Abstract
Cellular stress induced by the accumulation of misfolded proteins in the endoplasmic reticulum (ER) activates an elaborate signalling network termed the unfolded protein response (UPR). This adaptive response is mediated by the transmembrane signal transducers IRE1, PERK, and ATF6 to decide cell fate of recovery or death. In malignant cells, UPR signalling may be required to maintain ER homeostasis and survival in the tumor micro-environment characterized by oxidative stress, hypoxia, lactic acidosis and compromised protein folding. Here we provide an overview of the ER response to cellular stress and how the sustained activation of this network enables malignant cells to develop tumorigenic, meta-static and drug-resistant capacities to thrive under adverse conditions. Understanding the complexity of ER stress responses and how to target the UPR in disease will have significant potential for novel future therapeutics.
Keywords: ATF6, Oxidative stress, ER stress, UPR, IRE1α, XBP1, ATF4, PERK, Cancer, BiP, Chaperones
8.1. Adaptive Signalling for Endoplasmic Reticulum (ER) Protein Homeostasis
The endoplasmic reticulum (ER) is a highly dynamic stress-sensing organelle essential for cellular homeostasis that integrates different extracellular and intracellular stimuli to coordinate downstream translational and transcriptional responses. The ER is a major platform for secretory protein homeostasis, or proteostasis, that consists of the coordinated folding, processing and trafficking of at least a third of the proteome, coupled to quality control mechanisms. Understanding how cells ensure the conformational integrity of their proteome when challenged with acute and chronic stress is fundamental to health and disease. Research in the past decades has helped unravel the complexity of protein folding and revealed that aberrant folding and aggregation of proteins can lead to disease.
8.2. ER Protein Folding and Quality Control Mechanisms
The life cycle of a protein begins as a linear sequence of amino acids extending from the 80S ribosome. Proteins destined for intracellular organelles and secretion are co- or post- translationally translocated across the ER membrane in an unfolded state. The folding of a polypeptide in the ER allows it to acquire its native conformation and associated post-translational modifications and is often facilitated by ER resident chaperones such as immunoglobulin binding protein (BiP/GRP78), calnexin and calreticulin and enzymes including protein disulfide isomerases (PDIs) and cis-trans peptidyl-prolyl isomerases (PPIs). Correctly folded proteins are then transported to the Golgi compartment and subsequently sorted for trafficking to their ultimate cellular destination.
Due to the complexity of protein folding, it is the most error-prone step in gene expression (Wang and Kaufman 2016). As a result, quality-control machines engage terminally misfolded proteins for ER-associated degradation (ERAD) and/or autophagy to selectively degrade the mis-folded protein or to activate the unfolded protein response (UPR). The UPR is an adaptive mechanism that re-establishes proteostasis in the ER. However, if the adaptive UPR is overwhelmed by chronic or severe ER protein misfolding and is unable to preserve ER function, the UPR activates an apoptotic response. It is believed that apoptotic cell death prevents release of misfolded non-functional proteins from the cell.
8.3. The Unfolded Protein Response (UPR)
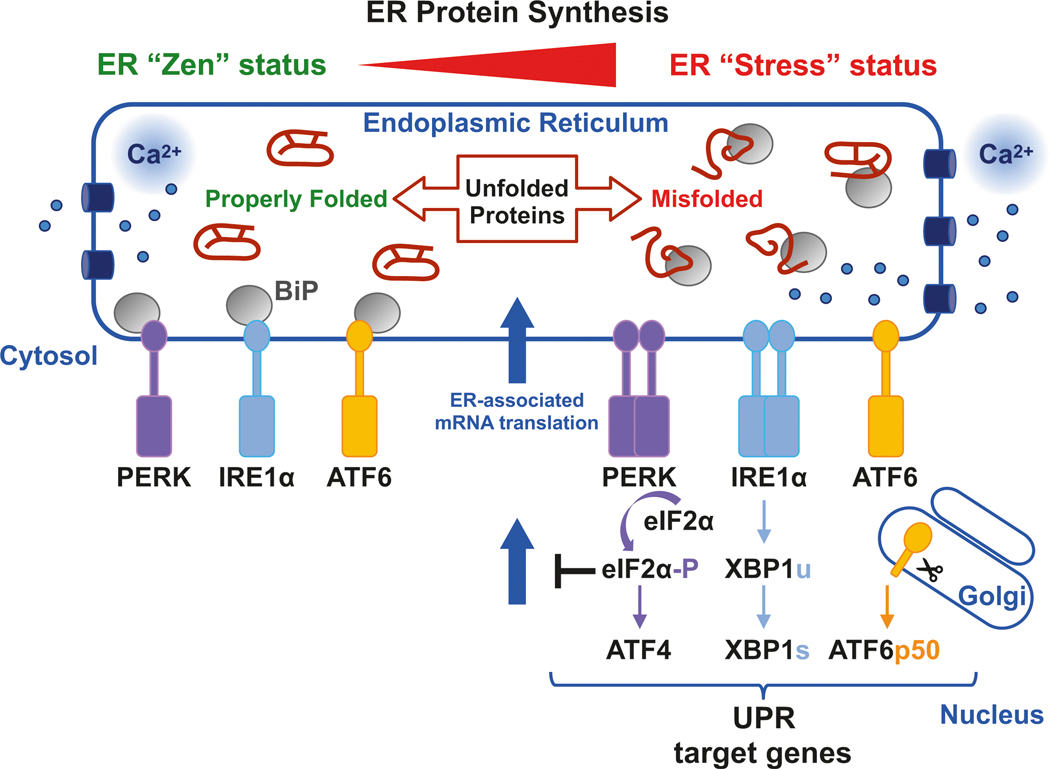
When misfolded proteins accumulate in the ER, the cell engages the UPR to increase the ER protein-folding capacity for its needs (Fig. 8.1). The UPR is a conserved signalling network evolved to restore ER homeostasis and in metazoans involves the activation of three transmembrane sensors: (i) inositol-requiring enzyme 1 (IRE1); (ii) PKR-like ER kinase (PERK); and (iii) activating transcription factor 6 (ATF6). These ER-localized UPR signal transducers convey information about the intensity and duration of the stress stimuli through the detection of mis-folded proteins in the ER and signal to the cytosol and nucleus to either restore proteostasis or induce apoptosis. Under unstressed conditions, IRE1α, PERK and ATF6 are maintained in inactive states by interaction with the luminal ER chaperone BiP. During ER stress, BiP binds to misfolded proteins promoting dissociation from the ER stress sensors, thereby permitting their activation to induce selective protein synthesis attenuation and gene transcription.
Fig. 8.1.
Schematic model for ER stress and UPR activation
In the ER “Zen” status, the ER maintains protein and Ca2+ homeostasis. In the ER “Stress” status, accumulation of misfolded proteins induces the release of BiP from PERK, IRE1α and ATF6. These arms can be subsequently activated in a signaling cascade termed the UPR, which involves the downregulation of translation and the activation of transcription factors that regulate target genes to promote ER homeostasis and cell survival
8.4. IRE1α
IRE1α is the most conserved UPR transducer, possessing catalytic serine/threonine kinase and endoribonuclease (RNase) activities. Following BiP dissociation from its ER luminal N-terminal domain, IRE1α dimerizes and oligomerizes while stimulating trans-autophosphorylation, inducing a conformational change that activates the cytosolic RNase domain (Han et al. 2009; Korennykh et al. 2009; Lee et al. 2008). Recent evidence also suggests that unfolded proteins may be sensed by binding directly to IRE1α and inducing an allosteric change that triggers IRE1α activation (Karagoz et al. 2017). Once ER protein homeostasis is restored, IRE1α oligomers disassemble concomitantly with IRE1α dephosphorylation to return to a basal IRE1α monomeric inactive state bound to BiP (Karagoz et al. 2017).
Activated IRE1α initiates the unconventional splicing of the mRNA encoding the X-box binding protein 1 (XBP1) and the regulated IRE1α- dependent decay (RIDD) (Han et al. 2009; Hollien and Weissman 2006; Hollien et al. 2009) of mRNAs and miRNAs. Spliced XBP1 (XBP1s) induces expression of genes that encode ER protein folding, secretion, ERAD and lipid synthesis functions (Hetz et al. 2011). Physiological RIDD activity appears to be an ancestral mechanism to ensure ER homeostasis by reducing the protein folding burden, while hyperactivated RIDD is associated with an apoptotic cellular output (Maurel et al. 2014). The mechanism controlling the switch from cytoprotective to cytotoxic RNase function remains to be identified, although it may involve oligomerization of activated IRE1α molecules (Han et al. 2009).
The IRE1α platform for signalling is described as a dynamic scaffold termed the “UPRosome” onto which regulatory components assemble to orchestrate crosstalk between the UPR and other signalling pathways (Hetz and Glimcher 2009). The direct binding of the ER chaperone HSP47 (Sepulveda et al. 2018), ERdj4/DNAJB9 (Amin-Wetzel et al. 2017), and the ER resident ER protein disulfide isomerase PDIA6 (Eletto et al. 2014; Groenendyk et al. 2014) to IRE1α may modulate its signalling behaviour. The recruitment of TRAF2 can also trigger JNK or NFκB activation, which may participate in the regulation of insulin resistance, inflammation and apoptosis (Marciniak and Ron 2006; Urano et al. 2000). IRE1α signalling may further be activated by pro-apoptotic BCL2 members BAX and BAK (Hetz et al. 2006), or negatively regulated by BI-1 (Bailly-Maitre et al. 2010; Lebeaupin et al. 2018b; Lisbona et al. 2009). Hence, the assembly of distinct adaptors and modulators on the cytosolic or luminal domains of IRE1α fine-tunes downstream signalling (Hetz et al. 2015). The physiological significance of these interactions remains to be explored.
8.5. PERK
During the early phase of ER stress, PERK is acutely activated to phosphorylate the heterotrimeric GTPase eukaryotic translation initiation factor 2 on the alpha subunit (eIF2α) at Serine residue 51 (Ser51) to attenuate protein synthesis and reduce the load on the ER to support protein folding (Kaufman 2004). Phosphorylated eIF2α halts the initiation of mRNA translation, while paradoxically increases the selective translation of numerous mRNAs that have upstream open reading frames (uORFs) in their 5′ untranslated region, including Atf4, Chop and Gadd34 (Blais et al. 2004; Harding et al. 1999). The transcription factor ATF4 transactivates a cluster of UPR target genes involved in amino acid synthesis and transport, protein synthesis and folding, autophagy, redox homeostasis, and apoptosis (Cullinan et al. 2003; Han et al. 2013; Harding et al. 2003). In particular, ATF4 induces expression of C/EBP homologous protein (CHOP) (Averous et al. 2004) and GADD34 that mediates eIF2α dephosphorylation and restores global mRNA translation (Ma and Hendershot 2003; Novoa et al. 2001). ATF4 may form heterodimers with CHOP, and transcriptional induction through ATF4 and CHOP leads to increased protein synthesis that causes oxidative stress and cell death (Han et al. 2013). Although it was originally proposed that PERK directly regulates redox homeostasis through phosphorylation of nuclear factor E2-related factor 2 (NRF2) to cause dissociation from KEAP1 permitting it to increase antioxidant gene expression, the physiological significance of this is yet to be demonstrated (Cullinan and Diehl 2004; Cullinan et al. 2003).
8.6. ATF6
ATF6 is synthesized as a type II ER-resident transmembrane protein bearing a large cytosolic N-terminal B-Zip transcriptional activation domain. Upon accumulation of unfolded proteins, ATF6 is released from BiP for trafficking to the Golgi apparatus for cleavage by the Golgi-resident proteases S1P and S2P, generating a cytosolic fragment that migrates to the nucleus to induce UPR target genes encoding functions in protein folding and ERAD (Haze et al. 1999; Nadanaka et al. 2004; Shen et al. 2002; Wu et al. 2007).
Together, the three main UPR pathways lead to activation of the major transcription factors XBP1s, ATF4, CHOP and ATF6 that govern the expression of a large range of genes encoding overlapping functions, creating a dynamic UPR network. Under conditions of chronic or severe ER stress, these UPR signalling pathways play critical roles in disease pathogenesis.
8.7. The ER Response to Cellular Stress
Despite the robustness of the ER, cells remain susceptible to various intracellular and extracellular insults that compromise protein folding or exert additional demands on the secretory path-way. Inefficient protein trafficking to the Golgi and the accumulation of misfolded proteins in the ER are responsible for the development and progression of many diseases. For example, Factor VIII expression, which is defective in the coagulation disorder hemophilia A, is limited due to unstable mRNA, polypeptide interaction with ER chaperones and inefficient transport of the primary translation product from the ER to the Golgi (Miao et al. 2004; Pipe et al. 2001). Moreover, the hallmark of many neurodegenerative diseases is the accumulation of protein aggregates. Understanding the causes of protein misfolding, protein aggregation and protein quality control is critical to developing novel therapeutic strategies for intervention in diseases of protein misfolding.
8.8. Oxidative Stress and ROS Production
Cellular stress can arise from an imbalance between reactive oxygen species (ROS) production and antioxidant defense mechanisms that lead to organelle dysfunction. Disulfide bond formation, mediated by ER oxidases (ERO1s) and protein disulphide isomerases (PDIs), can alter the redox status of the ER (Tu and Weissman 2004). Oxidative protein folding, detoxification and mitochondrial respiration are all processes that generate ROS such as the superoxide radical (O2−) and hydrogen peroxide (H2O2). If produced in excess, ROS lead to oxidative stress that damage proteins, lipids and DNA to alter cellular function and architecture and induce apoptosis (Cao and Kaufman 2014). The major pro-apoptotic factor of the UPR, CHOP, activates ERO1α transcription leading to increased ROS production and Ca2+-dependent apoptosis through inositol trisphosphate receptors (IP3R) (Li et al. 2009). Ca2+ released from the ER is taken up by mitochondria, which subsequently opens the permeability transition pore to release cytochrome c and activate the caspase cascade of apoptosis. Recently, IRE1α was found to physically interact with IP3R and control mitochondrial Ca2+ uptake at mitochondrial-associated membranes (MAMs) (Carreras-Sureda et al. 2019).
To protect against the deleterious impact of oxidative stress, antioxidant responses exist to restore cellular redox homeostasis. Certain antioxidant genes (Ngf, Ho-1, Txnrd1, xCT, p62) may be enhanced by NRF2 and ATF4 cooperation (Mimura et al. 2019). Nevertheless, in pathological conditions the antioxidant response may be impaired.
8.9. Cell Death Pathways Ensuing Prolonged or Severe ER Stress
Over the past thirty years, two fundamental mechanisms by which cells die in response to ER stress were discovered: (1) ER stress directly causes oxidative stress leading to cell death (Back et al. 2009; Han et al. 2015; Malhotra et al. 2008; Song et al. 2008); and (2) Chronic ER stress paradoxically increases protein synthesis leading to proteostatic stress that causes cell death (Back et al. 2009; Han et al. 2013; Nakagawa et al. 2014; Song et al. 2008).
There is ample experimental evidence indicating that misfolded protein accumulation in the ER causes oxidative stress generated by the mitochondrial electron transport chain (ETC), and the intimate functional cooperation between ER and mitochondria is likely causal for such an observation. Mitochondria provide ATP to the ER which is essential to support ATP-dependent protein chaperone functions, such as BiP/GRP78 (Dorner et al. 1990), for protein folding and trafficking. In fact, the level of cellular ATP determines which proteins are able to transit to the cell surface (Dorner et al. 1990, 1992). In response, the ER signals mitochondria to stimulate ATP production through ER-to-mitochondria Ca2+ trafficking. ER stress is associated with increased Ca2+ trafficking through IP3Rs on the ER membrane to enter the mitochondrial matrix (Carreras-Sureda et al. 2019; Kaufman and Malhotra 2014; Luciani et al. 2008; Yong et al. 2019). Although ER Ca2+ release channels have been known for decades and are relatively well-characterized (La Rovere et al. 2016), only recently has the mitochondrial Ca2+ uniporter (MCU) (Kwong 2017; Kwong et al. 2015; Pan et al. 2013) and the associated regulatory molecules been identified (Liu et al. 2016; Mallilankaraman et al. 2012; Sancak et al. 2013; Waldeck-Weiermair et al. 2015). Irrespective of the mechanism, Ca2+ trafficking from ER to mitochondria potentiates oxidative phosphorylation by activating NADH dehydrogenases (Kaufman and Malhotra 2014), which may further increase mitochondria-derived ROS production. In vivo, we hypothesize that a process of IP3R-mediated Ca2+ release, in response to IP3 generating hormonal receptor engagement, exacerbates ROS production due to mitochondrial Ca2+ overload. Supporting such a scenario, cardiomyocyte-specific Mcu deletion was protective in acute ischemia-reperfusion injury models (Kwong 2017; Pan et al. 2013).
Among the UPR transducers and downstream signalling molecules, the PERK-P-eIF2α path-way is intimately involved in mediating both cellular survival and death. Activated PERK phosphorylates eIF2α at Ser51, leading to rapid, reversible inhibition of mRNA translation initiation. PERK mutation in humans, and mice, is the cause of infantile onset diabetes associated with Wolcott-Rallison syndrome (Delepine et al. 2000). Experimentally, a Ser51 to Alanine (Ser51Ala) mutation introduced into the endogenous murine eIF2α gene generated mice that cannot phosphorylate eIF2α (Scheuner et al. 2001). Homozygous Ser51Ala mutant mice die perinatally due to hypoglycemia and defective hepatic gluconeogenesis. When fed a high fat diet (HFD) that triggers insulin resistance, heterozygous Ser51Ala mutant mice develop pancreatic β-cell failure and overt diabetes (Scheuner et al. 2005). In addition, since β-cell failure due to eIF2α-S51A mutation recapitulates all the effects of PERK deletion in mice and humans (Back et al. 2009), the findings support the model where PERK acts through P-eIF2α to attenuate protein synthesis (mainly proinsulin) and translocation into the ER, and thereby limits ER stress-induced β-cell death. Importantly, in the setting of increased proinsulin misfolding, CHOP protein, controlled primarily via the PERK branch of the UPR pathway contributes to β-cell death associated with unsuppressed protein synthesis and increased ROS production (Han et al. 2013; Song et al. 2008). Curiously, without ER stress, sole expression of CHOP does not promote cell death, in vitro or in vivo (Han et al. 2013; Southwood et al. 2016; Wang et al. 1998). Furthermore, ChIP-Seq and mRNA-Seq did not reveal any death-promoting genes directly transcriptionally activated by CHOP (Han et al. 2013). Instead, the death-promoting effect by CHOP expression in the context of ER stress was shown to be a consequence of increased protein synthesis in stressed cells, thereby further promoting oxidative stress leading to irreparable cellular damage (Han et al. 2013; Marciniak et al. 2004; Song et al. 2008). These observations suggested that the notorious “death- promoting” role of CHOP is more accurately attributed to its transcriptional role as a master regulator of ER-oriented anabolic metabolism (Yong et al. 2016). Indeed, deletion of the Chop gene delays the onset of hyperglycemia in the Akita mouse (Oyadomari 2002) and suppresses β-cell failure in response to insulin resistance in diet-induced obese mice and the leptin receptor mutant db/db mice (Song et al. 2008). The significance of ROS production was demonstrated by showing that antioxidant feeding prevents β-cell death in response to ER stress (Back et al. 2009; Han et al. 2015; Hassler et al. 2015; Malhotra et al. 2008).
To summarize the role of PERK branch in the UPR, although other factors (such as inflammation pathways) are proposed to cause cell failure, analysis of mice with eIF2α Ser51Ala mutation or Perk gene deletion supports the notion that increased protein synthesis is sufficient to generate ROS through ER-to-mitochondria crosstalk (Kaufman and Malhotra 2014; La Rovere et al. 2016) that initiates cell death.
8.10. ER Stress in Tumorigenesis
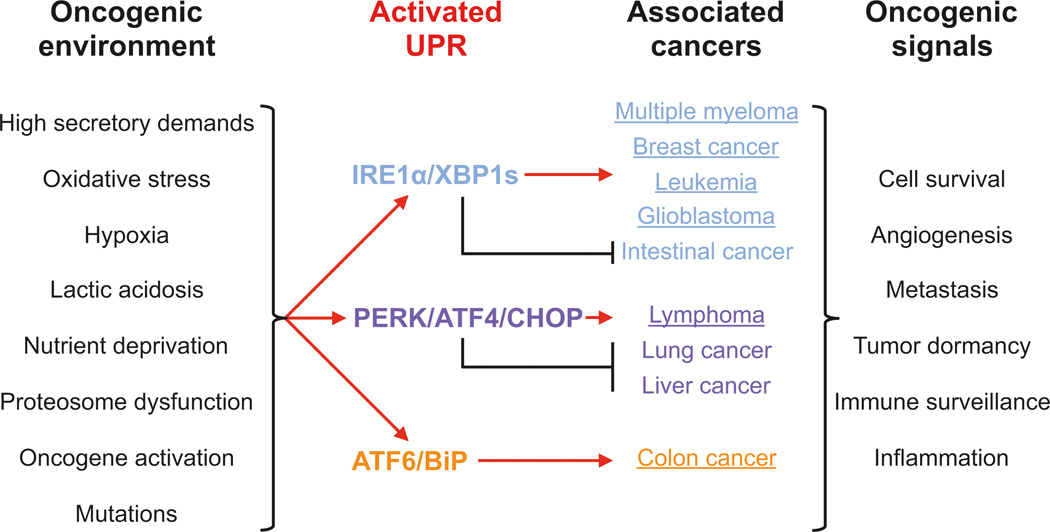
UPR activation is documented in many cancer types (Wang and Kaufman 2014) (Fig. 8.2). Accumulating evidence supports the notion that UPR signalling is integral to cell transformation and tumor progression, although the mechanisms remain poorly defined. As discussed above, protein misfolding in the ER is sufficient to cause oxidative stress (Nakagawa et al. 2014), and it was proposed that ER stress-induced oxidative stress may damage DNA, and further cause somatic gene mutations (Shalapour et al. 2017), further leading to oncogene activation or tumor suppressor gene inactivation. However, once a tumor microenvironment forms, the transformed cells likely depend on UPR signalling to promote cell growth under the unfavorable conditions of the microenvironment, e.g. hypoxia, nutrient deprivation (Milani et al. 2009; Mujcic et al. 2013; Rouschop et al. 2013; Rzymski et al. 2009), and to evade immune surveillance by the adaptive immune response.
Fig. 8.2.
UPR activation in the oncogenic environment Tumors frequently encounter stressors from the oncogenic environment that compromise protein folding and increase demands on the protein secretory pathway, activating the three branches of the UPR. UPR activation has been reported to promote or prevent cancer development in in a cell-specific manner. The combined outputs of the UPR can send oncogenic signals that influence cancer initiation and progression
8.11. ER Stress and UPR Activation in Cancer Cells
It is incompletely understood how ER homeostasis is perturbed in cancer cells, and how UPR signalling can impact tumorigenesis. After an initiating tumorigenic event, the UPR is likely essential to protect cells from ER stress-induced cell death and thereby promotes cancer progression. Under this scenario, UPR signalling may be required to maintain ER homeostasis to prevent ROS accumulation incurred from cancer cell damage and apoptosis. For example, previous studies demonstrated a severe hypoxic microenvironment activates the PERK-eIF2α arm of the UPR, leading to increased glutathione synthesis and consequently protection against ROS produced during periods of hypoxia, which is believed to contribute to therapy-resistance of cancer cells (Rouschop et al. 2013). Epithelial-to-mesenchymal transition (EMT) and the secretory-associated senescence phenotype (SASP) are cellular processes associated with metastasis and require increased protein secretion of cytoskeletal matrix proteins, metalloproteases and growth factors. The increased secretion can activate the UPR and thus prime the cells for preferential death upon pharmacological induction of the UPR (Denoyelle et al. 2006; Feng et al. 2014).
Cancer cells experience ER stress as identified by high level expression of UPR biomarkers (e.g., BiP/GRP78, CHOP, XBP1s and TRIB3). Some of these proteins provide protective and adaptive functions for survival of the cancer cell; most notably elevated BiP expression correlates with increased tumor grade (Dong et al. 2011). Alternatively, some of the UPR induced genes, most notably Chop, promote cell death. Due to the increased sensitivity of tumor cells to ER stress it may be possible to pharmacologically activate the UPR to kill tumor cells. Targeting the UPR may provide tumor-selective killing because normal cells, without basal ER stress, can mount an adaptive UPR and return to homeostasis. The majority of efforts to target the UPR in cancer are directed at compounds that activate the pro-apoptotic UPR (Flaherty et al. 2013, 2014) and will be discussed below.
8.12. The Role of IRE1 and XBP1s in Cancer
An early study discovered that IRE1α (Ern1) ranked 5th among 518 protein kinase genes that carry at least one driver mutation (Greenman et al. 2007), although it is unknown whether these mutations cause a gain or loss of function. In the same pathway, XBP1s was reported to drive multiple myeloma (Carrasco et al. 2007). IRE1α and XBP1 loss of function mutations were identified in tumor cells from multiple myeloma patients that were resistant to proteasome inhibitors (Hong and Hagen 2013; Leung-Hagesteijn et al. 2013). Inactivation of the IRE1α/XBP1s pathway reduced immunoglobulin (IgG) gene expression so the plasma cells dedifferentiated to preplasma-blasts that were resistant to proteasome inhibition because they did not express IgG which misfolds in the ER lumen. Furthermore, XBP1 splicing was suggested to cause human triple negative breast cancers, in part through its proposed role by increasing HIF1α expression (Chen et al. 2014). Increased XBP1s expression in luminal breast cancer mediates resistance to anti-hormonal therapy by stimulating Nuclear Receptor Coactivator 3 (NCOA3) (Gupta et al. 2016). Moreover, increased XBP1 expression is commonly observed in patients with acute myeloid leukemia (~70%) and leukemia cell lines (Sun et al. 2016). Along the same line, knocking-down XBP1 expression levels increased the susceptibility of multiple myeloma cells to ER stress-inducing reagents (Mimura et al. 2012). IRE1α was also suggested to be oncogenic in glioblastoma through its RNase activity to degrade selective mRNAs by RIDD (Dejeans et al. 2012; Pluquet et al. 2013). However, recent studies suggest a dual role of IRE1α RNase in glioblastoma development, where IRE1α-mediated splicing of XBP1 mRNA is pro-tumorigenic, while IRE1α-mediated RIDD is tumor suppressive (Lhomond et al. 2018).
In contrast, loss of XBP1 promotes tumorigenesis in mouse models of intestinal cancer induced by colitis or by APC mutation (Niederreiter et al. 2013). This appears due to hyperactivation of IRE1α because tumorigenesis actually required IRE1α (Niederreiter et al. 2013). These findings indicate that the IRE1α- XBP1 pathway plays a tumor-suppressor role in cancer in the intestine. Overall, while the under-lying role of IRE1α in cancer initiation is suspected, more recent findings do not fully support a uniform oncogenic role for IRE1α. To conclude, the above observations support the notion that IRE1α-XBP1 plays an oncogenic role in cancer, although the relative contribution of this pathway is highly cell-type-dependent.
8.13. The Role of PERK and ATF4 in Cancer
PERK activation promotes MYC-driven cell transformation and autophagy (Hart et al. 2012), the latter being a well-documented function of ATF4, the downstream signalling effector of PERK-P-eIF2α that initiates cell death. However, there are contradictory findings regarding whether ER stress-induced autophagy promotes tumor progression. While many studies support a cytoprotective role for autophagy in cancer cells (Cubillos-Ruiz et al. 2017; Ma et al. 2014; Ogata et al. 2006), others indicate that autophagy is important for cancer inhibition. For example, Cerezo et al. reported that BiP inhibition by the thiazole benzenesulfonamide HA15 exerts its cancer cell killing effect by causing ER stress to activate autophagy and apoptosis in a manner that requires the apoptosis factor CHOP (Cerezo et al. 2016). Similarly, other independent studies reported that Chop gene deletion increases K-RasG12V driven mouse lung cancer (Huber et al. 2013). In addition, Chop deletion increases hepatocellular carcinoma in a mouse model of hepatocyte-specific misfolding of urokinase (MUP-uPA transgenic mice) (Huber et al. 2013; Nakagawa et al. 2014), which is consistent with CHOP’s role in apoptosis induced by ER stress. It is unknown if CHOP plays a tumor-suppressor role in human tumors, although missense mutations in the Chop gene were reported in human lung adenocarcinoma (Kan et al. 2010).
8.14. The Role of ATF6 and BiP/ GRP78 in Cancer
Some studies suggest that ATF6α promotes tumorigenesis (Arai et al. 2006), possibly by its downstream effector ER chaperone, BiP. Elevated BiP expression is associated with benefits favoring cancer cell survival (Fu et al. 2007; Pyrko et al. 2007) and can prevent caspase activation (Reddy et al. 2003).
Inhibition of cell division due to G0/G1 cell cycle arrest and entry into quiescence are characteristic of cancer cell dormancy (Aguirre-Ghiso 2007). A main reason for cancer recurrence after radio-and chemotherapies is reactivation of dormant cells (Paez et al. 2012). There also appears to be a link between UPR activation and tumor dormancy as ATF6 is highly expressed in recurrent tumors (Ginos et al. 2004) and correlates with poor prognosis for colorectal cancer (Lin et al. 2007). ATF6 expression is elevated in metastatic lesions compared to the primary tumor (Ramaswamy et al. 2001). These findings suggest that ATF6 may be constitutively active in dormant cells, such as human squamous cell carcinoma (SCC) (Schewe and Aguirre-Ghiso 2008), but studies need to confirm this notion. Knock-down of ATF6 in SCC reduces cancer cell survival and tumor growth via downregulation of adaptive pathways, such as mTOR (Schewe and Aguirre-Ghiso 2008). ATF6 induces expression of proteins associated with tumor transformation (Arai et al. 2006) and chemo-resistance (Higa et al. 2014), which may explain why recurrent tumors are refractory to second rounds of chemotherapy. Finally, forced expression of the activated form of ATF6p50 caused spontaneous colitis and colon cancer in mice (Coleman et al. 2018).
Nevertheless, due to the mutual compensation of the UPR branches, it is challenging to dissect the exact requirement of each UPR sensor in cancer. For example, inactivation of any of the three UPR pathways may result in compensatory activation of alternative UPR pathways. Therefore, caution is necessary to interpret the causal role of individual UPR signalling events that promote or limit tumor initiation and progression.
8.15. The UPR and Immune Surveillance
The ER plays a pivotal role in the synthesis and assembly of the major histocompatibility complex (MHC). After its polypeptide synthesis and ER membrane translocation, the assembly of the heavy and macroglobulin β2m chains of MHC class I (MHC-I) takes place in the ER lumen. The assembly and the subsequent loading of a high-affinity peptide fragment for immune recognition are facilitated by the TAP channel, tapasin, and by the ER chaperones ERp57, calnexin and calreticulin (Blum et al. 2013). Under the stringent ER protein folding quality control, only appropriately folded MHC-I with a peptide cargo is allowed to be exported to the cell surface for antigen display under immune surveillance. Therefore, ER stress is associated with insufficient MHC-I assembly and cell surface presentation (Granados et al. 2009), which is speculated to cause impaired immune surveillance in the tumor microenvironment. Conversely, the ER chaperone GRP94, an HSP90 family member, is well known for its role in facilitating peptide pre- sentation by professional antigen-presenting cells (APCs) (Schild and Rammensee 2000). This peptide presentation function of GRP94 was further mapped to its N-terminal domain compromising the first 355 amino acids (Biswas et al. 2006). It is plausible that ER stress in tumor cells could interfere with the role of GRP94 in facilitating antigen uptake by APCs for its presentation class I and class II MHC molecules, as misfolded protein cargo in the ER lumen can sequester the HSP-like chaperones (Dorner et al. 1988).
8.16. Therapeutic Strategies to Target the UPR
The complexity of ER stress responses has significant potential for therapeutic intervention. The advantage of targeting the UPR is that tumor cells rely more on this pathway for survival than healthy cells, thus providing strategies for selective killing.
8.17. Chemical Chaperones
Broad spectrum chemical chaperones have been identified as low molecular weight compounds that alleviate ER stress by promoting protein folding, preventing protein aggregation and increasing ERAD. The most common ER stress alleviators are 4-phenylbutyric acid (PBA), a short-chain fatty acid also described as a potent histone deacetylase inhibitor, and tauroursode-oxycholic acid (TUDCA), a hydrophilic taurine conjugate of the bile salt ursodeoxycholic acid (Sarvani et al. 2017). It was first proposed that these chaperones may be potent therapeutic agents against metabolic disorders. Treatment with either PBA or TUDCA efficiently resolved hepatic steatosis and enhanced insulin action in the livers of ob/ob mice with type 2 diabetes (Özcan et al. 2006). PBA, and especially TUDCA, were also shown to protect against liver injury and regeneration failure as they reduced inflammation, apoptosis and necrosis in both steatotic and non-steatotic livers after partial hepatectomy and ischemia-reperfusion (Ben Mosbah et al. 2010). In HFD-fed mice and in non-alcoholic steatohepatitis (NASH)-prone transgenic mice that express high levels of misfolded urokinase in hepatocytes, treatment with TUDCA or hepatic overexpression of BiP abrogated the signs of NASH: protecting against hepatic steatosis and liver injury characterized by ballooned hepatocytes, increased ROS and cell death (Nakagawa et al. 2014). Similarly, administration of TUDCA to ob/ob leptin-deficient obese mice challenged with lipopolysaccharide protected against ER stress-dependent NLRP3 inflammasome activation and liver injury (Lebeaupin et al. 2015). Oral administration of either PBA or TUDCA in ER stress-prone mice with colitis significantly reduced signs of colonic inflammation by alleviating ER stress in colonic epithelial cells (Cao et al. 2013).
TUDCA and PBA are Food and Drug Administration-approved agents as orally active chemical chaperones that reduce ER stress and are being tested in extensive clinical trials. That being said, these chaperones usually require high concentrations due to their poor selectivity, often making them neglected as therapeutic agents. While PBA and TUDCA have proven their safety and potency in reducing ER stress, their direct mechanisms of action have yet to be clearly defined.
8.18. Pharmacological Inhibitors/ Activators
Many pharmacological compounds target distinct UPR signalling molecules (Hetz et al. 2013; Lebeaupin et al. 2018a) (Table 8.1). Nevertheless, many compounds have yet to prove their efficacy and safety in vivo to confirm their therapeutic potential.
Table 8.1.
Pharmacological compounds that target UPR pathways with therapeutic implications
| Name | Target | Mechanism | Potential therapies | References |
|---|---|---|---|---|
| 4μ8c | IRE1α RNase | Inhibits XBP1 mRNA splicing. Inhibits RIDD function. | Atherosclerosis | Tufanli et al. (2017) |
| NAFLD | Lebeaupin et al. (2018a, b) | |||
| STF-083010 | Diabetes | Lerner et al. (2012) | ||
| Inflammatory diseases | Kim et al. (2015) | |||
| Atherosclerosis | Tufanli et al. (2017) | |||
| NAFLD | Lebeaupin et al. (2018a, b) | |||
| MKC-3946 | Multiple Myeloma | Mimura et al. (2012) | ||
| KIRA6/ KIRA8 | IRE1α kinase and RNase | Promotes cell survival under ER stress. | Diabetes | Ghosh et al. (2014) |
| Diabetes | Morita et al. (2017) | |||
| APY29 | IRE1α kinase | ATP-competitive inhibitor that inhibits IRE1α kinase, but increases dimerization/oligomerization of IRE1α, enhancing RNase activity. | ND | Korennykh et al. (2009) |
| ND | Wang et al. (2012) | |||
| Kidney cancer | Kuo et al. (2017) | |||
| Selonsertib | ASK1 | Inhibits ASK1-JNK1 signaling with potential anti-inflammatory, and anti-fibrotic activities. | NAFLD with fibrosis | Loomba et al. (2017) |
| Multidrug resistance | Ji et al. (2019) | |||
| GSK2606414 GSK2656157 | PERK | Inhibits PERK autophosphorylation. | Cancer (tumor development and angiogenesis) | Axten et al. (2013) Atkins et al. (2013) |
| Parkinson’s disease | Mercado et al. (2018) | |||
| Salubrinal | P-eIF2α | Prevents eIF2α dephosphorylation, maintaining mRNA translation inhibition to limit the ER protein load. | Liver cancer | Vandewynckel et al. (2015) |
| Guanabenz | ND | Tsaytler et al. (2011) | ||
| Sephin1 | Charcot-Marie-Tooth disease and amyotrophic lateral sclerosis | Das et al. (2015) | ||
| ND | Crespillo-Casado et al. (2017) | |||
| Multiple sclerosis | Chen et al. (2019) | |||
| Raphin1 | Huntington’s disease | Krzyzosiak et al. (2018) | ||
| ISRIB 2BAct | P-eIF2α | Inhibits P-eIF2α to resume global mRNA translation. | ND | Sidrauski et al. (2015) |
| ND | Tsai et al. (2018) | |||
| ND | Zyryanova et al. (2018) | |||
| Vanishing white matter disease | Wong et al. (2019) | |||
| ML291 | CHOP | Selective CHOP inducer with anti-proliferative effects. | ND | Flaherty et al. (2013) and Flaherty et al. (2014) |
| PACMA 31 | PDI | PDI inhibitors increases ER stress to reduce cell viability with anti- proliferative effects. | Ovarian cancer | Badolato et al. (2017) |
| Bacitracin | Glioblastoma | Goplen et al. (2006) | ||
| AEBSF | ATF6 | Prevents ATF6 proteolytic cleavage in the Golgi and activation. | ND | Okada et al. (2003) |
| Ceapins | ATF6 | Traps ATF6 in the ER to prevent activation. | ND | Gallagher et al. (2016) |
| 147 | ATF6 | Localized metabolic activation of ATF6 to enhance proteostasis. | ND | Plate et al. (2016) |
| ND | Paxman et al.(2018) | |||
| Cardiac ischemia/reperfusion damage | Blackwood et al.(2019) |
ND: therapeutic function in vivo not determined
IRE1α, possessing both a catalytic core in its RNase domain and an ATP-binding pocket in its kinase domain, can be manipulated pharmacologically. The challenge lies in developing selective compounds that interact with one domain without affecting the other. Small molecule inhibitors of the RNase function of IRE1α include 4μ8c (Lebeaupin et al. 2018b; Tufanli et al. 2017), STF-083010 (Kim et al. 2015; Lebeaupin et al. 2018b; Lerner et al. 2012; Tufanli et al. 2017) and MKC-3946 (Mimura et al. 2012). IRE1α RNase function can also be modulated through its kinase domain by ATP-competitive ligands, forming a new pharmacological class of inhibitors called Kinase-Inhibiting RNase Attenuators (KIRAs) (Ghosh et al. 2014; Han et al. 2009; Morita et al. 2017). Through a conformational change, broad-acting kinase inhibitors APY29 (Kuo et al. 2017; Wang et al. 2012) and clinically-approved sunitinib (gastrointestinal, renal and pancreatic cancer) block IRE1α kinase activity, but allosterically activate IRE1α’s RNase domain in yeast (Korennykh et al. 2009). Another compound, selonsertib (GS-4997), showed potential in patients with NASH as a well-tolerated selective inhibitor of ASK1, an important intermediary of IRE1α-JNK1 signal- ling (Loomba et al. 2017), but recently failed a phase 3 clinical trial (Gilead Sciences), further exposing the unmet need for effective liver disease treatments. Selonsertib could nevertheless provide a treatment strategy against multidrug resistance in a variety of cancers (Ji et al. 2019).
Targeting the PERK pathway, the small molecules GSK2606414 (first generation) and GSK2656157 (preclinical development candidate) were developed as pharmacological inhibitors of PERK autophosphorylation (Atkins et al. 2013; Axten et al. 2013), but recent evidence showing off-target effects of these compounds questions their selectivity (Rojas-Rivera et al. 2017). In addition, these compounds destroy pancreatic β-cells, likely because β-cells require PERK-mediated phosphorylation of eIF2α (Mercado et al. 2018). At the expense of pancreatic β-cell survival, GSK2606414 may protect against ER stress-related neurodegenerative decline (Mercado et al. 2018). By preventing eIF2α dephosphorylation through the selective disruption of the PP1-PPP1R15A/GADD34 holoprotein P-eIF2α phosphatase complex, the compounds salubrinal (Vandewynckel et al. 2015), guanabenz (Tsaytler et al. 2011) and its derivative sephin1 (Chen et al. 2019; Das et al. 2015) maintain mRNA translational inhibition. Recent findings challenge the assumption that guanabenz and sephin1 interfere with eIF2α dephosphorylation (Crespillo-Casado et al. 2017), although both drugs do reduce ER stress. A newly discovered and selective phosphatase inhibitor raphin1 targeting PPP1R15B may have potential as an orally available and selective com- pound that improves proteostasis in neurodegen- erative diseases (Krzyzosiak et al. 2018).
While aiming to prevent eIF2α dephosphorylation may exert protective effects against misfolding and a protein overload in the ER in the short-term, persistent inhibition of global translation in cells is poorly tolerated in the long-term. On the contrary, a potent integrated stress response inhibitor (ISRIB, or the more recent 2BAct) was shown to render cells resistant to the effects of eIF2α phosphorylation via eIF2B activation (Sidrauski et al. 2015; Wong et al. 2019), likely a consequence of stabilizing a dimer of the pentameric eIF2B (Tsai et al. 2018; Zyryanova et al. 2018), permitting continued protein synthesis, protecting UPR function and promoting cell survival. In an effort to selectively activate the apoptotic versus the adaptive arm of the UPR, the chemical probe and sulfonamidebenzamide ML291 was developed (Flaherty et al. 2013). ML291 demonstrated efficacy in inducing CHOP-dependent cell death in a number of cell lines, making it a potential candidate for cancer therapy (Flaherty et al. 2014). PDI antagonists, such as PACMAs (Badolato et al. 2017) and bacitracin (Goplen et al. 2006), also represent important approaches for the development of targeted anticancer compounds.
Agents that specifically modulate ATF6 expression or activity are limited and general serine protease inhibitors, such as AEBSF (Okada et al. 2003), are commonly used. A new class of small molecule inhibitors called Ceapins trap full- length ATF6 in ER-resident foci, preventing ER stress-induced trafficking of ATF6 to the Golgi for proteolytic activation (Gallagher et al. 2016). Another strategy involves reprogramming the ER proteostasis environment through the genetic activation of ATF6α by the small molecule N-(2- hydroxy-5-methylphenyl)-3-phenylpropanamide (147), shown to attenuate secretion and aggregation of amyloidogenic proteins (Paxman et al. 2018; Plate et al. 2016). Treatment of ischemia/reperfused cardiomyocytes with 147 promoted proteostasis and reduced oxidative stress, while 147 administered in vivo improved cardiac performance in mice subjected to acute myocardial infarction (Blackwood et al. 2019).
8.19. Antioxidants
Accumulating evidence suggests that protein folding and production of ROS are closely linked, with persistent ER stress and oxidative stress synergistically initiating apoptotic cascades and playing significant roles in disease pathogenesis (Malhotra and Kaufman 2007). The application of antioxidants not only reduces oxidative stress, but also improves protein folding and secretion to prevent ER stress-induced apoptosis (Han et al. 2015; Malhotra et al. 2008). Accumulation of misfolded clotting FVIII in the ER lumen activates the UPR, causes oxidative stress and induces apoptosis. In mice injected with a vector that encodes aggregation-prone FVIII, feeding with butylated hydroxyl-anisole (BHA), a lipid-soluble antioxidant, reduces levels of UPR activation, oxidative stress and apoptosis and increases FVIII secretion (Malhotra et al. 2008). Mice deficient for P58IPK, an ER luminal co-chaperone for BiP, are susceptible to protein-folding defects, reduced pancreatic β-cell mass and function (Han et al. 2015), and multi- systemic neuropathy (Synofzik et al. 2014). In these mice the β-cell failure and diabetes were attenuated when fed with a BHA-supplemented diet (Han et al. 2015). Since efforts to reduce ROS are associated with improved protein folding and cell survival, antioxidant treatment may offer a feasible treatment perspective in protein misfolding diseases and metabolic diseases.
In detoxifying organs such as the liver, the antioxidant response is orchestrated by the highly expressed factor NRF2. NRF2 has been suggested to play a cytoprotective role in response to ER stress-dependent inflammation in animal models with NASH (Okada et al. 2012). Further, pharmacological activators of NRF2 signalling significantly reduced fibrosis in rats with diet-induced NASH, demonstrating a potential strategy to treat NASH patients with hepatic fibrosis (Shimozono et al. 2013). However, NRF2 also induces p62 gene expression through a self-amplifying auto-regulatory loop that creates an oncogenic environment, protecting hepatocellular carcinoma-initiating cells from oxidative stress-induced death (Jain et al. 2010; Umemura et al. 2016). Thus, antioxidants may promote rather than suppress certain cancer development.
8.20. Conclusion
Since tumor cells frequently exhibit a partially active UPR, there is the possibility to preferentially kill these cells by inducing low levels of ER stress, for which normal cells can tolerate and adapt. There are thus multiple ways to modulate ER physiology in an effort to treat diseases related to abnormal ER stress. To confirm their therapeutic potential, the specificity of different pharmacological compounds should be of particular concern, along with the potential side effects due to the artificial manipulation of the signalling pathways of the UPR, which can transition from adaptive to cell death programs. The application of our understanding of the UPR and downstream signalling will prove instrumental in developing novel therapies for a wide range of diseases.
Acknowledgements
This work was supported by the NIH grants R01HL052173, R37DK042394, R24DK110973, R01DK103185, R01DK113171, R01AG062190 and R01CA198103 to R.J.K. and the NCI Cancer Center Support Grant P30 CA030199. R.J.K. is a member of the UCSD DRC (P30 DK063491) and Adjunct Professor in the Department of Pharmacology, UCSD. C.L. is a member of the UCSD DRC and is supported by the NIH grant T32DK007494.
Contributor Information
Cynthia Lebeaupin, Degenerative Diseases Program, SBP Medical Discovery Institute, La Jolla, CA, USA; Department of Medicine, University of California San Diego, La Jolla, CA, USA.
Jing Yong, Degenerative Diseases Program, SBP Medical Discovery Institute, La Jolla, CA, USA.
Randal J. Kaufman, Degenerative Diseases Program, SBP Medical Discovery Institute, La Jolla, CA, USA
References
- Aguirre-Ghiso JA (2007) Models, mechanisms and clinical evidence for cancer dormancy. Nat Rev Cancer 7:834–846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amin-Wetzel N, Saunders RA, Kamphuis MJ, Rato C, Preissler S, Harding HP, Ron D (2017) A J-protein co-chaperone recruits BiP to monomerize IRE1 and repress the unfolded protein response. Cell 171:1625–1637.e1613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai M, Kondoh N, Imazeki N, Hada A, Hatsuse K, Kimura F, Matsubara O, Mori K, Wakatsuki T, Yamamoto M (2006) Transformation-associated gene regulation by ATF6alpha during hepatocarcinogenesis. FEBS Lett 580:184–190 [DOI] [PubMed] [Google Scholar]
- Atkins C, Liu Q, Minthorn E, Zhang SY, Figueroa DJ, Moss K, Stanley TB, Sanders B, Goetz A, Gaul N et al. (2013) Characterization of a novel PERK kinase inhibitor with antitumor and antiangiogenic activity. Cancer Res 73:1993–2002 [DOI] [PubMed] [Google Scholar]
- Averous J, Bruhat A, Jousse C, Carraro V, Thiel G, Fafournoux P (2004) Induction of CHOP expression by amino acid limitation requires both ATF4 expression and ATF2 phosphorylation. J Biol Chem 279:5288–5297 [DOI] [PubMed] [Google Scholar]
- Axten JM, Romeril SP, Shu A, Ralph J, Medina JR, Feng Y, Li WH, Grant SW, Heerding DA, Minthorn E et al. (2013) Discovery of GSK2656157: an optimized PERK inhibitor selected for preclinical development. ACS Med Chem Lett 4:964–968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Back SH, Scheuner D, Han J, Song B, Ribick M, Wang J, Gildersleeve RD, Pennathur S, Kaufman RJ (2009) Translation attenuation through eIF2alpha phosphorylation prevents oxidative stress and maintains the differentiated state in beta cells. Cell Metab 10:13–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badolato M, Carullo G, Aiello F, Garofalo A (2017) Synthesis and experimental validation of new PDI inhibitors with antiproliferative activity. J Chem 2017:1–9 [Google Scholar]
- Bailly-Maitre B, Belgardt BF, Jordan SD, Coornaert B, von Freyend MJ, Kleinridders A, Mauer J, Cuddy M, Kress CL, Willmes D et al. (2010) Hepatic Bax inhibitor-1 inhibits IRE1alpha and protects from obesity- associated insulin resistance and glucose intolerance. J Biol Chem 285:6198–6207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Mosbah I, Alfany-Fernandez I, Martel C, Zaouali MA, Bintanel-Morcillo M, Rimola A, Rodes J, Brenner C, Rosello-Catafau J, Peralta C (2010) Endoplasmic reticulum stress inhibition protects steatotic and non- steatotic livers in partial hepatectomy under ischemia- reperfusion. Cell Death Dis 1:e52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas C, Sriram U, Ciric B, Ostrovsky O, Gallucci S, Argon Y (2006) The N-terminal fragment of GRP94 is sufficient for peptide presentation via professional antigen-presenting cells. Int Immunol 18:1147–1157 [DOI] [PubMed] [Google Scholar]
- Blackwood EA, Azizi K, Thuerauf DJ, Paxman RJ, Plate L, Kelly JW, Wiseman RL, Glembotski CC (2019) Pharmacologic ATF6 activation confers global protec- tion in widespread disease models by reprograming cellular proteostasis. Nat Commun 10:187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blais JD, Filipenko V, Bi M, Harding HP, Ron D, Koumenis C, Wouters BG, Bell JC (2004) Activating transcription factor 4 is translationally regulated by hypoxic stress. Mol Cell Biol 24:7469–7482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum JS, Wearsch PA, Cresswell P (2013) Pathways of antigen processing. Annu Rev Immunol 31:443–473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao SS, Kaufman RJ (2014) Endoplasmic reticulum stress and oxidative stress in cell fate decision and human disease. Antioxid Redox Signal 21:396–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao SS, Zimmermann EM, Chuang BM, Song B, Nwokoye A, Wilkinson JE, Eaton KA, Kaufman RJ (2013) The unfolded protein response and chemical chaperones reduce protein misfolding and colitis in mice. Gastroenterology 144:989–1000.e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco DR, Sukhdeo K, Protopopova M, Sinha R, Enos M, Carrasco DE, Zheng M, Mani M, Henderson J, Pinkus GS et al. (2007) The differentiation and stress response factor XBP-1 drives multiple myeloma pathogenesis. Cancer Cell 11:349–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carreras-Sureda A, Jana F, Urra H, Durand S, Mortenson DE, Sagredo A, Bustos G, Hazari Y, Ramos-Fernandez E, Sassano ML et al. (2019) Non-canonical function of IRE1alpha determines mitochondria-associated endoplasmic reticulum composition to control calcium transfer and bioenergetics. Nat Cell Biol 21:755–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerezo M, Lehraiki A, Millet A, Rouaud F, Plaisant M, Jaune E, Botton T, Ronco C, Abbe P, Amdouni H et al. (2016) Compounds triggering ER stress exert anti-melanoma effects and overcome BRAF inhibitor resistance. Cancer Cell 29:805–819 [DOI] [PubMed] [Google Scholar]
- Chen X, Iliopoulos D, Zhang Q, Tang Q, Greenblatt MB, Hatziapostolou M, Lim E, Tam WL, Ni M, Chen Y et al. (2014) XBP1 promotes triple-negative breast cancer by controlling the HIF1alpha pathway. Nature 508:103–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Podojil JR, Kunjamma RB, Jones J, Weiner M, Lin W, Miller SD, Popko B (2019) Sephin1, which prolongs the integrated stress response, is a promising therapeutic for multiple sclerosis. Brain 142:344–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman OI, Lobner EM, Bierwirth S, Sorbie A, Waldschmitt N, Rath E, Berger E, Lagkouvardos I, Clavel T, McCoy KD et al. (2018) Activated ATF6 induces intestinal dysbiosis and innate immune response to promote colorectal tumorigenesis. Gastroenterology 155:1539–1552.e12 [DOI] [PubMed] [Google Scholar]
- Crespillo-Casado A, Chambers JE, Fischer PM, Marciniak SJ, Ron D (2017) PPP1R15A-mediated dephosphorylation of eIF2alpha is unaffected by Sephin1 or Guanabenz. Elife 6:pii: e26109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cubillos-Ruiz JR, Bettigole SE, Glimcher LH (2017) Tumorigenic and immunosuppressive effects of endo-plasmic reticulum stress in cancer. Cell 168:692–706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullinan SB, Diehl JA (2004) PERK-dependent activation of Nrf2 contributes to redox homeostasis and cell survival following endoplasmic reticulum stress. J Biol Chem 279:20108–20117 [DOI] [PubMed] [Google Scholar]
- Cullinan SB, Zhang D, Hannink M, Arvisais E, Kaufman RJ, Diehl JA (2003) Nrf2 is a direct PERK substrate and effector of PERK-dependent cell survival. Mol Cell Biol 23:7198–7209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das I, Krzyzosiak A, Schneider K, Wrabetz L, D’Antonio M, Barry N, Sigurdardottir A, Bertolotti A (2015) Preventing proteostasis diseases by selective inhibition of a phosphatase regulatory subunit. Science 348:239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejeans N, Pluquet O, Lhomond S, Grise F, Bouchecareilh M, Juin A, Meynard-Cadars M, Bidaud-Meynard A, Gentil C, Moreau V et al. (2012) Autocrine control of glioma cells adhesion and migration through IRE1α-mediated cleavage of SPARC mRNA. J Cell Sci 125:4278. [DOI] [PubMed] [Google Scholar]
- Delepine M, Nicolino M, Barrett T, Golamaully M, Lathrop GM, Julier C (2000) EIF2AK3, encoding translation initiation factor 2-alpha kinase 3, is mutated in patients with Wolcott-Rallison syndrome. Nat Genet 25:406–409 [DOI] [PubMed] [Google Scholar]
- Denoyelle C, Abou-Rjaily G, Bezrookove V, Verhaegen M, Johnson TM, Fullen DR, Pointer JN, Gruber SB, Su LD, Nikiforov MA et al. (2006) Anti-oncogenic role of the endoplasmic reticulum differentially activated by mutations in the MAPK pathway. Nat Cell Biol 8:1053–1063 [DOI] [PubMed] [Google Scholar]
- Dong D, Stapleton C, Luo B, Xiong S, Ye W, Zhang Y, Jhaveri N, Zhu G, Ye R, Liu Z et al. (2011) A criti- cal role for GRP78/BiP in the tumor microenvironment for neovascularization during tumor growth and metastasis. Cancer Res 71:2848–2857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorner AJ, Krane MG, Kaufman RJ (1988) Reduction of endogenous GRP78 levels improves secretion of a heterologous protein in CHO cells. Mol Cell Biol 8:4063–4070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorner AJ, Wasley LC, Kaufman RJ (1990) Protein dissociation from GRP78 and secretion are blocked by depletion of cellular ATP levels. Proc Natl Acad Sci U S A 87:7429–7432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorner AJ, Wasley LC, Kaufman RJ (1992) Overexpression of GRP78 mitigates stress induction of glucose regulated proteins and blocks secretion of selective proteins in Chinese hamster ovary cells. EMBO J 11:1563–1571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eletto D, Eletto D, Dersh D, Gidalevitz T, Argon Y (2014) Protein disulfide isomerase A6 controls the decay of IRE1alpha signaling via disulfide-dependent association. Mol Cell 53:562–576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng YX, Sokol ES, Del Vecchio CA, Sanduja S, Claessen JH, Proia TA, Jin DX, Reinhardt F, Ploegh HL, Wang Q et al. (2014) Epithelial-to-mesenchymal transition activates PERK-eIF2alpha and sensitizes cells to endoplasmic reticulum stress. Cancer Discov 4:702–715 [DOI] [PubMed] [Google Scholar]
- Flaherty D, Golden J, Liu C, Hedrick M, Gosalia P, Liou Y, Milewski M, Sugarman E, Suyama E, Nguyen K et al. (2013) Selective small molecule activator of the apoptotic arm of the UPR. In (Bethesda (MD): National Center for Biotechnology Information: Probe Reports from the NIH Molecular Libraries Program) [PubMed] [Google Scholar]
- Flaherty DP, Miller JR, Garshott DM, Hedrick M, Gosalia P, Li Y, Milewski M, Sugarman E, Vasile S, Salaniwal S et al. (2014) Discovery of sulfonamidebenzamides as selective apoptotic CHOP pathway activators of the unfolded protein response. ACS Med Chem Lett 5:1278–1283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y, Li J, Lee AS (2007) GRP78/BiP inhibits endoplasmic reticulum BIK and protects human breast cancer cells against estrogen starvation-induced apoptosis. Cancer Res 67:3734–3740 [DOI] [PubMed] [Google Scholar]
- Gallagher CM, Garri C, Cain EL, Ang KK, Wilson CG, Chen S, Hearn BR, Jaishankar P, Aranda-Diaz A, Arkin MR et al. (2016) Ceapins are a new class of unfolded protein response inhibitors, selectively targeting the ATF6alpha branch. Elife 5:pii: e11878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh R, Wang L, Wang ES, Perera BG, Igbaria A, Morita S, Prado K, Thamsen M, Caswell D, Macias H et al. (2014) Allosteric inhibition of the IRE1alpha RNase preserves cell viability and function during endoplasmic reticulum stress. Cell 158:534–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginos MA, Page GP, Michalowicz BS, Patel KJ, Volker SE, Pambuccian SE, Ondrey FG, Adams GL, Gaffney PM (2004) Identification of a gene expression signature associated with recurrent disease in squamous cell carcinoma of the head and neck. Cancer Res 64:55–63 [DOI] [PubMed] [Google Scholar]
- Goplen D, Wang J, Enger PO, Tysnes BB, Terzis AJ, Laerum OD, Bjerkvig R (2006) Protein disulfide isomerase expression is related to the invasive properties of malignant glioma. Cancer Res 66:9895–9902 [DOI] [PubMed] [Google Scholar]
- Granados DP, Tanguay PL, Hardy MP, Caron E, de Verteuil D, Meloche S, Perreault C (2009) ER stress affects processing of MHC class I-associated peptides. BMC Immunol 10:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenman C, Stephens P, Smith R, Dalgliesh GL, Hunter C, Bignell G, Davies H, Teague J, Butler A, Stevens C et al. (2007) Patterns of somatic mutation in human cancer genomes. Nature 446:153–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groenendyk J, Peng Z, Dudek E, Fan X, Mizianty MJ, Dufey E, Urra H, Sepulveda D, Rojas-Rivera D, Lim Y et al. (2014) Interplay between the oxidoreductase PDIA6 and microRNA-322 controls the response to disrupted endoplasmic reticulum calcium homeostasis. Sci Signal 7:ra54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A, Hossain MM, Miller N, Kerin M, Callagy G, Gupta S (2016) NCOA3 coactivator is a transcriptional target of XBP1 and regulates PERK–eIF2α– ATF4 signalling in breast cancer. Oncogene 35:5860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han D, Lerner AG, Vande Walle L, Upton JP, Xu W, Hagen A, Backes BJ, Oakes SA, Papa FR (2009) IRE1alpha kinase activation modes control alternate endoribonuclease outputs to determine divergent cell fates. Cell 138:562–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J, Back SH, Hur J, Lin YH, Gildersleeve R, Shan J, Yuan CL, Krokowski D, Wang S, Hatzoglou M et al. (2013) ER-stress-induced transcriptional regulation increases protein synthesis leading to cell death. Nat Cell Biol 15:481–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J, Song B, Kim J, Kodali VK, Pottekat A, Wang M, Hassler J, Wang S, Pennathur S, Back SH et al. (2015) Antioxidants complement the requirement for protein chaperone function to maintain β-cell function and glucose homeostasis. Diabetes 64:2892–2904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding HP, Zhang Y, Ron D (1999) Protein translation and folding are coupled by an endoplasmic-reticulum- resident kinase. Nature 397:271–274 [DOI] [PubMed] [Google Scholar]
- Harding HP, Zhang Y, Zeng H, Novoa I, Lu PD, Calfon M, Sadri N, Yun C, Popko B, Paules R et al. (2003) An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol Cell 11:619–633 [DOI] [PubMed] [Google Scholar]
- Hart LS, Cunningham JT, Datta T, Dey S, Tameire F, Lehman SL, Qiu B, Zhang H, Cerniglia G, Bi M et al. (2012) ER stress-mediated autophagy promotes Mycdependent transformation and tumor growth. J Clin Invest 122:4621–4634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassler JR, Scheuner DL, Wang S, Han J, Kodali VK, Li P, Nguyen J, George JS, Davis C, Wu SP et al. (2015) The IRE1alpha/XBP1s pathway is essential for the glucose response and protection of beta cells. PLoS Biol 13:e1002277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haze K, Yoshida H, Yanagi H, Yura T, Mori K (1999) Mammalian transcription factor ATF6 is synthesized as a transmembrane protein and activated by proteolysis in response to endoplasmic reticulum stress. Mol Biol Cell 10:3787–3799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hetz C, Glimcher LH (2009) Fine-tuning of the unfolded protein response: assembling the IRE1α interactome. Mol Cell 35:551–561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hetz C, Bernasconi P, Fisher J, Lee AH, Bassik MC, Antonsson B, Brandt GS, Iwakoshi NN, Schinzel A, Glimcher LH et al. (2006) Proapoptotic BAX and BAK modulate the unfolded protein response by a direct interaction with IRE1alpha. Science 312:572–576 [DOI] [PubMed] [Google Scholar]
- Hetz C, Martinon F, Rodriguez D, Glimcher LH (2011) The unfolded protein response: integrating stress signals through the stress sensor IRE1alpha. Physiol Rev 91:1219–1243 [DOI] [PubMed] [Google Scholar]
- Hetz C, Chevet E, Harding HP (2013) Targeting the unfolded protein response in disease. Nat Rev Drug Discov 12:703–719 [DOI] [PubMed] [Google Scholar]
- Hetz C, Chevet E, Oakes SA (2015) Proteostasis control by the unfolded protein response. Nat Cell Biol 17:829–838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higa A, Taouji S, Lhomond S, Jensen D, Fernandez-Zapico ME, Simpson JC, Pasquet JM, Schekman R, Chevet E (2014) Endoplasmic reticulum stress- activated transcription factor ATF6alpha requires the disulfide isomerase PDIA5 to modulate chemoresistance. Mol Cell Biol 34:1839–1849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollien J, Weissman JS (2006) Decay of endoplasmic reticulum-localized mRNAs during the unfolded protein response. Science 313:104. [DOI] [PubMed] [Google Scholar]
- Hollien J, Lin JH, Li H, Stevens N, Walter P, Weissman JS (2009) Regulated Ire1-dependent decay of messenger RNAs in mammalian cells. J Cell Biol 186:323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong SY, Hagen T (2013) Multiple myeloma Leu167Ile (c.499C>A) mutation prevents XBP1 mRNA splicing. Br J Haematol 161:898–901 [DOI] [PubMed] [Google Scholar]
- Huber AL, Lebeau J, Guillaumot P, Petrilli V, Malek M, Chilloux J, Fauvet F, Payen L, Kfoury A, Renno T et al. (2013) p58(IPK)-mediated attenuation of the proapoptotic PERK-CHOP pathway allows malignant pro- gression upon low glucose. Mol Cell 49:1049–1059 [DOI] [PubMed] [Google Scholar]
- Jain A, Lamark T, Sjottem E, Larsen KB, Awuh JA, Overvatn A, McMahon M, Hayes JD, Johansen T (2010) p62/SQSTM1 is a target gene for transcription factor NRF2 and creates a positive feedback loop by inducing antioxidant response element-driven gene transcription. J Biol Chem 285:22576–22591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji N, Yang Y, Cai CY, Lei ZN, Wang JQ, Gupta P, Shukla S, Ambudkar SV, Kong D, Chen ZS (2019) Selonsertib (GS-4997), an ASK1 inhibitor, antagonizes multidrug resistance in ABCB1- and ABCG2-overexpressing cancer cells. Cancer Lett 440–441: 82–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kan Z, Jaiswal BS, Stinson J, Janakiraman V, Bhatt D, Stern HM, Yue P, Haverty PM, Bourgon R, Zheng J et al. (2010) Diverse somatic mutation patterns and pathway alterations in human cancers. Nature 466:869–873 [DOI] [PubMed] [Google Scholar]
- Karagoz GE, Acosta-Alvear D, Nguyen HT, Lee CP, Chu F, Walter P (2017) An unfolded protein-induced conformational switch activates mammalian IRE1. Elife 6:pii: e30700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman RJ (2004) Regulation of mRNA translation by protein folding in the endoplasmic reticulum. Trends Biochem Sci 29:152–158 [DOI] [PubMed] [Google Scholar]
- Kaufman RJ, Malhotra JD (2014) Calcium trafficking integrates endoplasmic reticulum function with mitochondrial bioenergetics. Biochim Biophys Acta 1843:2233–2239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Joe Y, Kim HJ, Kim YS, Jeong SO, Pae HO, Ryter SW, Surh YJ, Chung HT (2015) Endoplasmic reticulum stress-induced IRE1alpha activation mediates cross-talk of GSK-3beta and XBP-1 to regulate inflammatory cytokine production. J Immunol 194:4498–4506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korennykh AV, Egea PF, Korostelev AA, Finer-Moore J, Zhang C, Shokat KM, Stroud RM, Walter P (2009) The unfolded protein response signals through high- order assembly of Ire1. Nature 457:687–693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krzyzosiak A, Sigurdardottir A, Luh L, Carrara M, Das I, Schneider K, Bertolotti A (2018) Target-based discovery of an inhibitor of the regulatory phosphatase PPP1R15B. Cell 174:1216–1228.e19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo CY, Lin CH, Hsu T (2017) VHL inactivation in precancerous kidney cells induces an inflammatory response via ER stress-activated IRE1alpha signaling. Cancer Res 77:3406–3416 [DOI] [PubMed] [Google Scholar]
- Kwong JQ (2017) The mitochondrial calcium uniporter in the heart: energetics and beyond. J Physiol 595:3743–3751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwong JQ, Lu X, Correll RN, Schwanekamp JA, Vagnozzi RJ, Sargent MA, York AJ, Zhang J, Bers DM, Molkentin JD (2015) The mitochondrial calcium uniporter selectively matches metabolic output to acute contractile stress in the heart. Cell Rep 12:15–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Rovere RM, Roest G, Bultynck G, Parys JB (2016) Intracellular Ca(2+) signaling and Ca(2+) microdomains in the control of cell survival, apoptosis and autophagy. Cell Calcium 60:74–87 [DOI] [PubMed] [Google Scholar]
- Lebeaupin C, Proics E, de Bieville CH, Rousseau D, Bonnafous S, Patouraux S, Adam G, Lavallard VJ, Rovere C, Le Thuc O et al. (2015) ER stress induces NLRP3 inflammasome activation and hepatocyte death. Cell Death Dis 6:e1879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebeaupin C, Vallee D, Hazari Y, Hetz C, Chevet E, Bailly-Maitre B (2018a) Endoplasmic reticulum stress signalling and the pathogenesis of non-alcoholic fatty liver disease. J Hepatol 69:927–947 [DOI] [PubMed] [Google Scholar]
- Lebeaupin C, Vallee D, Rousseau D, Patouraux S, Bonnafous S, Adam G, Luciano F, Luci C, Anty R, Iannelli A et al. (2018b) Bax inhibitor-1 protects from nonalcoholic steatohepatitis by limiting inositol-requiring enzyme 1 alpha signaling in mice. Hepatology 68:515–532 [DOI] [PubMed] [Google Scholar]
- Lee KP, Dey M, Neculai D, Cao C, Dever TE, Sicheri F (2008) Structure of the dual enzyme Ire1 reveals the basis for catalysis and regulation in nonconventional RNA splicing. Cell 132:89–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner AG, Upton JP, Praveen PV, Ghosh R, Nakagawa Y, Igbaria A, Shen S, Nguyen V, Backes BJ, Heiman M et al. (2012) IRE1alpha induces thioredoxin-interacting protein to activate the NLRP3 inflammasome and promote programmed cell death under irremediable ER stress. Cell Metab 16:250–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung-Hagesteijn C, Erdmann N, Cheung G, Keats JJ, Stewart AK, Reece DE, Chung KC, Tiedemann RE (2013) Xbp1s-negative tumor B cells and pre-plasmablasts mediate therapeutic proteasome inhibitor resistance in multiple myeloma. Cancer Cell 24:289–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lhomond S, Avril T, Dejeans N, Voutetakis K, Doultsinos D, McMahon M, Pineau R, Obacz J, Papadodima O, Jouan F et al. (2018) Dual IRE1 RNase functions dictate glioblastoma development. EMBO Mol Med 10(3):pii: e7929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Mongillo M, Chin KT, Harding H, Ron D, Marks AR, Tabas I (2009) Role of ERO1-alpha-mediated stimulation of inositol 1,4,5-triphosphate receptor activity in endoplasmic reticulum stress-induced apoptosis. J Cell Biol 186:783–792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin YH, Friederichs J, Black MA, Mages J, Rosenberg R, Guilford PJ, Phillips V, Thompson-Fawcett M, Kasabov N, Toro T et al. (2007) Multiple gene expression classifiers from different array platforms predict poor prognosis of colorectal cancer. Clin Cancer Res 13:498–507 [DOI] [PubMed] [Google Scholar]
- Lisbona F, Rojas-Rivera D, Thielen P, Zamorano S, Todd D, Martinon F, Glavic A, Kress C, Lin JH, Walter P et al. (2009) BAX inhibitor-1 is a negative regulator of the ER stress sensor IRE1alpha. Mol Cell 33:679–691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JC, Liu J, Holmstrom KM, Menazza S, Parks RJ, Fergusson MM, Yu ZX, Springer DA, Halsey C, Liu C et al. (2016) MICU1 serves as a molecular gatekeeper to prevent in vivo mitochondrial calcium overload. Cell Rep 16:1561–1573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loomba R, Lawitz E, Mantry PS, Jayakumar S, Caldwell SH, Arnold H, Diehl AM, Djedjos CS, Han L, Myers RP et al. (2017) The ASK1 inhibitor selonsertib in patients with nonalcoholic steatohepatitis: a randomized, phase 2 trial. Hepatology 67(2):549–559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luciani DS, Gwiazda KS, Yang TLB, Kalynyak TB, Bychkivska Y, Frey MHZ, Jeffrey KD, Sampaio AV, Underhill TM, Johnson JD (2008) Roles of IP3R and RyR Ca2+ channels in endoplasmic reticulum stress and beta-cell death. Diabetes 58:422–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Hendershot LM (2003) Delineation of a negative feedback regulatory loop that controls protein translation during endoplasmic reticulum stress. J Biol Chem 278:34864–34873 [DOI] [PubMed] [Google Scholar]
- Ma X-H, Piao S- F, Dey S, McAfee Q, Karakousis G, Villanueva J, Hart LS, Levi S, Hu J, Zhang G et al. (2014) Targeting ER stress–induced autophagy over- comes BRAF inhibitor resistance in melanoma. J Clin Invest 124:1406–1417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra JD, Kaufman RJ (2007) Endoplasmic reticulum stress and oxidative stress: a vicious cycle or a double-edged sword? Antioxid Redox Signal 9:2277–2293 [DOI] [PubMed] [Google Scholar]
- Malhotra JD, Miao H, Zhang K, Wolfson A, Pennathur S, Pipe SW, Kaufman RJ (2008) Antioxidants reduce endoplasmic reticulum stress and improve protein secretion. Proc Natl Acad Sci U S A 105:18525–18530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallilankaraman K, Cardenas C, Doonan PJ, Chandramoorthy HC, Irrinki KM, Golenar T, Csordas G, Madireddi P, Yang J, Muller M et al. (2012) MCUR1 is an essential component of mitochondrial Ca2+ uptake that regulates cellular metabolism. Nat Cell Biol 14:1336–1343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marciniak SJ, Ron D (2006) Endoplasmic reticulum stress signaling in disease. Physiol Rev 86:1133–1149 [DOI] [PubMed] [Google Scholar]
- Marciniak SJ, Yun CY, Oyadomari S, Novoa I, Zhang Y, Jungreis R, Nagata K, Harding HP, Ron D (2004) CHOP induces death by promoting protein synthesis and oxidation in the stressed endoplasmic reticulum. Genes Dev 18:3066–3077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurel M, Chevet E, Tavernier J, Gerlo S (2014) Getting RIDD of RNA: IRE1 in cell fate regulation. Trends Biochem Sci 39:245–254 [DOI] [PubMed] [Google Scholar]
- Mercado G, Castillo V, Soto P, Lopez N, Axten JM, Sardi SP, Hoozemans JJM, Hetz C (2018) Targeting PERK signaling with the small molecule GSK2606414 prevents neurodegeneration in a model of Parkinson’s disease. Neurobiol Dis 112:136–148 [DOI] [PubMed] [Google Scholar]
- Miao HZ, Sirachainan N, Palmer L, Kucab P, Cunningham MA, Kaufman RJ, Pipe SW (2004) Bioengineering of coagulation factor VIII for improved secretion. Blood 103:3412–3419 [DOI] [PubMed] [Google Scholar]
- Milani M, Rzymski T, Mellor HR, Pike L, Bottini A, Generali D, Harris AL (2009) The role of ATF4 stabilization and autophagy in resistance of breast cancer cells treated with Bortezomib. Cancer Res 69:4415–4423 [DOI] [PubMed] [Google Scholar]
- Mimura N, Fulciniti M, Gorgun G, Tai YT, Cirstea D, Santo L, Hu Y, Fabre C, Minami J, Ohguchi H et al. (2012) Blockade of XBP1 splicing by inhibition of IRE1alpha is a promising therapeutic option in multiple myeloma. Blood 119:5772–5781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimura J, Inose-Maruyama A, Taniuchi S, Kosaka K, Yoshida H, Yamazaki H, Kasai S, Harada N, Kaufman RJ, Oyadomari S et al. (2019) Concomitant Nrf2- and ATF4-activation by carnosic acid cooperatively induces expression of cytoprotective genes. Int J Mol Sci 20:pii: E1706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita S, Villalta SA, Feldman HC, Register AC, Rosenthal W, Hoffmann-Petersen IT, Mehdizadeh M, Ghosh R, Wang L, Colon-Negron K et al. (2017) Targeting ABL-IRE1alpha signaling spares ER-stressed pancreatic beta cells to reverse autoimmune diabetes. Cell Metab 25:883–897.e8 [DOI] [PubMed] [Google Scholar]
- Mujcic H, Nagelkerke A, Rouschop KM, Chung S, Chaudary N, Span PN, Clarke B, Milosevic M, Sykes J, Hill RP et al. (2013) Hypoxic activation of the PERK/eIF2alpha arm of the unfolded protein response promotes metastasis through induction of LAMP3. Clin Cancer Res 19:6126–6137 [DOI] [PubMed] [Google Scholar]
- Nadanaka S, Yoshida H, Kano F, Murata M, Mori K (2004) Activation of mammalian unfolded protein response is compatible with the quality control system operating in the endoplasmic reticulum. Mol Biol Cell 15:2537–2548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa H, Umemura A, Taniguchi K, Font-Burgada J, Dhar D, Ogata H, Zhong Z, Valasek MA, Seki E, Hidalgo J et al. (2014) ER stress cooperates with hypernutrition to trigger TNF-dependent spontaneous HCC development. Cancer Cell 26:331–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niederreiter L, Fritz TM, Adolph TE, Krismer AM, Offner FA, Tschurtschenthaler M, Flak MB, Hosomi S, Tomczak MF, Kaneider NC et al. (2013) ER stress transcription factor Xbp1 suppresses intestinal tumorigenesis and directs intestinal stem cells. J Exp Med 210:2041–2056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novoa I, Zeng H, Harding HP, Ron D (2001) Feedback inhibition of the unfolded protein response by GADD34-mediated dephosphorylation of eIF2α. J Cell Biol 153:1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogata M, Hino S, Saito A, Morikawa K, Kondo S, Kanemoto S, Murakami T, Taniguchi M, Tanii I, Yoshinaga K et al. (2006) Autophagy is activated for cell survival after endoplasmic reticulum stress. Mol Cell Biol 26:9220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada T, Haze K, Nadanaka S, Yoshida H, Seidah NG, Hirano Y, Sato R, Negishi M, Mori K (2003) A serine protease inhibitor prevents endoplasmic reticulum stress-induced cleavage but not transport of the membrane-bound transcription factor ATF6. J Biol Chem 278:31024–31032 [DOI] [PubMed] [Google Scholar]
- Okada K, Warabi E, Sugimoto H, Horie M, Tokushige K, Ueda T, Harada N, Taguchi K, Hashimoto E, Itoh K et al. (2012) Nrf2 inhibits hepatic iron accumulation and counteracts oxidative stress-induced liver injury in nutritional steatohepatitis. J Gastroenterol 47:924–935 [DOI] [PubMed] [Google Scholar]
- Oyadomari S (2002) Targeted disruption of the Chop gene delays endoplasmic reticulum stress-mediated diabetes. J Clin Investig 109:525–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Özcan U, Yilmaz E, Özcan L, Furuhashi M, Vaillancourt E, Smith RO, Görgün CZ, Hotamisligil GS (2006) Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science 313:1137–1140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paez D, Labonte MJ, Bohanes P, Zhang W, Benhanim L, Ning Y, Wakatsuki T, Loupakis F, Lenz HJ (2012) Cancer dormancy: a model of early dissemination and late cancer recurrence. Clin Cancer Res 18:645–653 [DOI] [PubMed] [Google Scholar]
- Pan X, Liu J, Nguyen T, Liu C, Sun J, Teng Y, Fergusson MM, Rovira II, Allen M, Springer DA et al. (2013) The physiological role of mitochondrial calcium revealed by mice lacking the mitochondrial calcium uniporter. Nat Cell Biol 15:1464–1472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxman R, Plate L, Blackwood EA, Glembotski C, Powers ET, Wiseman RL, Kelly JW (2018) Pharmacologic ATF6 activating compounds are metabolically activated to selectively modify endoplasmic reticulum proteins. Elife 7:pii: e37168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pipe SW, Saenko EL, Eickhorst AN, Kemball-Cook G, Kaufman RJ (2001) Hemophilia A mutations associated with 1-stage/2-stage activity discrepancy disrupt protein-protein interactions within the triplicated A domains of thrombin-activated factor VIIIa. Blood 97:685. [DOI] [PubMed] [Google Scholar]
- Plate L, Cooley CB, Chen JJ, Paxman RJ, Gallagher CM, Madoux F, Genereux JC, Dobbs W, Garza D, Spicer TP et al. (2016) Small molecule proteostasis regulators that reprogram the ER to reduce extracellular protein aggregation. Elife 5:pii: e15550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluquet O, Dejeans N, Bouchecareilh M, Lhomond S, Pineau R, Higa A, Delugin M, Combe C, Loriot S, Cubel G et al. (2013) Posttranscriptional regulation of PER1 underlies the oncogenic function of IREα. Cancer Res 73:4732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyrko P, Schonthal AH, Hofman FM, Chen TC, Lee AS (2007) The unfolded protein response regula- tor GRP78/BiP as a novel target for increasing chemosensitivity in malignant gliomas. Cancer Res 67:9809–9816 [DOI] [PubMed] [Google Scholar]
- Ramaswamy S, Tamayo P, Rifkin R, Mukherjee S, Yeang CH, Angelo M, Ladd C, Reich M, Latulippe E, Mesirov JP et al. (2001) Multiclass cancer diagnosis using tumor gene expression signatures. Proc Natl Acad Sci U S A 98:15149–15154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy RK, Mao C, Baumeister P, Austin RC, Kaufman RJ, Lee AS (2003) Endoplasmic reticulum chaperone protein GRP78 protects cells from apoptosis induced by topoisomerase inhibitors: role of ATP binding site in suppression of caspase-7 activation. J Biol Chem 278:20915–20924 [DOI] [PubMed] [Google Scholar]
- Rojas-Rivera D, Delvaeye T, Roelandt R, Nerinckx W, Augustyns K, Vandenabeele P, Bertrand MJM (2017) When PERK inhibitors turn out to be new potent RIPK1 inhibitors: critical issues on the specificity and use of GSK2606414 and GSK2656157. Cell Death Differ 24:1100–1110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouschop KM, Dubois LJ, Keulers TG, van den Beucken T, Lambin P, Bussink J, van der Kogel AJ, Koritzinsky M, Wouters BG (2013) PERK/eIF2alpha signaling protects therapy resistant hypoxic cells through induction of glutathione synthesis and protection against ROS. Proc Natl Acad Sci U S A 110:4622–4627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rzymski T, Milani M, Singleton DC, Harris AL (2009) Role of ATF4 in regulation of autophagy and resistance to drugs and hypoxia. Cell Cycle 8:3838–3847 [DOI] [PubMed] [Google Scholar]
- Sancak Y, Markhard AL, Kitami T, Kovacs-Bogdan E, Kamer KJ, Udeshi ND, Carr SA, Chaudhuri D, Clapham DE, Li AA et al. (2013) EMRE is an essential component of the mitochondrial calcium uniporter complex. Science 342:1379–1382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarvani C, Sireesh D, Ramkumar KM (2017) Unraveling the role of ER stress inhibitors in the context of metabolic diseases. Pharmacol Res 119:412–421 [DOI] [PubMed] [Google Scholar]
- Scheuner D, Song B, McEwen E, Liu C, Laybutt R, Gillespie P, Saunders T, Bonner-Weir S, Kaufman RJ (2001) Translational control is required for the unfolded protein response and in vivo glucose homeostasis. Mol Cell 7:1165–1176 [DOI] [PubMed] [Google Scholar]
- Scheuner D, Vander Mierde D, Song B, Flamez D, Creemers JW, Tsukamoto K, Ribick M, Schuit FC, Kaufman RJ (2005) Control of mRNA translation preserves endoplasmic reticulum function in beta cells and maintains glucose homeostasis. Nat Med 11:757–764 [DOI] [PubMed] [Google Scholar]
- Schewe DM, Aguirre-Ghiso JA (2008) ATF6alpha- Rheb-mTOR signaling promotes survival of dor- mant tumor cells in vivo. Proc Natl Acad Sci U S A 105:10519–10524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schild H, Rammensee HG (2000) gp96 – the immune system’s Swiss army knife. Nat Immunol 1:100–101 [DOI] [PubMed] [Google Scholar]
- Sepulveda D, Rojas-Rivera D, Rodriguez DA, Groenendyk J, Kohler A, Lebeaupin C, Ito S, Urra H, Carreras-Sureda A, Hazari Y et al. (2018) Interactome screening identifies the ER luminal chaperone Hsp47 as a regulator of the unfolded protein response transducer IRE1alpha. Mol Cell 69:238–252.e7 [DOI] [PubMed] [Google Scholar]
- Shalapour S, Lin XJ, Bastian IN, Brain J, Burt AD, Aksenov AA, Vrbanac AF, Li W, Perkins A, Matsutani T et al. (2017) Inflammation-induced IgA+ cells dismantle anti-liver cancer immunity. Nature 551:340–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen J, Chen X, Hendershot L, Prywes R (2002) ER Stress Regulation of ATF6 Localization by Dissociation of BiP/GRP78 Binding and Unmasking of Golgi Localization Signals. Dev Cell 3:99–111 [DOI] [PubMed] [Google Scholar]
- Shimozono R, Asaoka Y, Yoshizawa Y, Aoki T, Noda H, Yamada M, Kaino M, Mochizuki H (2013) Nrf2 activators attenuate the progression of nonalcoholic steatohepatitis-related fibrosis in a dietary rat model. Mol Pharmacol 84:62–70 [DOI] [PubMed] [Google Scholar]
- Sidrauski C, McGeachy AM, Ingolia NT, Walter P (2015) The small molecule ISRIB reverses the effects of eIF2alpha phosphorylation on translation and stress granule assembly. Elife 4:e05033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song B, Scheuner D, Ron D, Pennathur S, Kaufman RJ (2008) Chop deletion reduces oxidative stress, improves β cell function, and promotes cell survival in multiple mouse models of diabetes. J Clin Invest 118:3378–3389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southwood CM, Fykkolodziej B, Maheras KJ, Garshott DM, Estill M, Fribley AM, Gow A (2016) Overexpression of CHOP in myelinating cells does not confer a significant phenotype under normal or metabolic stress conditions. J Neurosci 36:6803–6819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H, Lin DC, Guo X, Kharabi Masouleh B, Gery S, Cao Q, Alkan S, Ikezoe T, Akiba C, Paquette R et al. (2016) Inhibition of IRE1alpha-driven pro-survival pathways is a promising therapeutic application in acute myeloid leukemia. Oncotarget 7:18736–18749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Synofzik M, Haack TB, Kopajtich R, Gorza M, Rapaport D, Greiner M, Schonfeld C, Freiberg C, Schorr S, Holl RW et al. (2014) Absence of BiP co-chaperone DNAJC3 causes diabetes mellitus and multisystemic neurodegeneration. Am J Hum Genet 95:689–697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai JC, Miller-Vedam LE, Anand AA, Jaishankar P, Nguyen HC, Renslo AR, Frost A, Walter P (2018) Structure of the nucleotide exchange factor eIF2B reveals mechanism of memory-enhancing molecule. Science 359:pii: eaaq0939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsaytler P, Harding HP, Ron D, Bertolotti A (2011) Selective Inhibition of a Regulatory Subunit of Protein Phosphatase 1 Restores Proteostasis. Science 332:91. [DOI] [PubMed] [Google Scholar]
- Tu BP, Weissman JS (2004) Oxidative protein folding in eukaryotes: mechanisms and consequences. J Cell Biol 164:341–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tufanli O, Telkoparan Akillilar P, Acosta-Alvear D, Kocaturk B, Onat UI, Hamid SM, Cimen I, Walter P, Weber C, Erbay E (2017) Targeting IRE1 with small molecules counteracts progression of atherosclerosis. Proc Natl Acad Sci U S A 114:E1395–E1404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umemura A, He F, Taniguchi K, Nakagawa H, Yamachika S, Font-Burgada J, Zhong Z, Subramaniam S, Raghunandan S, Duran A et al. (2016) p62, upregulated during preneoplasia, induces hepatocellular carcinogenesis by maintaining survival of stressed HCC-initiating cells. Cancer Cell 29:935–948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urano F, Wang X, Bertolotti A, Zhang Y, Chung P, Harding HP, Ron D (2000) Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science 287:664. [DOI] [PubMed] [Google Scholar]
- Vandewynckel Y-P, Laukens D, Bogaerts E, Paridaens A, Van den Bussche A, Verhelst X, Van Steenkiste C, Descamps B, Vanhove C, Libbrecht L et al. (2015) Modulation of the unfolded protein response impedes tumor cell adaptation to proteotoxic stress: a PERK for hepatocellular carcinoma therapy. Hepatol Int 9:93–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldeck-Weiermair M, Malli R, Parichatikanond W, Gottschalk B, Madreiter-Sokolowski CT, Klec C, Rost R, Graier WF (2015) Rearrangement of MICU1 multimers for activation of MCU is solely controlled by cytosolic Ca(2.). Sci Rep 5:15602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Kaufman RJ (2014) The impact of the endoplasmic reticulum protein-folding environment on cancer development. Nat Rev Cancer 14:581–597 [DOI] [PubMed] [Google Scholar]
- Wang M, Kaufman RJ (2016) Protein misfolding in the endoplasmic reticulum as a conduit to human disease. Nature 529:326–335 [DOI] [PubMed] [Google Scholar]
- Wang XZ, Kuroda M, Sok J, Batchvarova N, Kimmel R, Chung P, Zinszner H, Ron D (1998) Identification of novel stress-induced genes downstream of chop. EMBO J 17:3619–3630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Perera BG, Hari SB, Bhhatarai B, Backes BJ, Seeliger MA, Schurer SC, Oakes SA, Papa FR, Maly DJ (2012) Divergent allosteric control of the IRE1alpha endoribonuclease using kinase inhibitors. Nat Chem Biol 8:982–989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong YL, LeBon L, Basso AM, Kohlhaas KL, Nikkel AL, Robb HM, Donnelly-Roberts DL, Prakash J, Swensen AM, Rubinstein ND et al. (2019) eIF2B activator prevents neurological defects caused by a chronic integrated stress response. Elife 8:pii: e42940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Rutkowski DT, Dubois M, Swathirajan J, Saunders T, Wang J, Song B, Yau GD, Kaufman RJ (2007) ATF6alpha optimizes long-term endoplasmic reticulum function to protect cells from chronic stress. Dev Cell 13:351–364 [DOI] [PubMed] [Google Scholar]
- Yong J, Itkin-Ansari P, Kaufman RJ (2016) When less is better: ER stress and beta cell proliferation. Dev Cell 36:4–6 [DOI] [PubMed] [Google Scholar]
- Yong J, Bischof H, Siirin M, Murphy A, Malli R, Kaufman RJ (2019) Mitochondria supply ATP to the ER through a mechanism antagonized by cytosolic Ca2+. Elife 8:pii: e49682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zyryanova AF, Weis F, Faille A, Alard AA, Crespillo-Casado A, Sekine Y, Harding HP, Allen F, Parts L, Fromont C et al. (2018) Binding of ISRIB reveals a regulatory site in the nucleotide exchange factor eIF2B. Science 359:1533. [DOI] [PMC free article] [PubMed] [Google Scholar]