Abstract
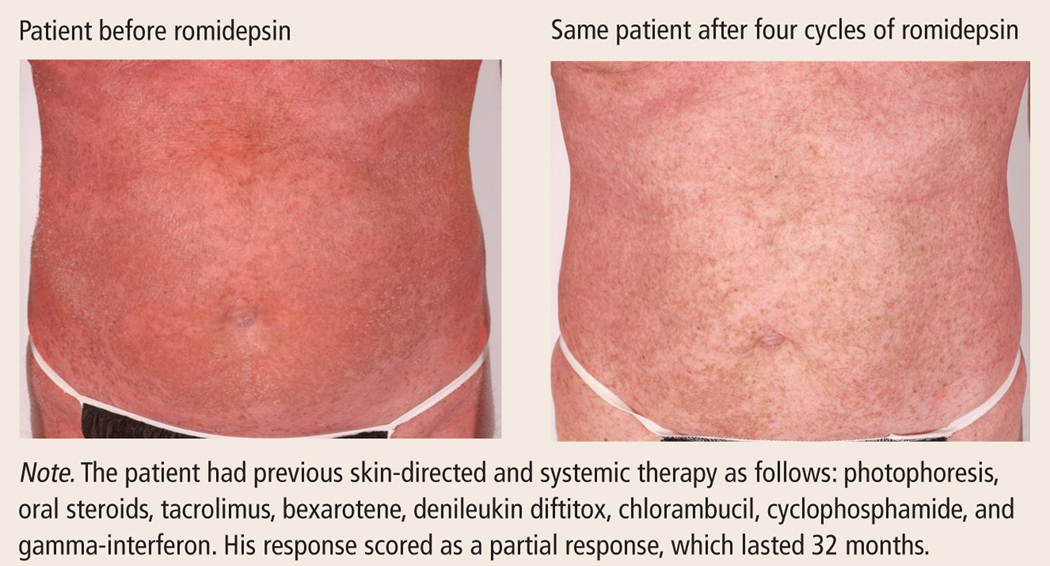
Patients with cutaneous T-cell lymphoma (CTCL) have a rare, disfiguring, and life-threatening subtype of non-Hodgkin lymphoma primarily localized to the skin. Their immune systems are altered and their skin is compromised. In addition, they are highly prone to infections—the most common cause of death in patients with this disease. Patients presenting with early-stage disease involvement typically are treated with topical therapies; patients with advanced-stage and recurrent disease require systemic treatment. Specialized knowledge is required by oncology healthcare providers to manage the wide array of symptoms experienced by these patients as a part of the natural course of this disease. A new drug, romidepsin, approved by the U.S. Food and Drug Administration, is indicated in the treatment of relapsed CTCL. The authors discuss use of romidepsin in the context of CTCL and the information needed to safely administer romidepsin and manage its side effects.
Graphical Abstract

cutaneous T-cell lymphoma (CTCL) is a heterogeneous category of non-Hodgkin lymphoma involving the skin as the primary site of malignant T-lymphocyte proliferation. The malignant skin-homing lymphocytes also invade and traffic between the lymph system, blood, and visceral organs, creating variable and complex clinical presentations. Appearance, degree of blood involvement, histology, immunophenotypic profile, and prognosis can vary widely among patients, making treatment and nursing care a challenge. Mycosis fungoides (MF) and its leukemic variant, Sézary syndrome (SS), are the most common types of CTCL. A review of the rare disease CTCL is presented, followed by a discussion of the clinical development for romidepsin, which was approved by the U.S. Food and Drug Administration (FDA) for treatment of CTCL. Finally, the article will summarize drug administration interventions and nursing considerations for this complicated patient population.
Because treatment of patients with CTCL often moves from topical in early stage to systemic therapies in more advanced-stage disease, both dermatology and oncology are involved in determining the course of treatment. The CTCL disease course can be indolent or it can demonstrate rapid progression. Of the systemic options available for CTCL, traditional therapy includes biologics and a wide array of chemotherapeutic agents, maintaining control with varying success. Strategies to improve outcomes are an important area of clinical research for this patient population.
The chronicle of a new drug starts as compounds are screened preclinically for potential therapeutic value. For every 5,000 compounds screened, about five agents reach clinical trials in human participants and one of those, on average, will eventually be approved by the FDA (DiMasi & Grabowski, 2007). In the United States, new drug development is controlled by the FDA through a series of laws and regulations. Drug development follows a multistep process from the investigational new drug application, to clinical testing, and then to submission of data to the FDA, which has the authority to determine whether a drug should be approved for general clinical use. Romidepsin followed this process.
Cutaneous T-Cell Lymphoma: An Orphan Disease
MF was first described more than 200 years ago by Jean Louise Alibert (1806). The term CTCL was created in the 1970s to unify all cutaneous-based lymphomas sharing a common T-cell phenotype, including MF and SS (Lutzner et al., 1975). To improve diagnosis and treatment, cutaneous lymphomas were defined and categorized according to clinical and pathological criteria by the World Health Organization and European Organisation for the Research and Treatment of Cancer (EORTC). This classification system differentiates cutaneous lymphomas from systemic lymphomas with cutaneous presentations (Willemeze et al., 2005). Of note, CTCL is a disease classification and MF and SS are distinct diagnoses within this classification and should not be used interchangeably (Booher, McCann, & Tawa, 2008).
MF typically is characterized by an indolent clinical course with disease progression taking years and occasionally decades to develop. MF has an array of cutaneous appearances including patches, plaques, and tumors, presenting singly or in combination. The cutaneous presentation often follows the “bathing suit” distribution, although the degree of body surface area affected varies. If a patient presents with tumors, differentiating between MF and non-MF subtypes of CTCL is fundamental (Olsen et al., 2007). Regardless of the skin appearance, histology of the epidermis and dermis reveals abnormal T cells with ceribriform or convoluted nuclei. Another presentation is erythroderma (cutaneous erythema on more than 80% of the body surface area), which can result as progression of preexisting MF or can occur spontaneously. Patients with erythroderma are at a higher risk of concurrent blood involvement than patients with limited skin involvement (Vonderheid et al., 2002).
SS is characterized by a more aggressive course relative to that of MF, and it typically presents with the classic triad of circulating Sézary cells in the peripheral blood (lymphocytes having ceribriform nuclei in the peripheral blood), erythroderma, and lymphadenopathy (Willemze et al., 2005). Trafficking of malignant T cells between the blood, lymphatic system, and skin occurs more easily in SS. A patient with SS may present with one or more of the following symptoms: alopecia, ectropian (a turning out of the eyelid), keratoderma (skin thickening) of the palms and soles, nail dystrophy, pruritus, extensive skin scaling and shedding, temperature dysregulation, and cutaneous edema of the lower extremities.
Epidemiology
Despite MF and SS being rare disease states, Criscione and Weinstock (2007) reported the incidence more than tripled from 1973–2002 in the United States and represents 3.9% of all non-Hodgkin lymphomas. MF occurs more frequently than SS, accounting for 72% and 2.5% of all CTCLs, respectively. The male to female ratio is 2:1, incidence increases with age, and an increased incidence of CTCL has been noted in African Americans. Increased incidence was found in areas with high physician density, high family income, higher education, and high home property values (Criscione & Weinstock, 2007). Those findings may represent a difference in access to medical care as well as increased medical awareness.
Etiology
The pathogenesis of MF and SS is unknown, and although evidence has been gathered to suggest that the disease may originate with an infectious process or environmental or genetic factor, no single theory has been proven (Hwang, Janik, Jaffe, & Wilson, 2008; Morales Suarez-Varela, Llopis Gonzalez, Marquina Vila, & Bell, 2000).
MF and SS are characterized by an altered immune environment. Neoplastic helper CD4-positive T cells home to the skin, proliferate through clonal expansion, and achieve clonal dominance (Girardi, Heald, & Wilson, 2004). The malignant T cells become activated and release cytokines and chemokines that attract antigen-presenting cells, causing pruritus, scaling, and thickening of the epidermis (Girardi et al., 2004).
Evaluation and Staging
An accurate patient assessment is essential for staging MF or SS; malignant T cells circulate between the skin, blood, and lymph system, and presence at each site is used to determine the stage of disease. The International Society for Cutaneous Lymphomas and the EORTC developed recommendations for a more accurate evaluation and staging of patients with MF and SS (Olsen et al., 2007), and those recommendations are supported by the National Comprehensive Cancer Network (Horwitz, Olsen, Duvic, Pierliugi, & Kim, 2008). The recommendations follow.
Complete history and physical, including determining the type of lesions and sum of affected skin surface as well as identifying palpable lymph nodes or organomegaly
Skin biopsy of suspected lesions, including immunophenotype for T cell markers CD2, CD3, CD4, CD5, CD7, and CD8
Blood tests, such as a comprehensive chemistry panel to include hepatic functions and complete blood count; assess for clonality and SS by T cell gene rearrangement and flow cytometry, respectively.
Radiologic tests; depends on assessment and suspected disease state
Lymph node biopsy; depends on assessment and suspected disease state
Staging for MF and SS is important for determining prognosis and treatment. Patients diagnosed with early-stage MF and with only limited skin involvement have a better prognosis than patients diagnosed with or evolving to advanced-stage disease and extracutaneous involvement (Kim, Liu, Mraz-Gernhard, Varghese, & Hoppe, 2003) (see Table 1 and Figure 1).
TABLE 1.
ISCL/EORTC Revision to the Staging of Mycosis Fungoides and Sézary Syndrome
| Stage | T | N | M | B |
|---|---|---|---|---|
| IA | 1 | 0 | 0 | 0, 1 |
| IB | 2 | 0 | 0 | 0, 1 |
| II | 1, 2 | 1, 2 | 0 | 0, 1 |
| IIB | 3 | 0–2 | 0 | 0, 1 |
| III | 4 | 0–2 | 0 | 0, 1 |
| IIIA | 4 | 0–2 | 0 | 0 |
| IIIB | 4 | 0–2 | 0 | 1 |
| IVA1 | 1–4 | 0–2 | 0 | 2 |
| IVA2 | 1–4 | 3 | 0 | 0–2 |
| IVB | 1–4 | 0–3 | 1 | 0–2 |
B—blood; EORTC—European Organisation for the Research and Treatment of Cancer; ISCL—International Society for Cutaneous Lymphomas; M—visceral; N—node; T—tumor
Note. From “Revisions to the Staging and Classification of Mycosis Fungoides and Sézary Syndrome: A Proposal of the International Society for Cutaneous Lymphomas (ISCL) and the Cutaneous Lymphoma Task Force of the European Organization of Research and Treatment of Cancer (EORTC),” by E. Olsen, E. Vonderheid, N. Pimpinelli, R. Willemze, Y. Kim, R. Knobler, … S. Whittaker, 2007, Blood, 110, p. 1719. Copyright 2007 by the American Society of Hematology. Reprinted with permission.
FIGURE 1. International Society for Cutaneous Lymphomas (ISCL) and European Organisation for the Research and treatment of Cancer Revision to the Classification of Mycosis Fungoides and Sézary Syndrome.
Note. From “Revisions to the Staging and Classification of Mycosis Fungoides and Sézary Syndrome: A Proposal of the International Society for Cutaneous Lymphomas (ISCL) and the Cutaneous Lymphoma Task Force of the European Organization of Research and Treatment of Cancer (EORTC), by E. Olsen, E. Vonderheid, N. Pimpinelli, R. Willemze, Y. Kim, R. Knobler, … S. Whittaker, 2007, Blood, 110, p. 1715. Copyright 2007 by the American Society of Hematology. Reprinted with permission.
Treatment
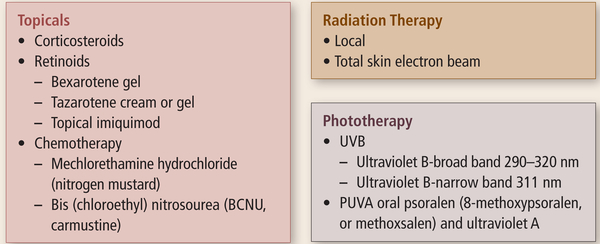
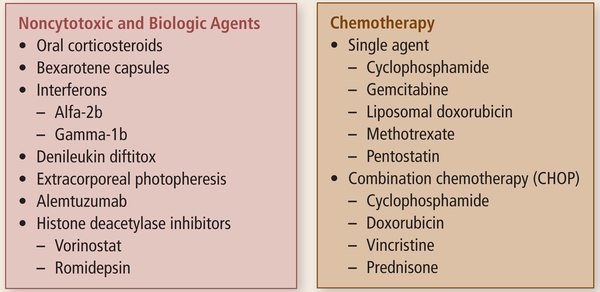
Treatment for MF and SS are contingent on the clinical stage and prognosis (Horwitz, 2008). Treatment decisions should include the patient’s clinical presentation, avoiding immunosuppression and related symptoms, reducing toxicities, and improving quality of life. A variety of unique therapies are used to treat MF and SS, including both skin-directed and systemic agents (Lansigan & Foss, 2010) (see Figures 2 and 3). Patients with stage I disease can be sufficiently treated with skin-directed therapies and may never require systemic treatment (Horwitz, 2008). One of the most effective and frequently used skin-direct therapies is phototherapy with psoralen plus ultraviolet A (PUVA). Psoralen is an oral agent administered to sensitize tumor cells; patients then are exposed to the effects of ultraviolet A light for 1.5–2 hours after ingestion of the tablet. In early-stage disease, most patients have a healthy cellular immune response. Remission is likely and occurs in about 60% of patients (Lansigan & Foss, 2010). Therapies with long-term treatment-related toxicities should be avoided in early-stage disease (Lansigan & Foss, 2010). Patients with worse prognostic factors, those with stage IIB disease or higher (Horwitz, 2008), or those with earlier stages for whom skin-directed therapies alone prove ineffective (Lansigan & Foss, 2010) will most likely need to use a combination of skin-directed and systemic therapies (Horwitz, 2008). As a patient’s disease advances into stage IV, more intensive systemic therapies may be required. Patients who have very advanced disease, who have bulky lymph node or visceral disease, and who are unresponsive to other treatment methods should follow chemotherapy regimens used for aggressive lymphomas. To date, the only curative therapy for MF and SS is an allogeneic stem cell transplantation (Lansigan & Foss, 2010), although research continues in an effort to discover novel agents to treat MF and SS. Before the approval of romidepsin, FDA-approved systemic therapies for CTCL included bexarotene, a retinoid administered as a daily oral medication; denileukin diftitox, a recombinant protein conjugate of interleukin-2 and diphtheria toxin fragments administered IV daily for five consecutive days every 21 days; and vorinostat, an orally administered agent that, like romidepsin, is a histone deacetylase (HDAC) inhibitor (Lansigan & Foss, 2010). Romidepsin, the focus of this article, was approved by the FDA for patients with CTCL who have received at least one prior systemic therapy.
FIGURE 2. Skin-Directed Therapies for Mycosis Fungoides and Sézary Syndrome.
Note. Cited with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines™) for Mycosis Fungoides/Sézary Syndrome V3.2011. © 2011 National Comprehensive Cancer Network, Inc. All rights reserved. The NCCN Guidelines™ and illustrations herein may not be reproduced in any form for any purpose without the express written permission of the NCCN. To view the most recent and complete assessment and treatment information from the NCCN Guideline, go online to NCCN.org. National Comprehensive Cancer Network®, NCCN®, NCCN Guidelines™, and all other NCCN content are trademarks owned by the National Comprehensive Cancer Network, Inc.
FIGURE 3. Systemic Therapies for Mycosis Fungoides and Sézary Syndrome.
Note. Cited with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines™) for Mycosis Fungoides/Sézary Syndrome V3.2011. © 2011 National Comprehensive Cancer Network, Inc. All rights reserved. The NCCN Guidelines™ and illustrations herein may not be reproduced in any form for any purpose without the express written permission of the NCCN. To view the most recent and complete assessment and treatment information from the NCCN Guideline, go online to NCCN.org. National Comprehensive Cancer Network®, NCCN®, NCCN Guidelines™, and all other NCCN content are trademarks owned by the National Comprehensive Cancer Network, Inc.
Histone Deacetylase Inhibitors
HDAC inhibitors work to remove acetyl groups from lysine during DNA synthesis to regulate gene expression in cells (Piekarz, Sackett, & Bates, 2007). An association exists between aberrant HDAC activity and cancer that occurs through translocation, amplification, overexpression, and mutation of genes (Richon, Garcia-Vargas, & Hardwick, 2009). HDAC inhibitors represent a new class of antineoplastic agents. Inhibition of HDAC enzymatic activity promotes the acetylation of histones, allowing relaxation of DNA from the neutralization of the positive charges on histone lysines. The uncoiled and loosened DNA is thought to promote the transcription of genes that inhibit cell growth (see Figure 4).
FIGURE 4. Mechanism of Action in HDAC Inhibitors.

In vitro, HDAC inhibitors have been shown to induce cell cycle arrest, cell maturation, and gene expression changes. Apoptosis of cancer cells also occurs, although whether modulation of gene expression is the event causing cell death is unclear (Johnstone & Licht, 2003). Some cellular effects of HDAC inhibitors may be attributed to the increased acetylation of some cytoplasmic proteins (Piekarz et al., 2009; Schrump, 2009).
Many HDAC inhibitors are at various stages of drug development (Marks & Xu, 2009). Vorinostat, panobinostat, belinostat, entinostat, and romidepsin are a partial list under investigation as monotherapy or in combination trials including studies in patients with T-cell lymphoma (Marks & Xu, 2009; Piekarz & Bates, 2009). Romidepsin originally was acquired from the bacteria Chromobacterium violaceum. In its first in vitro testing, romidepsin was shown to suppress the growth of tumors in mice (Ueda, Manda, et al., 1994). Additional in vitro data in human tumor cell lines showed that romidepsin strongly induced cell cycle arrest (Ueda, Nakajima, Hori, Goto, & Okuhara, 1994). Those studies led to additional preclinical work culminating in the submission of an investigational new drug (IND) to the FDA. The investigational new drug outlines the general investigative plan (Title 21 CFR, Part 312.5, 2010). A sponsor is defined as the party responsible for initiating a clinical investigation (Title 21 CFR, Part 312.5, 2010). Communication between the sponsor and the FDA, reporting significant events and updates, occurs throughout the course of clinical investigation (Title 21 CFR, Part 312.32, 2010).
Phase I Single-Agent Romidepsin Trial
Two phase I trials for patients with advanced, incurable cancers were carried out for romidepsin, both following a traditional phase I study design, and aimed at defining the maximum tolerated dose (MTD), identifying the safety profile, and determining the pharmacokinetic properties. Sandor et al. (2002) used a day 1 and 5 schedule every 21 days, and Marshall et al. (2002) investigated a romidepsin schedule on days 1, 8, and 15, every 28 days. Romidepsin was administered as a four-hour infusion through a central venous catheter, although it eventually was determined that this was not a critical mode of IV administration. Using Common Toxicity Criteria, version 2.0, dose-limiting toxicity (DLT) was defined as any grade 4 hematologic toxicity, or any grade 3 or 4 nonhematologic toxicity occurring during the first cycle (National Cancer Institute Cancer Therapy Evaluation Program, 1999). MTD was defined as the dose in which more than one subject out of six experienced DLTs. The study showed DLTs, including fatigue, nausea, vomiting, and thrombocytopenia. The MTD was found to be 17.8 mg/m2 on the day 1 and 5 schedule and 14 mg/m2 on the day 1, 8, and 15 schedule. Nonhematologic toxicities in patients treated with multiple cycles were similar to the first cycle toxicities (Sandor et. al., 2002). After the MTD had been defined, patients with T-cell lymphoma were accrued, and activity was observed (Piekarz et al., 2001). The activity observed in the Sandor et al. (2002) phase I study led to the development of a phase II trial in those diseases.
Phase II Romidepsin Monotherapy Trials
Phase II trials use the recommended dose determined during phase I to evaluate anti-cancer activity (i.e., response). Additional safety evaluations also are important endpoints of these trials. The phase II trial conducted by Sandor et al. (2002) evaluated the efficacy of romidepsin in patients with T-cell lymphoma and the long-term safety of romidepsin. Included were patients with relapsed, refractory, or advanced CTCL who had received two or less systemic cytotoxic chemotherapy agents or combinations. Responses occurring early in the study allowed the trial to add additional cohorts, including patients who received more than two cytotoxic regimens (see Figures 5, 6, and 7). Seventy-one patients were enrolled at the time of analysis; 87% had advanced disease (Piekarz et al., 2007). Patients had received a median of four prior regimens, including cytotoxic, topical, biologic, and radiologic therapies. The regimen initially given was 18 mg/m2 on days 1 and 5 of a 21-day cycle, as in the phase I trial conducted by Sandor et al. (2002), but was amended to the 14 mg/m2 schedule (which was thought to have somewhat fewer side effects) (Marshall et al., 2002). Treatment modifications included: doses held for absolute neutrophil count (ANC) below 0.5 K cells/mcl; platelet count below 50 K cells/mcl, or grade 3 nonhematologic toxicity on the day of treatment and initial dose reduction from 14 mg/m2 to 10.5 mg/m2 or second dose reduction from 10.5 mg/m2 to 8 mg/m2 for ANC of 0.5–1 K cells/mcl; or platelet count of 50–75 K cells/mcl. In all patients, 17% of doses required reductions. The protocol mandated permanent dose reductions for previously held doses. Dose reduction most commonly occurred from thrombocytopenia. Results from that study demonstrated an overall response rate of 34%, four complete responses, and 20 2007).
FIGURE 5. Patient With Sézary Syndrome Before and After Romidepsin.
Note. Photos courtesy of the National Institutes of Health. Used with permission.
FIGURE 6. Patient With Patch or Plaque-Stage Disease Before and After Romidepsin.
Note. Photos courtesy of the National Institutes of Health. Used with permission.
FIGURE 7. Patient With Tumor-Stage Disease Before and After Romidepsin.
Note. Photos courtesy of the National Institutes of Health. Used with permission.
A second phase II trial was sponsored by Gloucester Pharmaceuticals and confirmed the results from the Piekarz et al. (2007) study (Whittaker et al., 2010). Among 96 patients with treatment-refractory CTCL enrolled, the overall response rate was 34%, which included six patients with complete response. Interestingly, of 68 patients with advanced disease, 26 (38%) achieved a response, including five with complete response. A secondary finding of improvement in pruritus was observed in 28 of 65 patients (43%) who had moderate or severe symptoms at baseline irrespective of whether they achieved an objective response (Whittaker et al., 2010).
Safety Data
Side effects of romidepsin reflect those of HDAC inhibitors: primarily gastrointestinal disturbance, fatigue, and thrombocytopenia. To avert the nausea, patients in both trials were supported with prophylactic antiemetics. IV hydration was added for marked nausea, fever, or hypovolemia (Piekarz et al., 2007; Whittaker et al., 2010). Electrocardiogram changes also were noted but were not felt to be clinically significant (Piekarz et al., 2006, 2007; Whittaker et al., 2010). Patients who discontinued therapy because of adverse events did so because of infection, fatigue, QT prolongation, and dyspnea (Gloucester Pharmaceuticals, 2009). Deaths in both studies were primarily from progressive disease. In addition to progressive disease, Whittaker et al. (2010) reported deaths caused by cardiopulmonary failure and acute renal insufficiency. Potentially related deaths reported by Piekarz et al. (2009) were primarily from infection. In addition, one patient died with preexisting acute respiratory distress syndrome; another patient with valvular heart disease, who developed atrial fibrillation, started warfarin and digoxin and died unexpectedly one day after receiving romidepsin (Piekarz et al., 2009). That death led to the revision of the clinical trial entry criteria to exclude patients with cardiac disease.
Approval
Once enough evidence is gathered to support safety and efficacy, a new drug application (NDA) is submitted to the FDA for review and potential approval. The application contains all preclinical data, clinical study information, manufacturing plans, and labeling details collected during study investigations. The FDA reviews the application to determine whether a drug or biologic agent provides benefit by looking for evidence of improved survival, prolonged time to progression of disease, symptom palliation, and/or a favorable riskbenefit profile. Approval by the FDA gives the sponsor license to label, market, and transport a drug for a specific indication. Romidepsin received approval by the FDA on November 5, 2009, for patients with CTCL who have received at least one prior systemic chemotherapy agent or combination (Gloucester Pharmaceuticals, 2009). Package insert precautions include monitoring of laboratory tests for hematologic parameters and ensuring that potassium and magnesium levels remain within normal range. Those recommendations are in place to decrease the risk of QT prolongation, which can be eliminated or significantly decreased (Piekarz et al., 2006). A thorough medication history must be taken for evaluation of drugs that inhibit or induce CYP3A4 enzymes or prolong the QT, as those drugs may potentiate adverse effects when taken with romidepsin (Piekarz et al., 2009). Coumadin and coumadin derivatives may interact with romidepsin (Gloucester Pharmaceuticals, 2009). The FDA-approved dose of romidepsin for treatment of CTCL is 14 mg/m2 (14 mg x body surface area), on days 1, 8, and 15 of a 28-day cycle. Therapy should continue as long as the patient receives benefit and tolerates the drug (Gloucester Pharmaceuticals, 2009).
Nursing Considerations
Nursing assessments and interventions overlap between those constituting disease-specific care of a patient whose major protective barrier, the skin, is compromised, and those who are romidepsin-associated.
Managing skin-care issues: Patients with CTCL must have a meticulous assessment of their skin and associated lesions. Nurses need to follow a careful regimen that reduces the risk of bacterial infection and prevents sepsis (National Cancer Institute, 2010). Axelrod, Lorber, and Vonderheid (1992) performed a 12-year retrospective study and found the most common site of bacterial infection was skin, followed by bloodstream, lung, and genitourinary tract. They also found that bacterial infections were the major cause of morbidity and mortality. Basic wound care healing principles guide the topical treatment of the patient receiving romidepsin. First, the skin care goals for the patient are to prevent infection, moisturize, and prevent bleeding. To reduce the bacterial load of the skin and reduce infection, patients were administered a Dakin’s bath prior to any line placement. That consisted of one bottle of full-strength sodium hypochlorite solution in a whirlpool tub for 20–30 minutes (Cornwell, Arnold-Long, Barss, & Varnado, 2010). Dakin’s baths were administered on a daily basis if the patient was able to tolerate it. Hibiclens® is another antibacterial product used when patients bathe (Duvic, 2001). If debriding of the skin is warranted, surgical scrub brushes with Hibiclens in the bath or shower may help remove the dead and necrotic skin. Moisturizing the patient’s skin is very important to maintain elasticity and facilitate healing. Adhering to the rule of moisturizing within three minutes of leaving the bath, before water has a chance to evaporate, is suggested (Simpson, 2010). Skin moisturizing reduces flaking, which in turn reduces the bacterial count on the skin and, again, helps prevent infection. Increasing skin elasticity can prevent injury and decrease bleeding.
CTCL has various disease presentations—from a diffuse flare of reddened edematous skin, not unlike a bad sunburn, to multiple raised nodular lesions varying in size that can become ulcerated as they progress in severity or break down as a result of treatment. When those lesions become ulcerated, serous or sanguinous leakage occurs. Drainage must be managed with dressings while preventing wound adherence to the dressing. First, changing the bed linen at home and in the hospital, at least once daily, is considered part of the dressing change because drainage and debris attract bacteria. Second, the use of non-stick dressings such as petroleum-based product dressings with bismuth, silicone-backed dressings, and foams are suggested to control the drainage (Goldberg & McGinn-Byer, 2007). Reinforcing dressing and frequent changes often are necessary. Infected wounds may require topical metronidazole after soaks as an adjunct to systemic antibiotics (Goldberg & McGinn-Byer, 2007).
Patients with MF or SS often feel cold, which hinders skin and wound care. Baths can be an unpleasant experience if the atmosphere is not conducive to bathing. Pain is another major consideration. Ulcers often surface at the level of the skin where the nerves end, resulting in serious pain issues. Patients tolerate dressing changes better if pain medication is administered prior to dressing changes. Nursing care requires diligence with dressings, wound care, and daily hygiene, including linen changes.
Assessing Laboratory Data
Laboratory data should be assessed prior to romidepsin administration. Electrolyte levels, specifically the magnesium and potassium levels, should be in the high-normal range as a preventive strategy to mitigate potential cardiac effects (Piekarz et al., 2006). Hypoalbuminemia and hypocalcemia have been reported as possible side effects of romidepsin, although those findings also are common in patients with advanced CTCL. Romidepsin has been associated with thrombocytopenia, leucopenia, granulocytopenia, and anemia (Sandor et al., 2002). An ANC of less than 1.5 K/mcl, platelets less than 75 K/mcl, febrile neutropenia, or thrombocytopenia necessitating platelet transfusion are rare and require delay or dose reduction. Dosing may be reduced to 10 mg/m2 once counts return to baseline. If grade 3 or 4 toxicities recur after dose reduction, romidepsin should be discontinued (Gloucester Pharmaceuticals, 2009).
The most frequently reported side effects were fatigue, nausea, vomiting, dysguesia, dehydration, and loss of appetite (Gloucester Pharmaceuticals, 2009). Antiemetics are recommended prior to romidepsin administration and should begin from the start of therapy. Granisetron was used by Piekarz et al. (2009) for patients with CTCL, as it reportedly has less effect on the QT interval than other antiemetics (Keefe, 2002). Oral antiemetics for 24–48 hours postinfusion are generally necessary to prevent nausea in patients who experience it. Some patients do not require continuation of antiemetics once experience is gained with romidepsin. In addition, patients reported lessening of side effects after receiving post-treatment hydration (Piekarz et al., 2009). One liter of dextrose/saline IV fluid has been anecdotally found to aid in managing side effects of romidepsin administration. Patients also should be instructed to orally hydrate (see Figure 8).
FIGURE 8.
Education for Patients Receiving Romidepsin.
Establishing IV access
Careful attention to invasive lines is a priority for the notably high risk for infection. Avoid central lines if possible because of risk of infection. Romidepsin is not a vesicant, and may be infused via a peripheral line (Marshall et al., 2002).
Administration of romidepsin has been associated with electrocardiographic changes, including T wave flattening, T wave inversion, and QT prolongation (Sandor et al., 2002). It has been concluded that the electrocardiogram abnormalities observed are not indicative of myocardial dysfunction or myocardial damage (Piekarz et al., 2006). The package insert (Gloucester Pharmaceuticals, 2009) warns:
In patients with congenital long QT syndrome, patients with a history of significant cardiovascular disease, and patients taking antiarrhythmic medicines or medicinal products that lead to significant QT prolongation, appropriate cardiovascular monitoring precautions should be considered, such as the monitoring of electrolytes and ECGs at baseline and periodically during treatment.
Nurses must meticulously document all medications that could potentiate QT prolongation, updating this history and evaluation of current medications at regular intervals. Because of the risk of QT prolongation, the potassium and magnesium should be normal prior to dose administration (Gloucester Pharmaceuticals, 2009). During the course of Piekarz et al. (2006), a corrected potassium to greater than 4 mmol/L and magnesium greater than 0.85 mmol/L was required.
Evaluating concurrent medications
Romidepsin is metabolized by CYP3A4. Strong CYP3A4 inhibitors (e.g., ketoconazole, intraconazole, clarithromycin, atazamavir, indimavir, nefazodone, nelfinavir, ritonavir, saquinavir, telithromycin, voriconazole) may increase concentrations of romidepsin, and strong CYP3A4 inducers (e.g., dexamethasone, carbamazepine, phenytoin, rifampin, rifabutin, rifapentine, phenobarbital) may decrease concentrations (Gloucester Pharmaceuticals, 2009). Nurses need to perform ongoing assessment of the patient’s current medications and inform physicians of concomitant use of those medications.
Supporting patients and families
Nurses may initiate referrals to social work, nutrition, and outpatient laboratories. Antiemetic therapy, nutrition, hydration, and wound care regimens require continued reevaluation. Some patients experienced profound side effects, significantly affecting their ability to work or perform self-care activities of daily living. The calendar of treatment should be carefully reviewed by the patient and nurse. Caring for a patient with CTCL involves more than just administering the drug; care is required for the entire patient and family network. Patients can become exhausted from the rigors of treatment and supportive care, and caregiver exhaustion from extensive wound care regimens and other care requirements also is a concern. Nurses, recognizing this, must provide support and resources as appropriate.
Conclusions
Patients with CTCL display a complex clinical picture. Their clinical presentation and length of prior therapy will affect how they respond to current treatment and can exacerbate clinical symptoms. In addition, the stage of a patient’s disease and the degree of skin involvement will affect the supportive care needed for patients’ ongoing safety. Romidepsin is a valuable new drug with an FDA-approved indication for patients with CTCL. Nurses will initiate and monitor much of this care. Therefore, nurses should have a clear picture of patient’s disease-specific needs and a firm understanding of how to safely administer this effective new treatment.
Implications for Practice.
Skin care is the most important nursing consideration for patients with cutaneous T-cell lymphoma. Without vigorous prophylactic skin care measures, bacterial infections will lead to treatment stoppage, morbidity, and mortality.
Avoid central line IV access if possible during initial treatment when the skin barrier is compromised. To avoid lethal secondary infections, never leave in a central line between doses.
Normalized levels of serum potassium and magnesium are important to prevent QT prolongation.
Concurrent medications need to be discussed with the patient and evaluated for their interaction with romidepsin at each treatment interval—not just on day 1 of a cycle—to avoid potent interactions
Acknowledgments
The authors take full responsibility for the content of the article. Romidepsin, NSC 630176, was provided by the Cancer Therapy Evaluation Program. This research was supported, in part, by the Intramural Research Program of the NIH, NCI, Center for Cancer Research, and by a CRADA with Gloucester Pharmaceuticals. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government. The content of this article has been reviewed by independent peer reviewers to ensure that it is balanced, objective, and free from commercial bias. No financial relationships relevant to the content of this article have been disclosed by the independent peer reviewers or editorial staff.
Contributor Information
Robin Frye, Medical Oncology Branch at the Center for Cancer Research, National Cancer Institute (NCI), National Institutes of Health (NIH).
Mary Myers, Nursing and Patient Care Services at the Clinical Center at NIH.
Karen C. Axelrod, Nursing and Patient Care Services at the Clinical Center at NIH.
Elizabeth A. Ness, director of staff development at the Center for Cancer Research, NCI, NIHNIH, all in Bethesda, MD.
Richard L Piekarz, Cancer Therapy Evaluation Program at NCI, NIH, in Rockville, MD.
Susan E. Bates, Medical Oncology Branch.
Susan Booher, Dermatology Branch, both at NCI, NIH, in Bethesda..
References
- Alibert JL (1806). Description des maladies de le peau: observées a l’hôspital St Louis et exposition des meilleurs methodes suivies pour leur traitement. Paris, France: Barrois l’ainé et fils. [Google Scholar]
- Axelrod PI, Lorber B, & Vonderheid EC (1992). Infections complicating mycosis fungoides and Sézary Syndrome. JAMA, 267, 1354–1358. doi: 10.1001/jama.1992.03480100060031 [DOI] [PubMed] [Google Scholar]
- Booher S, McCann S, & Tawa M. (2008). Cutaneous T-cell lymphoma: Mycosis fungoides/Sézary Syndrome In Muehlbauer P. & McGowan C. (Eds.), Site-specific cancer series: Skin cancer (pp. 81–101). Pittsburgh, PA: Oncology Nursing Society. [Google Scholar]
- Cornwell P, Arnold-Long M, Barss SB, & Varnado MF (2010). The use of Dakin’s solution in chronic wounds. Journal of Wound Ostomy, 37, 94–104. doi: 10.1097/WON.0b013e3181c78874 [DOI] [PubMed] [Google Scholar]
- Criscione VD, & Weinstock MA (2007). Incidence of cutaneous T-cell lymphoma in the United States, 1973–2002. Archives of Dermatology, 143, 854–859. doi: 10.1001/archderm.143.7.854 [DOI] [PubMed] [Google Scholar]
- DiMasi JA, & Grabowski HG (2007). Economics of new oncology drug development. Journal of Clinical Oncology, 25, 209–216. doi: 10.1200/JCO.2006.09.0803 [DOI] [PubMed] [Google Scholar]
- Duvic M. (2001). Current treatment of cutaneous T-cell lymphoma. Retrieved from http://dermatology-s10.cdlib.org/DOJvol7num1 [PubMed]
- Girardi M, Heald P, & Wilson L. (2004). The pathogenesis of mycosis fungoides. New England Journal of Medicine, 350, 1978–1988. doi: 10.1056/NEJMra032810 [DOI] [PubMed] [Google Scholar]
- Gloucester Pharmaceuticals. (2009). Istodax® (romidepsin) [Package insert]. Cambridge, MA: Author. [Google Scholar]
- Goldberg MT, & McGinn-Byer M. (2007). Oncology related skin damage In Bryant RA & Nix DP (Eds.), Acute and chronic wounds: Current management concepts (3rd ed.). St. Louis, MO: Mosby/Elsevier, Inc. [Google Scholar]
- Horwitz SM (2008). Novel therapies for cutaneous T-cell lymphomas. Clinical Lymphoma and Myeloma, 8(Suppl. 5), S187–S192. doi: 10.3816/CLM.2008.s.015 [DOI] [PubMed] [Google Scholar]
- Horwitz SM, Olsen EA, Duvic M, Pierliugi P, & Kim YH (2008). Review of the treatment of mycosis fungoides and Sézary Syndrome: A stage-based approach. Journal of the National Comprehensive Cancer Network, 6, 436–442. [DOI] [PubMed] [Google Scholar]
- Hwang ST, Janik JE, Jaffe ES, & Wilson WH (2008). Mycosis fungoides and Sézary Syndrome. Lancet, 37, 945–957. doi: 10.1016/S0140-6736(08)60420-1 [DOI] [PubMed] [Google Scholar]
- Johnstone RW, & Licht JD (2003). Histone deacetylase inhibitors in cancer therapy: Is transcription the primary target? Cancer Cell, 4, 13–18. doi: 10.1016/S1535-6108(03)00165-X [DOI] [PubMed] [Google Scholar]
- Keefe DL (2002). The cardiotoxic potential of the 5-HT3 receptor antagonist antiemetics: Is there cause for concern? Oncologist, 7, 65–72. doi: 10.1634/theoncologist.7-1-65 [DOI] [PubMed] [Google Scholar]
- Kim YH, Liu HL, Mraz-Gernhard S, Varghese A, & Hoppe RT (2003). Long-term outcome of 525 patients with mycosis fungoides and Sézary Syndrome: Clinical prognostic factors and risk for disease progression. Archives of Dermatology, 139, 857–866. doi: 10.1001/archderm.139.7.857 [DOI] [PubMed] [Google Scholar]
- Lansigan F, & Foss FM (2010). Current and emerging treatment strategies for cutaneous T-cell lymphoma. Drugs, 70, 273–286. [DOI] [PubMed] [Google Scholar]
- Lutzner M, Edelson R, Schein P, Green I, Kirkpatrick C, & Ahmed A. (1975). Cutaneous T-cell lymphomas: The Sézary Syndrome, mycosis fungoides, and related disorders. Annals of Internal Medicine, 83, 534–552. [DOI] [PubMed] [Google Scholar]
- Marks PA, & Xu WS (2009). Histone deacetylase inhibitors: Potential in cancer therapy. Journal of Cellular Biochemistry, 107, 600–608. doi: 10.1002/jcb.22185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall JL, Rizvi N, Kauh J, Dahut W, Figuera M, Kang MH, … Hawkins MJ (2002). A phase I trial of depsipeptide (FR901228) in patients with advanced cancer. Journal of Experimental Therapeutics and Oncology, 2, 325–332. doi: 10.1046/j.1359-4117.2002.01039.x [DOI] [PubMed] [Google Scholar]
- Morales Suarez-Varela MM, Llopis Gonzalez A, Marquina Vila A, & Bell J. (2000). Mycosis fungoides: Review of epidemiological observations. Dermatology, 201, 21–28. doi: 10.1159/000018423 [DOI] [PubMed] [Google Scholar]
- National Cancer Institute. (2010). Mycosis fungoides and the Sezary Syndrome treatment (PDQ) [Health professional version]. Retrieved from http://www.cancer.gov/cancertopics/pdq/treatment/mycosisfungoides/HealthProfessional
- National Cancer Institute Cancer Therapy Evaluation Program. (1999). Common Toxicity Criteria [version 2.0]. Retrieved from http://www.eortc.be/services/doc/ctc/ctcv20_4-30-992.pdf
- Olsen E, Vonderheid E, Pimpinelli N, Willemze R, Kim Y, Knobler R, … Whittaker S. (2007). Revisions to the staging and classification of mycosis fungoides and Sézary Syndrome: A proposal of the International Society for Cutaneous Lymphomas (ISCL) and the Cutaneous Lymphoma Task Force of the European Organisation of Research and Treatment of Cancer (EORTC). Blood, 110, 1713–1722. doi: 10.1182/blood-2007-03-055749 [DOI] [PubMed] [Google Scholar]
- Piekarz RL, & Bates SE (2009). Epigenetic modifiers: Basic understanding and clinical development. Clinical Cancer Research, 15, 3918–3926. doi: 10.1158/1078-0432.CCR-08-2788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piekarz RL, Frye AR, Wright JJ, Steinberg SM, Liewehr DJ, Rosing DR, … Bates SE. (2006). Cardiac studies in patients treated with depsipeptide, FK228, in a phase II trial for T-cell lymphoma. Clinical Cancer Research, 12, 3762–3773. doi: 10.1158/1078-0432.CCR-05-2095 [DOI] [PubMed] [Google Scholar]
- Piekarz RL, Frye R, Turner M, Wright JJ, Allen SL, Kirschbaum MH, … Bates SE. (2009). Phase II multi-institutional trial of the histone deacetylase inhibitor romidepsin as monotherapy for patients with cutaneous T-cell lymphoma. Journal of Clinical Oncology, 27, 5410–5417. doi: 10.1200/JCO.2008.21.6150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piekarz RL, Robey R, Sandor V, Bakke S, Wilson WH, Dahmoush L, … Bates SE (2001). Inhibitor of histone deacetylation, depsipeptide (FR901228), in the treatment of peripheral and cutaneous T-cell lymphoma: A case report. Blood, 98, 2865–2868. doi: 10.1182/blood.V98.9.2865 [DOI] [PubMed] [Google Scholar]
- Piekarz RL, Sackett DL, & Bates SE (2007). Histone deacetylase inhibitors and demethylating agents: Clinical development of histone deacetylase inhibitors for cancer therapy. Cancer Journal, 13, 30–39. doi: 10.1097/PPO.0b013e31803c73cc [DOI] [PubMed] [Google Scholar]
- Richon VM, Garcia-Vargas J, & Hardwick JS (2009). Development of vorinostat: Current applications and future perspectives for cancer therapy. Cancer Letters, 280, 201–210. doi: 10.1016/j.canlet.2009.01.002 [DOI] [PubMed] [Google Scholar]
- Sandor V, Bakke S, Robey RW, Kang MH, Blagosklonny MV, Bender J, … Bates SE (2002). Phase I trial of the histone deacetylase inhibitor, depsipeptide (FR901228, NSC630176), in patients with refractory neoplasms. Clinical Cancer Research, 8, 718–728. [PubMed] [Google Scholar]
- Schrump DS (2009). Cytotoxicity mediated by histone deacetylase inhibitors in cancer cells: Mechanisms and potential clinical implications. Clinical Cancer Research, 15, 3947–3957. doi: 10.1158/1078-0432.CCR-08-2787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson E. (2010). Atopic dermatitis: A review of topical treatment options. Current Medical Research and Opinion, 26, 633–640. [DOI] [PubMed] [Google Scholar]
- Title 21, United States Code of Federal Regulations (CFR), Part 312, 2010.
- Ueda H, Manda T, Matsumoto S, Mukumoto S, Nishigaki F, Kawamura I, & Shimomura K. (1994). FR901228, a novel antitumor bicyclic depsipeptide produced by Chromobacterium violaceum No. 968. III. Antitumor activities on experimental tumors in mice. Journal of Antibiotics, 47, 315–323. [DOI] [PubMed] [Google Scholar]
- Ueda H, Nakajima H, Hori Y, Goto T, & Okuhara M. (1994). Action of FR901228, a novel antitumor bicyclic depsipeptide produced by Chromobacterium violaceum No. 968, on Ha-ras transformed NIH3T3 cells. Japan Society for Bioscience, Biotechnology, and Agrochemistry, 58, 1572–1583. [DOI] [PubMed] [Google Scholar]
- Vonderheid EC, Bernengo MG, Burg G, Duvic M, Heald P, Laroch L, … Willemze R. (2002). Update on erythrodermic cutaneous T-cell lymphoma: Report of the International Society for Cutaneous Lymphomas. Journal of the American Academy of Dermatology, 46, 95–106. doi: 10.1067/mjd.2002.118538 [DOI] [PubMed] [Google Scholar]
- Whittaker SJ, Demierre M, Kim EJ, Rook AH, Lerner A, Duvic M, … Kim YH. (2010). Final results from a multicenter, international, pivotal study of romidepsin in refractory cutaneous T-cell lymphoma. Journal of Clinical Oncology, 28, 4485–4491. doi: 10.1200/JCO.2010.28.9066 [DOI] [PubMed] [Google Scholar]
- Willemze R, Jaffe ES, Burg G, Cerroni L, Berti E, Swerdlow SH, … Meijer CJ. (2005). WHO-EORTC classification for cutaneous lymphomas. Blood, 105, 3768–3785. doi: 10.1182/blood-2004-09-3502 [DOI] [PubMed] [Google Scholar]