Abstract
Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has become a global health threat. Although most patients with COVID-19 manifest fever and respiratory tract symptoms, SARS-CoV-2 infection may also involve other organs/systems and present with extra-respiratory manifestations, including cardiac, gastrointestinal, hepatic, renal, neurological, olfactory, gustatory, ocular, cutaneous and haematological symptoms. Occasionally, these extra-respiratory symptoms/signs represent the initial presentation of SARS-CoV-2 infection, prior to fever or respiratory manifestations. Therefore, this comprehensive review of the extra-respiratory manifestations of COVID-19 is intended to help clinicians better understand the range of clinical presentations associated with SARS-CoV-2 infection, allowing the consideration of COVID-19 in differential diagnoses. A screening test for SARS-CoV-2 should be performed when patients have these extra-respiratory manifestations. In addition, clinicians should be alerted to the adverse effects of anti-SARS-CoV-2 agents that can mimic the extra-respiratory manifestations of COVID-19. Moreover, some extra-respiratory manifestations, such as ocular and gastrointestinal involvement, may be caused by direct invasion of SARS-CoV-2. Therefore, protective measures should be taken while managing the associated clinical specimens. Finally, several extra-respiratory manifestations, such as cardiac involvement, acute kidney injury, coagulation disorders and thrombotic complications, could be associated with a poor prognosis.
Keywords: COVID-19, SARS-CoV-2, Epidemiology, Extra-respiratory manifestations
1. Introduction
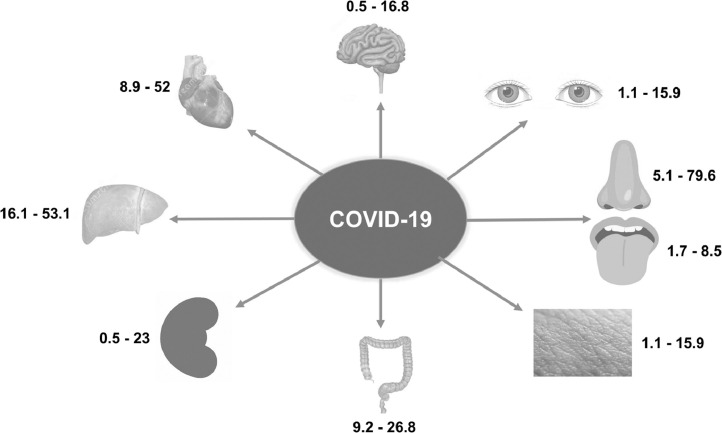
Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has become a global health threat, infecting 1 844 863 people and resulting in 117 021 deaths at the time of writing [1], [2], [1]. An infected person's lungs are the organs most affected because the virus accesses host cells via angiotensin-converting enzyme 2 (ACE2), which is most abundant on type II alveolar cells. The virus uses a surface glycoprotein, called a ‘spike’ (peplomer), to bind to ACE2 and enter the host cell [4]. Thus, respiratory manifestations such as cough, sputum production and shortness of breath remain the most common symptoms, following fever [5]. In addition, upper airway manifestations, including nasal congestion and sore throat, are observed in patients exhibiting mild disease. Furthermore, respiratory tract specimens (e.g. nasopharyngeal, nasal and oropharyngeal swabs, sputum, saliva and bronchoalveolar lavage fluid) are the most common clinical specimens obtained for real-time quantitative reverse transcription PCR (RT-qPCR) detection of SARS-CoV-2 [6]. Based on a preliminary understanding of COVID-19, patients with fever or airway symptoms and characteristic travel, occupation, contact or cluster histories were initially screened for SARS-CoV-2 infection [7]. However, extra-respiratory manifestations of SARS-CoV-2 infection have recently been observed in the rapidly increasing number of COVID-19 cases. To reduce the risk of overlooking patients with COVID-19 who manifest only extra-respiratory symptoms, clinicians need to better understand the range of SARS-CoV-2 infection-related extra-respiratory manifestations. Therefore, this article provides a comprehensive review of the extra-respiratory manifestations of COVID-19 to help clinicians better understand the clinical presentations of the disease (Table 1 ; Fig. 1 ).
Table 1.
Summary of the main extra-respiratory manifestations of patients with COVID-19.
| Organ/system | Symptoms/signs (reported prevalence of patients) | References |
|---|---|---|
| Cardiac | Acute cardiac injury (8–12%), heart failure (23–52%), arrhythmia (8.9–16.7%), shock, acute myocarditis, chest tightness | [8,[7], [8], [9], [10], [11], [12], [13], [14]] |
| Gastrointestinal | Anorexia (26.8%), diarrhoea (12.5%), nausea/vomiting (10.2%), abdominal pain/discomfort (9.2%) | [[18], [19], [20],24,25,27,28] |
| Hepatic | Abnormal aspartate aminotransferase or alanine aminotransferase values (16.1–53.1%) | [8,10,[21], [22], [23], [24], [25], [26]] |
| Kidney | Acute kidney injury (overall 0.5%; 2.9–23% in severe cases) | [10,24,25] |
| Neurological | Dizziness (16.8%), headache (13.1%), skeletal muscle injury (10.7%), impaired consciousness (7.5%), acute cerebrovascular disease (2.8%), ataxia (0.5%), seizures (0.5%), meningoencephalitis, Guillain–Barré syndrome | [31], [32], [33], [34] |
| Olfactory and gustatory | Hyposmia (5.1–20.4%), anosmia (79.6%), dysgeusia (8.5%), ageusia (1.7%) | [24,25,39,40] |
| Ocular | Acute conjunctivitis (31.6%) | [44,45] |
| Cutaneous | Erythematous rash (15.9%), hives rash (3.4%), vesicles (1.1%), acro-ischaemia, transient unilateral livedo reticularis | [25,[49], [50], [51], [52]] |
| Haematological | Lymphopenia (56.5%), thrombocytopenia (16.4–32.3%), coagulation disorders, thrombotic events, antiphospholipid antibody | [53], [54], [55], [56], [57], [58], [59] |
Fig. 1.
Reported ranges of prevalence (%) of extra-respiratory manifestations in patients with COVID-19, by organ/system.
2. Cardiac manifestations
COVID-19-associated cardiac complications have been reported frequently [6], [7], [8], [9], [10], [11], [12] and the mechanisms appear complicated, including direct viral injury, hypoxaemia, unstable haemodynamic status with hypoperfusion, enhanced systematic inflammation, ACE2 receptor downregulation, increased endogenous catecholamine production and medication toxicity [15,16]. In a series of 138 hospitalised patients with SARS-CoV-2 pneumonia in Wuhan, China, arrhythmia, shock and acute cardiac injury were observed in 16.7%, 8.7% and 7.2% of patients, respectively [8]. Another study of 41 patients with SARS-CoV-2-caused pneumonia showed similar findings, with 5 patients (12.2%) exhibiting acute cardiac injury [10]. Zhou et al. reported that 44 (23.0%) of 191 hospitalised patients had heart failure [11]. In addition, acute myocarditis and ventricular arrhythmia have been reported rarely [10], [11], [12]. Most importantly, patients with COIVD-19 and cardiac injuries more frequently require mechanical ventilation and demonstrate a higher mortality rate than those without cardiac injuries [9]. In summary, SARS-CoV-2 infection can cause acute cardiac injuries, chest pain and arrhythmic complications; cardiac involvement in patients with COVID-19 may be associated with poor outcomes.
3. Gastrointestinal manifestations
In addition to the respiratory tract, SARS-CoV-2 also affects gastrointestinal organs because ACE2 is abundantly expressed on glandular cells of the gastric, duodenal and rectal epithelia [17] as well as on endothelial cells and enterocytes of the small intestine. In addition, many reports show that SARS-CoV-2 can be detected in faecal specimens [18,19] as well as in oesophageal, stomach, duodenal and rectal samples [20]. A recent meta-analysis including 4234 COVID-19 patients from 60 studies reporting gastrointestinal symptoms showed that the prevalence of all gastrointestinal symptoms was 17.6% [95% confidence interval (CI) 12.3–24.5%] and that severe COVID-19 cases were more likely to have gastrointestinal symptoms than non-severe cases (17.1% vs. 11.8%) [21]. The pooled prevalence of anorexia, diarrhoea, nausea/vomiting and abdominal pain/discomfort were 26.8% (95% CI 16.2–40.8%), 12.5% (95% CI 9.6–16.0%), 10.2% (95% CI 6.6–15.3%) and 9.2% (95% CI 5.7–14.5%), respectively [21]. Moreover, the pooled prevalence of stool specimens that tested positive for SARS-CoV-2 was 48.1% (95% CI 38.3–57.9%), including 70.3% (95% CI 49.6–85.1%) of specimens collected after returning negative respiratory specimen results [21]. In addition, the duration of SAR-CoV-2 RNA detection in stool specimens from patients treated with steroids (20 days) was significantly longer than that for those not receiving steroids (11 days) (P < 0.001) [22]. Based on these findings, clinicians should be cognisant of the common gastrointestinal symptoms and understand that stool viral shedding may occur throughout the disease course. Healthcare providers should remain cautious during the management of patients with gastrointestinal manifestations and during the handling of faecal material to avoid potential faecal–oral transmission of SARS-CoV-2.
4. Hepatic manifestations
Liver impairment is a common complication of SARS-CoV-2 infection and may be caused by direct viral infection of liver cells [23]. Abnormal liver function and elevated levels of aspartate aminotransferase or alanine aminotransferase, which have developed in 16.1–53.1% of SARS-CoV-2-infected patients, are the most commonly reported manifestations of liver injury among patients with COVID-19 [8,10,[21], [22], [23], [24], [25], [26]]. In addition, one study of 56 patients reported elevated levels of gamma-glutamyl transferase (30 patients; 54%) and alkaline phosphatase (1 patient; 1.8%) [23].
5. Renal manifestations
The mechanisms of acute kidney injury (AKI) in COVID-19 could be multifactorial, such as cytokine damage, cardiorenal crosstalk, hypoxia, intra-abdominal hypertension, fluid imbalance, hypoperfusion, rhabdomyolysis-related tubular toxicity and endotoxin [29]. Chen et al. showed only three (3%) of 99 COVID-19 pneumonia case had AKI [24]. Huang et al. showed that three (7%) of 41 COVID-19 patients had AKI and that intensive care unit (ICU) patients were more likely to have AKI than non-ICU patients (23% vs. 0%; P = 0.027) [10]. A similar finding was shown in a large study which reported that the overall prevalence of AKI was only 0.5% (6/1099) but that severe cases had more AKI than non-severe cases (2.9% vs. 0%) [25]. All of these indicate that the prevalence of AKI is low but the risk of AKI could be increased with the severity of COVID-19.
6. Neurological manifestations
Previous studies have shown the presence of ACE2 receptors in the nervous system and in skeletal muscle, suggesting a mechanism for SARS-CoV-2-related neuromuscular injury [30,31]. Apart from ACE2, COVID-19-associated nervous system damage may also be caused by direct infection injury, hypoxic injury and immune responses [32]. A retrospective clinical study of 214 laboratory-confirmed COVID-19 cases reported that 78 patients (36.4%) had neurological manifestations, including 53 (24.8%) with central nervous system (CNS) injuries, 19 (8.9%) with peripheral nervous system (PNS) injuries and 23 (10.7%) with skeletal muscle injuries [33]. Dizziness (36 patients; 16.8%) and headache (28 patients; 13.1%) were the most commonly reported CNS symptoms; impairments of taste (12 patients; 5.6%) and smell (11 patients; 5.1%) were the most common PNS symptoms [33]. In addition, impaired consciousness, acute cerebrovascular disease, ataxia, seizures, vision impairment and nerve pain were reported in <10% of patients. Except for stroke and impaired consciousness, which developed within a median post-admission period of 8–10 days, most neurological manifestations occurred within a median period of 1–2 post-admission days. Moreover, neurological manifestations were more common in patients with severe COVID-19 infection than in those with non-severe disease (45.5% vs. 30.2%; P = 0.02); patients with severe disease were also more likely to have impaired consciousness, acute cerebrovascular disease and skeletal muscle injuries than those with non-severe infection. In addition, one case of meningoencephalitis associated with SARS-CoV-2 infection was reported in a patient with an initial neurological presentation of convulsions and unconsciousness in Japan [34]. Magnetic resonance imaging (MRI) of the brain showed hyperintensity along the right lateral ventricle wall and hyperintense signal changes in the right mesial temporal lobe and hippocampus, indicating meningitis; SARS-CoV-2 RNA was detected in the cerebral spinal fluid (CSF) [34]. Similarly, another case of COVID-19-associated encephalitis was reported in a male from Wuhan, China [35]. In Italy, Toscano et al. reported that five cases developed Guillain–Barré syndrome 5–10 days after the onset of COVID-19 with an initial presentation of lower limb weakness, paraesthesia and ataxia [36]. These data indicate that SARS-CoV-2 may infect the nervous system and skeletal muscle as well as the respiratory tract; thus, clinicians should consider COVID-19 as a differential diagnosis for patients with neurological manifestations.
7. Olfactory and gustatory manifestations
Olfactory and gustatory disorders are known to be associated with viral infections [37], and SARS-CoV-2 is no exception. An in vitro study showed that the ACE2 receptor is widely expressed on epithelial cells of the oral mucosa [38], suggesting a possible pathogenetic mechanism for the association between COVID-19 and olfactory and gustatory disorders. In a questionnaire-based, cross-sectional study conducted in Italy of 88 hospitalised COVID-19 patients, 59 patients were able to be interviewed, of whom 20 (33.9%) self-reported at least one olfactory or gustatory symptom, including the gustatory manifestations of dysgeusia (5 patients; 8.5%) and ageusia (1 patient; 1.7%) and the olfactory manifestation of hyposmia (3 patients; 5.1%) [39]. Another large surveillance study including patients with mild-to-moderate COVID-19 in 12 European hospitals reported that 85.6% (357/417) and 88.8% (342/385) of patients had olfactory and gustatory dysfunctions, respectively [40]. Anosmia was the most common olfactory symptom (284/357; 79.6%), followed by hyposmia (73/357; 20.4%). Interestingly, 11.8% of patients reported that their olfactory dysfunction began before their other symptoms; the short-term olfactory disorder recovery rate was 44.4% among the 59 clinically cured patients. Furthermore, 78.9% and 21.1% patients reported reduced/discontinued or distorted abilities to taste flavours, respectively. The same study also reported that other otolaryngologic symptoms, highly related to COVID-19, included facial pain (54 patients; 12.9%) and nasal obstruction (50 patients; 12.0%). Furthermore, rhinorrhoea, postnasal drip, sore throat and ear pain were possible COVID-19-related otolaryngological symptoms reported in 3.1–7.9% of patients [40]. In China, Chen et al. reported that 4 (4%) of 99 patients with COVID-19-related pneumonia had rhinorrhoea [24]. Guan et al reported a prevalence of nasal obstruction of 5% in a cohort study of 1099 patients with COVID-19 [25]. These findings indicate that olfactory and gustatory dysfunction are common manifestations of SARS-CoV-2 infection; occasionally, these disorders may even be the initial presentation of COVID-19. The results further suggest that screening for SARS-CoV-2 in patients with olfactory and taste manifestations should be considered during the pandemic.
8. Ocular manifestations
Although ACE2 receptors have been detected in ocular organs, including in the retina, choroid and conjunctival epithelia [39], [40], [41], ocular involvement associated with SARS-CoV-2 infection has rarely been reported [44,45]. Chen et al. reported a 30-year-old man who developed red eyes, tearing and foreign-body sensations (diagnosed as bilateral acute conjunctivitis) 13 days after the onset of COVID-19 [44]. Slit lamp examination demonstrated bilateral moderate conjunctival injection, water discharge and inferior palpebral conjunctival follicles. RT-qPCR assay of a conjunctival swab detected the presence of viral RNA, but routine bacterial and fungal culture results were negative. In addition to umifenovir and lopinavir/ritonavir, topical ribavirin eye drops were prescribed four times daily. On Day 19, the patient's ocular symptoms resolved and a repeated slit lamp examination showed improvements; the results of a follow-up conjunctival swab were negative for SARS-CoV-2. Another series showed that 12 (31.6%) of 38 clinically confirmed COVID-19 cases demonstrated ocular manifestations, including conjunctiva hyperaemia, chemosis, epiphora, and increased secretions that were indicative of acute conjunctivitis [45]. However, conjunctival specimens and nasopharyngeal swabs for only two patients tested positive for SARS-CoV-2 [45]. Moreover, four, two and six cases were classified as moderate, severe and critical novel coronavirus pneumonias, respectively. These preliminary data should remind clinicians of the potential for a rare ocular complication (acute conjunctivitis) in patients with COVID-19. The data also suggest that precautionary eye protection measures should be adopted when in contact with SARS-CoV-2-infected patients to prevent the spread of COVID-19 via an ocular route [46].
9. Cutaneous manifestations
Although many viral infections can be associated with skin manifestations [45], [46], [47], [48], cutaneous symptoms have rarely been reported in association with COVID-19 [51]. In China, 0.2–1.2% of 1099 COVID-19 patients had a rash [25]. In a series of 88 patients infected with SARS-CoV-2 in Italy, Recalcati showed that 18 patients (20.5%) developed cutaneous lesions, including 8 who developed lesions at disease onset [51]. Furthermore, erythematous rashes were the most common manifestation (n = 14), followed by hives rash (n = 3) and chickenpox-like vesicles (n = 1). In addition, acro-ischaemia presented with finger/toe cyanosis, skin bullae and dry gangrene in seven critically ill patients with COVID-19 in China and in a child in Brazil [52,53]. Transient unilateral livedo reticularis was observed in two non-severe COVID-19 cases in the USA [54]. However, these data are limited and further study is warranted to investigate the cutaneous manifestations of COVID-19.
10. Haematological manifestations
As for other viral infections, lymphopenia is common in patients with COVID-19. One meta-analysis of 24 studies comprising 2507 patients showed the prevalence of lymphopenia to be 56.5% (95% CI 46.5–66.4%) [55]. In addition, decreased platelet counts were observed in 32.3% and 16.4% of critically and non-critically ill COVID-19 patients, respectively [56]. Coagulation disorders are another common complication. Chen et al. showed that patients with COVID-19 pneumonia have increased d-dimer levels (36% of patients), activated partial thromboplastin times (6%) and prothrombin times (5%) [24]. Thrombotic complications have been observed in patients with COVID-19, especially in those who are critically ill [57,58]. The incidence of thrombotic complications among 148 patients with COVID-19 in an ICU was 31% (95% CI 20–41%), including a venous thromboembolism incidence of 27% (95% CI 17–37%) and an arterial thrombotic event incidence of 3.7% (95% CI 0–8.2%) [59]. Rarely, the presence of antiphospholipid antibodies was observed in COVID-19 patients with critical illness [60]. Most importantly, coagulation disorders and thrombotic complications may be associated with poor outcomes for patients with COVID-19 [61].
11. Treatment considerations
Some potentially effective drugs against SARS-CoV-2 have adverse effects that are difficult to differentiate from extra-respiratory manifestations of COVID-19. Table 2 lists the common adverse effects of commonly used agents for the treatment of COVID-19. Among them, an abnormality of hepatic function (23%) has been reported mostly for remdesivir, whereas no major adverse effects have been reported for favipiravir (both are RNA-dependent RNA polymerase inhibitors) [60], [61], [62], [63]. In contrast, the protease inhibitor lopinavir/ritonavir exerts adverse effects on the gastrointestinal tract, such as diarrhoea, nausea, vomiting and abdominal pain, as well as leading to asthenia, abnormal hepatic function, hyperglycaemia and hyperlipidaemia [66]. The potential for inducing ventricular dysrhythmia after QTc prolongation should be cautiously monitored for hydroxychloroquine, which alkalises the pH in the early endosomal pathway of SARS-CoV-2 entry [67]. Teicoplanin, which inhibits cathepsin L/B enzymes in the late endosomal pathway of SARS-CoV-2 entry, is actually an antibiotic agent active against methicillin-resistant Staphylococcus aureus (MRSA) [62]. Its dosage should be prescribed according to body weight and creatinine clearance rate [68]. In addition, tocilizumab, an anti-interleukin-6 monoclonal antibody originally prescribed in the treatment of acute exacerbation of rheumatoid arthritis and systemic lupus erythematosus, has been suggested to be used in the treatment of acute respiratory distress syndrome and the cytokine storm stage of COVID-19 [62,69]. However, this drug likely induces strong immunosuppression. Finally, depression, ataxia, psychosis and seizures induced by ivermectin, a broad-spectrum antiparasitic drug potentially effective against SARS-CoV-2, need to be monitored [70,71].
Table 2.
Summary of the main adverse effects of commonly used agents for the treatment of COVID-19.
| Agent | Adverse effects (reported prevalence of patients, if any) | Reference(s) |
|---|---|---|
| Remdesivir | Nausea, vomiting, abnormal hepatic function, skin rash, acute kidney injury and shock | [60], [61], [62] |
| Favipiravir | Potentially harmful to the baby during pregnancy (teratogenic and embryotoxic effects reported in animal experiments) | [65] |
| Lopinavir/ritonavir | Moderate to severe diarrhoea (27%), nausea (16%), vomiting, abdominal pain, asthenia, headache, abnormal hepatic function, hyperglycaemia and hyperlipidaemia | [66] |
| Hydroxychloroquine | Nausea, diarrhoea, dose-related retinopathy, altered eye pigmentation, acne, anaemia, hepatic dysfunction, loss of hair, muscle atrophy, tinnitus, vertigo, hypoglycaemia, (more severe) QTc prolongation on electrocardiograms, and life-threatening or fatal cardiomyopathy. Worsening psoriasis and porphyria reported in cases with these diseases | [67] |
| Teicoplanin | Rash, drug-related fever, pruritus, diarrhoea, nausea and vomiting, altered liver function, leukopenia, thrombocytopenia and impaired renal function (upon prolonged use) | [68] |
| Tocilizumab | Nasopharyngitis (10%), headache, hypertension (5%), asymptomatic alanine transaminase elevation (5%), hypercholesteremia, mouth ulcer, strong immunosuppression and (rare but severe) anaphylaxis (0.2%) | [69] |
| Ivermectin | Depression, consequent ataxia due to potentiation of inhibitory GABAergic synapses, (rare but severe) psychosis, and seizure | [70,71] |
12. Challenges
In addition to the characteristic manifestations of fever and respiratory tract symptoms/signs, SARS-CoV-2 infection can demonstrate many extra-respiratory symptoms including cardiac, gastrointestinal, renal, hepatic, neurological, olfactory, gustatory, ocular, cutaneous and haematological manifestations. Sometimes these extra-respiratory manifestations may be the initial or only symptom of COVID-19, prior to fever or respiratory manifestations. Many examinations can help clinicians in identifying the extra-respiratory manifestations. Tests for troponin, electrocardiogram and echocardiogram may help in identifying cardiac involvement in COVID-19 patients. Blood tests can help in the detection of abnormal liver function. To identify neurological involvement in COVID-19, brain imaging, analysis of CSF, nerve conduction studies and electromyography could be useful. Moreover, the development of acute cardiac injury, AKI and coagulation disorder can be associated with severe COVID-19 [61,72]. None the less, appropriate use of anticoagulant agents can help in managing these complications [73]. Overall, clinicians should be alert for these symptoms of SARS-CoV-2 infection and should consider COVID-19 as one of the differential diagnoses for patients with these symptoms.
Funding: None.
Competing interests: None declared.
Ethical approval: Not required.
References
- 1.Lai CC, Shih TP, Ko WC, Tang HJ, Hsueh PR. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): the epidemic and the challenges. Int J Antimicrob Agents. 2020;55 doi: 10.1016/j.ijantimicag.2020.105924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lai CC, Wang CY, Wang YH, Hsueh SC, Ko WC, Hsueh PR. Global epidemiology of coronavirus disease 2019 (COVID-19): disease incidence, daily cumulative index, mortality, and their association with country healthcare resources and economic status. Int J Antimicrob Agents. 2020;55 doi: 10.1016/j.ijantimicag.2020.105946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1.World Health Organization (WHO) April 2020. Coronavirus disease (COVID-19) pandemic.https://www.who.int/emergencies/diseases/novel-coronavirus-2019 [accessed 23] [Google Scholar]
- 2.Letko M, Marzi A, Munster V. Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses. Nat Microbiol. 2020;5:562–569. doi: 10.1038/s41564-020-0688-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lai CC, Liu YH, Wang CY, Wang YH, Hsueh SC, Yen MY, et al. Asymptomatic carrier state, acute respiratory disease, and pneumonia due to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): facts and myths. J Microbiol Immunol Infect. 2020 Mar 4 doi: 10.1016/j.jmii.2020.02.012. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tang YW, Schmitz JE, Persing DH, Stratton CW. The laboratory diagnosis of COVID-19 infection: current issues and challenges. J Clin Microbiol. 2020 Apr 3 doi: 10.1128/JCM.00512-20. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang YC, Lee PI, Hsueh PR. Evolving reporting criteria of COVID-19 in Taiwan during the epidemic. J Microbiol Immunol Infect. 2020 Mar 19 doi: 10.1016/j.jmii.2020.03.014. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shi S, Qin M, Shen B, Cai Y, Liu T, Yang F, et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020 Mar 25 doi: 10.1001/jamacardio.2020.0950. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet2020;395:1054–62. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed]
- 10.Hu H, Ma F, Wei X, Fang Y. Coronavirus fulminant myocarditis saved with glucocorticoid and human immunoglobulin. Eur Heart J. 2020 Mar 16 doi: 10.1093/eurheartj/ehaa190. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Inciardi RM, Lupi L, Zaccone G, Italia L, Raffo M, Tomasoni D, et al. Cardiac involvement in a patient with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020 Mar 27 doi: 10.1001/jamacardio.2020.1096. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guo T, Fan Y, Chen M, Wu X, Zhang L, He T, et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020 Mar 27 doi: 10.1001/jamacardio.2020.1017. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kochi AN, Tagliari AP, Forleo GB, Fassini GM, Tondo C. Cardiac and arrhythmic complications in patients with COVID-19. J Cardiovasc Electrophysiol. 2020;31:1003–1008. doi: 10.1111/jce.14479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bansal M. Cardiovascular disease and COVID-19. Diabetes Metab Syndr. 2020;14:247–250. doi: 10.1016/j.dsx.2020.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gu J, Han B, Wang J. COVID-19: gastrointestinal manifestations and potential fecal–oral transmission. Gastroenterology. 2020;158:1518–1519. doi: 10.1053/j.gastro.2020.02.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tang A, Tong ZD, Wang HL, Dai YX, Li KF, Liu JN, et al. Detection of novel coronavirus by RT-PCR in stool specimen from asymptomatic child, China. Emerg Infect Dis. 2020:26. doi: 10.3201/eid2606.200301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang J, Wang S, Xue Y. Fecal specimen diagnosis 2019 novel coronavirus-infected pneumonia. J Med Virol. 2020 Mar 3 doi: 10.1002/jmv.25742. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin L, Jiang X, Zhang Z, Huang S, Zhang Z, Fang Z, et al. Gastrointestinal symptoms of 95 cases with SARS-CoV-2 infection. Gut202069:997–1001. doi: 10.1136/gutjnl-2020-321013. [DOI] [PubMed]
- 19.Cheung KS, Hung IF, Chan PP, Lung KC, Tso E, Liu R, et al. Gastrointestinal manifestations of SARS-CoV-2 infection and virus load in fecal samples from the Hong Kong cohort and systematic review and meta-analysis. Gastroenterology. 2020 Apr 3 doi: 10.1053/j.gastro.2020.03.065. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ling Y, Xu SB, Lin YX, Tian D, Zhu ZQ, Dai FH, et al. Persistence and clearance of viral RNA in 2019 novel coronavirus disease rehabilitation patients. Chin Med J (Engl) 2020;133:1039–1043. doi: 10.1097/CM9.0000000000000774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang C, Shi L, Wang FS. Liver injury in COVID-19: management and challenges. Lancet Gastroenterol Hepatol. 2020;5:428–430. doi: 10.1016/S2468-1253(20)30057-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shi H, Han X, Jiang N, Cao Y, Alwalid O, Gu J, et al. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis. 2020;20:425–434. doi: 10.1016/S1473-3099(20)30086-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu XW, Wu XX, Jiang XG, Xu KJ, Ying LJ, Ma CL, et al. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-CoV-2) outside of Wuhan, China: retrospective case series. BMJ. 2020;368:m606. doi: 10.1136/bmj.m606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. Erratum in: Lancet Respir Med 2020;8:e26. doi: 10.1016/S2213-2600(20)30103-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ronco C, Reis T. Kidney involvement in COVID-19 and rationale for extracorporeal therapies. Nat Rev Nephrol. 2020 Apr 9 doi: 10.1038/s41581-020-0284-7. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hamming I, Timens W, Bulthuis ML, Lely AT, Navis G, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203:631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baig AM, Khaleeq A, Ali U, Syeda H. Evidence of the COVID-19 virus targeting the CNS: tissue distribution, host–virus interaction, and proposed neurotropic mechanisms. ACS Chem Neurosci. 2020;11:995–998. doi: 10.1021/acschemneuro.0c00122. [DOI] [PubMed] [Google Scholar]
- 30.Wu Y, Xu X, Chen Z, Duan J, Hashimoto K, Yang L, et al. Nervous system involvement after infection with COVID-19 and other coronaviruses. Brain Behav Immun. 2020 Mar 30 doi: 10.1016/j.bbi.2020.03.031. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mao L, Jin H, Wang M, Hu Y, Chen S, He Q, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020 Apr 10 doi: 10.1001/jamaneurol.2020.1127. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moriguchi T, Harii N, Goto J, Harada D, Sugawara H, Takamino J, et al. A first case of meningitis/encephalitis associated with SARS-coronavirus-2. Int J Infect Dis. 2020;94:55–58. doi: 10.1016/j.ijid.2020.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ye M, Ren Y, Lv T. Encephalitis as a clinical manifestation of COVID-19. Brain Behav Immun. 2020 Apr 10 doi: 10.1016/j.bbi.2020.04.017. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Toscano G, Palmerini F, Ravaglia S, Ruiz L, Invernizzi P, Cuzzoni MG, et al. Guillain–Barré syndrome associated with SARS-CoV-2. N Engl J Med. 2020 Apr 17 doi: 10.1056/NEJMc2009191. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van Riel D, Verdijk R, Kuiken T. The olfactory nerve: a shortcut for influenza and other viral diseases into the central nervous system. J Pathol. 2015;235:277–287. doi: 10.1002/path.4461. [DOI] [PubMed] [Google Scholar]
- 36.Xu H, Zhong L, Deng J, Peng J, Dan H, Zeng X, et al. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int J Oral Sci. 2020;12:8. doi: 10.1038/s41368-020-0074-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Giacomelli A, Pezzati L, Conti F, Bernacchia D, Siano M, Oreni L, et al. Self-reported olfactory and taste disorders in SARS-CoV-2 patients: a cross-sectional study. Clin Infect Dis. 2020 Mar 26 doi: 10.1093/cid/ciaa330. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lechien JR, Chiesa-Estomba CM, De Siati DR, Horoi M, Le Bon SD, Rodriguez A, et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur Arch Otorhinolaryngol. 2020 Apr 6 doi: 10.1007/s00405-020-05965-1. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Senanayake P, Drazba J, Shadrach K, Milsted A, Rungger-Brandle E, Nishiyama K, et al. Angiotensin II and its receptor subtypes in the human retina. Invest Ophthalmol Vis Sci. 2007;48:3301–3311. doi: 10.1167/iovs.06-1024. [DOI] [PubMed] [Google Scholar]
- 40.Wagner J, Jan Danser AH, Derkx FH, de Jong TV, Paul M, Mullins JJ, et al. Demonstration of renin mRNA, angiotensinogen mRNA, and angiotensin converting enzyme mRNA expression in the human eye: evidence for an intraocular renin–angiotensin system. Br J Ophthalmol. 1996;80:159–163. doi: 10.1136/bjo.80.2.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sun Y, Liu L, Pan X, Jing M. Mechanism of the action between the SARS-CoV S240 protein and the ACE2 receptor in eyes. Int J Ophthalmol. 2006;6:783–786. [Google Scholar]
- 42.Chen L, Liu M, Zhang Z, Qiao K, Huang T, Chen M, et al. Ocular manifestations of a hospitalised patient with confirmed 2019 novel coronavirus disease. Br J Ophthalmol. 2020 Apr 7 doi: 10.1136/bjophthalmol-2020-316304. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu P, Duan F, Luo C, Liu Q, Qu X, Liang L, et al. Characteristics of ocular findings of patients with coronavirus disease 2019 (COVID-19) in Hubei Province, China. JAMA Ophthalmol. 2020;138:575–578. doi: 10.1001/jamaophthalmol.2020.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li JO, Lam DSC, Chen Y, Ting DSW. Novel coronavirus disease 2019 (COVID-19): the importance of recognising possible early ocular manifestation and using protective eyewear. Br J Ophthalmol. 2020;104:297–298. doi: 10.1136/bjophthalmol-2020-315994. [DOI] [PubMed] [Google Scholar]
- 45.Drozd B, Andriescu E, Suarez A, De la Garza Bravo MM. Cutaneous cytomegalovirus manifestations, diagnosis, and treatment: a review. Dermatol Online J. 2019;25 pii: 13030/qt84f936cp. [PubMed] [Google Scholar]
- 46.Kimmis BD, Downing C, Tyring S. Hand-foot-and-mouth disease caused by coxsackievirus A6 on the rise. Cutis. 2018;102:353–356. [PubMed] [Google Scholar]
- 47.Martinez JD, Garza JAC, Cuellar-Barboza A. Going viral 2019: Zika, Chikungunya, and Dengue. Dermatol Clin. 2019;37:95–105. doi: 10.1016/j.det.2018.07.008. [DOI] [PubMed] [Google Scholar]
- 48.Neely G, Cabrera R, Hojman L. Parvovirus B19: a DNA virus associated with multiple cutaneous manifestations [in Spanish] Rev Chilena Infectol. 2018;35:518–530. doi: 10.4067/s0716-10182018000500518. [DOI] [PubMed] [Google Scholar]
- 49.Recalcati S. Cutaneous manifestations in COVID-19: a first perspective. J Eur Acad Dermatol Venereol. 2020 Mar 26 doi: 10.1111/jdv.16387. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 50.Zhang Y, Cao W, Xiao M, Li YJ, Yang Y, Zhao J, et al. Clinical and coagulation characteristics of 7 patients with critical COVID-2019 pneumonia and acro-ischemia [in Chinese] Zhonghua Xue Ye Xue Za Zhi. 2020;41:E006. doi: 10.3760/cma.j.issn.0253-2727.2020.0006. [DOI] [PubMed] [Google Scholar]
- 51.Mazzota F, Troccoli T. Acute acro-ischemia in the child at the time of COVID-19. Dermatologica Pediatrica. 2020 April 11 https://www.fip-ifp.org/wp-content/uploads/2020/04/acroischemia-ENG.pdf [accessed 20 May 2020] [Google Scholar]
- 52.Manalo IF, Smith MK, Cheeley J, Jacobs R. A dermatologic manifestation of COVID-19: transient livedo reticularis. J Am Acad Dermatol. 2020 Apr 10 doi: 10.1016/j.jaad.2020.04.018. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhu J, Ji P, Pang J, Zhong Z, Li H, He C, et al. Clinical characteristics of 3,062 COVID-19 patients: a meta-analysis. J Med Virol. 2020 Apr 15 doi: 10.1002/jmv.25884. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fu L, Wang B, Yuan T, Chen X, Ao Y, Fitzpatrick T, et al. Clinical characteristics of coronavirus disease 2019 (COVID-19) in China: a systematic review and meta-analysis. J Infect. 2020;80:656–665. doi: 10.1016/j.jinf.2020.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rotzinger DC, Beigelman-Aubry C, von Garnier C, Qanadli SD. Pulmonary embolism in patients with COVID-19: time to change the paradigm of computed tomography. Thromb Res. 2020 Apr 11;190:58–59. doi: 10.1016/j.thromres.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Panigada M, Bottino N, Tagliabue P, Grasselli G, Novembrino C, Chantarangkul V, et al. Hypercoagulability of COVID-19 patients in intensive care unit. A report of thromboelastography findings and other parameters of hemostasis. J Thromb Haemost. 2020 Apr 17 doi: 10.1111/jth.14850. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Klok FA, Kruip MJHA, van der Meer NJM, Arbous MS, Gommers DAMPJ, Kant KM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020 Apr 10 doi: 10.1016/j.thromres.2020.04.013. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang Y, Xiao M, Zhang S, Xia P, Cao W, Jiang W, et al. Coagulopathy and antiphospholipid antibodies in patients with COVID-19. N Engl J Med. 2020;382:e38. doi: 10.1056/NEJMc2007575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Henry BM, Santos de Oliveira MH, Benoit S, Plebani M, Lippi G. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): a meta-analysis. Clin Chem Lab Med. 2020 Apr 10 doi: 10.1515/cclm-2020-0369. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 60.Jean SS, Lee PI, Hsueh PR. Treatment options for COVID-19: the reality and challenges. J Microbiol Immunol Infect. 2020 Apr 4 doi: 10.1016/j.jmii.2020.03.034. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Medrxiv News. https://times.hinet.net/mobile/news/22831665[accessed 29 March 2020].
- 62.Grein J, Ohmagari N, Shin D, Diaz G, Asperges E, Castagna A, et al. Compassionate use of remdesivir for patients with severe COVID-19. N Engl J Med. 2020 Apr 10 doi: 10.1056/NEJMoa2007016. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shiraki K, Daikoku T. Favipiravir, an anti-influenza drug against life-threatening RNA virus infections. Pharmacol Ther. 2020;209 doi: 10.1016/j.pharmthera.2020.107512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.World Health Organization (WHO) World Health Organization model list of essential medicines: 21st list 2019. WHO; Geneva, Switzerland: 2019. Lopinavir/ritonavir.https://apps.who.int/iris/bitstream/handle/10665/325771/WHO-MVP-EMP-IAU-2019.06-eng.pdf?sequence=1&isAllowed=y WHO/MVP/EMP/IAU/2019.06. [accessed 24 April 2020]. [Google Scholar]
- 65.Drugs.com. Hydroxychloroquine sulfate. https://www.drugs.com/monograph/hydroxychloroquine-sulfate.html[accessed 25 April 2020].
- 66.NPS MEDICINEWISE New drug. Teicoplanin. Aust Prescr. 1995;18:7–9. doi: 10.18773/austprescr.1995.012. [DOI] [Google Scholar]
- 67.Genovese MC, McKay JD, Nasonov EL, Mysler EF, da Silva NA, Alecock E, et al. Interleukin-6 receptor inhibition with tocilizumab reduces disease activity in rheumatoid arthritis with inadequate response to disease-modifying antirheumatic drugs: the tocilizumab in combination with traditional disease-modifying antirheumatic drug therapy study. Arthritis Rheum. 2008;58:2968–2980. doi: 10.1002/art.23940. [DOI] [PubMed] [Google Scholar]
- 68.Wikipedia. Ivermectin. https://en.wikipedia.org/wiki/Ivermectin[accessed 20 May 2020].
- 69.Chandler RE. Serious neurological adverse events after ivermectin—do they occur beyond the indication of onchocerciasis? Am J Trop Med Hyg. 2018;98:382–388. doi: 10.4269/ajtmh.17-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zheng Y, Xu H, Yang M, Zeng Y, Chen H, Liu R, et al. Epidemiological characteristics and clinical features of 32 critical and 67 noncritical cases of COVID-19 in Chengdu. J Clin Virol. 2020;127 doi: 10.1016/j.jcv.2020.104366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tang N, Bai H, Chen X, Gong J, Li D, Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18:1094–1099. doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]