Abstract
Epidemiologic studies provide an insight into the etiology of lymphoplasmacytic lymphoma/Waldenström macroglobulinemia, which indicates that repetitive immune stimulation and genetic factors play an important role. Here, the current understanding on the causes of lymphoplasmacytic lymphoma/Waldenström macroglobulinemia are reviewed. Recent studies of the literature are discussed, and future population-based studies are proposed to further elucidate the molecular mechanisms that underlie these associations. Finally, the clinical implications of these data are outlined, and perspectives on clinical follow-up and counseling are provided.
Introduction
Waldenström macroglobulinemia (WM) is a lymphoplasmacytic lymphoma (LPL) that requires a detectable monoclonal immunoglobulin (Ig) M spike in serum for its diagnosis.1,2 It is characterized by a distinct population of small B lymphocytes, lymphocytes with plasmacytoid differentiation, and plasma cells that involve the bone marrow. Symptoms occur as a result of tissue involvement by this low-grade lymphoma, hyperviscosity, development of autoantibodies, and IgM deposition in tissues.3
LPL/WM is a very rare disease, which comprise <2% of all non-Hodgkin lymphomas (NHL), with an incidence rate of 3 cases per million people per year and with a higher incidence in men and whites.4,5 The median age at diagnosis is approximately 70 years old. Overall survival has improved in patients of all ages, which has been correlated with the introduction of novel agents and combination therapy,6–8 the strongest risk factor for WM is the precursor condition monoclonal gammopathy of undetermined significance (MGUS) of the IgM class, which is associated with an average 1.5% annual risk of developing WM, other lymphomas, or related disorders.9,10
Although the etiology of WM remains to be better clarified, analysis of emerging data suggests that there may be a link between the immune system and genetic factors that are involved in the development of WM. This review article discusses current insights along these lines.
Immune-related Factors
A growing number of studies have reported that most WM cells have somatic mutations in their immunoglobulin genes, which suggests that the cell of origin of WM is past the germinal center of the lymph nodes where antigenic stimulation and selection occurs.11–14 In relation to this, several epidemiologic studies have been conducted to determine the association between the risk of developing WM and immune conditions that favor chronic antigenic stimulation of B cells. In a small hospital-based study of 65 patients with WM and 213 hospital-based controls, a personal history of autoimmune disease was not associated with a subsequent risk of developing WM.15 Interestingly, patients with WM were more likely than controls to have first-degree relatives with a history of pneumonia, diphtheria, rheumatic fever, and diabetes mellitus. An exploratory evaluation of immunologic profiles revealed that relatives of 2 patients with WM had IgM MGUS, and approximately 40% had diverse immunologic abnormalities.15 To further study the role of antigenic stimulation in the etiology of WM, we conducted 2 large nationwide studies based on U.S. veterans that explored the role of antigenic stimulation in the pathogenesis of WM. In the first study, including 146,394 individuals infected with hepatitis C virus and 572,293 controls, we found that hepatitis C virus infection conferred a 20% to 30% increased risk of NHL overall and a 3-fold higher risk of WM.16 In the second study, we assessed the WM risk in relation to a variety of chronic immune stimulatory conditions based on 4 million U.S. veterans; among 361 patients with WM with up to 27 years of follow-up, we found a 2-to 3-fold elevated risk of WM in individuals with a personal history of an autoimmune disease and notably elevated risks associated with hepatitis, human immunodeficiency virus, and rick-ettsiosis.17 We also conducted 2 large population-based studies in Sweden. The first was based on 2470 patients with LPL/WM, 9698 matched controls, and almost 30,000 first-degree relatives of patients and controls from Sweden, we analyzed whether a personal or family history of a wide range of autoimmune, infectious, allergic, and inflammatory conditions were associated with LPL/WM.18 An increased risk of LPL/WM was associated with a personal history of the following autoimmune diseases: systemic sclerosis, Sjögren syndrome, autoimmune hemolytic anemia, polymyalgia rheumatica, and giant cell arteritis. An increased risk of LPL/WM was associated with a personal history of the following infectious diseases: pneumonia, septicemia, pyelonephritis, sinusitis, herpes zoster, and influenza. Interestingly, an increased risk of LPL/WM was associated with a family history of Sjögren syndrome, autoimmune hemolytic anemia, Guillain-Barré syndrome, cytomegalovirus, gingivitis and periodontitis, and chronic prostatitis.18
These findings are further supported by a recent, large, population-based, case-control study that evaluated 5403 patients with MGUS and 21,209 matched controls with their respective first-degree relatives (14,535 for MGUS and 58,164 for controls) to assess for the association of MGUS with a personal or family history of infections and autoimmune and/or inflammatory conditions. In this study, both a personal history (odds ratio [OR] 2.1) and a family history (OR 1.1) of autoimmune disease were independently associated with an increased risk of MGUS. Furthermore, a personal history of infections (OR 1.6) and inflammatory conditions (OR 1.4) also was associated with an increased risk of MGUS, which supports a role for shared susceptibility for these conditions.19
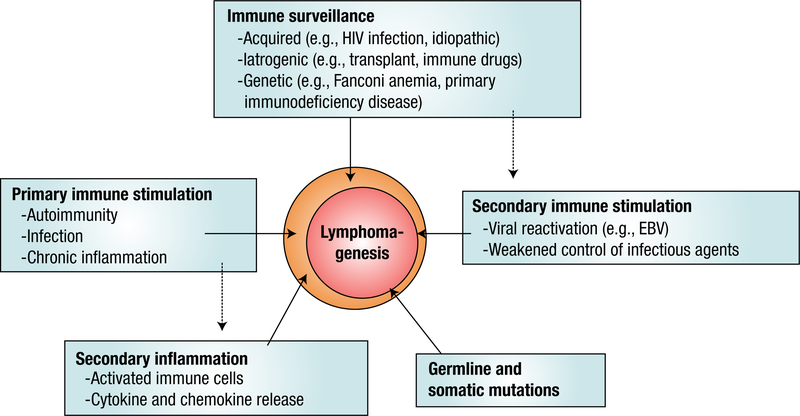
Taken as a whole, our outcomes provide further evidence that supported the hypothesis that chronic stimulation of the immune system is linked to the etiology of WM. The fact that both a personal and family history of autoimmune conditions, such as giant cell arteritis or polymyalgia rheumatica, and a personal history of infections and inflammation are associated with an increased risk of WM, which suggests that there may be shared immune-related susceptibility genes underlying these conditions. A model for immune-related factors and their role in lymphomagenesis is presented in Figure 1. Future research is required to unveil the mechanisms that account for an increased risk of WM in patients with immune-stimulating conditions.
Figure 1.
Immune-related and Genetic Factors, and Their Role in Lymphomagenesis. Proposed Model for the Assocation of Immune-related Factors and Lymphomagenesis. Failure of Immune Surveillance, Repetitive immune Stimulation, Germ Line and Somatic Mutations and Primary and Secondary Inflammation All can Lead to Uncontrolled B-cell Growth (Through Either Overstimulation or Defective Apoptosis) and Lymphomagenesis
Genetic Factors
Familial clustering of LPL/WM is well documented and has been published in case-control cohort studies and multiply affected families.15,20–23 One of these studies, a clinic-based series of 257 patients with WM, characterized the clinicopathologic patterns of familial disease. The investigators found that 19% of the patients had at least one first-degree relative affected with WM or another B-cell disorder.23 In 2008, we performed a large study that included 2144 patients with LPL/WM (1539 [72%] WM and 605 [28%] LPL) diagnosed in Sweden, 8279 population-based matched controls, and linkable first-degree relatives of patients (n = 6177) and controls (n = 24,609).24 We found that first-degree relatives of patients with LPL/WM had a significantly increased risk of developing LPL/WM, other subtypes of NHL (including chronic lymphocytic leukemia), and MGUS compared with first-degree relatives of the controls. However, we found no excess risk of multiple myeloma or Hodgkin lymphoma. Our results expand on previous studies that suggest that there is a role for shared common susceptibility genes that predispose to LPL/WM and certain lymphoproliferative disorders.25–27 A dominant or codominant gene signature, rather than a recessive one, is more likely given that we observed similar patterns of excess risk among parents, siblings, and offspring. In a questionnaire-based study of 103 patients with WM and of 272 unaffected relatives from 35 patients with multiple cases of WM and 46 with mixed WM/ related B-cell disorders kindred, and 28 patients with sporadic WM, the WM disease process appeared similar among patients regardless of family history.28 Patients with familial WM were more likely than unaffected relatives to report a history of autoimmune disease and infections. Patients with familial WM also were more likely to report exposure to farming, pesticides, wood dust, and organic solvents compared with unaffected family members.28 These results are in accordance with our previous results from Sweden.18
A few potential candidate genes may underlie familial LPL/WM and related conditions. For example, certain genetic polymorphisms (in germ line DNA) that affect the immune system, cell regulation, and DNA repair pathways have been associated with an increased risk of lymphomas and small lymphocytic lymphoma/chronic lymphocytic leukemia.29–32 By using germ line DNA, McMaster et al21 performed the first genome-wide linkage analysis of families at high risk for WM. In that study, 11 families with high-risk WM (a total of 122 individuals, including 34 patients with WM and 10 patients with IgM MGUS) were informative for linkage.21 The strongest evidence of linkage was found on chromosomes 1q and 4q when patients with WM and with IgM MGUS were both considered affected. Other locations suggestive of linkage were found on chromosomes 3 and 6.
Very recently, Treon et al,33 by using tumor cell (somatic) DNA extracted from LPL cells in the bone marrow, performed whole-genome sequencing on 54 patients with WM and found that 91% of these patients had a recurrent somatic mutation in chromosome 3 that corresponded to MYD88 L265P, a gene that encodes an adapter molecule involved in signal transduction in innate immunity. This finding provides novel clues on WM pathogenesis.
Clinical Implications
Understanding the underlying mechanisms of LPL/WM may have important clinical implications for its treatment and diagnosis. At the same time, the fact that both a history of chronic inflammation and autoimmune conditions are associated with an increased risk of developing LPL/WM and other lymphomas already has had implications in the treatment of these conditions. Pretreatment evaluation and long-term surveillance of our patients who received therapy for autoimmune diseases is recommended, while keeping in mind that the absolute lifetime risk of LPL/WM is very low. Thus, although the relative risk of LPL/WM is elevated for individual patients affected by immune-related conditions, the public health impact of these findings is small.
Familial clustering and the increased risk of developing LPL/WM and NHL/chronic lymphocytic leukemia in first-degree relatives of affected patients pointed toward a genetic link in the etiology of WM, very recently, a widely expressed somatic mutation in MYD88 has been identified. Despite this, confirmatory studies and the search for other putative genes are needed to fully understand the pathogenesis of LPL/WM, which may also include germ line mutations and individual polymorphisms.
On a clinical note, one has to keep in mind that the baseline risk of LPL/WM and other lymphoproliferative malignancies is very low in the general population. Consequently, despite the observed in-creased relative risk among first-degree relatives of patients with LPL/ WM, the absolute risk for a relative of a patient with LPL/WM to develop LPL/WM or another lymphoproliferative malignancy still remains very low. In addition, at the present time, early detection of LPL/WM is not likely to affect the outcome because asymptomatic LPL/WM is typically not treated and no increased medical surveillance is necessary at this time. However, in the future, it is possible that early intervention of defined targets will change the watch-and-wait paradigm. Overall, these insights might trigger complex medical considerations and imply ethical dilemmas. As treating physicians, we need to handle this information with care and provide and counsel our patients with clinically relevant information.
Future Directions
There is emerging evidence that supports a role for germ line genetic polymorphisms, somatic mutations, and even environmental factors (ie, a personal history of exposure to certain infectious diseases) to be intrinsically linked to the etiology of LPL/WM and other lymphoproliferative disorders (Figure 1). Results of recent studies have found an important somatic mutation in chromosome 3 that links the innate immune system to the lymphomagenesis of WM. Further confirmatory studies are needed to identify the exact role of certain mutations in WM as well as to identify other candidate genes and polymorphisms and their link to the immune system.
There also is a need to better define the role of immune-related conditions in the development of LPL/WM (Figure 1) by incorporating molecular and laboratory components to validate diagnoses and to evaluate more biologically homogeneous groupings of autoimmune disorders. Other areas of interest might be to study the natural history and pathogenesis of autoimmune disorders in individuals who subsequently develop LPL/WM. In addition, we need to better define the role of immune-related conditions on prognosis and survival in LPL/WM because this may have clinical implications for the treatment of patients with LPL/WM with autoimmune conditions.
In a note of novel drug development in WM, future studies are needed to characterize the molecular underpinnings of the findings by Treon et al.33 Their investigations show that the vast majority of patients with WM have a recurrent somatic mutation in chromosome 3 that corresponds to MYD88 L265P, a gene that encodes an adapter molecule involved in signal transduction in innate immunity.33 Their discovery provides novel clues on both pathogenesis and rational intervention strategies. For example, it seems reasonable to develop WM treatment trials by using inhibitors of IRAK4 kinase and other components of the MYD88 pathway.33
Acknowledgment
This research was supported by grants from the Swedish Cancer Society, Stockholm County Council, the Karolinska Institutet Foundations, and the Intramural Research Program of the National Institutes of Health, National Cancer Institute.
Footnotes
Disclosure
The authors have no conflict of interest to declare.
References
- 1.Gertz M. Waldenstrom macroglobulinemia: my way. Leuk Lymphoma 2013; 54: 464–71. [DOI] [PubMed] [Google Scholar]
- 2.Owen RG, Treon SP, Al-Katib A, et al. Clinicopathological definition of Walden-strom’s macroglobulinemia: consensus panel recommendations from the Second International Workshop on Waldenstrom’s Macroglobulinemia. Semin Oncol 2003; 30:110–5. [DOI] [PubMed] [Google Scholar]
- 3.Shaheen SP, Talwalkar SS, Lin P, et al. Waldenström macroglobulinemia: a review of the entity and its differential diagnosis. Adv Anat Pathol 2012; 19:11–27. [DOI] [PubMed] [Google Scholar]
- 4.Wang H, Chen Y, Li F, et al. Temporal and geographic variations of Waldenstrom macroglobulinemia incidence: a large population-based study. Cancer 2012; 118: 3793–800. [DOI] [PubMed] [Google Scholar]
- 5.Sekhar J, Sanfilippo K, Zhang Q, et al. Waldenstrom macroglobulinemia: a surveillance, epidemiology, and end results database review from 1988 to 2005. Leuk Lymphoma 2012; 53:1625–6. [DOI] [PubMed] [Google Scholar]
- 6.Dimopoulos MA, Kyle RA, Anagnostopoulos A, et al. Diagnosis and management of Waldenstrom’s macroglobulinemia. J Clin Oncol 2005; 23:1564–77. [DOI] [PubMed] [Google Scholar]
- 7.Björkholm M, Johansson E, Papamichael D, et al. Patterns of clinical presentation, treatment, and outcome in patients with Waldenstrom’s macroglobulinemia: a two-institution study. Semin Oncol 2003; 30:226–30. [DOI] [PubMed] [Google Scholar]
- 8.Kristinsson SY, Eloranta S, Dickman PW, et al. Patterns of survival in lymphoplasmacytic lymphoma/Waldenstrom macroglobulinemia: a population-based study of 1,555 patients diagnosed in Sweden from 1980 to 2005. Am J Hematol 2013; 88:60–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kyle RA, Therneau TM, Rajkumar SV, et al. Long-term follow-up of IgM monoclonal gammopathy of undetermined significance. Blood 2003; 102:3759–64. [DOI] [PubMed] [Google Scholar]
- 10.Landgren O, Kyle RA, Rajkumar SV. From myeloma precursor disease to multiple myeloma: new diagnostic concepts and opportunities for early intervention. Clin Cancer Res 2011; 17:1243–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aoki H, Takishita M, Kosaka M, et al. Frequent somatic mutations in D and/or JH segments of Ig gene in Waldenstrom’s macroglobulinemia and chronic lymphocytic leukemia (CLL) with Richter’s syndrome but not in common CLL. Blood 1995; 85:1913–9. [PubMed] [Google Scholar]
- 12.Wagner SD, Martinelli V, Luzzatto L. Similar patterns of V kappa gene usage but different degrees of somatic mutation in hairy cell leukemia, prolymphocytic leukemia, Waldenstrom’s macroglobulinemia, and myeloma. Blood 1994; 83: 3647–53. [PubMed] [Google Scholar]
- 13.Walsh SH, Laurell A, Sundström G, et al. Lymphoplasmacytic lymphoma/Walden-strom’s macroglobulinemia derives from an extensively hypermutated B cell that lacks ongoing somatic hypermutation. Leuk Res 2005; 29:729–34. [DOI] [PubMed] [Google Scholar]
- 14.Martín-Jiménez P, García-Sanz R, Balanzategui A, et al. Molecular characterization of heavy chain immunoglobulin gene rearrangements in Waldenstrom’s macroglobulinemia and IgM monoclonal gammopathy of undetermined significance. Haema-tologica 2007; 92:635–42. [DOI] [PubMed] [Google Scholar]
- 15.Linet MS, Humphrey RL, Mehl ES, et al. A case-control and family study of Waldenstrom’s macroglobulinemia. Leukemia 1993; 7:1363–9. [PubMed] [Google Scholar]
- 16.Giordano TP, Henderson L, Landgren O, et al. Risk of non-Hodgkin lymphoma and lymphoproliferative precursor diseases in US veterans with hepatitis C virus. JAMA 2007; 297:2010–7. [DOI] [PubMed] [Google Scholar]
- 17.Koshiol J, Gridley G, Engels EA, et al. Chronic immune stimulation and subsequent Waldenstrom macroglobulinemia. Arch Intern Med 2008; 168:1903–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kristinsson SY, Koshiol J, Björkholm M, et al. Immune-related and inflammatory conditions and risk of lymphoplasmacytic lymphoma or Waldenstrom macroglobulinemia. J Natl Cancer Inst 2010; 102:557–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lindqvist EK, Goldin LR, Landgren O, et al. Personal and family history of immune-related conditions increase the risk of plasma cell disorders: a population-based study. Blood 2011; 118:6284–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fine JM, Lambin P, Massari M, et al. Malignant evolution of asymptomatic monoclonal IgM after seven and fifteen years in two siblings of a patient with Walden-strom’s macroglobulinemia. Acta Med Scand 1982; 211:237–9. [DOI] [PubMed] [Google Scholar]
- 21.McMaster ML, Goldin LR, Bai Y, et al. Genomewide linkage screen for Walden-strom macroglobulinemia susceptibility loci in high-risk families. Am J Hum Genet 2006; 79:695–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ogmundsdóttir HM, Jóhannesson GM, Sveinsdóttir S, et al. Familial macroglobulinaemia: hyperactive B-cells but normal natural killer function. Scand J Immunol 1994; 40:195–200. [DOI] [PubMed] [Google Scholar]
- 23.Treon SP, Hunter ZR, Aggarwal A, et al. Characterization of familial Walden-strom’s macroglobulinemia. Ann Oncol 2006; 17:488–94. [DOI] [PubMed] [Google Scholar]
- 24.Kristinsson SY, Björkholm M, Goldin LR, et al. Risk of lymphoproliferative disorders among first-degree relatives of lymphoplasmacytic lymphoma/Waldenstrom macroglobulinemia patients: a population-based study in Sweden. Blood 2008; 112: 3052–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goldin LR, Landgren O, McMaster ML, et al. Familial aggregation and heterogeneity of non-Hodgkin lymphoma in population-based samples. Cancer Epidemiol Biomarkers Prev 2005; 14:2402–6. [DOI] [PubMed] [Google Scholar]
- 26.Goldin LR, Pfeiffer RM, Li X, et al. Familial risk of lymphoproliferative tumors in families of patients with chronic lymphocytic leukemia: results from the Swedish family-cancer database. Blood 2004; 104:1850–4. [DOI] [PubMed] [Google Scholar]
- 27.Goldin LR, Pfeiffer RM, Gridley G, et al. Familial aggregation of Hodgkin lymphoma and related tumors. Cancer 2004; 100:1902–8. [DOI] [PubMed] [Google Scholar]
- 28.Royer RH, Koshiol J, Giambarresi TR, et al. Differential characteristics of Walden-strom macroglobulinemia according to patterns of familial aggregation. Blood 2010; 115:4464–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rudd MF, Sellick GS, Webb EL, et al. Variants in the ATM-BRCA2-CHEK2 axis predispose to chronic lymphocytic leukemia. Blood 2006; 108:638–44. [DOI] [PubMed] [Google Scholar]
- 30.Hohaus S, Massini G, D’Alo F, et al. Association between glutathione S-transferase genotypes and Hodgkin’s lymphoma risk and prognosis. Clin Cancer Res 2003; 9:3435–40. [PubMed] [Google Scholar]
- 31.Skibola CF, Curry JD, Nieters A. Genetic susceptibility to lymphoma. Haemato-logica 2007; 92:960–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Slager SL, Camp NJ, Conde L, et al. Common variants within 6p21.31 locus are associated with chronic lymphocytic leukaemia and, potentially, other non-Hodgkin lymphoma subtypes. Br J Haematol 2012; 159:572–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Treon SP, Xu L, Yang G, et al. MYD88 L265P somatic mutation in Waldenstrom’s macroglobulinemia. N Engl J Med 2012; 367:826–33. [DOI] [PubMed] [Google Scholar]