Abstract
Plasmacytoid dendritic cells (pDC) compose 0.2–0.5% of circulating leukocytes but play a significant role in mounting host immune responses. Elevated and chronic activation of pDC are implicated in autoimmune disease like systemic lupus erythematosus and rheumatoid arthritis. Δ9-tetrahydrocannabinol (THC) is a well characterized cannabinoid with potent anti-inflammatory activity, but acceptance of THC as a treatment for autoimmune disorders has been hindered due to psychotropic activity. The psychotropic effects of THC are mediated through cannabinoid receptor 1 (CB1) expressed in the central nervous system while the immunomodulatory effects of THC result from THC binding to CB1 and CB2 on immune cells. Synthetic CB2-selective agonists have been developed to explore immune modulation by cannabinoids in the absence of psychotropic effects. The goal of these studies was to determine if the CB2-selective agonists, JWH-015 and JWH-133, have comparable efficacy to THC in modulating IFNα and TNFα responses by primary human pDC. Treatment with JWH-133 and JWH-015 inhibited CpG-induced IFNα and TNFα responses by pDC. Further, the phosphorylation of IRF7, TBK1, NFκB, and IKKγ, key events in pDC activation, were suppressed by THC, JWH-133, and JWH-015. Likewise, the phosphorylation of AKT at the S473 and T308 residues were differentially modulated by treatment with THC and both JWH compounds. Collectively, these results demonstrate the potential for CB2 targeted therapeutics for treatment of inflammatory conditions involving aberrant pDC activity.
Keywords: Plasmacytoid dendritic cells, Cannabinoids, Δ9-Tetrahydrocannabinol, Interferon α, Tumor necrosis factor α, Toll-like receptors
1. Introduction
Plasmacytoid dendritic cells (pDC) are a minor population (0.2–0.5%) of peripheral blood mononuclear cells (PBMC), but they play a significant role in mounting host anti-viral responses by sensing viral pathogen-associated molecular patterns (PAMPs) (Colonna et al., 2004). Specifically, pDC respond to non-host genomic material through toll like receptors (TLR)7/TLR8 (Prinz et al., 2011) and TLR9 (Henriquez et al., 2017). Following stimulation, pDC produce up to 1000-fold more IFNα than other leukocytes (Cisse et al., 2008). Although the robust IFNα response of pDC is well documented, pDC are also known secretors of tumor-necrosis factor α (TNFα) (Gibson et al., 2002). TNFα is part of the acute phase response (Baumann and Gauldie, 1994) and has various effects during viral (Ramshaw et al., 1997) and bacterial infection (Takeuchi et al., 1999) including enhancement of dendritic cell function (Pasparakis et al., 1996), patterning of T cell responses (Chen and Oppenheim, 2011), and pathogen clearance (Wang et al., 1996).
During the acute phase of infection, the immediate and vigorous secretion of both IFNα and TNFα promote a robust immune response. Inappropriate activation of pDC and sustained IFNα and TNFα secretion can be deleterious by promoting autoimmunity. For example, systemic lupus erythematosus (SLE) develops as host cells die and pDC respond to host genomic material (Lande et al., 2007; Crispín et al., 2010). Elevated TNFα (Gabay et al., 1997; Davas et al., 1999) and more so IFNα (Crow, 2007; Rönnblom, 2010) are a hallmark of lupus and believed to be closely associated with the etiology of disease progression. Furthermore, activation of pDC may expediate T cell exhaustion during HIV infection (Berghöfer et al., 2006) while chronic activation of pDC may play a role in mediating monocyte activation, a contributing factor to the development of HIV-associated neurocognitive disorders (HAND) (Ancuta et al., 2008; Gannon et al., 2011).
The use of Cannabis sativa for the remediation of inflammatory conditions is well documented (Lu and Clarke, 1995; Klein, 2005). C. sativa contains over 400 known compounds and of those over 100 have been identified as phytocannabinoids (Dewey, 1986; Pertwee, 2006). Of these compounds, Δ9-tetrahydrocannabinol (THC) exhibits potent immunomodulatory activity (Massi et al., 2006; Tanasescu and Constantinescu, 2010). THC is also psychotropic, inducing a variety of effects including paranoia (Englund et al., 2013), psychosis (Thacore and Shukla, 1976), hypothermia (Bhargava, 1980), and memory deficiencies (Hampson and Deadwyler, 1999; Englund et al., 2013).
THC mediates its activity through two canonical cannabinoid receptors (CB), CB1 and CB2. CB1 is most highly expressed by cells within the central nervous system (Domenici et al., 2006) while also expressed at lower levels across many different tissues. By contrast, CB2 is most highly expressed by cells of the immune system, in many other peripheral tissues, and very minimally expressed within the CNS (Galiegue et al., 1995). CB1 is responsible for the aforementioned psychotropic effects (Izzo et al., 2009). For this reason, CB1-selective agonists have not been widely pursued as therapeutics (Every-Palmer, 2010). By contrast, CB2 selective binding is devoid of psychotropic effects while capable of mediating anti-inflammatory and immune modulating effects (Basu and Dittel, 2011). Indeed, CB2-specific agonists have been and continue to be explored for treatment of inflammatory conditions (Ashton, 2007) like neuroinflammation (Ashton and Glass, 2007).
The objective of this study was to compare the efficacy of JWH-015, a moderately selective CB2-selecitive agonists (CB1:CB2 selectivity of 1:27), and JWH-133, a highly selective CB2 agonist (CB1:CB2 selectivity of 1:200), with THC, a non-selective CB2 partial-agonist (CB1:CB2 selectivity of 2:1) in suppressing the CpG-ODN (CpG)-induced IFNα and TNFα responses in pDC. Furthermore, these studies aimed to measure the modulation of key phosphorylation events downstream of TLR9 activation via CpG by THC and the CB2-selective agonists.
2. Materials and methods
2.1. Peripheral Blood Mononuclear Cell (PBMC) isolation and cell identification
Leukocyte packs were purchased from the Gulf Coast Regional Blood Center (Houston, TX) without differentiating between male and female donors. Blood was diluted 1:1 with Hanks Balanced Salt Solution from Gibco™ (Grand Island, NY). Diluted blood was layer on 15 ml Ficoll Paque Plus (GE Healthcare Life Sciences, Pittsburgh, PA) using SepMate 50 ml conical tubes by StemCell Technologies (Vancouver, BC, Canada) and centrifuged at 1300 ×g for 25 min at 4 °C. The layer of PBMC, aka “Buffy coat”, was carefully removed from the plasma and resuspended in complete Roswell Park Memorial Institute (C-RPMI) Media from Gibco™ containing 5% Human AB Serum (Sigma-Aldrich, St. Louis, MO), 1% Penicillin-Streptomycin (Gibco™), and 0.035% β-mercaptoethanol. PBMC were seeded in 96 well plates at a density of 1 × 106 cells/well in 200 μl of complete RPMI media. pDC were identified using mouse anti-human antibodies by Miltenyi Biotec GmgH© (Bergisch Gladbach, Germany) as CD303+ CD123+ cells.
2.2. Treatment with cannabinoids or vehicle control and cell stimulation
(6aR,10aR)-delta-9-tetrahydrocannabinol (Δ9-Tetrahydrocannabinol or THC) and cannabidiol (CBD) were supplied by the National Institute of Drug Abuse (NIDA) and 3-(1,1-dimethylburyl)-6aR,7,10,10aR-tetrahydro-6–6-9-trimethyl-6H-dibenzo[b,d]pyran (JWH-133) and (2-methyl-1-propyl-1H-indol-3-yl)-1-naphthalenyl-methanone (JWH-015) were purchased from Cayman Chemicals (Ann Arbor, MI). PBMCs were treated with either THC, CBD, JWH-133, JWH-015 or vehicle (VH – 0.03% Ethanol). The cannabinoids were prepared in Complete-RPMI to a final EtOH concentration of 0.03%. The concentrations of THC, JWH-133, and JWH-015 were selected based on preliminary experiments that demonstrated a linear relationship between cannabinoid concentration and suppression of the IFNα response. Furthermore, concentrations of JWH-133, and JWH-015 were used that produced approximately the same magnitude of suppression of the IFNα response as observed with THC. CBD has not been shown to suppress the IFNα response and therefore a single concentration was used for comparison to the highest concentration of THC (10 μM) as an additional treatment control for CB receptor involvement. The concentrations for JWH are represented in log10 on all figures and are as follows: JWH-133–0.001, 0.01, 0.1 μM (in log10: −3, −2, −1 μM); JWH-015–0.01, 0.1, 1 μM (in log10: −2, −1, 0 μM); THC – 0.1, 1, 10 μM (in log10: −1, 0, 1), and CBD – 10 μM (in log10: 1 μM). The prepared cell suspensions and appropriate treatments were added to flat bottom 96 well tissue culture plates and incubated at 37 °C and 5% CO2 for 30 min. Cells were stimulated with CpG-ODN Type A 2216 (15 μg/ml) (InvivoGen©, San Diego, CA) following treatment with cannabinoids.
2.3. Intracellular detection of IFNα and TNFα
IFNα+ and TNFα+ pDC were determined by intracellular staining with antibodies by Biolegend®. In brief, harvested cells were stained for CD303 and CD123 as indicated above and fixed using CytoFix™ buffer by BD Biosciences (San Jose, CA). Fixed cells were permeabilized using PermWash™ buffer (BD Biosciences) by washing with 1 × PermWash™ and preparing IFNα/TNFα master mix in PermWash™ buffer with 7% Human AB serum to reduce non-specific staining. Cells were stained for 30 min at 4 °C, washed with PermWash™, resuspended in FACS. IFNα+ and TNFα+ pDC were determined by flow cytometric analysis.
2.4. Phosphoprotein detection
Treated PBMCs were washed and pDCs were stained as described. CpG-mediated induction of pIRF7, pTBK1, p65 (NFκB), pIKKγ, pS473-AKT, and pT308-AKT were determined using Phosflow™ antibodies and the harsh detergent method by BD Biosciences©. In brief, cells were fixed using BD cytofix buffer for 10 min at 37 °C then permeabilized using 1 × of perm buffer IV™, stained for 1 h under continuous motion using FACS buffer and 5% Human AB serum, washed 3× with 0.5× perm buffer, and analyzed by flow cytometry.
2.5. Data acquisition and analysis
Flow cytometry data were acquired on a BD FACS Canto II using DIVA software and analyzed using FlowJo 8.0 (TreeStar Inc., Ashland, OR). To determine changes in phosphorylation for each intracellular protein, gates were set based upon the non-activated vehicle with each donor serving as their own control for normalization due to inherent variability in human leukocyte responses. GraphPad© Prism 5.0™ was used for statistical analysis. Where appropriate, samples were normalized to 0 μM THC + CpG, which was considered 100% maximum response for each individual donor and the appropriate statistical test was performed (see figures).
3. Results
3.1. Treatment with THC, JWH-015 and JWH-133 suppressed CpG-induced IFNα and TNFα responses in pDC
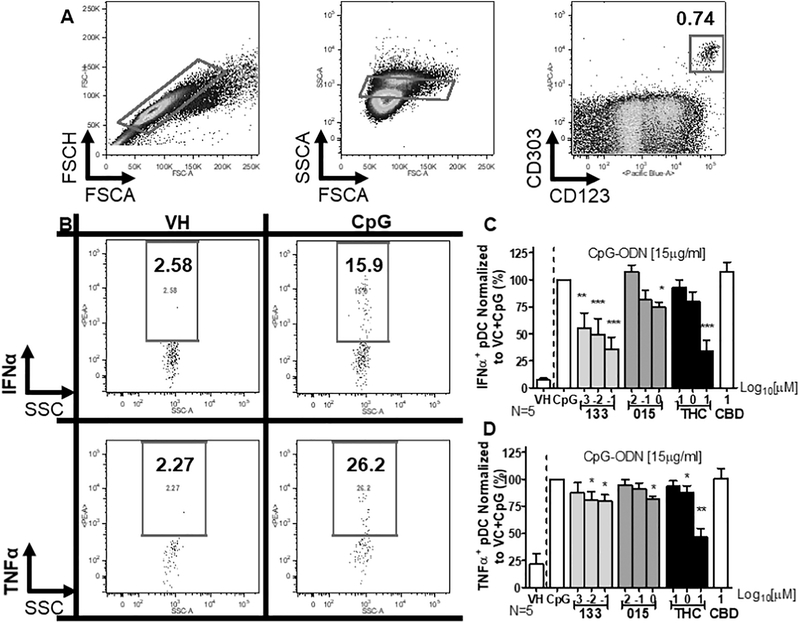
Suppression of the IFNα response in pDC by THC has been previously reported (Henriquez et al., 2017). THC exhibits approximately equal binding affinity for both CB1 and CB2. Despite the 10–40-fold higher expression of CB2 compared to CB1 by leukocytes (Galiegue et al., 1995; Pertwee, 1999) the possible influence of CB1 on THC-mediated suppression of pDC activity is unclear. Here PBMC were treated with JWH-015, JWH-133, or THC and stimulated with CpG for 5 h. pDC were identified as CD303+/CD123+ cells (Fig. 1A). Treatment with CpG induced expression of IFNα and TNFα in pDC within 5 h post stimulation (Fig. 1B). Treatment with JWH-133, JWH-015, and THC suppressed the CpG-mediated IFNα response by pDC, producing an IC50 (±standard error) concentration of 0.63 ± 0.58 μM, 3.26 ± 0.30 μM, and 11.15 ± 5.62 μM, respectively (Fig. 1C).
Fig. 1.
Treatment with THC, JWH-015 and JWH-133 inhibited CpG-induced IFNα and TNFα responses in pDC. Isolated human PBMCs were treated with either Vehicle (VH; 0.03% Ethanol), CBD (10 μM), THC (0.1, 1, or 10 μM), JWH-015 (10−2, 10−1, 100 μM) or JWH-133 at (10−3, 10−2, 10−1 μM) for 30 min, stimulated with CpG-ODN at 15 μg/ml for 6 h, and intracellularly stained for either IFNα or TNFα (N = 5 donors). pDC > 90% viability within 6 h of treatment. (A) pDC were identified by first gating on singlets, then focusing on the interface of leukocytes and monocytes based on forward and side scatter area, and then on CD303+ CD123+ cells. (B) Example of gating for IFNα and TNFα positive pDC in resting cells cultured in the presence of VH and following CpG stimulation. (C) CpG induced intracellular expression of IFNα which was suppressed by THC, JWH-015, and JWH-133. (D) CpG induced intracellular expression of TNFα which was suppressed by THC, JWH-015, and JWH-133. Asterisks indicate statistically significant differences in IFNα or TNFα expression compared to 0 THC + CpG (*** = P < .001, ** = P < .01, * = P < .05) using a 1-Way ANOVA with Dunnett’s posttest.
THC has been reported to suppress TNFα secretion in both human and animal models while JWH-133 suppresses TNFα in animal models of inflammatory disease (Xu et al., 2007). Here PBMC were treated with JWH-015, JWH-133, or THC and stimulated with CpG for 5 h. Treatment with JWH-015, JWH-133, and THC suppressed the TNFα response in a concentration-dependent manner (Fig. 1D). Treatment with THC produced an IC50 for the IFNα response of 8.71 ± 5.98 μM. Inhibition of the TNFα response by the JWH compounds plateaued, precluding determination of an IC50. Finally, cannabidiol (CBD), a phytocannabinoid possessing minimal binding affinity for CB1 and CB2, was used as a control for CB receptor involvement. Treatment with CBD did not impair CpG-induced IFNα and TNFα production (Fig. 1C & D).
3.2. Treatment with THC, JWH-015 and JWH-133 impaired phosphorylation of IRF7 and TBK1, key signaling events in the IFNα response
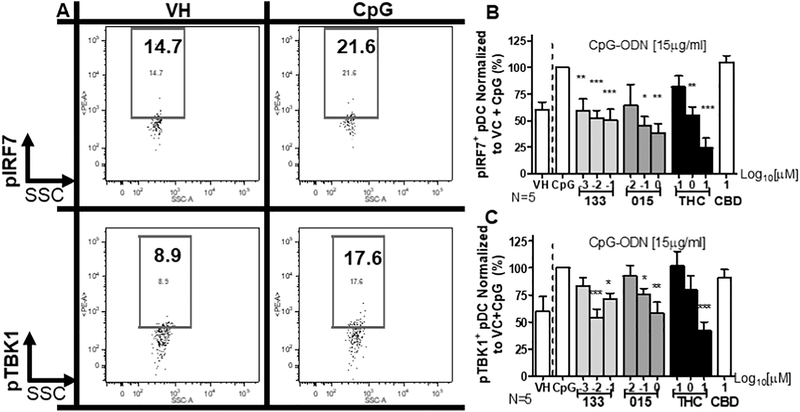
It has been previously reported that THC treatment impaired CpG-induced IRF7 phosphorylation (Henriquez et al., 2017). IRF7 is considered the “master regulator” of the IFNα response by pDC (Honda et al., 2005). Therefore, to determine if either of the CB2-selective agonists, JWH-015 or JWH-133, ablated the phosphorylation of IRF7, PBMC were treated with JWH-015, JWH-133, or THC and stimulated with CpG for 5 h and then measured for pIRF7 using flow cytometric analysis. Treatment with both JWH-015 and JWH-133 significantly reduced pIRF7 (Fig. 2A) in a concentration-dependent manner (Fig. 2B) which was comparable to effect by THC. Treatment with JWH-133, JWH-015, and THC produced IC50 (±standard error) concentrations of 0.53 ± 0.45 μM, 1.02 ± 0.65 μM, and 2.47 ± 0.93 μM, respectively.
Fig. 2.
Treatment with THC, JWH-015, and JWH-133 inhibited the CpG-induced phosphorylation of IRF7 and TBK1 in pDC. Isolated human PBMCs were treated with either Vehicle (VH; 0.03% Ethanol), CBD (10 μM), THC (0.1, 1, or 10 μM), JWH-015 (10−2, 10−1, 100 μM) or JWH-133 at (10−3, 10−2, 10−1 μM) for 30 min, stimulated with CpG-ODN at 15 μg/ml for 5 h and intracellularly stained for pIRF7 or pTBK1 (N = 5 donors). pDC > 90% viability within 5 h of treatment (A) Example of gating for pIRF7 and pTBK1 in the presence of VH, with and without CpG stimulation. (B) CpG induced intracellular expression of pIRF7 was suppressed by THC, JWH-015, and JWH-133. (C) CpG induced intracellular expression of pTBK1 was suppressed by THC, JWH-015, and JWH-133. Asterisks indicate statistically significant differences in IRF7 and TBK1 phosphorylation compared to VC + CpG (*** = P < .001, ** = P < .01, * = P < .05) using a 1-Way ANOVA with Dunnett’s posttest.
TBK1 plays a significant role in the induction of IFNα (Barton and Medzhitov, 2003; Oganesyan et al., 2006) by phosphorylating both IRF3 and IRF7 (Guo and Cheng, 2007). Therefore, to determine whether JWH-015, JWH-133, or THC modulated the phosphorylation of TBK1, PBMC were treated with JWH-015, JWH-133, or THC and stimulated with CpG for 5 h and then assayed for pTBK1 by flow cytometry. In these experiments, treatment with JWH-015, JWH-133, and THC significantly diminished CpG-induced pTBK1 (Fig. 2C). Treatment with JWH-133, JWH-015, and THC produced IC50 (±standard error) concentrations of 0.32 ± 0.18 μM, 4.49 ± 2.95 μM, and 4.53 ± 1.77 μM, respectively. Once again, CBD had no effect on CpG-induced pIRF7 or pTBK1 responses (Fig. 2B & C).
3.3. Treatment with THC, JWH-015 and JWH-133, inhibit the phosphorylation of NFκB and IKKγ, key signaling events in TNFα response
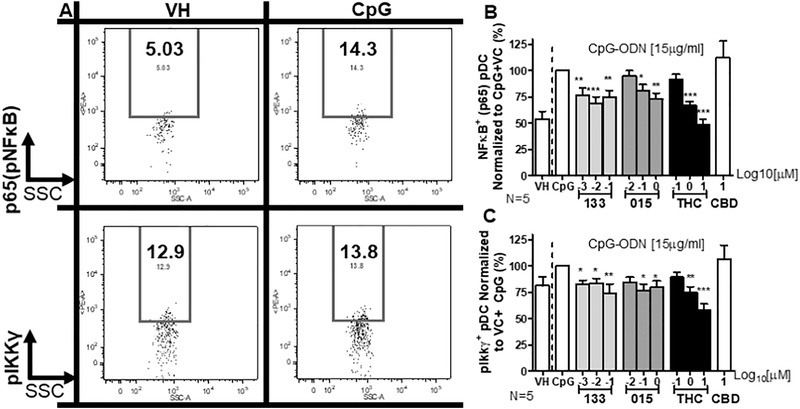
Stimulation of TLR9 can induce TNFα via NFκB activation (Kumar et al., 2009). While cannabinoid-modulation of NFκB activity is documented in immune cells (Kozela et al., 2010), cannabinoid-mediated modulation of NFκB activation in pDC has not been previously investigated. To determine if CpG stimulation induced NFκB activation in pDC and whether that activation was sensitive to modulation by cannabinoids, PBMC were treated with JWH-015, JWH-133, or THC, stimulated with CpG for 5 h, and then assayed by flow cytometry for phospho-p65, a key event in NFκB activation (Fig. 3A). JWH-015, JWH-133, and THC all significantly diminished CpG-induced phopho-p65 in a concentration-dependent manner (Fig. 3B). Treatment with THC produced an IC50 for NFκB p65 phosphorylation of 7.58 ± 3.44 μM. Inhibition of TNFα response by the JWH compounds plateaued precluding determination of an IC50.
Fig. 3.
Treatment with THC, JWH-015, and JWH-133 inhibited the CpG-induced phosphorylation of the p65 subunit of NFκB and IKKγ (NEMO) in pDC. Isolated human PBMCs were treated with either Vehicle (VH; 0.03% Ethanol), CBD (10 μM), THC (0.1, 1, or 10 μM), JWH-015 (10−2, 10−1, 100 μM) or JWH-133 at (10−3, 10−2, 10−1 μM) for 30 min, stimulated with CpG-ODN at 15 μg/ml for 5 h and intracellularly stained for p65 (pNFκB) or IKKγ (N = 5 donors). pDC > 90% viability within 5 h of treatment. (A) Example of gating for p65 (pNFκB) and pIKKγ with VH treated resting and CpG stimulated pDC. (B) CpG induced intracellular expression of pNFκB was suppressed by THC, JWH-015, and JWH-133. (C) CpG induced intracellular expression of IKKγ was suppressed by THC and both CB2-selective agonists. Asterisks indicate statistically significant differences in p65 and IKKγ phosphorylation compared to VC + CpG (*** = P < .001, ** = P < .01, * = P < .05) using a 1-Way ANOVA with Dunnett’s posttest.
Though effects of cannabinoids on the activation of IKKγ, also known as NFκB essential modulator (NEMO), have been postulated (Gertsch et al., 2004; Gertsch, 2008), few studies have directly shown cannabinoid-mediated modulation of IKKγ phosphorylation. Therefore, studies were conducted to determine if treatment with JWH-015, JWH-133 or THC would modulate phospho-IKKγ (pIKKγ), a key event in the activation of NFκB. These studies revealed that stimulation by CpG-induced pIKKγ in pDC (Fig. 3A), which was suppressed by treatment with JWH-015, JWH-133, and THC (Fig. 3C). Once again, CBD had no effect on CpG-induced pNKκB and pIKKγ (Fig. 3B & C). Treatment with THC produced an IC50 for IKKγ phosphorylation just outside the range of concentrations tested, at 10.76 ± 2.90 μM. Suppression of the TNFα response by the JWH compounds plateaued, precluding derivation of an IC50.
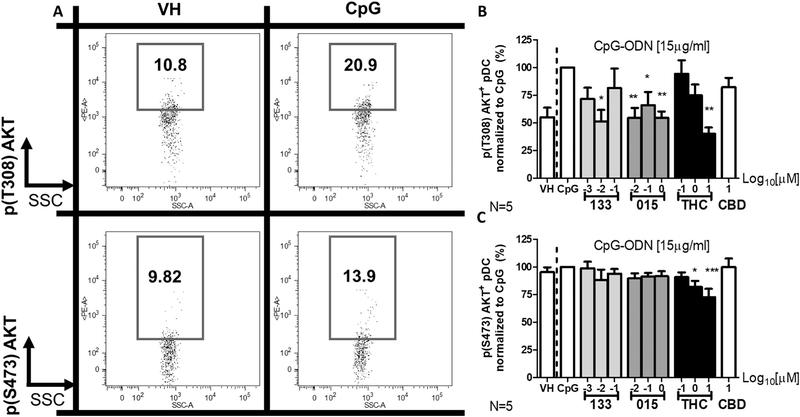
3.4. Treatment with THC, JWH-015, and JWH-133 differentially affects AKT phosphorylation at two key sites
Protein kinase B (PKB), also known as AKT, is a serine/threonine kinase which plays a critical role in both anti-apoptotic and activation processes (Weichhart and Säemann, 2008). Cannabinoid-mediated modulation of AKT-related signaling has been suggested as a target for immune modulation in autoimmune disorders (Molina-Holgado et al., 2002; Ellert-Miklaszewska et al., 2005; Badr et al., 2010; Gomez et al., 2011). AKT activation is principally controlled by phosphorylation of two residues, S473 and T308. To determine the effects of CpG-mediated activation and cannabinoid treatment on the phosphorylation of S473 (pS473) and T308 (pT308), PBMC were treated with JWH-015, JWH-133, or THC, stimulated with CpG for 5 h, and then quantified for pS473 and pT308 by flow cytometry. These studies revealed that pT308 was induced by CpG activation while pS473 was not (Fig. 4A). Further, pT308 was reduced by treatment with JWH-015, JWH-133, and THC (Fig. 4B). While the reduction in pT308 by THC was concentration-dependent, the suppressive effects of the CB2 selective agonists were not (Fig. 4B). Treatment with THC inhibited 50% of maximum AKT T308 phosphorylation at a calculated concentration of 5.79 ± 3.41 μM. Interestingly, treatment with the JWH compounds did not have a concentration-dependent effect. Additionally, treatment with THC significantly reduced S473 phosphorylation while neither of the CB2-selective agonist affected S473 phosphorylation (Fig. 4C). Though significant, the IC50 for the inhibition of AKT T308 phosphorylation by the JWH compounds and THC could not be calculated to a physiologically relevant value. As with other endpoints discussed above, CBD had no effect on the phosphorylation of either AKT residue (Fig. 4B & C).
Fig. 4.
Treatment with THC, JWH-015, and JWH-133 differentially affected the phosphorylation of the AKT at the T308 and S473 residues. Isolated human PBMCs were treated with either Vehicle (VC; 0.03% Ethanol), CBD (10 μM), THC (0.1, 1, or 10 μM), JWH-015 (10−2, 10−1, 100 μM) or JWH-133 at (10−3, 10−2, 10−1 μM) for 30 min, stimulated with CpG-ODN at 15 μg/ml for 5 h and intracellularly stained for either pT308 or pS473 AKT (N = 5 donors). pDC > 90% viability within 5 h of treatment. (A) Example of gating for pT308 and pS473 with VH treated resting and CpG stimulated pDC. (B) CpG induced intracellular expression of pT308 which was suppressed by THC, JWH-015, and JWH-133. (C) CpG had no significant effect on pS478 expression and treatment with THC reduced pS478 while neither JWH-015 or JWH-133 had a significant effect. Asterisks indicate statistically significant differences in p65 and IKKγ phosphorylation compared to VC + CpG (*** = P < .001, ** = P < .01, * = P < .05) using a 1-Way ANOVA with Dunnett’s posttest.
4. Discussion
The suppression of IFNα and TNFα by THC, JWH-015, and JWH-133 support the potential for cannabinoid-based therapies in treating inflammatory conditions. While the presented studies focus on pDC specifically, the utilization of cannabinoids for the treatment of inflammation has been suggested previously and medicinal cannabinoids are already recommended for some inflammatory conditions (Aggarwal et al., 2009). To better understand how these compounds, reduce cytokine responses, phosphorylation of key regulators in the pathway from TLR-9 ligation to the induction of IFNα and TNFα were investigated.
Evidence here is presented showing that signaling through CB2 can lead to the suppression of pIRF7. Further, IRF7 can be phosphorylated by TBK1. pTBK1, in turn, is inhibited by JWH-015, JWH-133 and THC. These results are congruent with the literature (Kozela et al., 2010; Downer et al., 2011) and suggest that signaling through CB2 can suppress IRF7 phosphorylation, at least in part, by reducing the activation of TBK1.
Similar to results for the pathway leading to IFNα secretion, the phosphorylation of key intermediates for TNFα production, NFκB and IKKγ, were also impaired by THC, JWH-133, and JWH-015. While the modulation of NFκB by cannabinoids has been previously reported (Ngaotepprutaram et al., 2013), the suppression of IKKγ is a novel finding. IKKγ is also a member of an activation complex that includes TBK1 (Huang et al., 2005; Guo and Cheng, 2007). Therefore, cannabinoid suppression of IKKγ may play a role in the suppression of other cytokines, including IFNα. Taken together, these findings provide support for CB2 as a therapeutic target since NFκB has a range of effects in cells (Zhang et al., 2017)
In this set of experiments, treatment with THC resulted in a greater degree of suppression of NFκB and IKKγ phosphorylation than did treatment with either JWH-133 or JWH-015. While direct comparison between the compounds is not possible due to differences in the effective concentrations, the suppressive effect of the JWH compounds appeared to plateau at the concentrations used in studying the NFκB-associated signaling pathways but not the IRF7-associated pathways. This indicates that signaling through CB1, and other orphan cannabinoid receptors, by THC may contribute to suppression of immune cell activation. The possibility of orphan-receptor involvement in immune modulation by cannabinoids has been previously suggested (Kozela et al., 2010; Karmaus et al., 2012).
The most informative results from these experiments came from investigations of AKT. AKT plays a role in many cellular processes which are key to proper cell function and persistence (Manning and Cantley, 2007). Modulation of the AKT-PI3K-mTOR pathways by cannabinoids has already been reported in various cell models and suggested as a putative target of cannabinoid therapy in immune disorders (Ellert-Miklaszewska et al., 2005; Weichhart and Säemann, 2008; Chappell et al., 2011; Gomez et al., 2011). The CpG-induced phosphorylation of T308 was impaired by treatment with THC and CB2 selective agonists. Interestingly, while inhibition of pT308 by THC was concentration-dependent, the effects by JWH-015 and JWH-133 were concentration-independent. It is tempting to speculate that signaling through CB2 is critical for modulating the CpG-induced phosphorylation of AKT at the T308 residue, which was saturated by CB2 selective agonists at the concentrations employed. The phosphorylation of both the T308 and S473 residues are needed for optimum AKT activity (Manning and Cantley, 2007). Though treatment with CpG induced no significant change to S473 phosphorylation, treatment with THC alone reduced the phosphorylation of the S473 residue. These results suggest that THC suppresses the phosphorylation of a constitutively phosphorylated residue on AKT in pDC. Moreover, these findings may help to explain why the effects of the CB2-selective agonists plateaued regarding inhibition of IKKγ and NFκB (p65) phosphorylation while the effect with THC did not. Specifically, by reducing the phosphorylation of both the S473 and T308 residues, THC was more effective at reducing the subsequent activation of IKKγ and NFκB, which control the TNFα response. Likewise, JWH-015 and JWH-133-mediated suppression of the TNFα response also plateaued while inhibition of TNFα by THC did not, thereby confirming the downstream effects of AKT modulation by both the CB2 selective agonists and THC.
There are several limitations associated with our studies that warrant discussion. First, all responses were evaluated at 5 h after pDC stimulation as this was determined as the peak time of response in pilot studies. Therefore, the effects of cannabinoid treatment on the distinct pathways investigated are specific to that time point. This point is noteworthy as pDC are a dynamic cellular population capable of a variety of effector functions including cytokine production and antigen presentation (Colonna et al., 2004). Therefore, cannabinoid-mediated modulation of pDC beyond cytokine production, are needed to more completely understand the impact of cannabinoids on pDC effector functions. The results presented in this article have been acquired using human leukocytes isolated from peripheral blood and focused on the cannabinoid-mediated modulation of TLR9 activation via CpG (Guiducci et al., 2009). While this study is the first to demonstrate differential sensitivity to different cannabinoid receptor agonists in pDC, cannabinoid-mediated suppression of other TLR agonist-mediated activation in pDC is known. Specifically, one study demonstrated significant suppression by JWH-015 of the IFNα and TNFα response in pDC stimulated with R848, a TLR7/8 agonist (Chiurchiù et al., 2013). These results indicate that while there are shared signaling events downstream of endosomal TLRs, how cannabinoids modulate these signaling events vary. Further studies are needed to determine how treatment with different cannabinoid receptor agonists suppresses pDC activation via different TLRs.
The studies presented in this article demonstrate that the CB2-selective agonists, JWH-015 and JWH-133, and THC significantly inhibit CpG-mediated IFNα and TNFα responses in pDC. Interesting, while significant, the degree of inhibition by the CB2-selective agonist of the TNFα response was modest when compared to treatment by THC. Further studies revealed that the JWH compounds and THC equivalently reduced the phosphorylation of key proteins in the IFNα pathway, but the phosphorylation of proteins associated with the TNFα response was differentially modulated by treatment with the CB2-agonists compared to THC, including specific residues on AKT. Collectively, our present findings offer further support for CB2 targeted therapies in the treatment of inflammatory conditions involving pDC and suggest a possible role of CB1, or orphan cannabinoid receptors, in immunomodulation by THC.
Supplementary Material
Acknowledgements
The authors would like to thank Jiajun Zhou for assistance with figure formatting, Mrs. Kimberly Hambleton for submission of this article, and Lance Blevins, PhD, for suggestions which aided in the interpretation of the results.
Funding
All funds for these studies were supplied through the National Institute on Drug Abuse grant DA007908 and DA047180; and the National Institute of Environmental Health Sciences Training grant T32-ES007255. The authors report no conflict of interest.
Footnotes
Conflict of interest
The authors report no conflict of interest.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.taap.2019.02.013.
References
- Aggarwal SK, Carter GT, Sullivan MD, ZumBrunnen C, Morrill R, Mayer JD, 2009. Medicinal use of cannabis in the United States: historical perspectives, current trends, and future directions. J. Opioid Manag. 5, 153–168. [DOI] [PubMed] [Google Scholar]
- Ancuta P, Kamat A, Kunstman KJ, Kim E-Y, Autissier P, Wurcel A, Zaman T, Stone D, Mefford M, Morgello S, 2008. Microbial translocation is associated with increased monocyte activation and dementia in AIDS patients. PLoS One 3, e2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashton JC, 2007. Cannabinoids for the treatment of inflammation. Curr. Opin. Investig. Drugs 2000 (8), 373–384 London, England. [PubMed] [Google Scholar]
- Ashton JC, Glass M, 2007. The cannabinoid CB2 receptor as a target for inflammation-dependent neurodegeneration. Curr. Neuropharmacol. 5, 73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badr G, Saad H, Waly H, Hassan K, Abdel-Tawab H, Alhazza IM, Ahmed EA, 2010. Type I interferon (IFN-α/β) rescues B-lymphocytes from apoptosis via PI3Kδ/Akt, Rho-A, NFκB and Bcl-2/BclXL. Cell. Immunol. 263, 31–40. [DOI] [PubMed] [Google Scholar]
- Barton GM, Medzhitov R, 2003. Linking toll-like receptors to IFN-α/β expression. Nat. Immunol. 4, 432. [DOI] [PubMed] [Google Scholar]
- Basu S, Dittel BN, 2011. Unraveling the complexities of cannabinoid receptor 2 (CB2) immune regulation in health and disease. Immunol. Res. 51, 26–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann H, Gauldie J, 1994. The acute phase response. Immunol. Today 15, 74–80. [DOI] [PubMed] [Google Scholar]
- Berghöfer B, Frommer T, Haley G, Fink L, Bein G, Hackstein H, 2006. TLR7 ligands induce higher IFN-α production in females. J. Immunol. 177, 2088–2096. [DOI] [PubMed] [Google Scholar]
- Bhargava HN, 1980. The effects of thyrotropin releasing hormone and histidyl-proline diketopiperazine on delta-9-tetrahydrocannabinol-induced hypothermia. Life Sci. 26, 845–850. [DOI] [PubMed] [Google Scholar]
- Chappell WH, Steelman LS, Long JM, Kempf RC, Abrams SL, Franklin RA, Bäsecke J, Stivala F, Donia M, Fagone P, 2011. Ras/Raf/MEK/ERK and PI3K/PTEN/Akt/mTOR inhibitors: rationale and importance to inhibiting these pathways in human health. Oncotarget 2, 135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Oppenheim JJ, 2011. Contrasting effects of TNF and anti-TNF on the activation of effector T cells and regulatory T cells in autoimmunity. FEBS Lett. 585, 3611–3618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiurchiù V, Cencioni MT, Bisicchia E, De Bardi M, Gasperini C, Borsellino G, Centonze D, Battistini L, Maccarrone M, 2013. Distinct modulation of human myeloid and plasmacytoid dendritic cells by anandamide in multiple sclerosis. Ann. Neurol. 73, 626–636. [DOI] [PubMed] [Google Scholar]
- Cisse B, Caton ML, Lehner M, Maeda T, Scheu S, Locksley R, Holmberg D, Zweier C, den Hollander NS, Kant SG, 2008. Transcription factor E2–2 is an essential and specific regulator of plasmacytoid dendritic cell development. Cell 135, 37–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colonna M, Trinchieri G, Liu Y-J, 2004. Plasmacytoid dendritic cells in immunity. Nat. Immunol. 5, 1219–1226. [DOI] [PubMed] [Google Scholar]
- Crispín JC, Liossis S-NC, Kis-Toth K, Lieberman LA, Kyttaris VC, Juang Y-T, Tsokos GC, 2010. Pathogenesis of human systemic lupus erythematosus: recent advances. Trends Mol. Med. 16, 47–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crow M, 2007. Type I Interferon in Systemic Lupus Erythematosus, Interferon: The 50th Anniversary. Springer, pp. 359–386. [DOI] [PubMed] [Google Scholar]
- Davas E, Tsirogianni A, Kappou I, Karamitsos D, Economidou I, Dantis P, 1999. Serum IL-6, TNFα, p55 srTNFα, p75 srTNFα, srIL-2α levels and disease acitivity in systemic lupus erythematosus. Clin. Rheumatol. 18, 17–22. [DOI] [PubMed] [Google Scholar]
- Dewey WL, 1986. Cannabinoid pharmacology. Pharmacol. Rev. 38, 151–178. [PubMed] [Google Scholar]
- Domenici MR, Azad SC, Marsicano G, Schierloh A, Wotjak CT, Dodt H-U, Zieglgänsberger W, Lutz B, Rammes G, 2006. Cannabinoid receptor type 1 located on presynaptic terminals of principal neurons in the forebrain controls glutamatergic synaptic transmission. J. Neurosci. 26, 5794–5799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downer EJ, Clifford E, Gran B, Nel HJ, Fallon PG, Moynagh PN, 2011. Identification of the synthetic cannabinoid R (+) WIN55, 212–2 as a novel regulator of IFN regulatory factor 3 activation and IFN-β expression relevance to therapeutic effects in models of multiple sclerosis. J. Biol. Chem. 286, 10316–10328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellert-Miklaszewska A, Kaminska B, Konarska L, 2005. Cannabinoids down-regulate PI3K/Akt and Erk signalling pathways and activate proapoptotic function of bad protein. Cell. Signal. 17, 25–37. [DOI] [PubMed] [Google Scholar]
- Englund A, Morrison PD, Nottage J, Hague D, Kane F, Bonaccorso S, Stone JM, Reichenberg A, Brenneisen R, Holt D, 2013. Cannabidiol inhibits THC-elicited paranoid symptoms and hippocampal-dependent memory impairment. J. Psychopharmacol. 27, 19–27. [DOI] [PubMed] [Google Scholar]
- Every-Palmer S, 2010. Warning: legal synthetic cannabinoid-receptor agonists such as JWH-018 may precipitate psychosis in vulnerable individuals. Addiction 105, 1859–1860. [DOI] [PubMed] [Google Scholar]
- Gabay C, Cakir N, Moral F, Roux-Lombard P, Meyer O, Dayer J, Vischer T, Yazici H, Guerne P, 1997. Circulating levels of tumor necrosis factor soluble receptors in systemic lupus erythematosus are significantly higher than in other rheumatic diseases and correlate with disease activity. J. Rheumatol. 24, 303–308. [PubMed] [Google Scholar]
- Galiegue S, Mary S, Marchand J, Dussossoy D, Carriere D, Carayon P, Bouaboula M, Shire D, Fur G, Casellas P, 1995. Expression of central and peripheral cannabinoid receptors in human immune tissues and leukocyte subpopulations. FEBS J. 232, 54–61. [DOI] [PubMed] [Google Scholar]
- Gannon P, Khan MZ, Kolson DL, 2011. Current understanding of HIV-associated neurocognitive disorders pathogenesis. Curr. Opin. Neurol. 24, 275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gertsch J, 2008. Anti-inflammatory cannabinoids in diet. Commun. Integr. Biol. 1, 26–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gertsch J, Schoop R, Kuenzle U, Suter A, 2004. Echinacea alkylamides modulate TNF-α gene expression via cannabinoid receptor CB2 and multiple signal transduction pathways. FEBS Lett. 577, 563–569. [DOI] [PubMed] [Google Scholar]
- Gibson SJ, Lindh JM, Riter TR, Gleason RM, Rogers LM, Fuller AE, Oesterich JL, Gorden KB, Qiu X, McKane SW, 2002. Plasmacytoid dendritic cells produce cytokines and mature in response to the TLR7 agonists, imiquimod and resiquimod. Cell. Immunol. 218, 74–86. [DOI] [PubMed] [Google Scholar]
- Gomez O, Sanchez-Rodriguez A, Le M, Sanchez-Caro C, Molina-Holgado F, Molina-Holgado E, 2011. Cannabinoid receptor agonists modulate oligodendrocyte differentiation by activating PI3K/Akt and the mammalian target of rapamycin (mTOR) pathways. Br. J. Pharmacol. 163, 1520–1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guiducci C, Coffman R, Barrat F, 2009. Signalling pathways leading to IFN-α production in human plasmacytoid dendritic cell and the possible use of agonists or antagonists of TLR7 and TLR9 in clinical indications. J. Intern. Med. 265, 43–57. [DOI] [PubMed] [Google Scholar]
- Guo B, Cheng G, 2007. Modulation of the interferon antiviral response by the TBK1/IKKi adaptor protein TANK. J. Biol. Chem. 282, 11817–11826. [DOI] [PubMed] [Google Scholar]
- Hampson RE, Deadwyler SA, 1999. Cannabinoids, hippocampal function and memory. Life Sci. 65, 715–723. [DOI] [PubMed] [Google Scholar]
- Henriquez JE, Rizzo MD, Schulz MA, Crawford RB, Gulick P, Kaminski NE, 2017. Δ9-Tetrahydrocannabinol suppresses secretion of IFNα by plasmacytoid dendritic cells from healthy and HIV-infected individuals. JAIDS J. Acquir. Immune Defic. Syndr. 75, 588–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda K, Yanai H, Negishi H, Asagiri M, Sato M, Mizutani T, Shimada N, Ohba Y, Takaoka A, Yoshida N, 2005. IRF-7 is the master regulator of type-I interferon-dependent immune responses. Nature 434, 772–777. [DOI] [PubMed] [Google Scholar]
- Huang J, Liu T, Xu LG, Chen D, Zhai Z, Shu HB, 2005. SIKE is an IKKε/TBK1-associated suppressor of TLR3-and virus-triggered IRF-3 activation pathways. EMBO J. 24, 4018–4028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izzo AA, Borrelli F, Capasso R, Di Marzo V, Mechoulam R, 2009. Non-psychotropic plant cannabinoids: new therapeutic opportunities from an ancient herb. Trends Pharmacol. Sci. 30, 515–527. [DOI] [PubMed] [Google Scholar]
- Karmaus PW, Chen W, Kaplan BL, Kaminski NE, 2012. Δ 9-Tetrahydrocannabinol suppresses cytotoxic T lymphocyte function independent of CB 1 and CB 2, disrupting early activation events. J. NeuroImmune Pharmacol. 7, 843–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein TW, 2005. Cannabinoid-based drugs as anti-inflammatory therapeutics. Nat. Rev. Immunol. 5, 400–411. [DOI] [PubMed] [Google Scholar]
- Kozela E, Pietr M, Juknat A, Rimmerman N, Levy R, Vogel Z, 2010. Cannabinoids Δ9-tetrahydrocannabinol and cannabidiol differentially inhibit the lipopolysaccharide-activated NF-κB and interferon-β/STAT proinflammatory pathways in BV-2 microglial cells. J. Biol. Chem. 285, 1616–1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar H, Kawai T, Akira S, 2009. Toll-like receptors and innate immunity. Biochem. Biophys. Res. Commun. 388, 621–625. [DOI] [PubMed] [Google Scholar]
- Lande R, Gregorio J, Facchinetti V, Chatterjee B, Wang Y-H, Homey B, Cao W, Wang Y-H, Su B, Nestle FO, 2007. Plasmacytoid dendritic cells sense self-DNA coupled with antimicrobial peptide. Nature 449, 564–569. [DOI] [PubMed] [Google Scholar]
- Lu X, Clarke RC, 1995. The cultivation and use of hemp (Cannabis sativa L.) in ancient China. J. Int. Hemp Assoc. 2, 26–30. [Google Scholar]
- Manning BD, Cantley LC, 2007. AKT/PKB signaling: navigating downstream. Cell 129, 1261–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massi P, Vaccani A, Parolaro D, 2006. Cannabinoids, immune system and cytokine network. Curr. Pharm. Des. 12, 3135–3146. [DOI] [PubMed] [Google Scholar]
- Molina-Holgado E, Vela JM, Arévalo-Martín A, Almazán G, Molina-Holgado F, Borrell J, Guaza C, 2002. Cannabinoids promote oligodendrocyte progenitor survival: involvement of cannabinoid receptors and phosphatidylinositol-3 kinase/Akt signaling. J. Neurosci. 22, 9742–9753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngaotepprutaram T, Kaplan BL, Kaminski NE, 2013. Impaired NFAT and NFκB activation are involved in suppression of CD40 ligand expression by Δ9-tetrahydrocannabinol in human CD4+ T cells. Toxicol. Appl. Pharmacol. 273, 209–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oganesyan G, Saha SK, Guo B, He JQ, Shahangian A, Zarnegar B, Perry A, Cheng G, 2006. Critical role of TRAF3 in the toll-like receptor-dependent and-independent antiviral response. Nature 439, 208–211. [DOI] [PubMed] [Google Scholar]
- Pasparakis M, Alexopoulou L, Episkopou V, Kollias G, 1996. Immune and inflammatory responses in TNF alpha-deficient mice: a critical requirement for TNF alpha in the formation of primary B cell follicles, follicular dendritic cell networks and germinal centers, and in the maturation of the humoral immune response. J. Exp. Med. 184, 1397–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertwee RG, 1999. Pharmacology of cannabinoid receptor ligands. Curr. Med. Chem. 6, 635–664. [PubMed] [Google Scholar]
- Pertwee RG, 2006. Cannabinoid pharmacology: the first 66 years. Br. J. Pharmacol. 147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prinz N, Clemens N, Strand D, Pütz I, Lorenz M, Daiber A, Stein P, Degreif A, Radsak M, Schild H, 2011. Antiphospholipid antibodies induce translocation of TLR7 and TLR8 to the endosome in human monocytes and plasmacytoid dendritic cells. Blood 118, 2322–2332. [DOI] [PubMed] [Google Scholar]
- Ramshaw IA, Ramsay AJ, Karupiah G, Rolph MS, Mahalingam S, Ruby JC, 1997. Cytokines and immunity to viral infections. Immunol. Rev. 159, 119–135. [DOI] [PubMed] [Google Scholar]
- Rönnblom L, 2010. Potential role of IFNα in adult lupus. Arthritis Res. Ther. 12 (S3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi O, Hoshino K, Kawai T, Sanjo H, Takada H, Ogawa T, Takeda K, Akira S, 1999. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity 11, 443–451. [DOI] [PubMed] [Google Scholar]
- Tanasescu R, Constantinescu CS, 2010. Cannabinoids and the immune system: an overview. Immunobiology 215, 588–597. [DOI] [PubMed] [Google Scholar]
- Thacore VR, Shukla S, 1976. Cannabis psychosis and paranoid schizophrenia. Arch. Gen. Psychiatry 33, 383–386. [DOI] [PubMed] [Google Scholar]
- Wang C-Y, Mayo MW, Baldwin AS Jr., 1996. TNF-and cancer therapy-induced apoptosis: potentiation by inhibition of NF-kB. Science 274, 784. [DOI] [PubMed] [Google Scholar]
- Weichhart T, Säemann M, 2008. The PI3K/Akt/mTOR pathway in innate immune cells: emerging therapeutic applications. Ann. Rheum. Dis. 67, iii70–iii74. [DOI] [PubMed] [Google Scholar]
- Xu H, Cheng CL, Chen M, Manivannan A, Cabay L, Pertwee RG, Coutts A, Forrester JV, 2007. Anti-inflammatory property of the cannabinoid receptor-2-selective agonist JWH-133 in a rodent model of autoimmune uveoretinitis. J. Leukoc. Biol. 82, 532–541. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Lenardo MJ, Baltimore D, 2017. 30 years of NF-κB: a blossoming of relevance to human pathobiology. Cell 168, 37–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.