Abstract
Hospitals are complex environments that rely on clinicians working together to provide appropriate care to patients. These clinical teams adapt their interactions to meet changing situational needs. Venous thromboembolism (VTE) prophylaxis is a complex process that occurs throughout a patient’s hospitalisation, presenting five stages with different levels of complexity: admission, interruption, re-initiation, initiation, and transfer. The objective of our study is to understand how the VTE prophylaxis team adapts as the complexity in the process changes; we do this by using social network analysis (SNA) measures. We interviewed forty-five clinicians representing 9 different cases, creating 43 role networks. The role networks were analysed using SNA measures to understand team changes between low and high complexity stages. When comparing low and high complexity stages, we found two team adaptation mechanisms: (1) relative increase in the number of people, team activities, and interactions within the team, or (2) relative increase in discussion among the team, reflected by an increase in reciprocity.
Keywords: team adaptation, social network analysis, complexity, patient safety
Practitioner Summary
The reason for this study was to quantify team adaptation to complexity in a process using social network analysis (SNA). The VTE prophylaxis team adapted to complexity by two different mechanisms, by increasing the roles, activities, and interactions among the team or by increasing the two-way communication and discussion throughout the team. We demonstrated the ability for SNA to identify adaptation within a team.
1. Introduction
Hospitals are complex sociotechnical systems that rely on multidisciplinary teams to provide appropriate, timely care to patients (Carayon et al. 2006; Mitchell and Golden 2012). In complex environments, such as hospitals, situational requirements and demands are often changing (Flach 2012). Teams adapt to complex environments (Hollnagel 2012) with changing situational requirements by adjusting their interactions and structure to coordinate their activities and effectively meet the demands of the situation (Baard et al. 2014; Burke et al. 2006; Salas et al. 2007). In this study, we examine healthcare team adaptation to complex situations related to venous thromboembolism (VTE) prophylaxis (i.e., prevention of VTE). The objective of the study is to quantitatively assess team adaptation in response to different levels of complexity in the VTE prophylaxis process throughout a patient’s hospital stay; we do this using social network analysis (SNA) measures.
1.1. Venous thromboembolism
VTE chemical prophylaxis (i.e., administering medication to prevent VTE) is a complex process that occurs throughout a patient’s hospital stay. VTE is made up of deep vein thrombosis (DVT) and pulmonary embolism (PE), which together account for 10% of in-hospital mortality each year in the US (Heit et al. 2005). Approximately 900,000 VTE cases occur each year, with two-thirds of cases being hospital-acquired (Heit et al. 2005). VTE is a complex clinical condition due to the multiple factors that impact a patient’s risk of developing VTE, including age, medical condition, surgery type, duration of immobilization, and underlying hypercoaguable state (e.g., cancer) (Shojania et al. 2001). In an effort to reduce mortality and complications due to VTE, the American College of Physicians recommends all patients be prescribed chemical prophylaxis with blood thinning medications during hospitalisation unless contraindicated (Qaseem et al. 2011). Chemical prophylaxis is contraindicated when the risk of significant bleeding with administration of prophylaxis is assessed to outweigh the risk of developing VTE. These situations may be present on admission (i.e., a patient admitted for gastrointestinal bleeding) or may occur after admission (i.e., a patient needs to stop prophylaxis due to anticipated bleeding risk during a surgical procedure) (Qaseem et al. 2011). While VTE prophylaxis guidelines exist, the process of implementing the guidelines is complex and continues to be a concern for patient safety (Fiumara et al. 2010; Galanter et al. 2010; Mahan and Spyropoulos 2010; Piazza et al. 2009; Shojania et al. 2001). Significant effort has focused on the admission process where the VTE risk of a patient is evaluated and appropriate chemical prophylaxis is ordered, often through an electronic order set in the electronic health record (Haut et al. 2012; Maynard and Stein 2008; Maynard et al. 2010; Streiff et al. 2012). These efforts have produced benefit in increasing the use of appropriate chemical prophylaxis and reducing VTE. However, less attention has been on the rest of the patient’s hospitalisation and how to ensure appropriate management of VTE prophylaxis during the entire hospital stay. Missed doses of VTE prophylaxis during hospitalisation occur for various types of patients (Popoola et al. 2018), which increases the risk of developing VTE (Louis et al. 2014; Ramanathan et al. 2015). Through a better understanding of the work system associated with the VTE prophylaxis process, in particular the roles, activities and interactions involved in VTE prophylaxis during the entire hospitalisation, solutions can be developed to improve VTE prophylaxis management for hospitalised patients (Hundt et al. 2017).
1.2. The VTE prophylaxis process
Management of VTE prophylaxis is a dynamic, collaborative process involving multiple team members and activities throughout a patient’s hospitalisation. The VTE prophylaxis team is comprised of multiple individuals (e.g., physician, pharmacist, nurse) who interact interdependently, dynamically, and adaptively to make decisions about VTE prophylaxis and implement and monitor these decisions (Salas et al. 1992). The roles involved in the VTE prophylaxis team may change depending on the context (e.g., academic medical center with residents) and patient clinical situation (e.g., preparation for surgical procedure). The team members must coordinate their activities in the VTE prophylaxis process by communicating with one another in order to provide appropriate and timely VTE prophylaxis care to hospitalised patients (Salas et al. 2008).
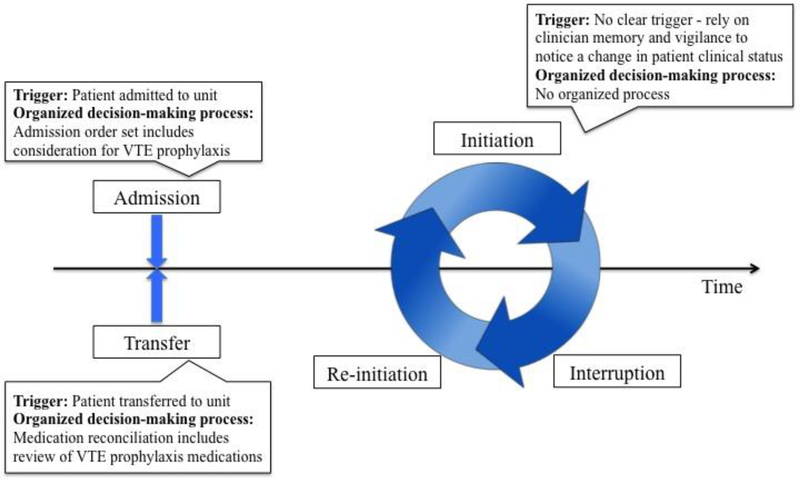
The VTE prophylaxis process exhibits dynamic complexity (Patterson et al. 2010; Woods 1988) and occurs over five stages of a patient’s hospitalisation (see Figure 1): admission, interruption of VTE prophylaxis, re-initiation of VTE prophylaxis, initiation of VTE prophylaxis (if not started at admission), and re-evaluation at the time of transfer into a unit (Hundt et al. 2017). Complexity in a process such as VTE prophylaxis can be characterized by many factors, e.g. uncertainty, large problem spaces and disturbances (Carayon 2006; Vicente 1999). In the context of VTE prophylaxis, a major source of process complexity is related to uncertainty of action and decision-making. First, there has to be a ‘trigger’, i.e. an event or thing that causes the team to consider whether to put the patient on VTE prophylaxis or discontinue the treatment (Dix et al. 2004). Second, there needs to be a way for the team to use the new information from the trigger and make decisions about VTE prophylaxis. The complexity of the VTE prophylaxis stages is, therefore, based on two factors: (1) whether there is a clear trigger for action in evaluating VTE prophylaxis, and (2) if there is an organised decision-making process for the VTE prophylaxis stage. For example, on admission to the hospital, the patient workup process provides a clear trigger for considering VTE prophylaxis; clinicians know that they need to consider VTE prophylaxis as they complete the admission order set in the electronic health record (EHR). This organised process includes completing an admission order set and placing a medication order for VTE prophylaxis if it is clinically indicated. Similarly, when a patient is transferred into a unit, the physical movement of the patient serves as a trigger to consider VTE prophylaxis. The transfer process requires medication reconciliation, which includes reviewing VTE prophylaxis orders in the EHR. Unlike admission and transfer, the stages of interruption, re-initiation and initiation have limited physical triggers and no clear temporal triggers for action as they occur continuously throughout the patient’s stay (rather than at a discrete point in time such as admission or transfer). Therefore, these stages rely on the memory and vigilance of clinicians to recognise the need to interrupt, re-initiate or initiate VTE prophylaxis based on the patient’s clinical status and treatment plan. In addition, if a team member recognises the need for a change in a patient’s VTE prophylaxis management plan, there is no clearly organised process to follow.
Figure 1.
Stages of the VTE prophylaxis process.
The two factors, trigger for action and organised decision-making process, characterise the complexity of the 5 stages of VTE prophylaxis. Based on these two factors and our above description of the 5 stages on these two factors, we grouped the 5 stages into (1) low-uncertainty, less complex stages: admission and transfer, and (2) high-uncertainty, complex stages: interruption, re-initiation and initiation. Understanding the team’s response and ability to adapt to the complexity is clinically significant because dynamic complexity, such as in the VTE prophylaxis process, creates cognitive demands that increase clinicians’ workload and risks for error (Patterson et al. 2010; Salas et al. 2007).
1.3. Team adaptation and social network analysis
When faced with uncertain situations that have varying levels of complexity, teams, such as the VTE prophylaxis clinical team, adapt their communication and structure in order to meet changing situational needs (Hollnagel 2012; Salas et al. 2007). These team changes represent team adaptation, or “the innovation of new or modification of existing structures, capacities, and/or behavioural or cognitive goal-directed actions” (page 1190) (Burke et al. 2006). In light of the Interactive Team Cognition approach (Cooke et al. 2013), team adaptation can be examined by evaluating interactions among team members. In our study, we look at interactions among roles that participate in the VTE prophylaxis team (not specific individuals in those roles). This interaction-focused perspective to team adaptation fits the “domain-specific approach” described in the performance adaptation taxonomy proposed by Baard et al. (2014). Team adaptation in the VTE prophylaxis process relies on interaction (and changes in interaction) in the team that responds to changing situational demands throughout a patient’s stay in the hospital. These domain-specific processes can be evaluated with quantitative measures such as SNA measures (Barth et al. 2015).
Team adaptation has been studied using multiple different methods including analysis of team performance in simulated environments (Burtscher et al. 2011), team cognitive work analysis (Ashoori et al. 2014; Pfautz and Pfautz 2009), and direct team observations utilizing questionnaires (Schraagen 2011). Social network analysis (SNA) is another method that can be used to measure team adaptation as the complexity of a situation changes (Baber et al. 2013; Barth et al. 2015; Houghton et al. 2006; Schraagen and Post 2014). SNA, using social network metrics based on various data (e.g., survey, observation, interview)(Valente 2010; Wasserman and Faust 1994), provides a way to quantitatively evaluate team adaptation (Houghton et al. 2006).
SNA has been used in various fields such as public health, computer science, psychology and marketing (Valente 2010). Within human factors and ergonomics, SNA has been used to understand organisational and team processes, such as information sharing and coordination. For example, SNA was used to study command and control operations within military and emergency services (Baber et al. 2013; Houghton et al. 2006; Houghton et al. 2015; Roberts et al. 2018); to evaluate and characterise naval team readiness (Schraagen and Post 2014); to explore changes in the transportation system from the introduction of Connected and Autonomous Vehicles (Banks et al. 2018); to measure knowledge across a team and its effect on cohesion and performance (Espinosa and Clark 2014); to measure interruptions (McCurdie et al. 2018) and influential team members (Fong et al. 2017) in intensive care units; and to demonstrate a positive relationship between the number of nurse-to-nurse advice-seeking interactions about safe patient handling and frequency of patient-handling equipment use (Hurtado et al. 2018). Euerby and Burns (2014) used SNA to measure information sharing in an online community comprised of university students, university faculty, community activists and members of local government, finding that human factors design changes to the website increased communication and connections between the website members. Various methods were used in these studies to collect SNA data including survey (Espinosa and Clark 2014; Fong et al. 2017; Hurtado et al. 2018), observations (Houghton et al. 2006; McCurdie et al. 2018; Roberts et al. 2018; Schraagen and Post 2014), cognitive work analysis (Houghton et al. 2015), review of documents (Baber et al. 2013), and website activity logs (Euerby and Burns 2014).
Barth et al. (2015) used SNA to examine team adaptation to varying levels of task complexity during paediatric cardiac surgeries. Forty surgical team observations produced data on real-time communication throughout various surgeries and their different phases. SNA methods helped to characterise the team members’ communication and adaptation to phases of surgery for complex and non-complex procedures. Complexity of procedures was determined using the Aristotle® risk assessment scoring system, which is based on the difficulty of the procedure and the potential morbidity and mortality for the patient. Researchers used the following SNA measures: density (measure of the number of connections and information sharing in a network), reciprocity (measure of two-way communications and discussions in a network), degree of centralisation (measure of the variation in centrality in a network), betweenness centralisation (measure of cohesion in a network), and closeness centralisation (measure of how compact a network is). Researchers found higher density and reciprocity during more complex procedures and during more complex phases of surgeries such as transitional phases (e.g., when a patient is being put on or off cardio-pulmonary bypass). This provided confirmation that more discussion, two-way communication and information sharing occurred when the team was involved in complex procedures. The surgical teams became less centralised during complex procedures, therefore, producing a flatter team structure. Overall, this study showed that team structure changes and adapts with changing complexity; this adaptation can be observed with the use of SNA measures.
The studies used several different SNA measures: centrality and centralisation (degree, betweenenss, and closeness), density, diameter, hierarchy, isolation, reciprocity, and sociometric status. Degree centrality and density were the most commonly used SNA measures (Baber et al. 2013; Banks et al. 2018; Barth et al. 2015; Espinosa and Clark 2014; Euerby and Burns 2014; Houghton et al. 2006; Houghton et al. 2015; Hurtado et al. 2018; Roberts et al. 2018; Schraagen and Post 2014; Stanton and Roberts 2018). Since degree centrality focuses on individuals within a team rather than the team as a whole, we decided not to include this metric. Instead, we included the measure of degree centralisation, which provides information at the team level about the distribution of centrality and interdependence between team members. We decided to use degree centralisation instead of betweenness or closeness centralisation because it takes into account how connected a role is regardless of the location or position of the role in the network; it is also the most commonly used centralisation metric (from now on referred to only as “centralisation”) (Valente 2010). In addition, we included the SNA measures of reciprocity and density, which provide insight into the level of discussion and interactions among the team, respectively, and have been shown to change as the complexity in a process increases (Barth et al. 2015). Finally, we included basic SNA measures on the number of roles (number of people actively involved in the VTE prophylaxis process), number of team activities (total number of activities performed jointly by two VTE prophylaxis team members), and number of interactions (total number of one-way and two-way interactions between members of the VTE prophylaxis team) to better understand each team.
1.4. Hypotheses
We propose using SNA as a method of assessing adaptation of clinical teams to different levels of complexity (i.e., stages) in the VTE prophylaxis process. To understand how the clinical team involved in VTE prophylaxis changes as the complexity in the process changes, we developed six hypotheses. According to Barth et al. (2015), teams in high-uncertainty complex situations need more communication, more interaction and more people contributing to the discussion and decision. Therefore, we hypothesise that, as compared to low complexity stages (administration and transfer), in the high complexity stages of VTE prophylaxis (interruption, re-initiation, and initiation), there will be:
More expertise needed as reflected by an increase in the number of roles present on the team (Salas et al. 2007);
More activities requiring collaboration as reflected by an increase in team activities (Salas et al. 2007);
More communication between team members as reflected by an increase in the number of interactions (Marlow et al. 2018);
More discussion about the patient’s care plan as reflected by an increase in reciprocity (Barth et al. 2015);
Higher information sharing between all team members as reflected by an increase in density (Barth et al. 2015).
Increased teamwork and interdependence reflected by a decrease in centralisation (Barth et al. 2015).
2. Methods
This study was conducted as a part of a larger study with the aim of developing sociotechnical system design requirements to support VTE prophylaxis (https://cqpi.wisc.edu/vte-and-health-it/). Our study used a multiple case study design: each hospital and service represented a case for a total of 9 cases (Table 1). For example, the critical care medicine service at hospital A represented one case.
Table 1.
Description of 9 cases.
| Hospital | A | B | C | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Hospital type | Quaternary, teaching | Community tertiary, teaching | Quaternary, teaching | ||||||
| Location | Rural | Urban | Urban | ||||||
| Service | CC-M | Hospitalist | Cardiology | CC-M | CC-S | Hospitalist | CC-M | CC-S | Hospitalist |
| Number of beds | 24 | 58 | 60 | 41 | 41 | 87 | 24 | 24 | 110 |
| Average length of stay | 3.5 days | 5.3 days | 3.4 days | 4.3 days | 6.5 days | 4.8 days | 6.1 days | 8.2 days | 5.1 days |
| Annual admissions | 5,548 | 4,314 | 399 | 3,303 | 491 | 4,867 | 943 | 1,255 | 2,652 |
| Nurse to patient ratio | 1:2 | 1:5 | 1:3 | 1:2 | 1:2 | 1:5 | 1:2 | 1:2 | 1:4 |
| EHR-based VTE risk assessment tool | Yes | Yes | Yes | Yes | Yes | Yes | No | No | No |
| EHR-based order set | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes |
2.1. Sample and setting
Three hospitals were included in the study: two quaternary teaching hospitals and one community tertiary, teaching hospital. We collected data from 4 services that care for different types of patients: critical care medicine (CC-M) (in 3 hospitals), critical care surgery (CC-S) (in 2 hospitals), hospitalist (in 3 hospitals), and cardiology (in 1 hospital). We conducted 43 interviews with a total of 45 clinicians: 29 attending physicians, 1 fellow, 14 residents, and 1 advanced practice provider (APP); two interviews were paired (2 clinicians participated). We conducted one focus group with 7 residents. The study was approved by all associated institutional review boards.
2.2. Data collection
The 43 semi-structured interviews and one focus group lasted a total of 33 hours (http://cqpi.wisc.edu/wp-uploads/2016/07/VTE-prophylaxis-VTE-Interview-Guide.pdf). The average interview time was 45 minutes with standard deviation of 12 minutes and a range from 24 minutes to 81 minutes. The focus group was 95 minutes long. Two human factors engineers conducted each interview, one leading the interview and the other helping with logistics; three human factors engineers facilitated the focus group. We asked interviewees about the activities they perform temporally throughout a patient’s stay: first asking about admission, then during stay activities including interruption, re-initiation, and initiation, and finally activities relating to a patient’s transfer. We audio-recorded, transcribed and coded the interview and focus group data using a qualitative data analysis software, Dedoose®. After coding the qualitative data, one researcher created summaries of the coded excerpts according to the work system model (Carayon 2009; Carayon et al.2006; Carayon et al. 2014; Smith and Carayon-Sainfort 1989), including information on the role of the interviewee, the VTE prophylaxis activity, the tool(s) and technology(ies) being used, the physical environment, and any other organisational, locational, or service-specific information relevant to the excerpt.
2.3. Development of role networks
Using the interview data summaries, we created a total of 43 role networks for the nine cases/services and the associated VTE prophylaxis stages at the three hospitals (Hundt et al. 2017). We are missing two role networks: the transfer stage in hospital B, critical care surgery and the initiation stage in hospital A, cardiology. Patient transfer does not exist for critical care surgical patients in hospital B because the surgeon follows their patients throughout their hospital stay. Initiation is not relevant for cardiology patients since all patients are either already on blood thinning medications or are prescribed VTE prophylaxis medications on admission. Throughout the interview analysis, we relied on multiple clinicians and iterations to develop the role networks. After the role networks were developed, we validated them using member checking in an iterative, reliable process (Hundt et al. 2017); this helps to finalize the role networks that accurately reflected standard practice in the VTE prophylaxis process. The use of analyst triangulation and member checking contributes to the credibility and validity of our analysis (Devers 1999). We built the role networks in Lucidchart®, a diagramming software. We standardised the location of each role on the role networks to facilitate comparison between role networks; for example, the resident was always located in the upper left hand corner of the network.
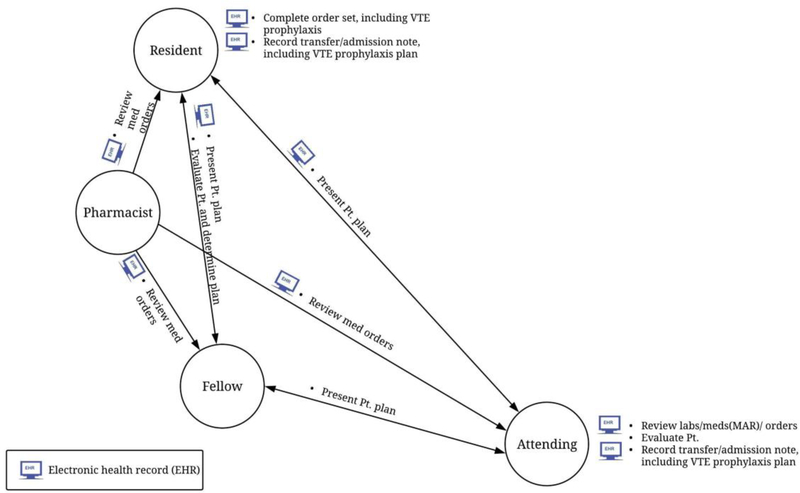
Each role network contains information on the 5 elements of the work system (Carayon 2009; Carayon et al.2006; Smith and Carayon-Sainfort 1989): people, tasks, tools/technologies, physical environment, and organisation (Table 2). Figure 2 shows an example of a role network from the VTE prophylaxis stage of transfer for one service at one hospital.
Table 2.
Work system elements represented on role networks.
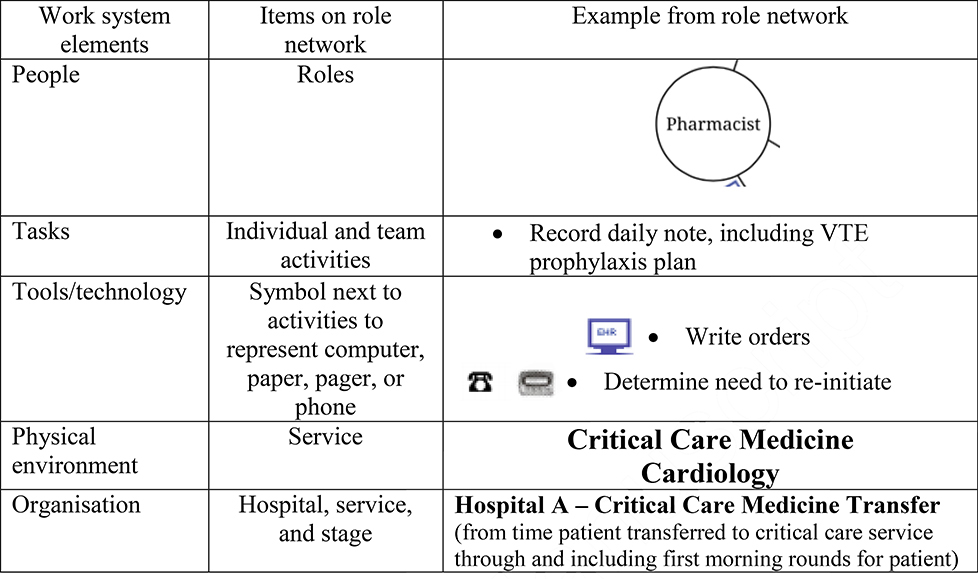 |
Figure 2.
Example of role network – Transfer stage of VTE prophylaxis in the critical care medicine service at hospital A.
2.4. Description of SNA measures
To evaluate each role network, we used 6 SNA measures: number of roles, number of team activities, number of interactions, reciprocity, density, and centralisation. These dependent variables are network-level measures as they represent SNA data for the entire network rather than the individual roles (represented by circles) in the network. The number of roles is defined as the number of people actively involved in the VTE prophylaxis process for the stage and is calculated by counting how many roles are present per role network (i.e., pharmacist, resident, nurse). A team activity is defined as an activity performed jointly by two members of the team (represented by a description preceded by a bullet above or below a line connecting roles); the number of team activities in the role network is a sum of the team activities in the role network. The number of interactions is calculated by adding the number of one-way and two-way interactions between the roles in the network. As an example, the role network in figure 2 contains 4 roles, 7 team activities and 6 interactions (3 one-way interactions and 3 two-way interactions).
Reciprocity, density, and centralisation are measured on a scale from 0 (low) to 1 (high). Reciprocity is a measure of the two-way communications within the network and is calculated by dividing the number of two-way interactions per network by the sum of one-way and two-way interactions in that network (Valente 2010). A high reciprocity score indicates there is a lot of communication and discussion among the team members. Density is a measure of the connectedness in the network and is calculated by dividing the total interactions in the network by the total possible interactions between all roles (Valente 2010). A high density score indicates that there is a large amount of information sharing between all team members. Centralisation is the degree with which one or a few nodes (roles) hold power in the network and is calculated by comparing the highest role centrality score in the network to all the other centrality scores. A low centralisation score indicates there is a lot of interdependence between team members, whereas a high centralisation indicates that one or a few roles are central to the team. As an example, the role network in figure 2 has a reciprocity score of 0.50 (3/6), a density score of 1.0 (6/6), and a centralisation score of 0 (0/6).
2.5. Data analysis of SNA measures
We entered data from each role network into an Excel spreadsheet and grouped the data by service, hospital, and complexity (low or high) of the VTE prophylaxis stage (admission and transfer were grouped together as low complexity stages; interruption, re-initiation and initiation were grouped together as high complexity stages) (Tables 3–6). We did not aggregate the data across hospital and service in order to maintain role network contextual information.
Table 3.
SNA data for the critical care medicine services.
| Hospital | A | B | C | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Stage complexity | Low | High | Low | High | Low | High | |||||||||
| SNA measure | Admission | Transfer | Interruption | Re-initiation | Initiation | Admission | Transfer | Interruption | Re-initiation | Initiation | Admission | Transfer | Interruption | Re-initiation | Initiation |
| Number of roles | 4 | 4 | 6 | 6 | 6 | 5 | 5 | 6 | 6 | 6 | 6 | 6 | 8 | 8 | 7 |
| Number of team activities | 7 | 7 | 11 | 11 | 12 | 12 | 12 | 14 | 14 | 13 | 12 | 11 | 28 | 17 | 10 |
| Number of interactions | 6 | 6 | 10 | 10 | 11 | 8 | 8 | 8 | 8 | 8 | 11 | 11 | 22 | 17 | 10 |
| Reciprocity | 0.50 | 0.50 | 0.70 | 0.40 | 0.45 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0.82 | 0.71 | 1 |
| Density | 1 | 1 | 0.67 | 0.67 | 0.73 | 0.80 | 0.80 | 0.53 | 0.53 | 0.53 | 0.73 | 0.73 | 0.78 | 0.61 | 0.48 |
| Centralisation | 0 | 0 | 0.09 | 0.09 | 0.08 | 0.083 | 0.083 | 0.14 | 0.14 | 0.14 | 0.08 | 0.08 | 0.04 | 0.07 | 0.04 |
Table 6.
SNA data for the cardiology service.
| Hospital | A | ||||
|---|---|---|---|---|---|
| Stage complexity | Low | High | |||
| SNA measure | Admission | Transfer | Interruption | Re-initiation | Initiation |
| Number of roles | 5 | 4 | 5 | 5 | - |
| Number of team activities | 8 | 5 | 8 | 7 | - |
| Number of interactions | 8 | 5 | 8 | 7 | - |
| Reciprocity | 0.63 | 1 | 1 | 0.71 | - |
| Density | 0.80 | 0.83 | 0.80 | 0.70 | - |
| Centralisation | 0.80 | 0.11 | 0.08 | 0.13 | - |
We compared the SNA measures’ values between the low and high complexity stages. Then, for each hospital, service and SNA measure, we calculated the proportion of cases supporting our hypotheses; this is summarised in table 7. For example, within one hospital and service (i.e., a case), if the VTE prophylaxis low-complexity stages of admission and transfer had fewer team activities compared to the high-complexity stages of interruption, re-initiation and initiation, the hypothesis was supported in 6/6 cases, or 100% (e.g., hospital A in critical care medicine service; Tables 3 and 7). On the other hand, if admission consistently had more team activities involved, compared to the stages of interruption, re-initiation and initiation, but transfer always had fewer team activities, the hypothesis was supported in 3/6 cases, or 50% (e.g., hospital C in hospitalist service; Tables 5 and 7). If the SNA measures’ values were the same between low and high complexity stages, they were not counted as supporting the hypothesis. Therefore, if, for example, the number of interactions was the same for all stages in a hospital and service, the hypothesis was supported in 0/6 cases or 0% (e.g., hospital B in critical care medicine service; Tables 3 and 7). Using a cut-off level agreed upon by the research team, we evaluated the cases when the hypothesis was supported by at least 2/3 or 67% of the cases as supporting our hypothesis for the hospital and service (table 7).
Table 7.
Percent of cases supporting hypotheses.
| Service | Critical care medicine | Critical care surgery | Hospitalist | Cardiology | |||||
|---|---|---|---|---|---|---|---|---|---|
| SNA measure | Hospital A | Hospital B | Hospital C | Hospital B | Hospital C | Hospital A | Hospital B | Hospital C | Hospital A |
| Number of roles | 100 | 100 | 100 | 33 | 0 | 83 | 33 | 100 | 50 |
| Number of team activities | 100 | 100 | 67 | 33 | 0 | 50 | 50 | 50 | 50 |
| Number of interactions | 100 | 0 | 67 | 33 | 0 | 100 | 50 | 67 | 50 |
| Reciprocity | 33 | 0 | 0 | 100 | 67 | 0 | 67 | 50 | 50 |
| Density | 0 | 0 | 33 | 0 | 33 | 33 | 67 | 0 | 0 |
| Centralisation | 0 | 0 | 100 | 0 | 0 | 50 | 17 | 17 | 25 |
% Supports hypothesis
Table 5.
SNA data for the hospitalist services.
| Hospital | A | B | C | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Stage complexity | Low | High | Low | High | Low | High | |||||||||
| SNA measure | Admission | Transfer | Interruption | Re-initiation | Initiation | Admission | Transfer | Interruption | Re-initiation | Initiation | Admission | Transfer | Interruption | Re-initiation | Initiation |
| Number of roles | 5 | 4 | 6 | 6 | 5 | 5 | 4 | 4 | 5 | 5 | 5 | 4 | 6 | 6 | 6 |
| Number of team activities | 9 | 3 | 6 | 6 | 7 | 9 | 4 | 5 | 7 | 7 | 10 | 3 | 8 | 6 | 7 |
| Number of interactions | 5 | 3 | 6 | 6 | 6 | 7 | 4 | 5 | 7 | 7 | 6 | 3 | 6 | 6 | 7 |
| Reciprocity | 0.80 | 0.67 | 0.67 | 0.67 | 0.67 | 0.43 | 0 | 0.60 | 0.29 | 0.43 | 0.50 | 1 | 0.83 | 0.67 | 0.57 |
| Density | 0.50 | 0.50 | 0.40 | 0.40 | 0.60 | 0.70 | 0.67 | 0.83 | 0.70 | 0.70 | 0.60 | 0.50 | 0.40 | 0.40 | 0.47 |
| Centralisation | 0.21 | 0.11 | 0.18 | 0.18 | 0.16 | 0.13 | 0 | 0.11 | 0.13 | 0.13 | 0.17 | 0.11 | 0.18 | 0.18 | 0.16 |
3. Results
Across all hospitals and services, the values of the SNA measures varied. The number of roles ranged from 3 to 8, the number of team activities ranged from 3 to 28, and the number of interactions ranged from 3 to 22 per role network. Reciprocity scores ranged from the lowest possible score of 0 (e.g., transfer for hospital B in hospitalist service) to the highest possible score of 1 (e.g., initiation for hospital B in critical care surgery service), with the mean reciprocity of 0.69 (standard deviation 0.25). Density had a smaller range than reciprocity, from 0.40 to 1, with a similar mean of 0.67 (standard deviation 0.17). The centralisation scores were low ranging from 0 to 0.21, with a mean of 0.10 (standard deviation 0.05). The remaining results are organised by service: first discussing critical care medicine, then critical care surgery, next, hospitalist, and finally, cardiology.
3.1. SNA measure values
As shown in table 3, the critical care medicine services demonstrated an increase in the number of roles, number of team activities and number of interactions from the low-complexity stages of admission and transfer, to the high-complexity stages of interruption, re-initiation and initiation. In low-complexity stages, there were 4 to 6 roles involved, compared to high-complexity stages that had 6 to 8 roles. The number of team activities increased from low-complexity, ranging from 7 to 12, to high-complexity stages, ranging from 10 to 28. In low-complexity stages, there were 6 to 11 interactions. In comparison, high-complexity stages had 10 to 22 interactions. The proportion of two-way interactions, indicated by the reciprocity values, did not increase from low compared to high complexity stages. For hospital A, reciprocity increased, compared to the low-complexity stages, for one of the high-complexity stages, interruption. In hospitals B and C, the reciprocity values either stayed the same (i.e., 1) or decreased to 0.71 or 0.82 from low to high complexity stages. The density values decreased from low to high complexity stages. Centralisation decreased from low (0.08) to high (0.04 or 0.07) complexity stages for one hospital, C, but increased in hospitals A and B.
Within the critical care surgery services (table 4), there were fewer increases in the number of roles, number of team activities or number of interactions from low to high complexity stages. In hospital B, the number of roles increased from admission (4 roles) to interruption (6 roles); however, the number of roles did not increase in the stages of re-initiation (3 roles) and initiation (4 roles). In hospital C, the number of roles stayed the same or decreased from low to high-complexity stages, changing from 6 to 5 or 6 roles. Hospital B had 6 team activities and interactions during the low-complexity stages compared to 3 to 8 team activities and interactions during the high-complexity stages. Hospital C had 14 team activities and 10 interactions during admission and transfer with 7 to 13 team activities and interactions during interruption, re-initiation and initiation. Unlike the critical care medicine services, reciprocity increased from low (0.33–0.50) compared to high (0.50–1) complexity stages for hospitals B and C. Density varied from low (0.67–1) to high complexity stages (0.47–1). Similar to critical care medicine, the centralisation scores did not decrease, ranging from 0–0.10 in the low-complexity stages to 0–0.16 in high-complexity stages.
Table 4.
SNA data for the critical care surgery services.
| Hospital | B | C | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Stage complexity | Low | High | Low | High | ||||||
| SNA measure | Admission | Transfer | Interruption | Re-initiation | Initiation | Admission | Transfer | Interruption | Re-initiation | Initiation |
| Number of roles | 4 | - | 6 | 3 | 4 | 6 | 6 | 6 | 5 | 6 |
| Number of team activities | 6 | - | 8 | 3 | 5 | 14 | 14 | 8 | 8 | 13 |
| Number of interactions | 6 | - | 8 | 3 | 5 | 10 | 10 | 7 | 7 | 10 |
| Reciprocity | 0.33 | - | 0.63 | 1 | 1 | 0.50 | 0.50 | 0.57 | 0.57 | 0.50 |
| Density | 1 | - | 0.53 | 1 | 0.83 | 0.67 | 0.67 | 0.47 | 0.70 | 0.67 |
| Centralisation | 0 | - | 0.08 | 0 | 0.11 | 0.10 | 0.10 | 0.16 | 0.13 | 0.10 |
In the hospitalist services (table 5), the number of roles ranged from 4 to 5 during admission and transfer and ranged from 4 to 6 roles during interruption, re-initiation and initiation. The number of team activities increased from the low-complexity stage of transfer (3–4 team activities) compared to the high-complexity stages of interruption, re-initiation and initiation (5–8 team activities); for all three hospitals, there were a large number of team activities (9 or 10) during the stage of admission. The number of interactions increased from low (3–6 interactions) compared to high (6–7 interactions) complexity stages for hospitals A and C. The number of interactions did not increase for hospital B between low (4 and 7 interactions) and high (5 and 7 interactions) complexity stages. Reciprocity decreased for hospital A between low and high-complexity stages, changing from 0.80 to 0.67; density also decreased from low compared to high complexity stages (0.50 to 0.40), except for the stage of initiation where there was an increase in density (0.60). Reciprocity and density both increased for hospital B from the low-complexity stages of admission (0.43; 0.70) and transfer (0; 0.67) to the high-complexity stages of interruption (0.60; 0.83), re-initiation (0.29; 0.70) and initiation (0.43; 0.70). For hospital C, reciprocity increased from admission (0.50) to the more complex stages (0.57 to 0.83), but reciprocity was the highest for transfer (1); density decreased from the low-complexity stages (0.50 and 0.60) to the high-complexity stages (0.40 and 0.47). The centralisation scores stayed about the same between low and high complexity stages ranging from 0.11–0.21 in hospital A, 0–0.13 in hospital B, and 0.11–0.18 in hospital C.
In the cardiology service, the number of roles was the same for the stages of admission, interruption and re-initiation (5 roles), with the stage of transfer having one fewer role present on the team (4 roles). The number of team activities and interactions changed from 8 and 5 during low-complexity stages to 8 and 7 during high-complexity stages. The reciprocity values increased from the low complexity stage of admission (0.63) to the high-complexity stages of interruption (1) and re-initiation (0.71). The low-complexity stage of transfer had a high reciprocity value of 1. The density and centralisation values did not vary much from low to high complexity stages, ranging between 0.70–0.83 and 0.08–0.13, respectively.
3.2. Hypothesis checking
We hypothesised that the number of roles, number of team activities, number of interactions, reciprocity and density would increase from low compared to high complexity stages of VTE prophylaxis, with centralisation decreasing. Based on a group consensus, we decided that any instance with at least 2/3 (i.e., 67%) of cases increasing from low to high complexity would indicate support for the hypothesis. As shown in table 7, our data show varying levels of support for the 6 hypotheses.
In the critical care medicine services, 3 of the 6 hypotheses were supported. We found 100% support for hypothesis 1 that there would be more roles involved in the high complexity stages of VTE prophylaxis. Additionally, hypothesis 2 regarding an increase in team activities was supported at all three hospitals (67–100%). Hypothesis 3 was almost always supported, except for hospital B (0% support) where the number of interactions remained unchanged between low and high complexity stages. Reciprocity (hypothesis 4) and density (hypothesis 5) did not increase as the complexity in the process increased in the critical care medicine services (0–33% support). Only one hospital, hospital C, supported hypothesis 6 (100%) that the centralisation would decrease from low to high complexity.
Unlike critical care medicine, data in the critical care surgery services did not support our hypotheses that there would be more roles, activities and interactions in the high-complexity stages of VTE prophylaxis (0–33% support). On the other hand, our hypothesis that there would be increased two-way communications (i.e., reciprocity) was supported (67–100%). Our hypothesis that the density would increase (0–33%) and that centralisation would decrease (0%) in more complex stages was not supported.
Data in the hospitalist service of hospitals A and C supported our first and third hypotheses that there would be an increase in the number of roles (83–100% support) and the number of interactions (67–100% support) from low to high complexity stages. Data in hospital B supported hypothesis 4 (67%) and 5 (67%), reflecting an increase in reciprocity and density from low to high complexity stages. The other hypotheses demonstrated low and varying levels of support (0–50%) in the hospitalist services of the 3 hospitals.
In the cardiology service of hospital A, there was 50% support for hypotheses 1 through 4 showing an increase in the number of roles, team activities, interactions and reciprocity in the more complex VTE prophylaxis stages. There was 0% support for hypothesis 5 that there would be an increase in density from low to high complexity stages of VTE prophylaxis. Our hypothesis that centralisation would decrease was supported 25%.
4. Discussion
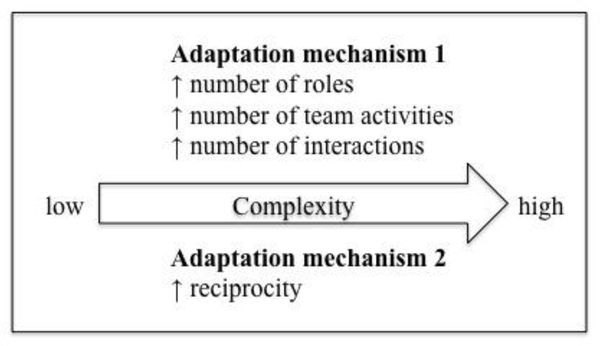
In this study, we expanded on research using SNA to study teams and team adaptation (Baber et al. 2013; Barth et al. 2015; Espinosa and Clark 2014; Houghton et al. 2006; Houghton et al. 2015; Hurtado et al. 2018; McCurdie et al. 2018; Schraagen and Post 2014). We used SNA to quantitatively measure team adaptation to changes in complexity in the VTE prophylaxis process. The VTE prophylaxis process occurs over five stages during a patient’s stay in the hospital that represent low complexity (admission and transfer) or high complexity (interruption, re-initiation and initiation). The complexity of each stage is based on whether there is a clear trigger for action and whether there is an organised decision-making process for the patient’s VTE prophylaxis plan. To evaluate the VTE prophylaxis team’s adaptation to stages with varying complexity, we suggested 6 hypotheses regarding number of roles, number of team activities, number of interactions, reciprocity, density, and centralisation during high complexity stages of the VTE prophylaxis process compared to the low complexity stages. Similar to Barth et al. (2015), we found changes in those SNA measures in response to changes in complexity. Whereas Barth and colleagues (2015) conducted an in-depth analysis of communication activities within one type of team (paediatric surgical team) in one hospital, we collected data on multiple cases, i.e. 9 different contexts and services. This allowed us to identify patterns of team adaptation across contexts. We found that VTE prophylaxis teams adapted to changes in complexity in the VTE prophylaxis process through two mechanisms, either by increasing the number of roles actively involved in the VTE prophylaxis process, the number of team activities and the number of interactions in the team or by increasing the number of two-way communications between team members (i.e., reciprocity) as compared to the low-complexity stages. Figure 3 illustrates the two adaptation mechanisms. Adaptation mechanism #1 occurred in the critical care medicine services and two hospitalist services, and adaptation mechanism #2 occurred in the critical care surgery services and one hospitalist service.
Figure 3.
Team adaptation to increase in complexity in the VTE prophylaxis process.
4.1. Team adaptation to process complexity
We found that the critical care medicine teams adapted by increasing the number of roles, number of team activities, and number of interactions in response to an increase in complexity in the VTE prophylaxis process (adaptation mechanism 1). Contrary to Barth et al. (2015), these critical care medicine teams did not increase reciprocity (i.e., the proportion of two-way communications), density (i.e., the proportion of connections between roles), or decrease centralisation (i.e., the difference in centrality between roles) in response to the increase in complexity. As a patient’s clinical status changed regarding the VTE prophylaxis plan, the team relied on additional people, expertise and interactions in order to assess the patient and determine the appropriate care plan. In all 3 hospitals, the nurse was added to the team when the situation changed from low to high complexity. Two of the critical care medicine services also added a specialist (e.g., neurosurgeon) during the high-complexity stages. As the complexity in the process increased, the critical care medicine teams adapted by adding expertise to the team, by performing more patient care activities together, and by increasing interactions between the team in order to respond to a patient’s changing clinical status and provide appropriate VTE prophylaxis care.
Alternatively, we found that in the critical care surgery services, the teams adapted to an increase in complexity in the VTE prophylaxis process by increasing their two-way communication and discussion, indicated by an increase in reciprocity (adaptation mechanism 2). Unlike the critical care medicine services, these teams did not adapt by increasing the number of roles involved on the team, the number of team activities performed, or their total interactions. The nurse was always involved in the low complexity stages within the critical care surgery services, however the nurse was not always involved in the high complexity stages. Additionally, the pharmacist and surgeon were sometimes involved in the critical care surgery team, but not consistently between low and high complexity stages. Compared to critical care medicine, the critical care surgery teams are less structured, as team members may be in the operating room, preparing for or completing a surgical case. Due to the variability in the team members involved throughout low and high complexity stages, the critical care surgery teams did not respond to an increase in complexity by adding members to the team. Instead, the team responded by increasing the number of two-way communications in the network (i.e., between the resident and APP) and by decreasing the number of one-way communications between the team (i.e., between the nurse and fellow). This allows for more information sharing across the team so an appropriate decision can be made regarding the patient’s VTE prophylaxis plan. These results are similar to those of Barth et al. (2015): when the size of the team is relatively fixed (8–9 team members), such as the paediatric surgical team (Barth study) and the critical care surgery team in our study, teams adapt to increases in complexity by increasing their discussion and two-way communications, reflected by an increase in reciprocity.
In the hospitalist services, the teams at hospitals A and C adapted according to mechanism 1 (by increasing the number of roles, number of team activities, and number of interactions) whereas the VTE prophylaxis team at hospital B adapted through mechanism 2 (by increasing reciprocity). Hospitals A and C are both academic, teaching hospitals, and therefore, the hospitalist service uses a more structured team similar to a critical care medicine service. Like the critical care medicine teams, the hospitalist teams from hospitals A and C often involved the nurse and a specialist as the complexity in the process increased. These teams involved more people and performed more activities together to handle the increasing complexity in the VTE prophylaxis process. Hospital B is a community hospital that does not have as many roles to rely on and, therefore, did not add team members as the complexity increased. Instead, the team increased two-way communication among team members and increased how many people on the team participated in the communication to adjust to increasing situational demands. This resulted in increases in reciprocity and density during high complexity stages of VTE prophylaxis. The hospitalist service in hospital B is the only case that supported hypothesis 5 that the density of the team will increase as complexity in the VTE prophylaxis stage increases. Overall, the hospitalist service differed from the other services as the teams all had a large number of team activities in the low complexity stage, admission. This is likely because patients in the hospitalist service have shorter overall lengths of stay and therefore, the majority of activities relating to the patient, including their VTE prophylaxis plan, plus their hospital and discharge care plans occur during admission, even though it is a low-complexity stage.
The cardiology team did not follow either of the adaptation mechanisms found in the other 3 services. The team did not significantly vary as the complexity in the stages of VTE prophylaxis changed. The main difference in the team between the stages of VTE prophylaxis is that the pharmacist was not present in the team during the transfer of patients into the cardiology unit. Unlike other services, VTE prophylaxis treatment for cardiology patients is typically determined on admission, and will only be interrupted, re-initiated or initiated for very specific patient situations. Therefore, the team may not need to adapt like other services in the hospital, given the unique (compared to other services) patient population.
4.2. Research and practical implications
We identified two adaptation mechanisms that teams use to respond to changing complexity in the VTE prophylaxis process: either the team adapted by increasing the number of roles, activities, and interactions involved or the team adapted by increasing reciprocity. Unlike the work of Barth et al. (2015), most of the teams we studied changed in size from low to high complexity stages of the VTE prophylaxis process. Many SNA measures, such as density, are dependent on the number of people in the team (Valente 2010); therefore, if the team size changes, it is difficult to compare SNA values between teams (Schraagen and Post 2014). For example, the density in a network is related to how many roles are in the network and how many people in that network are connected to each other. In a network of 3 people, it is more likely that all people will be connected, resulting in a high density score, compared to a network of 10 people. In our study, with increasing complexity we found an almost mutually exclusive relationship between an increase in the number of people on the team and an increase in reciprocity and density. Researchers need to be careful using SNA measures when the team size varies and should carefully consider what SNA measures to use when comparing teams.
Our findings provide insight for design of health information technology (IT) to support the VTE prophylaxis process. Not only does the VTE prophylaxis team vary between different hospitals and services, but the teams also vary in how they adapt throughout the different stages of VTE prophylaxis care. This shows the importance of understanding the context when designing health IT to support the VTE prophylaxis process. A technology designed to support a low-complexity stage such as admission will not have the same design requirements as a technology designed for a high-complexity stage such as interruption, as the team members involved, activities, interactions, and communication between team members vary as the complexity in the process changes. In addition, the VTE prophylaxis process relies heavily on team activities and interactions to provide appropriate VTE prophylaxis care to patients. A technology to support the VTE prophylaxis process, especially for high-complexity stages, should be designed to support the work of teams rather than only supporting tasks performed by individuals. This is consistent with the challenge posed by Walker and Carayon (2009) to go beyond individual tasks and consider processes and teams when designing health IT. For instance, health IT could be designed to send messages to the team about an imminent surgical procedure, which may require interruption of VTE prophylaxis. Upon completion of the surgical procedure, the health IT could alert the team and remind them to re-initiate VTE prophylaxis.
Previous studies have used SNA to quantitatively evaluate team structure (Baber et al., 2013; Barth et al., 2015; Espinosa & Clark, 2014; Houghton et al., 2006; Houghton et al., 2015; Hurtado et al., 2018; McCurdie et al., 2018; Schraagen & Post, 2014). Our study expands upon this work by using SNA to measure team adaptation as the complexity in a situation changes. Teams adapt to situations with changing complexity by adjusting their interactions and structure to meet situational demands (Burke et al. 2006; Hollnagel 2012). Our study as well as the work of Barth et al. (2015) show that SNA can be used to identify team adaptation mechanisms in response to changes in complexity. While we believe the changes observed in the team are due to adaptations to complexity, it is worth mentioning two alternative explanations: (1) the SNA measures may actually be measuring the complexity of the clinical VTE prophylaxis process rather than measuring adaptations to complexity, and (2) the larger number of roles involved on the team during high-complexity stages is what makes the process complex. Despite these alternatives, we believe our results reflect the team’s adaptation to changes in complexity since there are inherent differences in complexity in the VTE prophylaxis process between admission and transfer, and interruption, re-initiation, and initiation due to the trigger for action and organised decision-making process at each stage (see figure 1).
4.3. Study limitations
One limitation in our study is that the role networks only represent activities and interactions relating to the VTE prophylaxis process, and, therefore, the data do not necessarily represent how the team adapts to specific clinical processes or patient scenarios. Another limitation is that the data only come from three hospitals, with a limited number of services, and therefore results may not apply for VTE prophylaxis teams in other settings. Because of the small (but diverse) number of services, we had to define a cut-off level for our hypotheses, which was set at 67% based on group consensus. A different level would have led to slightly different conclusions. Future research can use SNA to understand if different types of healthcare teams or if other hospitals and services adapt according to similar mechanisms as these VTE prophylaxis teams when facing situations that change in complexity.
5. Conclusion
Teams adapt to situations with varying levels of complexity. SNA is a method that can be used to study team structure and adaptation as the complexity in a process changes. In our study, we found that VTE prophylaxis care teams adapted according to two mechanisms based on the stages of VTE prophylaxis representing different levels of complexity: either by increasing the roles, activities, and interactions among the team or by increasing two-way communication and discussion between team members. Caution is needed when using SNA measures to compare team adaptation to complex situations, especially when the team size varies.
Acknowledgements
This project was supported by grant number R01HS022086 from the Agency for Healthcare Research and Quality. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Agency for Healthcare Research and Quality. The project was also partially supported by the Clinical and Translational Science Award (CTSA) program, through the NIH National Center for Advancing Translational Sciences (NCATS), grant UL1TR000427. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Declaration of interest statement:
No potential conflict of interest was reported by the authors.
References
- Ashoori M, Burns CM, D’entremont B and Momtahan K 2014. Using team cognitive work analysis to reveal healthcare team interactions in a birthing unit. Ergonomics 57, no 7: 973–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baard SK, Rench TA and Kozlowski SW 2014. Performance adaptation: A theoretical integration and review. Journal of Management 40, no 1: 48–99. [Google Scholar]
- Baber C, Stanton N, Atkinson J, Mcmaster R and Houghton RJ 2013. Using social network analysis and agent-based modelling to explore information flow using common operational pictures for maritime search and rescue operations. Ergonomics 56, no 6: 889–905. [DOI] [PubMed] [Google Scholar]
- Banks VA, Stanton NA, Burnett G and Hermawati S 2018. Distributed cognition on the road: Using east to explore future road transportation systems. Applied Ergonomics 68: 258–66. [DOI] [PubMed] [Google Scholar]
- Barth S, Schraagen JM and Schmettow M 2015. Network measures for characterising team adaptation processes. Ergonomics 58, no 8: 1287–302. [DOI] [PubMed] [Google Scholar]
- Burke CS, Stagl KC, Salas E, Pierce L and Kendall D 2006. Understanding team adaptation: A conceptual analysis and model. Journal of Applied Psychology 91, no 6: 1189. [DOI] [PubMed] [Google Scholar]
- Burtscher M, Manser T, Kolbe M, Grote G, Grande B, Spahn D and Wacker J 2011. Adaptation in anaesthesia team coordination in response to a simulated critical event and its relationship to clinical performance. British Journal of Anaesthesia 106, no 6: 801–06. [DOI] [PubMed] [Google Scholar]
- Carayon P 2006. Human factors of complex sociotechnical systems. Applied Ergonomics 37, no 4: 525–35. [DOI] [PubMed] [Google Scholar]
- Carayon P 2009. The balance theory and the work system model… twenty years later. Intl. Journal of Human–Computer Interaction 25, no 5: 313–27. [Google Scholar]
- Carayon P, Hundt AS, Karsh BT, Gurses AP, Alvarado CJ, Smith M and Brennan PF 2006. Work system design for patient safety: The seips model. BMJ Quality & Safety 15, no suppl 1: i50–i58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carayon P, Wetterneck T, Rivera-Rodriguez AJ, Hundt AS, Hoonakker P, Holden R and Gurses AP 2014. Human factors systems approach to healthcare quality and patient safety. Applied Ergonomics 45, no 1: 14–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke NJ, Gorman JC, Myers CW and Duran JL 2013. Interactive team cognition. Cognitive science 37, no 2: 255–85. [DOI] [PubMed] [Google Scholar]
- Devers KJ 1999. How will we know “good” qualitative research when we see it? Beginning the dialogue in health services research. Health Services Research 34, no 5: 1153–88. [PMC free article] [PubMed] [Google Scholar]
- Dix A, Ramduny-Ellis D and Wilkinson J 2004. Trigger analysis: Understanding broken tasks. The handbook of task analysis for human-computer interaction: 381–400. [Google Scholar]
- Espinosa JA and Clark MA 2014. Team knowledge representation: A network perspective. Human Factors 56, no 2: 333–48. [DOI] [PubMed] [Google Scholar]
- Euerby A and Burns CM 2014. Improving social connection through a communities-of-practice-inspired cognitive work analysis approach. Human Factors 56, no 2: 361–83. [DOI] [PubMed] [Google Scholar]
- Fiumara K, Piovella C, Hurwitz S, Piazza G, Niles CM, Fanikos J, Paterno M, Labreche M, Stevens L-A and Baroletti S 2010. Multi-screen electronic alerts to augment venous thromboembolism prophylaxis. Thrombosis and haemostasis 103, no 02: 312–17. [DOI] [PubMed] [Google Scholar]
- Flach JM 2012. Complexity: Learning to muddle through. Cognition, Technology & Work 14, no 3: 187–97. [Google Scholar]
- Fong A, Clark L, Cheng T, Franklin E, Fernandez N, Ratwani R and Parker SH 2017. Identifying influential individuals on intensive care units: Using cluster analysis to explore culture. Journal of nursing management 25, no 5: 384–91. [DOI] [PubMed] [Google Scholar]
- Galanter WL, Thambi M, Rosencranz H, Shah B, Falck S, Lin F-J, Nutescu E and Lambert B 2010. Effects of clinical decision support on venous thromboembolism risk assessment, prophylaxis, and prevention at a university teaching hospital. American Journal of Health-System Pharmacy 67, no 15: 1265–73. [DOI] [PubMed] [Google Scholar]
- Haut ER, Lau BD, S. KF, Hobson DB, Kraus PS, Carolan HT, Haider AH, Holzmueller CG, Efron DT, Pronovost PJ and Streiff MB 2012. Improved prophylaxis and decreased rates of preventable harm with the use of a mandatory computerized clinical decision support tool for prophylaxis for venous thromboembolism in trauma. Archives of Surgery 147, no 10: 901–07. [DOI] [PubMed] [Google Scholar]
- Heit JA, Cohen AT and Anderson FA 2005. Estimated annual number of incident and recurrent, non-fatal and fatal venous thromboembolism (vte) events in the us: Am Soc Hematology. [Google Scholar]
- Hollnagel E 2012. Coping with complexity: Past, present and future. Cognition, Technology & Work 14, no 3: 199–205. [Google Scholar]
- Houghton RJ, Baber C, Mcmaster R, Stanton NA, Salmon P, Stewart R and Walker G 2006. Command and control in emergency services operations: A social network analysis. Ergonomics 49, no 12–13: 1204–25. [DOI] [PubMed] [Google Scholar]
- Houghton RJ, Baber C, Stanton NA, Jenkins DP and Revell K 2015. Combining network analysis with cognitive work analysis: Insights into social organisational and cooperation analysis. Ergonomics 58, no 3: 434–49. [DOI] [PubMed] [Google Scholar]
- Hundt AS, Carayon P, Yang Y, Stamm J, Agrawal V, Kleinschmidt P and Hoonakker P 2017. Role network analysis of team interactions and individual activities: Application to vte prophylaxis In Proceedings of the Human Factors and Ergonomics Society Annual Meeting, 896–900: SAGE Publications Sage CA: Los Angeles, CA. [Google Scholar]
- Hurtado DA, Dumet LM, Greenspan SA and Rodriguez YI 2018. Social network analysis of peer-specific safety support and ergonomic behaviors: An application to safe patient handling. Applied Ergonomics 68: 132–37. [DOI] [PubMed] [Google Scholar]
- Louis SG, Sato M, Geraci T, Anderson R, Cho SD, Van PY, Barton JS, Riha GM, Underwood S and Differding J 2014. Correlation of missed doses of enoxaparin with increased incidence of deep vein thrombosis in trauma and general surgery patients. JAMA surgery 149, no 4: 365–70. [DOI] [PubMed] [Google Scholar]
- Mahan CE and Spyropoulos AC 2010. Venous thromboembolism prevention: A systematic review of methods to improve prophylaxis and decrease events in the hospitalized patient. Hospital Practice 38, no 1: 97–108. [DOI] [PubMed] [Google Scholar]
- Marlow SL, Lacerenza CN, Paoletti J, Burke CS and Salas E 2018. Does team communication represent a one-size-fits-all approach?: A meta-analysis of team communication and performance. Organizational behavior and human decision processes 144: 145–70. [Google Scholar]
- Maynard G and Stein J 2008. Preventing hospital-acquired venous thromboembolism: A guide for effective quality improvement. Rockville, MD: Agency for Healthcare Research and Quality. [Google Scholar]
- Maynard GA, Morris TA, Jenkins IH, Stone S, Lee J, Renvall M, Fink E and Schoenhaus R 2010. Optimizing prevention of hospital-acquired venous thromboembolism (vte): Prospective validation of a vte risk assessment model. Journal of hospital medicine 5, no 1: 10–18. [DOI] [PubMed] [Google Scholar]
- Mccurdie T, Sanderson P and Aitken LM 2018. Applying social network analysis to the examination of interruptions in healthcare. Applied Ergonomics 67: 50–60. [DOI] [PubMed] [Google Scholar]
- Mitchell P and Golden R 2012. Core principles & values of effective team-based health care: National Academy of Sciences. [Google Scholar]
- Patterson E, Roth E and Woods D 2010. Facets of complexity in situated work. Macrocognition Metrics and Scenarios: Design and Evaluation for Real-World Teams Ashgate Publishing ISBN 978. [Google Scholar]
- Pfautz JD and Pfautz SL 2009. Methods for the analysis of social and organizational aspects of the work domain. Applications of cognitive work analysis: 175–228. [Google Scholar]
- Piazza G, Rosenbaum EJ, Pendergast W, Jacobson JO, Pendleton RC, Mclaren GD, Elliott CG, Stevens SM, Patton WF and Dabbagh O 2009. Physician alerts to prevent symptomatic venous thromboembolism in hospitalized patients. Circulation 119, no 16: 2196–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popoola VO, Lau BD, Tan E, Shaffer DL, Kraus PS, Farrow NE, Hobson DB, Aboagye JK, Streiff MB and Haut ER 2018. Nonadministration of medication doses for venous thromboembolism prophylaxis in a cohort of hospitalized patients. The Bulletin of the American Society of Hospital Pharmacists 75, no 6: 392–97. [DOI] [PubMed] [Google Scholar]
- Qaseem A, Chou R, Humphrey LL, Starkey M and Shekelle P 2011. Venous thromboembolism prophylaxis in hospitalized patients: A clinical practice guideline from the american college of physicians. Annals of internal medicine 155, no 9: 625–32. [DOI] [PubMed] [Google Scholar]
- Ramanathan R, Gu Z, Limkemann AJ, Chandrasekhar S, Rensing E, Mays C and Duane TM 2015. Association between interruptions in chemical prophylaxis and vte formation. The American Surgeon 81, no 7: 732–37. [PubMed] [Google Scholar]
- Roberts AP, Stanton NA and Fay DT 2018. Go deeper, go deeper: Understanding submarine command and control during the completion of dived tracking operations. Applied Ergonomics 69: 162–75. [DOI] [PubMed] [Google Scholar]
- Salas E, Dickinson TL, Converse SA and Tannenbaum S 1992. Toward an understanding of team performance and training In Teams: Their training and performance, eds Swezey RW and Salas E, 3–29. Westport, CT, US: Ablex Publishing. [Google Scholar]
- Salas E, Rosen MA and King H 2007. Managing teams managing crises: Principles of teamwork to improve patient safety in the emergency room and beyond. Theoretical Issues in Ergonomics Science 8, no 5: 381–94. [Google Scholar]
- Salas E, Wilson KA, Murphy CE, King H and Salisbury M 2008. Communicating, coordinating, and cooperating when lives depend on it: Tips for teamwork. The Joint Commission Journal on Quality and Patient Safety 34, no 6: 333–41. [DOI] [PubMed] [Google Scholar]
- Schraagen JM 2011. Dealing with unforeseen complexity in the or: The role of heedful interrelating in medical teams. Theoretical Issues in Ergonomics Science 12, no 3: 256–72. [Google Scholar]
- Schraagen JM and Post W 2014. Characterizing naval team readiness through social network analysis In Proceedings of the Human Factors and Ergonomics Society Annual Meeting, 325–29: SAGE Publications Sage CA: Los Angeles, CA. [Google Scholar]
- Shojania KG, Duncan BW, Mcdonald K, Wachter R and Markowitz A 2001. Making health care safer: A critical analysis of patient safety practices. Evid Rep Technol Assess (Summ) 43, no 1: 668. [PMC free article] [PubMed] [Google Scholar]
- Smith MJ and Carayon-Sainfort P 1989. A balance theory of job design for stress reduction. International Journal of Industrial Ergonomics 4, no 1: 67–79. [Google Scholar]
- Stanton NA and Roberts APJ 2018. Examining social, information, and task networks in submarine command and control. IEEE Transactions on Human-Machine Systems 48, no 3: 252–65. [Google Scholar]
- Streiff MB, Carolan HT, Hobson DB, Kraus PS, Holzmueller CG, Demski R, Lau BD, Biscup-Horn P, Pronovost PJ and Haut ER 2012. Lessons from the johns hopkins multi-disciplinary venous thromboembolism (vte) prevention collaborative. BMJ 344: e3935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valente TW 2010. Social networks and health: Models, methods, and applications: Oxford University Press. [Google Scholar]
- Vicente KJ 1999. Cognitive work analysis. Mahwah, New Jersey: Lawrence Erlbaum Associates, Publishers. [Google Scholar]
- Walker J and Carayon P 2009. From tasks to processes: The case for changing health information technology to improve health care. Health Affairs 28, no 2: 467–77. [DOI] [PubMed] [Google Scholar]
- Wasserman S and Faust K 1994. Social network analysis: Methods and applications. Vol. 8 of: Cambridge university press. [Google Scholar]
- Woods DD 1988. Coping with complexity: The psychology of human behaviour in complex systems In Tasks, errors, and mental models, 128–48: Taylor & Francis, Inc. [Google Scholar]