 4 |
| NCT04351191 | Prophylaxis of exposed COVID-19 individuals with mild symptoms using chloroquine compounds | 400 | 4 |
| NCT04333732 | CROWN CORONATION: chloroquine repurposing to health workers for novel coronavirus mitigation | 55 000 | 3 |
At ClinicalTrials.gov (https://www.clinicaltrials.gov/).
5. Modes of action of chloroquine/hydroxychloroquine
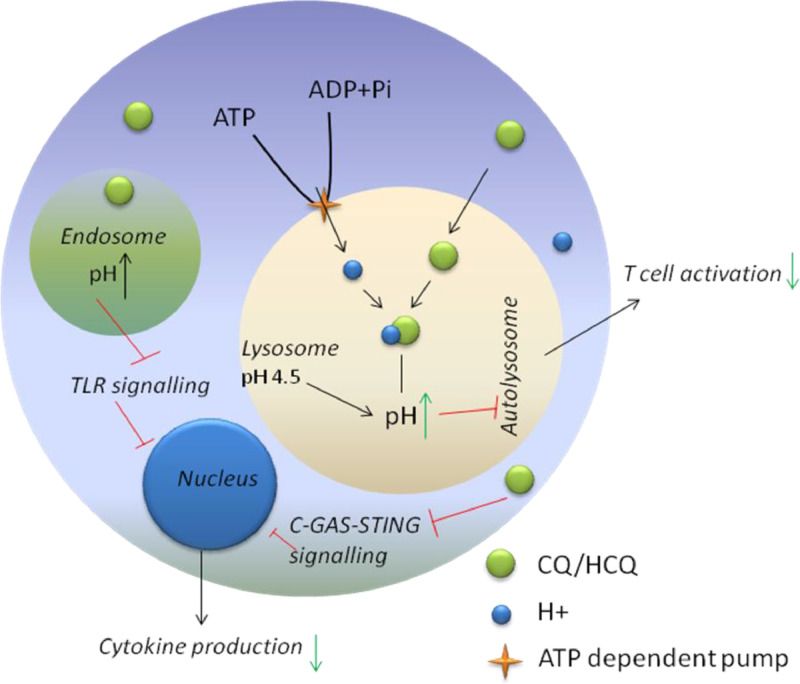
CQ in its unprotonated form can easily diffuse across cell membranes to acidic vesicles in the cytoplasm [lysosomes, late endosomes, trans-Golgi network (TGN) vesicles] and becomes trapped in the vesicles after being protonated. Protonated CQ is unable to diffuse out of lysosome or endosomes, being retained in the cellular compartments with hydrolases. As CQ and its analogues are diprotic weak bases and its unprotonated form can selectively enter lysosomes and become protonated in a manner inversely proportional to pH (according to the Henderson–Hasselbalch law) [41], [42], they are known as lysosomotropic agents [43].
The optimal activity of hydrolases is maintained in lysosomes and/or the TGN with the help of H+-ATPase (proton pump) activity, which maintains a pH of 5.0 inside these compartments [44]. The lysosomal H+-ATPase influxes H+ ions through ATP-dependent pumps, resulting in irreversible drug accumulation inside the lysosome (Fig. 2 ) from the cytoplasm because of the difference in pH. The drug alters the acidic environment in the lysosome and, as a result, the cell cannot proceed systematically with endocytosis, exosome release or phagolysosomal fusion [45]. In addition, An et al. [46] have reported that CQ/HCQ can also hamper the interaction between cytosolic DNA and the nucleic acid sensor cyclic GMP-AMP synthase (cGAS) when in the cytosol [47].
Fig. 2.
Schematic diagram of the role of chloroquine (CQ) and hydroxychloroquine (HCQ) in the intracellular space. The drugs increase the pH of endosomes and lysosomes. As a result, activation of T-cells and other cytokines is repressed.
Elevation of the lysosomal pH by CQ/HCQ hinders antigen presentation, chemotaxis and proteolysis by the cell. The reduction of antigen presentation owing to the elevation of pH by CQ/HCQ decreases the antigen–major histocompatibility complex (MHC), as autoantigenic peptides have low affinity for self-MHC. The reduced amount of self-peptide–MHC on antigen-presenting cells results in subordinate activity of other target cells as well as release of cytokines by immune cells such as T-cells and other antigen-presenting cells [48].
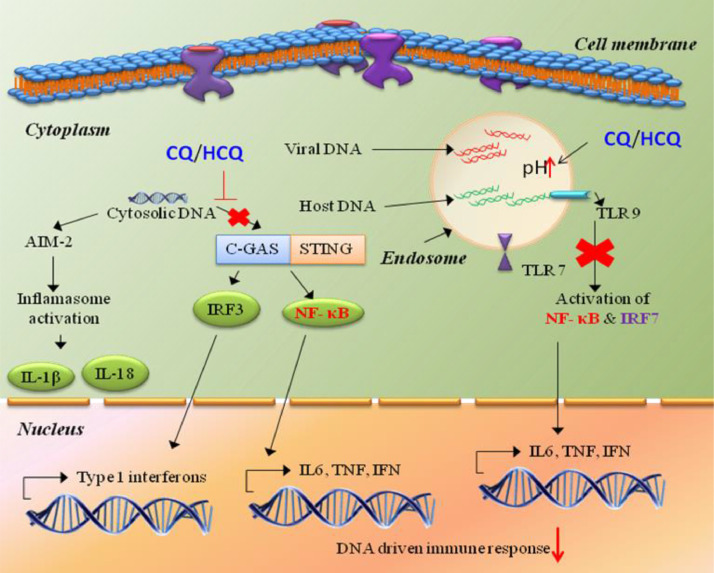
Moreover, CQ/HCQ causes a sporadic obligation between Toll-like receptor 7 (TLR7) and TLR9 and their RNA/DNA ligands owing to changing of pH towards the basic in the cellular endosomal environment, which suppresses TLR signalling [47], [49], [50]. The impending cGAS-STING (stimulator of interferon genes) pathway results in attenuation of pro-inflammatory cytokines such as tumour necrosis factor (TNF), interleukin-6 (IL-6) and IL-1 (Fig. 3 ) [47], [51].
Fig. 3.
Chloroquine (CQ) and hydroxychloroquine (HCQ) suppress DNA sensing by the cGAS-STING pathway and Toll-like receptor (TLR) signalling.
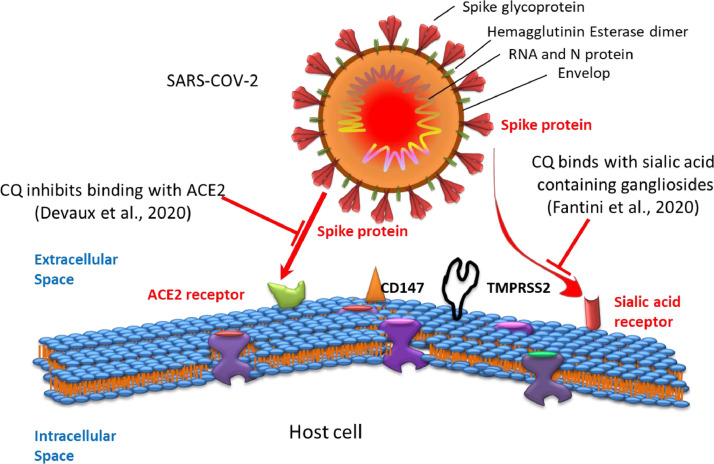
On the other hand, enveloped viruses are trapped within the endoplasmic and TGN vesicles for post-translational modification of their envelope glycoproteins. This process involves proteases and glycosyltransferases, some of which require a low-pH environment. By neutralising the acidic pH, CQ/HCQ is responsible for the deactivation of several enzymes in the vesicles, such as glycosyltransferases, which in turn is responsible for inhibition of glycosylation. Inhibition of glycosylation results in the host developing an adaptive immune response against the infection [48], [52] and impairs the cellular receptor angiotensin-converting enzyme 2 (ACE2) for SARS-CoV-1 binding and blocks fusion with the host cell [22], [53]. It is believed that SARS-CoV-2 also employs the ACE2 receptor to enter the host cell [53], [54], [55]. Colson et al. [55] reported that the spike (S) protein of SARS-CoV-2 is cleaved in the autophagosome by host cell proteases such as cathepsins, which can be inhibited owing to the increased pH in the lysosome as a result of CQ accumulation.
In addition, several studies have shown that many human viruses, e.g. influenza virus [56] and coronavirus [57], [58], that affect the respiratory tract enter cells with the help of sialic acid-linked gangliosides [59]. In addition to the sialic acid receptor [58] and ACE2 [60], the transmembrane serine protease 2 (TMPRSS2) [61], [62] and extracellular matrix metalloproteinase inducer CD147 (also known as basigin) [63] are under investigation regarding the entry of SARS-CoV into human cells (Fig. 4 ).
Fig. 4.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) entry occurs via the angiotensin-converting enzyme 2 (ACE2), the sialic acid receptor, the transmembrane serine protease 2 (TMPRSS2) and CD147. The viral spike protein binds to receptor(s) present on the cell surface of host cells and enters the cell. CQ, chloroquine.
CQ inhibits human coronavirus HCoV-O43 and orthomyxoviruses by inhibiting sialic acid biosynthesis through inhibiting quinine reductase-2. The viruses use sialic acid moieties as receptors [23]. Considering the higher transmissibility of SARS-CoV-2, Fantini et al. have observed sialic acid-containing glycoproteins and gangliosides as a cell surface attachment factor by a molecular modelling study [64]. The results support that CQ and HCQ bind readily to sialic acids with high affinity [58] and also bind to sialic-acid containing gangliosides [64].
In view of the ACE2 receptor as a regulator of the contagiousness both for SARS-CoV [65] and SARS-CoV-2 [60], CQ may interfere with effective binding of the spike protein to the host cell by reducing glycosylation of ACE2 [66].
6. Clinical efficacy of chloroquine/hydroxychloroquine against SARS-CoV-2
As infections and deaths due to the SARS-CoV-2 pandemic have overwhelmed the globe faster than science can respond, hospitals and doctors are prescribing CQ/HCQ as a treatment for COVID-19. The proposed use of HCQ for treating COVD-19 is based on preliminary preclinical in vitro evidence where this drug showed antiviral properties against SARS-CoV-2 [67], [68]. In these studies, CQ/HCQ appears to work by interfering with entry of the virus into human cells and inhibition of virus replication. There are different views held by clinicians on the safety and efficacy of HCQ for treating COVID-19. CQ/HCQ causes a multitude of serious complications including retinopathy, cardiomyopathy, neuromyopathy and myopathy [2]. Use of CQ and HCQ has not been reported in acute prevention, thus their efficacy is unknown, but they are indicated for therapeutic use.
The beneficial impact of HCQ (200 mg three times a day for 10 days) or HCQ plus azithromycin (AZM) (500 mg on Day 1 followed by 250 mg/day for the next 4 days) on viral negativity at Day 6 was reported in a non-randomised open-label clinical trial in France enrolling COVID-19 patients treated with HCQ (20 patients) or untreated controls (16 patients) [69]. In another study, Gautret et al. reported that at Day 6 post-inclusion, 70% of patients were cured with 600 mg HCQ (200 mg three times a day for 10 days) compared with 12.5% with patients in the control group (P = 0.001) [70]. Recently, two trials in China have released their findings, namely NCT04261517 (www.ClinicalTrials.gov) (HCQ 400 mg/day for 5 days) and ChiCTR2000029559 (Chinese Clinical Trials Registry; http://www.chictr.org.cn/enindex.aspx) (HCQ 200 mg twice daily). Based on details from a Chinese clinical trial (ChiCTR2000029741), 50% efficacy of CQ phosphate (5 days) was observed in COVID-19 compared with 20% efficacy of combination lopinavir/ritonavir (5 days) in Chinese patients with pneumonia [71]. In a clinical study, Huang et al. found that CQ-treated patients (500 mg orally twice daily for 10 days) do better than patients treated with lopinavir/ritonavir [72]. Consequently, CQ-treated patients have already been released from hospital. Another clinical trial conducted with 100 Chinese patients with COVID-19 infection reported that CQ phosphate showed remarkable results both in terms of clinical outcome and viral clearance compared with the control group [73]. Gao et al. also reported that the drug was effective in inhibiting the exacerbation of pneumonia, improving lung imaging findings, promoting virus negativity and shortening the disease course of COVID-19 [73]. They recommended addition of the drug to guidelines for the prevention, diagnosis and treatment of pneumonia caused by COVID-19 [74].
7. Limitations of chloroquine/hydroxychloroquine against SARS-CoV-2
Some preliminary non-peer-reviewed scientific reports suggest that CQ/HCQ may not be beneficial for use in COVID-19. A report from Brazil warned that patients receiving high-dose CQ had more severe QT prolongation (heart rhythm disorder causing arrhythmias) and a trend towards higher mortality compared with the low-dose CQ group [75]. At the time of their reporting, the overall fatality rate across both arms of the study was 13.5% [75], similar to historical data from similar patients not taking CQ. In their study of 84 adults patients with SARS-CoV-2 treated with HCQ+AZM combination at New York University's Langone Medical Center, Chorin et al. found a statistically significant change in QT prolongation from baseline; in 30% of patients the QTc increased by >40 ms and in 11% of patients the QTc increased to >500 ms [76]. They concluded that these patients represent a high-risk group for arrhythmia. They further concluded that the development of renal failure in these patients was a cause of the extreme QT prolongation. The group is advising against the use of HCQ as a treatment for COVID-19 [https://www.washingtonpost.com/health/2020/05/22/hydroxychloroquine-coronavirus-study/].
Magagnoli et al. performed a retrospective analysis of data from SARS-CoV-2-infected patients hospitalised in US Veterans Health Administration Medical Centres in which a total of 368 patients were followed [77]. In this study, patients were grouped into those receiving HCQ alone or HCQ+AZM combination in addition to a group that received no treatment but standard supportive management for COVID-19. The analysis studied two primary outcomes, namely death and the requirement for mechanical ventilation. The authors of this study concluded that there was no evidence of the efficacy of HCQ alone or in combination with AZM. They found that mortality was higher in patients receiving HCQ alone (27.8%) and the combination of HCQ+AZM (22.1%) compared with the group receiving no drug treatment (11.4%) [77]. According to the authors, they found an association with mortality in patients treated with HCQ alone. Although these studies are small and retrospective, they highlight important decision-making to not rush into widespread recommendation of these drugs for COVID-19.
8. Conclusion
Considering the global health crisis of the COVID-19 pandemic, the option of repurposing CQ and HCQ, especially HCQ, in the treatment of SARS-CoV-2 might be a logical approach to follow. The available scientific evidence points to the diverse mode of actions of CQ and/or HCQ that places them as a favourable choice for COVID-19 regarding their effect both pre- and post-infection, but of course we must pay attention to doses as well the negative effects of CQ/HCQ on human health. However, with new evidence emerging, there is need for precautionary measures to be taken not to rush in recommending CQ/HCQ for use in COVID-19 patients. We must wait for results from larger prospective, randomised, dose-determining controlled clinical trials before making clinical recommendations for HCQ and indicating it for COVID-19. In the meantime, whilst looking for a vaccine, which may only be preventative, in-depth but expedited responsive discovery and clinical research is needed, including looking at traditional medicine-based therapies.
Acknowledgments
The authors are grateful to the Department of Pharmacology of the University of the Free State (Bloemfontein, South Africa) for providing the opportunity and time to prepare this review. ST and BD are grateful to the Department of Pharmacology and Directorate for Research Development (DRD), University of the Free State, for giving them the opportunity of their postdoctoral fellowships.
Funding: None.
Competing interests: None declared.
Ethical approval: Not required.
References
- 1.Rainsford KD, Parke AL, Clifford-Rashotte M, Kean WF. Therapy and pharmacological properties of hydroxychloroquine and chloroquine in treatment of systemic lupus erythematosus, rheumatoid arthritis and related diseases. Inflammopharmacology. 2015;23:231–269. doi: 10.1007/s10787-015-0239-y. [DOI] [PubMed] [Google Scholar]
- 2.Al-Bari MA. Chloroquine analogues in drug discovery: new directions of uses, mechanisms of actions and toxic manifestations from malaria to multifarious diseases. J Antimicrob Chemother. 2015;70:1608–1621. doi: 10.1093/jac/dkv018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jutten B, Keulers TG, Schaaf MB, Savelkouls K, Theys J, Span PN, et al. EGFR overexpressing cells and tumors are dependent on autophagy for growth and survival. Radiother Oncol. 2013;108:479–483. doi: 10.1016/j.radonc.2013.06.033. [DOI] [PubMed] [Google Scholar]
- 4.Kim EL, Wüstenberg R, Rübsam A, Schmitz-Salue C, Warnecke G, Bücker EM, et al. Chloroquine activates the p53 pathway and induces apoptosis in human glioma cells. Neuro Oncol. 2010;12:389–400. doi: 10.1093/neuonc/nop046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Verbaanderd C, Maes H, Schaaf MB, Sukhatme VP, Pantziarka P, Sukhatme V, et al. Repurposing Drugs in Oncology (ReDO)—chloroquine and hydroxychloroquine as anti-cancer agents. Ecancermedicalscience. 2017;11:781. doi: 10.3332/ecancer.2017.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sotelo J, Briceno E, López-González MA. Adding chloroquine to conventional treatment for glioblastoma multiforme: a randomized, double-blind, placebo-controlled trial. Ann Intern Med. 2006;144:337–343. doi: 10.7326/0003-4819-144-5-200603070-00008. [DOI] [PubMed] [Google Scholar]
- 7.Maes H, Kuchnio A, Carmeliet P, Agostinis P. Chloroquine anticancer activity is mediated by autophagy-independent effects on the tumor vasculature. Mol Cell Oncol. 2016;3 doi: 10.4161/23723548.2014.970097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ratikan JA, Sayre JW, Schaue D. Chloroquine engages the immune system to eradicate irradiated breast tumors in mice. Int J Radiat Oncol Biol Phys. 2013;87:761–768. doi: 10.1016/j.ijrobp.2013.07.024. [DOI] [PubMed] [Google Scholar]
- 9.Yun CW, Lee SH. The roles of autophagy in cancer. Int J Mol Sci. 2018;19:3466. doi: 10.3390/ijms19113466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kimura T, Takabatake Y, Takahashi A, Isaka Y. Chloroquine in cancer therapy: a double-edged sword of autophagy. Cancer Res. 2013;73:3–7. doi: 10.1158/0008-5472.CAN-12-2464. [DOI] [PubMed] [Google Scholar]
- 11.Maes H, Rubio N, Garg AD, Agostinis P. Autophagy: shaping the tumor microenvironment and therapeutic response. Trends Mol Med. 2013;19:428–446. doi: 10.1016/j.molmed.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 12.Newman SL, Gootee L, Brunner G, Deepe GS. Chloroquine induces human macrophage killing of Histoplasma capsulatum by limiting the availability of intracellular iron and is therapeutic in a murine model of histoplasmosis. J Clin Invest. 1994;93:1422–1429. doi: 10.1172/JCI117119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levitz SM, Harrison TS, Tabuni A, Liu X. Chloroquine induces human mononuclear phagocytes to inhibit and kill Cryptococcus neoformans by a mechanism independent of iron deprivation. J Clin Invest. 1997;100:1640–1646. doi: 10.1172/JCI119688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gellin B, Modlin JF, Casadevall A, Pirofski LA. Adjunctive immune therapy for fungal infections. Clin Infect Dis. 2001;33:1048–1056. doi: 10.1086/322710. [DOI] [PubMed] [Google Scholar]
- 15.Shinde RB, Raut JS, Chauhan NM, Karuppayil SM. Chloroquine sensitizes biofilms of Candida albicans to antifungal azoles. Braz J Infect Dis. 2013;17:395–400. doi: 10.1016/j.bjid.2012.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. Erratum in: Lancet 2020;395:496. doi: 10.1016/S0140-6736(20)30252-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.World Health Organization (WHO) May 2020. Novel coronavirus (2019-nCoV) situation reports.https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports/ [accessed 24]. [Google Scholar]
- 19.Chen C. Development of antimalarial drugs and their application in China: a historical review. Infect Dis Poverty. 2014;3:9. doi: 10.1186/2049-9957-3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rodriguez-Caruncho C, Marsol IB. Antimalarials in dermatology: mechanism of action, indications, and side effects. Actas Dermosifiliográf. 2014;105:243–252. doi: 10.1016/j.adengl.2012.10.021. [DOI] [PubMed] [Google Scholar]
- 21.Marmor MF, Kellner U, Lai TY, Melles RB, Mieler WF. Recommendations on screening for chloroquine and hydroxychloroquine retinopathy (2016 revision) Ophthalmology. 2016;123:1386–1394. doi: 10.1016/j.ophtha.2016.01.058. [DOI] [PubMed] [Google Scholar]
- 22.Singh AK, Singh A, Shaikh A, Singh R, Misra A. Chloroquine and hydroxychloroquine in the treatment of COVID-19 with or without diabetes: a systematic search and a narrative review with a special reference to India and other developing countries. Diabetes Metab Syndr. 2020;14:241–246. doi: 10.1016/j.dsx.2020.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moore BR, Page-Sharp M, Stoney JR, Ilett KF, Jago JD, Batty KT. Pharmacokinetics, pharmacodynamics, and allometric scaling of chloroquine in a murine malaria model. Antimicrob Agents Chemother. 2011;55:3899–3907. doi: 10.1128/AAC.00067-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Müller F, König J, Glaeser H, Schmidt I, Zolk O, Fromm MF, et al. Molecular mechanism of renal tubular secretion of the antimalarial drug chloroquine. Antimicrob Agents Chemother. 2011;55:3091–3098. doi: 10.1128/AAC.01835-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.González-Hernández I, Aguirre-Cruz L, Sotelo J, López-Arellano R, Morales-Hipólito A, Jung-Cook H. Distribution of hydroxychloroquine in lymphoid tissue in a rabbit model for HIV infection. Antimicrob Agents Chemother. 2014;58:584–586. doi: 10.1128/AAC.01440-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Silva Farias KJ, Lima Machado PR, Lopes da Fonseca BA. Chloroquine inhibits dengue virus type 2 replication in Vero cells but not in C6/36 cells. ScientificWorldJournal. 2013;2013 doi: 10.1155/2013/282734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Silva Farias KJ, Lima Machado PR, Ferreira de Almeida Junior R, de Aquino AA, Lopes da Fonseca BA. Chloroquine interferes with dengue‐2 virus replication in U937 cells. Microbiol Immunol. 2014;58:318–326. doi: 10.1111/1348-0421.12154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boonyasuppayakorn S, Reichert ED, Manzano M, Nagarajan K, Padmanabhan R. Amodiaquine, an antimalarial drug, inhibits dengue virus type 2 replication and infectivity. Antiviral Res. 2014;106:125–134. doi: 10.1016/j.antiviral.2014.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Long J, Wright E, Molesti E, Temperton N, Barclay W. Antiviral therapies against Ebola and other emerging viral diseases using existing medicines that block virus entry. F1000Res. 2015;4:30. doi: 10.12688/f1000research.6085.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Madrid PB, Chopra S, Manger ID, Gilfillan L, Keepers TR, Shurtleff AC, et al. A systematic screen of FDA-approved drugs for inhibitors of biological threat agents. PLoS One. 2013;8:e60579. doi: 10.1371/journal.pone.0060579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Keyaerts E, Vijgen L, Maes P, Neyts J, Van Ranst M. In vitro inhibition of severe acute respiratory syndrome coronavirus by chloroquine. Biochem Biophys Res Commun. 2004;323:264–268. doi: 10.1016/j.bbrc.2004.08.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vincent MJ, Bergeron E, Benjannet S, Erickson BR, Rollin PE, Ksiazek TG, et al. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virology J. 2005;2:69. doi: 10.1186/1743-422X-2-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.De Wilde AH, Jochmans D, Posthuma CC, Zevenhoven-Dobbe JC, van Nieuwkoop S, Bestebroer TM, et al. Screening of an FDA-approved compound library identifies four small-molecule inhibitors of Middle East respiratory syndrome coronavirus replication in cell culture. Antimicrob Agents Chemother. 2014;58:4875–4884. doi: 10.1128/AAC.03011-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stock I. Chikungunya fever—expanded distribution of a re-emerging tropical infectious disease [in German] Med Monatsschr Pharm. 2009;32:17–26. [PubMed] [Google Scholar]
- 35.Kaur P, Chu JJ. Chikungunya virus: an update on antiviral development and challenges. Drug Discov Today. 2013;18:969–983. doi: 10.1016/j.drudis.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leroux-Roels G, Bourguignon P, Willekens J, Janssens M, Clement F, Didierlaurent AM, et al. Immunogenicity and safety of a booster dose of an investigational adjuvanted polyprotein HIV-1 vaccine in healthy adults and effect of administration of chloroquine. Clin Vaccine Immunol. 2014;21:302–311. doi: 10.1128/CVI.00617-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Martinson JA, Montoya CJ, Usuga X, Ronquillo R, Landay AL, Desai SN. Chloroquine modulates HIV-1-induced plasmacytoid dendritic cell alpha interferon: implication for T-cell activation. Antimicrob Agents Chemother. 2010;54:871–881. doi: 10.1128/AAC.01246-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mizuguchi T, Ohashi N, Nomura W, Komoriya M, Hashimoto C, Yamamoto N, et al. Anti-HIV screening for cell-penetrating peptides using chloroquine and identification of anti-HIV peptides derived from matrix proteins. Bioorg Med Chem. 2015;23:4423–4427. doi: 10.1016/j.bmc.2015.06.020. [DOI] [PubMed] [Google Scholar]
- 39.Savarino A, Shytaj IL. Chloroquine and beyond: exploring anti-rheumatic drugs to reduce immune hyperactivation in HIV/AIDS. Retrovirology. 2015;12:51. doi: 10.1186/s12977-015-0178-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang M, Cao R, Zhang L, Yang X, Liu J, Xu M, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Savarino A, Boelaert JR, Cassone A, Majori G, Cauda R. Effects of chloroquine on viral infections: an old drug against today's diseases. Lancet Infect Dis. 2003;3:722–727. doi: 10.1016/S1473-3099(03)00806-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chan JF, Chan KH, Kao RY, To KK, Zheng BJ, Li CP, et al. Broad-spectrum antivirals for the emerging Middle East respiratory syndrome coronavirus. J Infect. 2013;67:606–616. doi: 10.1016/j.jinf.2013.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Martin RE, Marchetti RV, Cowan AI, Howitt SM, Bröer S, Kirk K. Chloroquine transport via the malaria parasite's chloroquine resistance transporter. Science. 2009;325:1680–1682. doi: 10.1126/science.1175667. [DOI] [PubMed] [Google Scholar]
- 44.Saftig P, Klumperman J. Lysosome biogenesis and lysosomal membrane proteins: trafficking meets function. Nat Rev Mol Cell Biol. 2009;10:623–635. doi: 10.1038/nrm2745. [DOI] [PubMed] [Google Scholar]
- 45.Kaufmann AM, Krise JP. Lysosomal sequestration of amine-containing drugs: analysis and therapeutic implications. J Pharm Sci. 2007;96:729–746. doi: 10.1002/jps.20792. [DOI] [PubMed] [Google Scholar]
- 46.An J, Woodward JJ, Sasaki T, Minie M, Elkon KB. Cutting edge: antimalarial drugs inhibit IFN-β production through blockade of cyclic GMP–AMP synthase–DNA interaction. J Immunol. 2015;194:4089–4093. doi: 10.4049/jimmunol.1402793. [DOI] [PubMed] [Google Scholar]
- 47.Zhou D, Dai SM, Tong Q. COVID-19: a recommendation to examine the effect of hydroxychloroquine in preventing infection and progression. J Antimicrob Chemother. 2020 Mar 20 doi: 10.1093/jac/dkaa114. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Al‐Bari MA. Targeting endosomal acidification by chloroquine analogs as a promising strategy for the treatment of emerging viral diseases. Pharmacol Res Perspect. 2017;5:e00293. doi: 10.1002/prp2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kužnik A, Benčina M, Švajger U, Jeras M, Rozman B, Jerala R. Mechanism of endosomal TLR inhibition by antimalarial drugs and imidazoquinolines. J Immunol. 2011;186:4794–4804. doi: 10.4049/jimmunol.1000702. [DOI] [PubMed] [Google Scholar]
- 50.Ewald SE, Lee BL, Lau L, Wickliffe KE, Shi GP, Chapman HA, et al. The ectodomain of Toll-like receptor 9 is cleaved to generate a functional receptor. Nature. 2008;456:658–662. doi: 10.1038/nature07405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dijkmans BA, Verweij CL. Chloroquine and hydroxychloroquine equally affect tumor necrosis factor-alpha, interleukin 6, and interferon-gamma production by peripheral blood mononuclear cells. J Rheumatol. 1997;24:55–60. [PubMed] [Google Scholar]
- 52.Baize S, Leroy EM, Georges-Courbot MC, Capron M, Lansoud-Soukate J, Debré P, et al. Defective humoral responses and extensive intravascular apoptosis are associated with fatal outcome in Ebola virus-infected patients. Nat Med. 1999;5:423–426. doi: 10.1038/7422. [DOI] [PubMed] [Google Scholar]
- 53.Wang M, Cao R, Zhang L, Yang X, Liu J, Xu M, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lai CC, Liu YH, Wang CY, Wang YH, Hsueh SC, Yen MY, et al. Drug treatment options for the 2019-new coronavirus (2019-nCoV) Biosci Trends. 2020;14:69–71. doi: 10.5582/bst.2020.01020. [DOI] [PubMed] [Google Scholar]
- 55.Colson P, Rolain JM, Lagier JC, Brouqui P, Raoult D. Chloroquine and hydroxychloroquine as available weapons to fight COVID-19. Int J Antimicrob Agents. 2020;55 doi: 10.1016/j.ijantimicag.2020.105932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Verma DK, Gupta D, Lal SK. Host lipid rafts play a major role in binding and endocytosis of influenza A virus. Viruses. 2018;10:E650. doi: 10.3390/v10110650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lu Y, Liu DX, Tam JP. Lipid rafts are involved in SARS-CoV entry into Vero E6 cells. Biochem Biophys Res Commun. 2008;369:344–349. doi: 10.1016/j.bbrc.2008.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tortorici MA, Walls AC, Lang Y, Wang C, Li Z, Koerhuis D, et al. Structural basis for human coronavirus attachment to sialic acid receptors. Nat Struct Mol Biol. 2019;26:481–489. doi: 10.1038/s41594-019-0233-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Matrosovich M, Herrler G, Klenk HD. Vol. 367. Springer; Cham, Switzerland: 2013. Sialic acid receptors of viruses. In: Gerardy-Schahn R, Delannoy P, von Itzstein M, editors; pp. 1–28. (SialoGlyco chemistry and biology II. Topics in current chemistry, Vol). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang PH. Increasing host cellular receptor—angiotensin-converting enzyme 2 (ACE2) expression by coronavirus may facilitate 2019-nCoV infection. bioRxiv. 2020 Feb 27 doi: 10.1101/2020.02.24.963348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Matsuyama S, Nao N, Shirato K, Kawase M, Saito S, Takayama I, et al. Enhanced isolation of SARS-CoV-2 by TMPRSS2-expressing cells. Proc Natl Acad Sci U S A. 2020;117:7001–7003. doi: 10.1073/pnas.2002589117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181 doi: 10.1016/j.cell.2020.02.052. 271–80.e8 doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chen Z, Mi L, Xu J, Yu J, Wang X, Jiang J, et al. Function of HAb18G/CD147 in invasion of host cells by severe acute respiratory syndrome coronavirus. J Infect Dis. 2005;191:755–760. doi: 10.1086/427811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fantini J, Di Scala C, Chahinian H, Yahi N. Structural and molecular modelling studies reveal a new mechanism of action of chloroquine and hydroxychloroquine against SARS-CoV-2 infection. Int J Antimicrob Agents. 2020 Apr 3 doi: 10.1016/j.ijantimicag.2020.105960. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li W, Moore MJ, Vasilieva N, Sui J, Wong SK, Berne MA, et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Devaux CA, Rolain JM, Colson P, Raoult D. New insights on the antiviral effects of chloroquine against coronavirus: what to expect for COVID-19? Int J Antimicrob Agents. 2020 Mar 12 doi: 10.1016/j.ijantimicag.2020.105938. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liu J, Cao R, Xu M, Wang X, Zhang H, Hu H, et al. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discov. 2020;6:16. doi: 10.1038/s41421-020-0156-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yao X, Ye F, Zhang M, Cui C, Huang B, Niu P, et al. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Clin Infect Dis. 2020 Mar 9 doi: 10.1093/cid/ciaa237. ciaa237 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gautret P, Lagier JC, Parola P, Meddeb L, Mailhe M, Doudier B, et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. 2020 Mar 20 doi: 10.1016/j.ijantimicag.2020.105949. 105949 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 70.Gautret P, Lagier J-C, Parola P, Hoang VT, Meddeb L, Sevestre J, et al. Clinical and microbiological effect of a combination of hydroxychloroquine and azithromycin in 80 COVID-19 patients with at least a six-day follow up: a pilot observational study. Travel Med Infect Dis. 2020;34 doi: 10.1016/j.tmaid.2020.101663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rana DR, Dulal S.Therapeutic application of chloroquine and hydroxychloroquine in clinical trials for COVID-19: a systematic review. medRxiv2020 Apr 10. doi: 10.1101/2020.03.22.20040964.
- 72.Huang M, Tang T, Pang P, Li M, Ma R, Lu J, et al. Treating COVID-19 with chloroquine. J Mol Cell Biol. 2020;12:322–325. doi: 10.1093/jmcb/mjaa014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gao J, Tian Z, Yang X. Breakthrough: Chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci Trends. 2020;14:72–73. doi: 10.5582/bst.2020.01047. [DOI] [PubMed] [Google Scholar]
- 74.Meo SA, Klonoff DC, Akram J. Efficacy of chloroquine and hydroxychloroquine in the treatment of COVID-19. Eur Rev Med Pharmacol Sci. 2020;24:4539–4547. doi: 10.26355/eurrev_202004_21038. [DOI] [PubMed] [Google Scholar]
- 75.Silva Borba MG, de Almeida Val F, Sousa Sampaio V, Almeida Araújo Alexandre M, Cardoso Melo G, Brito M, et al. Chloroquine diphosphate in two different dosages as adjunctive therapy of hospitalized patients with severe respiratory syndrome in the context of coronavirus (SARS-CoV-2) infection: preliminary safety results of a randomized, double-blinded, phase IIb clinical trial (CloroCovid-19 Study) medRxiv. 2020 Apr 16 doi: 10.1101/2020.04.07.20056424. [DOI] [Google Scholar]
- 76.Chorin E, Dai M, Shulman E, Wadhwani L, Bar Cohen R, Barbhaiya C, et al. The QT interval in patients with SARS-CoV-2 infection treated with hydroxychloroquine/azithromycin. medRxiv. 2020 Apr 3 doi: 10.1101/2020.04.02.20047050. [DOI] [PubMed] [Google Scholar]
- 77.Magagnoli J, Narendran S, Pereira F, Cummings T, Hardin JW, Sutton SS, et al. Outcomes of hydroxychloroquine usage in United States veterans hospitalized with Covid-19. medRxiv. 2020 Apr 23 doi: 10.1101/2020.04.16.20065920. [DOI] [PMC free article] [PubMed] [Google Scholar]