Abstract
Background
Multiple techniques for delivering partial breast radiotherapy are available. We have previously presented the technical details of our procedure (ASO, 2007) of delivering partial breast irradiation with a single fraction of intraoperative radiotherapy (IORT) targeting the tumor in situ prior to partial mastectomy. This study details our completed, single institution stage II trial, including local control rates.
Methods
An IRB-approved, DSMB-monitored phase II trial was performed with the following inclusion criteria: women age ≥48, ultrasound-visible invasive ductal cancers <3 cm, clinically negative axillary nodes. IORT was delivered using a mobile electron irradiator, including a 1.5 cm radial and 1 cm deep margin, received 15 Gy and immediately underwent partial mastectomy. Ipsilateral breast recurrence was classified as true/marginal or elseware. Kaplan-Meier methods were used to estimate survival functions and exact 95% confidence intervals are reported.
Results
Between 2003–2007, 71 women underwent IORT (median follow-up: 3.5 years). For patients with tumor-involved or close margins, additional therapy was required; 7 patients underwent total mastectomy and 11 received whole breast radiation. Four women experienced invasive ipsilateral breast failures (1 new primary, 3 margin recurrences) for a three-year local control rate of 49/53 (94.8%; 95% CI: 84.2% - 98.3%), and breast cancer-specific survival was 100%.
Conclusions
Intraoperative radiotherapy delivered to an in situ tumor is feasible, but our local control rate at 3.5 years is concerning. Possible changes to this technique to improve local control rates include better preoperative imaging (MRI), routine intraoperative ultrasound and improved IORT delivery (larger cone size, increased dose).
BACKGROUND
The standard of care for women undergoing breast conservation therapy (BCT) is segmental mastectomy followed by whole breast radiotherapy (WBRT). Despite multiple randomized trials demonstrating equivalent overall survival for patients choosing BCT as compared to mastectomy, an increasing number of women eligible for BCT still undergo mastectomy [1, 2]. Furthermore, 15–20% of breast cancer patients undergoing a partial mastectomy do not receive WBRT [3, 4]. Potential explanations for these two factors include physician counseling methods [5], cost of WBRT [6], and logistical difficulties including the distance from home to radiation oncology centers [7–9].
It has been estimated that up to 85% of in-breast recurrences for women undergoing BCT without radiation occur in the region of the segmental mastectomy [10]. Because only ≤ 15% of the in breast recurrences occur outside of the segmental mastectomy site, many have hypothesized that irradiation of less than the entire breast would result in acceptable tumor control rates. Accelerated partial breast irradiation (APBI) aims to deliver radiation to a portion of the breast at a higher dose per fraction over a significantly shorter time frame than standard WBRT. Whether APBI is equivalent in terms of breast cancer control and survival to WBRT is being explored in several randomized trials, such as NSABP B-39/RTOG 0413 (http://www.nsabp.pitt.edu/B-39.asp) and TARGIT-A (http://www.targittrial.net/), but definitive results are still several years away. It is hoped that APBI will offer equivalent survival, comparable local control, and improved cosmesis when compared with WBRT, yet the optimal technique for delivering APBI is not known. Each technique requires specific expertise that may not be available in all centers [11, 12] making it likely that a “best” technique will not be determined.
Intraoperative radiotherapy (IORT) can be used to deliver APBI in a single fraction at the time of tumor excision. The largest experience with this technique is from the Milan group where Veronesi and colleagues first perform a standard quadrantectomy. Following this, the breast parenchyma is reapproximated using sutures, and IORT is delivered with a mobile linear accelerator [13, 14]. Originally used to deliver the tumor bed “boost,” the Milan group has subsequently extended the use of IORT to the sole modality for small, low risk tumors [15]. This approach to IORT has been criticized for problems regarding radiation dose distribution and confirmation of target coverage. In order to address these issues, we modified the IORT technique of Veronesi to deliver radiation prior to segmental mastectomy. This allowed us to more accurately define the target volume using ultrasound planning and to adjust the dosimetry appropriately. We have previously reported the technical details and our cosmetic outcomes with this [16] technique and now present the initial local control results of our single-institution, phase II trial.
METHODS
Patient selection
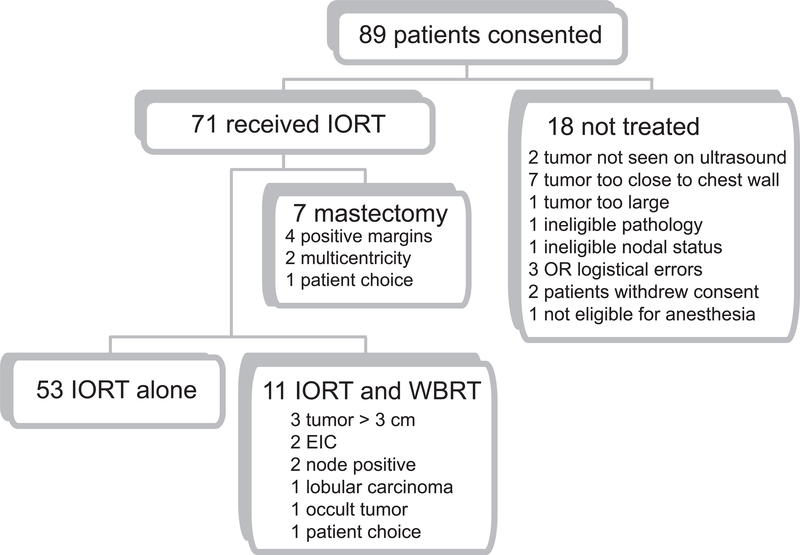
This study was approved by the University of North Carolina at Chapel Hill (UNC) Committee on the Protection of the Rights of Human Subjects, UNC Institutional Review Board (IRB), and the Lineberger Comprehensive Cancer Center Protocol Review Committee. A single institution study was approved by the University of North Carolina, Chapel Hill Institutional Review Board and a data safety monitoring board was established. Eligibility requirements included patients age 48 or older with clinically node negative, infiltrating ductal carcinoma less than three centimeters in greatest diameter and visible by pre-operative breast ultrasound (Figure 1). Patients with multicentric disease, bilateral breast cancer, contraindications to BCT, skin or chest wall involvement, ductal carcinoma in situ, invasive lobular carcinoma, or who had received neoadjuvant chemotherapy or prior irradiation to the involved breast were not eligible. The primary endpoint was to determine the rate of ‘Good or Excellent’ cosmesis and has previously been reported [16]. The secondary endpoint was an ipsilateral breast recurrence and is reported here.
Figure 1.
Enrollment flow diagram.
Pre-operative, surgical and intra-operative methods
We have previously described our procedure in detail [16, 17]. Briefly, a focused breast ultrasound simulation is performed at the time of ultrasound-guided needle localization to determine the optimal angle of approach, minimize the skin-to-tumor distance, and maximize the tumor-to-lung distance. The width of the tumor and the depth from the skin to: 1) anterior tumor edge; 2) posterior tumor edge; and, 3) pleural surface are measured. An electron energy and cone size necessary to deliver at least 1,500 cGy to the 90% isodose line covering the tumor with a 1 cm anterior-posterior margin and a 2 cm radial margin is chosen [16, 17].
Following lymphatic mapping and sentinel lymphadenectomy, breast tissue overlying the tumor is exposed by making very thin skin flaps to adequately allow placement of the radiation cone. The thickener of the Kopans (Cook Medical Inc., Indiana) needle localization wire is identified and a surgical suture is added to enhance visualization of the target. Skin edges are retracted from the radiation field and the radiation cone is locked into position at the angle of approach pre-determined by ultrasound simulation. To ensure correlation with ultrasound measurements, care is taken to not compress or distort the breast tissue. The tumor is centered in the radiation field and placement is verified by both the surgical and radiation oncologist prior to positioning the patient underneath the Mobetron (Intraop Medical Inc., Norcross, GA, USA), a mobile, self-shielded, linear accelerator. All operating room personnel exit the room and the radiation dose is delivered. Following completion of the IORT, the patient is moved from underneath the Mobetron, the cone is removed from the breast, and the segmental mastectomy is performed in the standard fashion.
Post-operative treatment
If the final pathologic review reveals a priori defined features suggesting that the patient is not a good candidate for APBI, additional therapy is recommended. Patients with tumor-involved margins are recommended to undergo re-excision to tumor-free (≥ 1 mm) margins or mastectomy. If an extensive intraductal component is present (i.e., DCIS representing >25% of the tumor and admixed-with and away-from the invasive component), final tumor size is greater than 3 cm, or histology demonstrates infiltrating lobular carcinoma the patient is recommended to undergo WBRT (with IORT serving as boost) or mastectomy. In addition, all patients with a tumor-involved sentinel lymph node underwent completion axillary lymph node dissection and either received WBRT or underwent a mastectomy. Systemic therapy was recommended independent of the local therapy delivered.
Statistical methods
Ipsilateral breast recurrence was classified as true/marginal, elsewhere, or regional. Local control and survival were analyzed by the method of Kaplan-Meier to estimate survival functions. Statistical analyses were performed with SAS statistical software, Version 9.2, SAS Institute Inc., Cary, NC.
RESULTS
Between March 2003 and July 2007, 89 patients were consented for this IRB approved study and 71 patients underwent IORT and form the basis for this report. Reasons for not undergoing IORT are indicated (Figure 1). IORT alone was given in 53 patients, 11 received WBRT and 7 underwent mastectomy. Baseline demographic and clinical characteristics (Table 1) were similar to those for other trials of APBI. ER-positive disease was seen in 79% of patients, while 15% were triple negative (i.e., negative for ER, PR, and HER2). Patients with both high and low grade tumors were included. As of December 31, 2009 median follow-up time was 3.5 years.
Table 1.
Patient characteristics
| Characteristic | Received IORT n=71 (%) | no IORT n=18(%) |
|---|---|---|
| Mean age (range) | 64.8 (48–92) | 65.9 (48–83) |
| Clinical Staging | ||
| cT1a | 2 (2.8%) | 3 (17%) |
| cT1b | 18 (25%) | 9 (50%) |
| cT1c | 35 (49%) | 4 (22%) |
| cT2 (<3cm) | 16 (23%) | 2 (11%) |
| cN0 | 89 (100%) | |
| Grade | ||
| I | 25 (35%) | 5 (28%) |
| II | 24 (34%) | 9 (50%) |
| III | 22 (31%) | 4 (22%) |
| Markers | ||
| ER+ | 56 (79%) | 15 (83%) |
| PR+ | 49 (69%) | 10 (56%) |
| HER2+ | 7 (10%) | 1 (5.6%) |
| ER+, PR+, HER2− | 46 (65%) | 10 (56%) |
| “triple negative” | 12 (17%) | 2 (11%) |
Parameters of IORT delivery are shown (Table 2). On ultrasound, median tumor diameter was 1.2 cm which corresponds to a median cone size of 5.5 cm. Median tumor size on pathologic review was 1.5 cm. Seven patients had a tumor-involved sentinel node. Twenty women went re-excision for close or positive margins following final pathologic review. Thirty-nine (39/71, 55.9%) received adjuvant hormonal therapy and 17 (17/71, 23.9%) received systemic adjuvant chemotherapy.
Table 2.
Treatment characteristics
| Characteristic | Median | Range |
| Tumor diameter on ultrasound (cm) | 1.2 | 0.5 – 2.4 |
| Skin to anterior tumor depth (cm) | 1.0 | 0.3 – 2.6 |
| Skin to posterior tumor depth (cm) | 2.2 | 0.15 – 5.1 |
| Skin to pleura depth (cm) | 3.9 | 1.6 – 6.7 |
| Dose (Gy) dmax | 15 | 14.3 – 23.7 |
| Pathologic tumor size (cm) | 1.5 | 0.4 – 4.0 |
| Pathologic Staging | all (n=71) | IORT alone (n=53) |
| pT1a | 2 (3%) | 2 (4%) |
| pT1b | 15 (21%) | 12 (23%) |
| pT1c | 38 (54%) | 30 (56%) |
| pT2 (≥3cm) | 13 (18%) | 9 (17%) |
| pT2 (>3cm) | 3 (4%) | 0 |
| pN0 | 64 (90%) | 53 (100%) |
| pN(+) | 7 (10%) | 0 |
| median number positive nodes | 2 | 0 |
| Additional therapy | all (n=71) | IORT alone (n=53) |
| Re-excision | 20 (28%) | 14 (26%) |
| Systemic chemotherapy | 17 (24%) | 8 (15%) |
| Hormonal therapy | 39 (55%) | 30 (56%) |
Local tumor recurrences were seen in 4 of 53 patients undergoing IORT alone and in 0 of 11 patients recommended to undergo WBRT following IORT (Fisher’s exact test p = ns). Actuarial 3 year local control was 92% (95% CI: 82% - 98%) (Figure 2a). Local tumor recurrence was classified as true/marginal in 3 of 4 patients and as an in breast elsewhere failure in 1 patient.
Figure 2.
Local recurrence free survival (a) and mastectomy free survival (b) for women treated with IORT alone. The number of patients included in the analysis at each year is indicated in the inset table.
No discernable pattern of patient characteristics predictive of local recurrence could be identified (Table 3).The 3 true marginal recurrences occurred 0.5, 2.9 and 4.9 years following IORT. All 3 patients chose a re-operative partial mastectomy and WBRT, and all are disease-free at last follow-up. The 1 patient who had an in breast elsewhere failure initially had a triple-negative breast cancer and the recurrence was ER positive, PR positive, HER2 negative. She underwent mastectomy, was started on aromasin, and received chest wall radiation. She remains breast cancer free 1 year after mastectomy.
Table 3.
Characteristics of ipsilateral breast tumor recurrences.
| Patient | age (y) | ultrasound size (cm) | Tumor size (cm) | ER | PR | HER2 | Grade | Chemotherapy | Hormonal therapy | LRFS (y) | OS (y) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 60 | 0.6 | 2.6 | − | − | − | 3 | no | no | 1.1 | 2.2 |
| 2 | 66 | 1.7 | 1.8 | − | − | − | 2 | yes | no | 2.9 | 3.1 |
| 3 | 82 | 0.9 | 1.7 | + | + | − | 2 | no | yes | 0.5 | 3.5 |
| 4 | 56 | 0.8 | 0.8 | + | + | − | 1 | no | yes | 5.0 | 5.3 |
Overall, following ipsilateral breast failure 3 women underwent breast conserving therapy and 1 underwent mastectomy for an actuarial three-year mastectomy free survival rate of 52/53 (98%, 95% CI: 87% - 100%, Figure 2b). Among the women who received IORT alone, no breast cancer-related deaths have been observed and overall survival is 52/53 or 98%.
DISCUSSION
A careful pathologic evaluation of small breast tumors by Holland and colleagues [18] showed that the probability of seeing additional tumor decreases as a function of distance from the known lesion and that only 15% of patients had invasive cancer located more than 2 cm from the reference mass. Similarly, the majority of in-breast cancer recurrences following BCT occur in the same quadrant as the primary tumor [10, 19–21]. These data suggest that the benefit of WBRT is primarily due to sterilization of residual microscopic disease in the region of the primary tumor. We [17], and others [13, 22, 23], have adopted the approach of using intraoperative radiation directed to the region of the breast at highest risk of residual disease. That may or may not be identified with standard histopathologic evaluation of the segmental mastectomy specimen.
A significant challenge when using either IORT [13, 17], brachytherapy [24], or external beam radiotherapy tumor bed boost is accurate delineation of the tumor bed [25]. After tumor removal, the tumor bed is imaged using CT or ultrasound yet the accuracy of each of these approaches for tumor bed definition varies even among experts. Delivery of IORT following tumor removal is challenging due to our limited ability to achieve a uniform dose distribution in the cavity that remains after resection. The Milan group has attempted to overcome this obstacle by re-approximating the normal tissue to fill the tumor bed cavity [13, 14]. However, this can distort the tissue, and thus the target, to a variable extent. The UK and Memorial Sloan Kettering groups use applicators that fill the cavity [22, 23], but due to the use of low energy photons there is limited tissue penetration and associated uncertainties regarding adequate target coverage.
When compared with delivery of IORT following tumor resection, our technique allows the use of pre-planning to select an electron energy and cone size to deliver the intended dose of radiation to the tumor and an appropriate margin. Based on the data of Holland et al. [18], we chose to utilize a 2 cm radial margin and prescribe the radiation dose to cover 1 cm deep to the tumor with the prescribed dose. It was hypothesized that this would result in more uniform coverage of tumor margins. It also results in irradiation of a smaller volume of remaining irradiated normal breast parenchyma as some of the irradiated tissue is subsequently excised with the tumor specimen. This is in contrast to postoperative techniques in which the entire irradiated volume should contain at most microscopic disease and in which the entire irradiated tissue remains in the breast.
One significant advantage to IORT delivered at the time of surgery is immediate completion of therapy in select cases. Recommending WBRT for a larger subset of patients, perhaps those with tumors larger than 1 cm, may be a reasonable approach to optimize ever scarcer resources. Several randomized trials are ongoing comparing APBI with WBRT [26, 27], if these eventually show equivalent outcomes between APBI and WBRT, the optimal treatment approach will likely remain an unanswered question. Even if APBI is found to be inferior to WBRT, the use of IORT to deliver a tumor bed boost at the time of surgery and prior to WBRT, an approach validated in several cohorts [15, 28–30], may have both logistical and biologic advantages. This approach may both decrease treatment time and has been shown to inhibit proliferation, migration and invasion of breast cancer cell lines by altering wound fluid composition [31].
Three year actuarial local control of 92% was lower than anticipated. There are several potential explanations for this finding. First, the dose of radiation delivered may have been inadequate. The Milan group used a dose of 21 Gy in the majority of their patients and saw ipsilateral breast tumors in 1% of patients [13]. Groups using the Intrabeam system (Carl Zeiss Meditec, Germany) have utilized a dose of 20 Gy to the applicator surface [27, 30]; further follow-up is needed for to determine the efficacy of this approach. We chose a dose of 15 Gy based on calculation of a biologically equivalent dose (BED) according to a standard formula (Table 4) and assuming an α/β ratio of 4 Gy. While the application of the α/β model for doses of radiation exceeding 10 Gy has been questioned [32], this modeling gives some indication that our radiation dose is in an appropriate range if, the α/β value chose for our model is correct. Several groups have suggested that the α/β for breast cancer is around 4 Gy [33, 34], however, if the α/β ratio is closer to 10 Gy as is the case for many tumor types, the chosen dose may have been inadequate (Table 4). Second, the volume of irradiated normal tissue may be inadequate. While we utilized a 2 cm margin in choosing our cone size median tumor size was a 0.3 cm larger on pathology than on ultrasound suggesting that a 2 cm margin may have been inadequate. In addition, we utilized a 1 cm margin deep to the tumor bed for our target coverage which may have resulted in underdosing of tissue near the chest wall. This compromise was made to minimize dose to the chest wall and avoid potential complications. Finally, the inclusion of patients with higher risk profiles including triple negative disease, ER negative disease, young age, and large tumors (>2 cm) may be contraindicated. Recently published ASTRO consensus guidelines recommend cautious consideration of APBI for women less than 60 years old, tumors > 2 cm, and ER negative disease [35]. Our patient numbers were too small for valid multivariate analyses to be performed.
Table 4.
Biologically equivalent dose (BED) calculations according to BED = D(1+d(α/β))
| Regimen | α/β = 4 | α/β = 10 |
|---|---|---|
| 15 Gy x 1 | 71.3 | 37.5 |
| 2.67 Gy x 16 | 71.2 | 54.1 |
| 2 Gy x 25 | 75.0 | 60.0 |
We recognize several limitations of our study. With a median follow-up of 3 years, it is still too early to draw firm conclusions regarding efficacy. In addition, use of improved preoperative imaging such as breast magnetic resonance imaging (MRI) may aid in selection of patients who are appropriate for partial breast irradiation [36]. Or perhaps routine intra-operative ultrasound may aid the surgical and radiation oncologist in identifying the target in the operating room. Other possibilities may include using differential gene-expression analyses or other biomarkers of local recurrence risk to identify appropriate patients for APBI.
CONCLUSIONS
Despite a higher than expected in breast failure rate, IORT as sole radiation therapy in BCT may still be a reasonable treatment option for appropriately selected patients. Additional follow-up is needed for this and other ongoing studies. Continued enrollment of patients on thoughtfully designed clinical studies may help identify an optimal technique for delivering APBI.
SYNOPSIS.
This is the first report of local control using intraoperatie radiotherapy prior to partial mastectomy in patients with early stage breast cancer.
ACKNOWLEDGEMENTS
Supported by UNC/Lineberger Comprehensive Cancer Center, Sisko, Rodney and Ruth James Foundation. RJK has been designated a B. Leonard Holman Pathway Fellow by the American Board of Radiology and is supported by a 2007-08 Phillips Medical Systems/Radiological Society of North America Research Resident Grant.
REFERENCES
- 1.McGuire KP, Santillan AA, Kaur P, et al. Are mastectomies on the rise? A 13-year trend analysis of the selection of mastectomy versus breast conservation therapy in 5865 patients. Ann Surg Oncol. October 2009;16(10):2682–2690. [DOI] [PubMed] [Google Scholar]
- 2.Katipamula R, Degnim AC, Hoskin T, et al. Trends in mastectomy rates at the Mayo Clinic Rochester: effect of surgical year and preoperative magnetic resonance imaging. J Clin Oncol. September 1 2009;27(25):4082–4088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morrow M, White J, Moughan J, et al. Factors predicting the use of breast-conserving therapy in stage I and II breast carcinoma. J Clin Oncol. April 15 2001;19(8):2254–2262. [DOI] [PubMed] [Google Scholar]
- 4.Nattinger AB, Hoffmann RG, Kneusel RT, Schapira MM. Relation between appropriateness of primary therapy for early-stage breast carcinoma and increased use of breast-conserving surgery. Lancet. September 30 2000;356(9236):1148–1153. [DOI] [PubMed] [Google Scholar]
- 5.Hawley ST, Griggs JJ, Hamilton AS, et al. Decision involvement and receipt of mastectomy among racially and ethnically diverse breast cancer patients. J Natl Cancer Inst. October 7 2009;101(19):1337–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McGinnis LS, Menck HR, Eyre HJ, Bland KI, Scott-Conner CE, Morrow M, Winchester DP. National Cancer Data Base survey of breast cancer management for patients from low income zip codes. Cancer. February 15 2000;88(4):933–945. [PubMed] [Google Scholar]
- 7.Mandelblatt JS, Hadley J, Kerner JF, et al. Patterns of breast carcinoma treatment in older women: patient preference and clinical and physical influences. Cancer. August 1 2000;89(3):561–573. [PubMed] [Google Scholar]
- 8.Nattinger AB, Kneusel RT, Hoffmann RG, Gilligan MA. Relationship of distance from a radiotherapy facility and initial breast cancer treatment. J Natl Cancer Inst. September 5 2001;93(17):1344–1346. [DOI] [PubMed] [Google Scholar]
- 9.Athas WF, Adams-Cameron M, Hunt WC, Amir-Fazli A, Key CR. Travel distance to radiation therapy and receipt of radiotherapy following breast-conserving surgery. J Natl Cancer Inst. February 2 2000;92(3):269–271. [DOI] [PubMed] [Google Scholar]
- 10.Veronesi U, Marubini E, Mariani L, et al. Radiotherapy after breast-conserving surgery in small breast carcinoma: long-term results of a randomized trial. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. July 2001;12(7):997–1003. [DOI] [PubMed] [Google Scholar]
- 11.Sauer R, Sautter-Bihl M, Budach W, et al. Accelerated partial breast irradiation. Cancer. 15 September 2007. 2007;110(6):1187–1194. [DOI] [PubMed] [Google Scholar]
- 12.Sanders ME, Scroggins T, Ampil FL, Li BD. Accelerated partial breast irradiation in early-stage breast cancer. J Clin Oncol. March 10 2007;25(8):996–1002. [DOI] [PubMed] [Google Scholar]
- 13.Veronesi U, Orecchia R, Luini A, et al. Full-dose intraoperative radiotherapy with electrons during breast-conserving surgery: experience with 590 cases. Ann Surg. July 2005;242(1):101–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Intra M, Gatti G, Luini A, et al. Surgical technique of intraoperative radiotherapy in conservative treatment of limited-stage breast cancer. Arch Surg. June 2002;137(6):737–740. [DOI] [PubMed] [Google Scholar]
- 15.Luini A, Orecchia R, Gatti G, et al. The pilot trial on intraoperative radiotherapy with electrons (ELIOT): update on the results. Breast Cancer Res Treat. September 2005;93(1):55–59. [DOI] [PubMed] [Google Scholar]
- 16.Kimple RJ, Klauber-DeMore N, Kuzmiak CM, et al. Cosmetic outcomes for accelerated partial breast irradiation prior to surgical excision of early stage breast cancer using single dose intraoperative radiotherapy. Int J Radiat Oncol Biol Phys. In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ollila DW, Klauber-DeMore N, Tesche LJ, et al. Feasibility of breast preserving therapy with single fraction in situ radiotherapy delivered intraoperatively. Ann Surg Oncol. February 2007;14(2):660–669. [DOI] [PubMed] [Google Scholar]
- 18.Holland R, Veling SH, Mravunac M, Hendriks JH. Histologic multifocality of Tis, T1–2 breast carcinomas. Implications for clinical trials of breast-conserving surgery. Cancer. September 1 1985;56(5):979–990. [DOI] [PubMed] [Google Scholar]
- 19.Liljegren G, Holmberg L, Bergh J, Lindgren A, Tabar L, Nordgren H, Adami HO. 10-Year results after sector resection with or without postoperative radiotherapy for stage I breast cancer: a randomized trial. J Clin Oncol. August 1999;17(8):2326–2333. [DOI] [PubMed] [Google Scholar]
- 20.Vicini F, Arthur D, Polgar C, Kuske R. Defining the efficacy of accelerated partial breast irradiation: the importance of proper patient selection, optimal quality assurance, and common sense. Int J Radiat Oncol Biol Phys. December 1 2003;57(5):1210–1213. [DOI] [PubMed] [Google Scholar]
- 21.Smith TE, Lee D, Turner BC, Carter D, Haffty BG. True recurrence vs. new primary ipsilateral breast tumor relapse: an analysis of clinical and pathologic differences and their implications in natural history, prognoses, and therapeutic management. Int J Radiat Oncol Biol Phys. December 1 2000;48(5):1281–1289. [DOI] [PubMed] [Google Scholar]
- 22.Vaidya JS, Baum M, Tobias JS, et al. Targeted intra-operative radiotherapy (Targit): an innovative method of treatment for early breast cancer. Ann Oncol. August 2001;12(8):1075–1080. [DOI] [PubMed] [Google Scholar]
- 23.Sacchini V, Beal K, Goldberg J, Montgomery L, Port E, McCormick B. Study of quadrant high-dose intraoperative radiation therapy for early-stage breast cancer. Br J Surg. September 2008;95(9):1105–1110. [DOI] [PubMed] [Google Scholar]
- 24.Wazer DE, Kaufman S, Cuttino L, DiPetrillo T, Arthur DW. Accelerated partial breast irradiation: an analysis of variables associated with late toxicity and long-term cosmetic outcome after high-dose-rate interstitial brachytherapy. Int J Radiat Oncol Biol Phys. February 1 2006;64(2):489–495. [DOI] [PubMed] [Google Scholar]
- 25.Li XA, Tai A, Arthur DW, et al. Variability of target and normal structure delineation for breast cancer radiotherapy: an RTOG Multi-Institutional and Multiobserver Study. Int J Radiat Oncol Biol Phys. March 1 2009;73(3):944–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sanghani M, Wazer DE. Patient selection for NSABP B-39/RTOG 0413: have we posed the right questions in the right way? Brachytherapy. Apr-Jun 2007;6(2):119–122. [DOI] [PubMed] [Google Scholar]
- 27.Holmes DR, Baum M, Joseph D. The TARGIT trial: targeted intraoperative radiation therapy versus conventional postoperative whole-breast radiotherapy after breast-conserving surgery for the management of early-stage invasive breast cancer (a trial update). Am J Surg. October 2007;194(4):507–510. [DOI] [PubMed] [Google Scholar]
- 28.Vaidya JS, Baum M, Tobias JS, et al. Targeted intraoperative radiotherapy (TARGIT) yields very low recurrence rates when given as a boost. Int J Radiat Oncol Biol Phys. December 1 2006;66(5):1335–1338. [DOI] [PubMed] [Google Scholar]
- 29.Reitsamer R, Peintinger F, Kopp M, Menzel C, Kogelnik HD, Sedlmayer F. Local recurrence rates in breast cancer patients treated with intraoperative electron-boost radiotherapy versus postoperative external-beam electron-boost irradiation. A sequential intervention study. Strahlenther Onkol. January 2004;180(1):38–44. [DOI] [PubMed] [Google Scholar]
- 30.Kraus-Tiefenbacher U, Bauer L, Scheda A, et al. Long-term toxicity of an intraoperative radiotherapy boost using low energy X-rays during breast-conserving surgery. Int J Radiat Oncol Biol Phys. October 1 2006;66(2):377–381. [DOI] [PubMed] [Google Scholar]
- 31.Belletti B, Vaidya JS, D’Andrea S, et al. Targeted intraoperative radiotherapy impairs the stimulation of breast cancer cell proliferation and invasion caused by surgical wounding. Clin Cancer Res. March 1 2008;14(5):1325–1332. [DOI] [PubMed] [Google Scholar]
- 32.Story M, Kodym R, Saha D. Exploring the possibility of unique molecular, biological, and tissue effects with hypofractionated radiotherapy. Semin Radiat Oncol. October 2008;18(4):244–248. [DOI] [PubMed] [Google Scholar]
- 33.Plataniotis GA, Dale RG. Biologically effective dose-response relationship for breast cancer treated by conservative surgery and postoperative radiotherapy. Int J Radiat Oncol Biol Phys. October 1 2009;75(2):512–517. [DOI] [PubMed] [Google Scholar]
- 34.Williams MV, Denekamp J, Fowler JF. A review of alpha/beta ratios for experimental tumors: implications for clinical studies of altered fractionation. Int J Radiat Oncol Biol Phys. January 1985;11(1):87–96. [DOI] [PubMed] [Google Scholar]
- 35.Smith BD, Arthur DW, Buchholz TA, et al. Accelerated partial breast irradiation consensus statement from the American Society for Radiation Oncology (ASTRO). J Am Coll Surg. August 2009;209(2):269–277. [DOI] [PubMed] [Google Scholar]
- 36.Tendulkar RD, Chellman-Jeffers M, Rybicki LA, Rim A, Kotwal A, Macklis R, Obi BB. Preoperative breast magnetic resonance imaging in early breast cancer: implications for partial breast irradiation. Cancer. April 15 2009;115(8):1621–1630. [DOI] [PubMed] [Google Scholar]