Abstract
The Ewing sarcoma family of tumors or Ewing sarcoma (ES) is the second most common malignant bone tumor of childhood. The prognosis for localized Ewing sarcoma has improved through the development of intense multimodal therapy over the past several decades. Unfortunately, patients with recurrent or metastatic disease continue to have a poor prognosis. Therefore, a number of complementary approaches are being developed in both the preclinical and clinical arenas to improve these outcomes. In this review, we will discuss efforts to directly target the biologic drivers of this disease and relate these efforts to the experience with several different agents both in the clinic and under development. We will review the data for compounds that have shown excellent activity in the clinic, such as the camptothecins, and summarize the biological data that supports this activity. In addition, we will review the clinical experience with IGF1 targeted agents, ET-743 and epigenetically targeted therapies, the substantial amount of literature that supports their activity in Ewing sarcoma and the challenges remaining translating these therapies to the clinic. Finally, we will highlight recent work aimed at directly targeting the EWS-FLI1 transcription factor with small molecules in Ewing tumors.
Keywords: Ewing sarcoma, Camptothecin, Insulin like growth factor (IGF), Poly ADP ribose polymerase (PARP), Trabectedin, Mithramycin
1. Introduction
Ewing sarcoma (ES) is a bone and soft tissue tumor that encompasses tumors formerly known as Askin’s tumor, Peripheral Neuroectodermal Tumor (PNET) and the Ewing Sarcoma Family of Tumors (ESFT) (Hawkins et al., 2011). This disease is most common in the second decade of life with an annual incidence of about 225 cases in North America in children between 1 and 20 years of age (Esiashvili et al., 2008). Patients with Ewing sarcoma who present with localized disease have a favorable 5-year event free survival of around 70% due to the advent of multimodal therapy that includes chemotherapy, surgery and/or radiation (Grier et al., 2003; Paulussen et al., 2008). Unfortunately, patients with bone and bone marrow metastatic disease or recurrent disease have a less than 20% overall survival rate (Bernstein et al., 2006; Esiashvili et al., 2008; Leavey et al., 2008). Therefore, there is a great need to develop new approaches or therapies for the treatment of this tumor that target the biology of the disease.
The defining molecular feature of Ewing sarcoma is the characteristic EWSR1/ETS fusion protein, most commonly involving EWSR1 and FLI1 (Delattre et al., 1992; May et al., 1993). EWS-FLI1 causes global changes in gene expression both by directly regulating gene expression and by causing changes in chromatin structure influenced by EWS-FLI1 downstream targets (Bertolotti et al., 1996; Owen et al., 2008; Kinsey et al., 2009; Patel et al., 2012). The end result is a transcriptional program that mediates malignant transformation, maintains the cells in a de-differentiated state and helps to evade the toxicity associated with DNA damaging agents (Riggi & Stamenkovic, 2007; Ban et al., 2008; Riggi et al., 2008; Awad et al., 2010; von Levetzow et al., 2011; Lawlor & Thiele, 2012).
In addition, silencing of EWS-FLI1 using antisense DNA, siRNA or dominant negative methods markedly impairs Ewing sarcoma cell growth (Maksimenko & Malvy, 2005).
From a therapeutic standpoint, the challenge is integrating this data into the preclinical and clinical development of new agents that target the biological drivers of this disease. In order to accomplish this, work continues to understand the mechanism of drugs that have proven to be active in the clinic, such as the camptothecins. Conversely, other preclinical data is aimed at understanding the reasons for disappointing clinical results with promising agents that have a strong preclinical rationale such as ET-743 and Insulin like Growth Factor (IGF) pathway inhibitors (Scotlandi et al., 2002; Lau et al., 2005; Grohar, Griffin et al., 2011; Pappo et al., 2011; Wagner, 2011; Malempati & Hawkins, 2012). Finally, the clinical translation of therapies that directly target EWS-FLI1, associated epigenetic changes as well as the DNA damage response presents unique challenges to both preclinical and clinical investigators (Stegmaier et al., 2007; Erkizan et al., 2009; Grohar, Woldemichael et al., 2011; Lawlor & Thiele, 2012).
The focus of this review will be to discuss recent advances in experimental therapeutics in both the clinical and preclinical arena for Ewing sarcoma. In particular, we will highlight efforts to link clinical results to the biology of Ewing sarcoma. The goal will be to summarize challenges remaining to translate these therapies to Ewing sarcoma patients.
2. The camptothecins
As with many tumors, the favorable survival rates for patients with localized Ewing sarcoma are the direct result of decades of skill-ful clinical research. In recent years, these efforts have focused on identifying and developing other active agents in both the relapsed and up-front setting. Among the most well studied compounds in the clinic in Ewing sarcoma are the camptothecins, irinotecan and topotecan (reviewed in Wagner, 2011).
The camptothecins are a class of compounds originally isolated from the bark of a Chinese tree called Camptotheca acuminate. The primary mechanism of action of these compounds involves stabilization of the topoisomerase I cleavage complex leading to replication fork collision, DNA damage and cell death (Pommier, 2006). In Ewing sarcoma, the camptothecin derivatives, topotecan and irinotecan have been investigated both as up-front windows in high-risk patients and in the relapsed setting. These compounds are structural derivatives of the parent compound substituted at the 7, 9 and 10 positions of the camptothecin backbone (Fig. 1). The importance of these compounds is suggested by a series of clinical studies that show good activity in Ewing sarcoma both as single agents and in combination therapies with either cyclophosphamide or temozolamide (Pappo et al., 2011; Wagner, 2011).
Fig. 1.
Chemical structures of the common camptothecins. Topotecan is substituted at the 9 and 10 positions while irinotecan is substituted at the 7 and 10 positions. Both have been extensively evaluated in Ewing sarcoma.
Topotecan is hydroxylated at the 10 position and contains an alkylamine at the 9 position of the camptothecin backbone. This agent has been evaluated in a broad range of both pediatric and adult tumors. The Pediatric Preclinical Testing Program (PPTP) did a thorough investigation of this compound across 45 different xenograft models of pediatric cancer and found an increase in event free survival in 86% of the solid tumors evaluated with complete responses observed in Ewing sarcoma, Wilm’s tumor and rhabdomyosarcoma (Carol et al., 2010). In Ewing sarcoma patients, a COG phase II evaluated topotecan as an up-front window in high-risk patients as a single agent (n=36) and in combination with cyclophosphamide (n=40) and showed partial response rates of 8% and 57% respectively (Bernstein et al., 2006). It is notable that these response rates are higher than what has been observed in the relapsed setting where partial response rates of 4% as a single agent and 33% as a combination have been seen although a 21-day infusion may offer slightly better results (Blaney et al., 1998; Hawkins et al., 2006; Hunold et al., 2006). The major dose limiting toxicity of this drug has been myelosuppression, occurring in more than 50% of patients treated with cyclophosphamide and topotecan (Saylors et al., 2001).
In contrast, irinotecan has shown much less myelosuppression and may be more active in Ewing sarcoma both alone and in combination with temozolamide. One phase II study of 13 heavily pretreated Ewing sarcoma patients demonstrated a response rate of 38% including 2 complete responses (Bisogno et al., 2006). Irinotecan has also shown significant activity in combination with temozolamide. A single center retrospective review of 19 pretreated Ewing sarcoma patients demonstrated an overall response rate of 63% with 5 documented complete responses (Casey et al., 2009). The most common toxicity observed in pediatric patients is diarrhea likely related to metabolism of the drug by intestinal flora, an effect that can be at least partially mitigated by administration of oral cephalosporins (Furman et al., 1999; Wagner et al., 2008; McGregor et al., 2012). It is notable that there is significant schedule dependence to the activity of these compounds consistent with their activity in S-phase that mitigates the myelosuppression particularly for irinotecan (Houghton et al., 1995).
Together these studies suggest that further prospective evaluation of camptothecins and in particular irinotecan as both a single agent and in combination therapies is warranted. This notion is further supported by recent preclinical work that suggests a particular sensitivity of Ewing sarcoma to these agents. In one study, gene expression data, chromosomal copy number and sequencing data were cross-referenced with novel pharmacological evaluations of 24 different agents in 479 human cancer cell lines. The Ewing sarcoma cell lines were among the most sensitive cell lines tested to camptothecin and showed the highest expression of SLFN11, a gene identified as a predictor of camptothecin sensitivity (Barretina et al., 2012). In addition, a recent drug screen to identify inhibitors of the EWS-FLI1 transcription factor (described below), identified among the active inhibitors of EWS-FLI1 camptothecin (Boro et al., 2012). It is notable that we independently identified camptothecins in our high throughput screen described below although we did not fully characterize this compound in lieu of other more effective inhibitors (unpublished data).
In summary, the camptothecins have an emerging role in Ewing sarcoma. Although it is not known to what extent the interference with EWS-FLI1 contributes to the clinical activity, clinical evidence has firmly established the importance of these compounds in this tumor type. Future preclinical and clinical efforts will likely focus on the further development of biomarkers of activity, novel combination therapies as well as the optimal time in Ewing sarcoma therapy to administer these drugs.
3. ET-743 (Ecteinascidin 743, Trabectedin, Yondelis™)
ET-743 is a natural product isolated from the sea squirt Ecteinascidia turbinate. The drug has been extensively studied in phase I and II studies in sarcomas in both the United States and Europe and found to have activity in leiomyosarcoma, liposarcoma, malignant fibrous histiocytoma, and synovial sarcoma (Chuk et al., 2009; Thornton, 2010). However, the most impressive response rates have occurred in myxoid liposarcoma where an almost 50% response rate has been observed in some series (Grosso et al., 2009; Cesne et al., 2012). Elegant preclinical work has demonstrated that this particular sensitivity of myxoid liposarcoma may be due to the drug’s ability to block the activity of the FUS-CHOP oncogene, allowing the tumor to differentiate into benign lipoblasts (Forni et al., 2009; Frapolli et al., 2010). This theory was further supported by work that showed that in an animal model of FUS-CHOP tumorigenesis, the drug is able to release the FUS-CHOP mediated block in differentiation again allowing the tumor to become benign lipoblasts (Charytonowicz et al., 2012). Given the broad range of responses in sarcoma and activity against ML in particular, it seemed as though the drug may have particular activity against translocation positive sarcomas, a theory being tested in a randomized phase III trial. Since Ewing sarcoma is a translocation positive sarcoma and FUS and EWS are family members, it follows that Ewing sarcoma might also display a particular sensitivity to the drug.
The evaluation of ET-743 in children initially lent credence to this theory. In a phase I study of 13 patients, 2 out of 3 Ewing sarcoma patients treated with the drug responded, one patient with widely metastatic disease had a complete response (Lau et al., 2005). In another case series of Ewing sarcoma patients, a 25% progression free survival rate was observed (Dileo et al., 2007). In addition, preclinical reports have shown that Ewing sarcoma cells are among the most sensitive to the drug in vitro with sub-nanomolar IC50s (Scotlandi et al., 2002; Aune et al., 2008). Furthermore, we have shown that the drug interferes with the activity of EWS-FLI1 at the promoter and mRNA level and effectively reverses a gene signature of EWS-FLI1 in vitro (Grohar, Griffin et al., 2011). Unfortunately, a recent phase II did not support the activity of this agent in Ewing sarcoma as 1 out of 11 patients with Ewing sarcoma who received ET-743 had stable disease and the rest progressed on therapy. A recent phase I trial at the National Cancer Institute also did not show activity in Ewing sarcoma, although only one patient with ES was enrolled in this study (Chuk et al., 2012).
Nevertheless, because of the preclinical work and the interference with EWS-FLI1 transcriptional activity, we believe that there may be a role for ET-743 in Ewing sarcoma. An analysis of the pharmacokinetic data published in the phase II clinical trial shows that at most the drug spent 5 to 6 days out of 21 above the IC50 of Ewing sarcoma cells with wide confidence intervals. In addition, our preclinical data has shown strong suppression of EWS-FLI1 at concentrations above 1 nM, serum levels that would only have been achieved for 48 h in the patient in this report (Lau et al., 2005). In addition, recent work has demonstrated limited sensitivity of xenograft models as a single agent on a similar schedule (manuscript in preparation). Nevertheless, in the COG phase I trial, a patient with widely metastatic Ewing sarcoma achieved a complete response. Therefore, more work needs to be done in the clinical and preclinical arena to study the dose and schedule of the drug in Ewing sarcoma. In addition, it is likely that the role of this drug in Ewing sarcoma will be in the setting of combination therapies (Scotlandi et al., 2002). Alternatively, new analogs of ET-743 such as Zalipsys™ and PM001183 are currently entering the clinic and may provide more potent or less toxic inhibition of EWS-FLI1 due to differences in effects on DNA damage pathways (Guirouilh-Barbat et al., 2008).
4. PARP inhibitors
There is a growing enthusiasm in the Ewing sarcoma community to evaluate poly ADP ribose polymerase (PARP) inhibitors in the clinic. This is the direct result of two manuscripts that suggest an inherent sensitivity of ES cells to PARP inhibition (Brenner et al., 2012; Garnett et al., 2012). In the first study, 639 cell lines were evaluated to determine a relationship between sensitivity to 130 different drugs as a function of 64 fully sequenced cancer genes, genome wide analysis of copy number, and expression profiling. Among the most statistically significant relationships was the relationship between EWS-FLI1 expression and sensitivity to the PARP inhibitor AZD2281 (olaparib) (Garnett et al., 2012). This relationship approximated the statistical significance of the known relationship between imatinib and nilotinib and BCR-ABL as well as PLX4720 and SB590885 and mutation of BRAF. The authors went on to show a relative increased sensitivity of some but not all ES cell lines to olaparib with a geometric mean IC50 of 4.7 μM relative to 64 μM for the 291 other cell lines.
The other manuscript utilized a candidate gene approach to evaluate olaparib in Ewing sarcoma cells (Brenner et al., 2012). The authors again showed increased sensitivity of 3 ES cell lines to olaparib as opposed to an osteosarcoma and rhabdomyosarcoma cell line. In addition, EWS-FLI1 gene transfer experiments seemed to increase the sensitivity to olaparib, an effect that was recapitulated in the other manuscript (Brenner et al., 2012). Of note, the drug was not effective as a single agent in xenograft experiments. However, this experiment was performed with the goal of demonstrating synergy so the drug may have single agent activity at higher doses. In addition, a combination with temozolamide showed an impressive regression of tumor growth (Brenner et al., 2012).
The challenge in moving PARP inhibitors forward lies in understanding the biology behind these effects, the context that determines sensitivity, as well as determining the optimal combination therapy for clinical translation. It has been known since the early 1990s that Ewing sarcoma cells express high levels of PARP mRNA, protein and have an inherently high PARP activity potentially related to an increase in copy number (Prasad et al., 1990). This increased PARP activity led to the demonstration that inhibition of PARP with chemical tool compounds sensitized ES cells to ionizing radiation (IR) (Prasad et al., 1990).
Subsequent studies confirmed the high level of PARP expression and showed that the promoter of PARP1 contained several ETS binding sites (Soldatenkov et al., 1999). However, it was shown that in A4573 Ewing sarcoma cells, PARP expression was driven by the FLI1 family member ETS1 and EWS-FLI1 actually suppressed PARP promoter activity and caused a minimal decrease in PARP expression (Soldatenkov et al., 1999; Soldatenkov et al., 2002). In addition, this report showed that silencing ETS1 with an antisense DNA, decreased PARP expression and was associated with resistance to the DNA damaging agent etoposide, while the suppression of EWS-FLI1 caused a minor increase in PARP that nonetheless sensitized cells to etoposide, presumably by interfering with other DNA damage pathways (Soldatenkov et al., 2002). This is in contrast to recent reports that show that in RD-ES cells PARP expression is driven by EWS-FLI1 and siRNA silencing of EWS-FLI1 markedly decreases PARP expression (Soldatenkov et al., 2002). Since both studies evaluated both promoter activity and total protein expression, there are clearly yet to be determined indirect effects that mediate the relationship between EWS-FLI1 and PARP.
Together, the data suggests that more work needs to be done to establish the role of PARP inhibitors in Ewing sarcoma. As a single agent, there is some activity in Ewing sarcoma cell lines and a highly statistically significant relationship between olaparib sensitivity and EWS-FLI1 expression. However, the sensitivity does not occur in all the ES cell lines and predictors of sensitivity to PARP inhibition are clearly needed. There appears to be impressive activity in an ES xenograft when PARP inhibition is combined with temozolamide (Brenner et al., 2012). However, it is clear that combination of PARP inhibitors with other chemotherapeutic agents may substantially enhance myelosuppression and will require careful study to determine optimal dose and schedules (Kummar et al., 2011). It is clear that the activities of PARP are complex and it participates in multiple DNA repair pathways (reviewed in Wang et al., 2012). This biology is likely only further complicated by EWS-FLI1 that impacts the expression of multiple genes in various pathways. Therefore, careful studies of these agents in Ewing sarcoma will be required to allow for optimal utilization of these agents in treatment strategies.
5. IGF signaling pathway inhibitors
The importance of the IGF pathway to sarcoma biology including Ewing sarcoma has been the subject of decades of investigation. A number of important studies have established the importance of this pathway to the biology of Ewing sarcoma. In particular, IGF has been shown to mediate EWS-FLI1 driven malignant transformation of NIH3T3 cells as measured by soft agar assay (Toretsky et al., 1997). EWS-FLI1 in turn drives expression of the IGF1R receptor and suppresses expression of the negative regulator of IGF, IGFBP3 (Prieur et al., 2004; Cironi et al., 2008; Herrero-Martin et al., 2009). In addition, recent work shows that EWS-FLI1 regulates a series of miRNAs that perturb multiple members of the IGF pathway (McKinsey et al., 2011). From a therapeutic standpoint, critical preclinical studies have established the sensitivity of ES cells to inhibition of IGF both in vitro and in animal models of Ewing sarcoma (Scotlandi et al., 1996; Scotlandi et al., 1998). These studies have established the importance of this pathway to the biology of Ewing sarcoma mitigating everything from angiogenesis to drug resistance to a variety of agents active in Ewing sarcoma including ET-743 (Hofbauer et al., 1993; Benini et al., 2001; Strammiello et al., 2003; Manara et al., 2005).
In recent years, agents have become available to target this pathway in the clinic. In particular, work has been done to evaluate antibodies targeting the receptor, small molecule kinase inhibitors of the receptor and downstream target inhibitors such as mTOR. In the preclinical setting, these agents were successful at inhibiting Ewing sarcoma tumor growth both in vitro and in animal models (Scotlandi et al., 2005; Martins et al., 2006; Manara et al., 2007; Kolb et al., 2008; Kurmasheva et al., 2009; Houghton et al., 2010; Kang et al., 2010; Beltran et al., 2011; Kolb et al., 2011). In addition, the agents were linked to critical biological processes such as the establishment of blood vessels in the growing tumor and even experimental models of metastasis (Krishnan et al., 2006; Manara et al., 2007; Liu et al., 2010). Therefore, there was considerable excitement when these agents moved into the clinic.
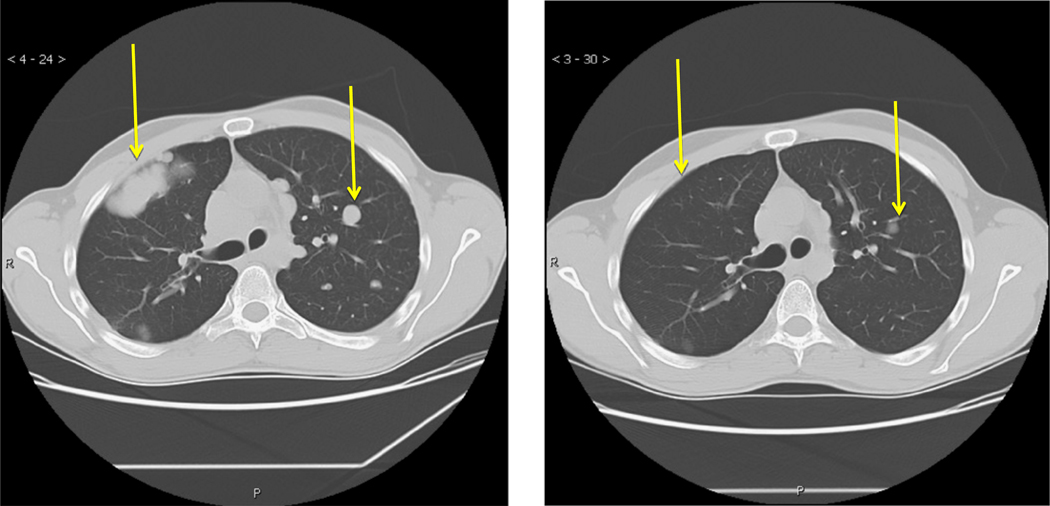
Early phase I work established the safety of the IGFIR antibodies in a variety of tumors, including Ewing sarcoma in children (Malempati et al., 2012). As the agents moved into phase II, the excitement led to robust accrual. In a study sponsored by the Sarcoma Alliance for Research through Collaboration (SARC) using a fully human IGFIR antibody, 115 Ewing sarcoma patients were accrued in 31 different institutions in just over two years (Pappo et al., 2011). Again dramatic responses were observed with this agent such as that seen in Fig. 2. However the overall response rates were in the range of 10–15%. A similar study with an alternative IGFIR antibody demonstrated response rates of 17% and again some dramatic responses were observed (Malempati et al., 2012).
Fig. 2.
Response of 26 year old Ewing sarcoma patient treated with IGF1R antibody R1507. CT scan showing response to treatment of lung metastasis at baseline (on left) and following 6 weeks of treatment with 9 mg/kg/week of R1507. The yellow arrows highlight specific metastatic lesions.
The relatively low overall response rates suggest the need for better predictive markers for response. The patient demographics in the SARC trial did not reflect typical Ewing sarcoma demographics as the patients tended to be older and had primarily soft tissue tumors. In addition, although accrual favored older children with soft tissue primary tumors, bone based primary tumors, more common in younger children, appeared to have a more robust response to the inhibitor. Finally, the dose and schedule of the drug in the early part of the study were designed to favor AUC pharmacokinetics, while later data suggested that drug peak was more important to activity. This led to a trial design that allowed both schedules. Unfortunately, the trial was forced to close prior to completing accrual due to a loss of corporate support (Pappo et al., 2011).
There remains considerable enthusiasm for IGF therapy due to the dramatic responses observed and the plurality of preclinical data. Therefore, efforts moving forward have been focused on addressing mechanisms of resistance as well as developing methods to try and identify those patients that are likely to respond to the drug. These efforts have met with some success. It is known that downstream blockade of mTOR leads to feedback activation of AKT via the IGF receptor originally described in myeloma and rhabdomyosarcoma (Shi et al., 2005; Wan et al., 2007). Therefore, combining IGF inhibition and mTOR inhibitors in preclinical sarcoma models has led to improved activity of both agents (Wan et al., 2007). This improved activity has also been observed in the clinic. A recent study that combined the IGF-1R inhibitor cixutumumab with temsirolimus found tumor regressions in 30% of Ewing sarcoma patients treated with this combination including a complete response in a patient previously refractory to IGF-1R targeting (Naing et al., 2012).
Finally, there have been a number of important studies that attempt to identify those patients that might respond to the antibody. For example, proteomic profiling has led to the development of a proteomic signature of likely responders that could be used to preselect patients once validated (Subbiah et al., 2011). In addition, a recent report suggests that nuclear staining of IGF may reflect pathway activation and predict those patients that might respond to the antibody (Asmane et al., 2012). Other important investigations have shown a wide range of IGF receptor expression that predicts response in rhabdomyosarcoma perhaps detectable with imaging methods (Cao et al., 2008; Fleuren et al., 2011). Finally, an analysis of over 200 ES patients has shown that patients with Ewing sarcoma have variable expression of circulating IGF and IGFBP3 (Borinstein et al., 2011). It is not known how these variable levels would predict a response to IGF blockade but it is notable that in preclinical models circulating IGF has been shown to block the anti-angiogenic effects of the drug (Bid et al., 2012).
In summary, the importance of IGF signaling to Ewing sarcoma is widely recognized. Despite this, early clinical investigations have obtained modest response rates. Therefore, the challenge to an IGF therapy is preselecting those patients likely to achieve dramatic responses and/or directly target the mechanisms of resistance.
6. Epigenetic targeting
Since Ewing sarcoma is a transcription factor driven disease, agents that target chromatin structure would be expected to have activity in this tumor type. In addition, a variety of protein complexes involved in the regulation of chromatin structure have been shown to be dysregulated in Ewing sarcoma (reviewed in Lawlor & Thiele, 2012). The resulting changes in the epigenome play a major role in the biology of ES and have been linked to alterations in gene expression, malignant transformation and even drug resistance (Lawlor & Thiele, 2012).
It is known that EWS-FLI1 blocks the expression of more genes then it induces (Kauer et al., 2009). This suppression is a critical component of the tumorigenicity of EWS-FLI1 as it likely silences critical tumor suppressor genes. The mechanism of EWS-FLI1 gene suppression is both direct, as with TGFBR2, and indirect by driving the expression of genes that in turn blocks expression of portions of the EWS-FLI1 gene signature, an effect that is at least partially mediated by changes in the epigenome (Hahm et al., 1999; Owen et al., 2008; Kinsey et al., 2009). For example, it has been shown that EWS-FLI1 drives the expression of NKX2.2, a gene that silences a portion of the EWS-FLI1 repressed signature (Owen et al., 2008). NKX2.2 binds to the promoters of target genes and facilitates TLE associated HDAC recruitment to suppress expression of these genes. Treatment of A673 ES cells with the HDAC inhibitor vorinostat reverses this suppression and leads to a dose dependent inhibition of Ewing sarcoma cell growth (Owen et al., 2008). This marked sensitivity to HDAC inhibitors mirrors an earlier report that evaluated the HDAC inhibitor, MS-275, in the TC71 xenograft model of Ewing sarcoma and found an impressive response with single agent treatment by oral gavage (Jaboin et al., 2002).
Other studies have investigated the role of HDAC inhibitors in particular signaling pathways mediated by EWS-FLI1 (Matsumoto et al., 2001). A recent investigation, showed a physical association of EWS-FLI1 and HDAC1 that helps mediate a disruption in the p53 activation of downstream targets (Li et al., 2012). Disruption of HDAC1 with the HDAC inhibitor trichostatin A (TSA) restored the competency of this pathway, potentially providing the basis for combination therapies involving cytotoxic agents and TSA.
Other epigenetic complexes have also been linked to important features in the biology of Ewing sarcoma. For example, EWS-FLI1 has been shown to directly regulate the polycomb complex gene EZH2 to help maintain a block in the differentiation of the tumors and facilitate metastatic spread (Richter et al., 2009). These results mirrored an earlier finding that showed that expression of EWS-FLI1 in mesenchymal stem cells upregulates EZH2 and triggers a transcriptional program that is similar to Ewing tumors (Riggi et al., 2008). Similarly, the expression of EWS-FLI1 in neural crest cells leads to the induction of EZH2 and another polycomb gene, BMI1 potentially creating a program permissive for EWS-FLI1 mediated transformation (von Levetzow et al., 2011).
In the clinic, the HDAC inhibitor vorinostat has been evaluated in children in the relapsed refractory setting. Unfortunately, single agent activity in Ewing sarcoma has not been observed, although only 2 patients with Ewing sarcoma received the drug (Fouladi et al., 2010). However, abnormal chromatin structure in Ewing sarcoma cells is a component of both the genotype and phenotype of this tumor. EWS-FLI1 drives the expression of genes that in turn suppress a large percentage of the genome by modifying the availability of chromatin to the transcriptional machinery. These genes allow Ewing sarcoma cells to evade senescence and apoptosis in the setting of genetic damage and help the cells maintain a more de-differentiated state. It follows that ES cells are in turn sensitive to these agents both alone and in combination with other agents in preclinical models. Therefore, further investigation of these agents in Ewing sarcoma in a prospective fashion is warranted. In addition, work continues to develop more specific small molecules and combination therapies that recapitulate the preclinical success of these agents in the clinic.
7. Targeting the EWS-FLI1 transcription factor
The defining molecular feature of Ewing sarcoma is the characteristic EWSR1 and ETS fusion protein (Delattre et al., 1992). The most common fusion transcription involves EWSR1 and FLI1 and is derived from the t(11;22)(q24;q12) chromosomal translocation (Delattre et al., 1992; May et al., 1993). The biology of this tumor revolves around this molecular feature (Riggi & Stamenkovic, 2007). Experiments that silence EWS-FLI1 have shown clearly that the translocation influences the expression of over 500 to 1000 genes (Kauer et al., 2009). The resulting genetic program is believed to mitigate all aspects of oncogenesis and progression (Riggi & Stamenkovic, 2007). In addition, silencing of EWS-FLI1 using antisense DNA, siRNA and dominant negatives markedly impairs Ewing sarcoma cell growth (Maksimenko & Malvy, 2005). Unfortunately, the fusion protein is a transcription factor and therefore a challenging drug target. However, given the importance of the target, a number of strategies have been employed to try to identify small molecule inhibitors of EWS-FLI1.
The first report of an EWS-FLI1 inhibitor employed functional drug screening and a novel gene signature approach that utilized branched chain technology to screen a library of clinically relevant small molecules to identify inhibitors of EWS-FLI1 (Stegmaier et al., 2007). The lead compound identified from this study was cytarabine, which depressed EWS-FLI1 protein expression, interfered with anchorage independent growth and impaired A673 xenograft growth (Stegmaier et al., 2007). Furthermore, older literature supported the in vitro sensitivity of Ewing sarcoma cells to cytarabine with IC50s that paralleled that of leukemia cell lines (Hofbauer et al., 1993). Unfortunately, the clinical translation of this compound was not successful. The phase II study evaluated 10 patients and found only one patient to have stable disease (DuBois et al., 2009). The limitations of the clinical study may explain this outcome and provide insight into future trials. First, although the screen was for a defined target, EWS-FLI1, only 5 of 10 patients treated had this documented translocation. Second, although historical pharmacokinetic data indicates that concentrations of cytarabine sufficient to inhibit EWS-FLI1 were achieved, it is not known if 5 days of these concentrations in a 21-day cycle are enough to impair tumor growth or if the metabolism of this drug in patients allows active concentrations of drug to be achieved in Ewing sarcoma cells. Finally, there is no good pharmacodynamic marker of EWS-FLI1 activity and so it is not known if downstream target expression was suppressed in the tumors.
We recently employed a functional drug screening approach to identify mithramycin as an inhibitor of EWS-FLI1. In our study, we employed a stepwise approach involving a luciferase primary screen and a novel multiplex PCR screen to evaluate over 50,000 compounds to identify mithramycin as an inhibitor of EWS-FLI1 (Grohar, Woldemichael et al., 2011). We showed that mithramycin does not interfere with EWS-FLI1 expression and instead works downstream at the promoter level to block activity, effectively reversing two independent gene signatures of EWS-FLI1 and well-characterized downstream targets such as ID2 and NR0B1 both in vitro and in vivo. Work is ongoing to establish the mechanism of transcriptional interference with EWS-FLI1. It is known that mithramycin blocks the binding of transcription factors to DNA by binding and distorting the minor groove (Banville et al., 1990; Sastry & Patel, 1993; Aich & Dasgupta, 1995). It is established that mithramycin inhibits the SP1 transcription factor, but it is not known what additional transcription factors are specifically blocked by the drug (Ray et al., 1989; Blume et al., 1991; Chatterjee et al., 2001; Remsing et al., 2003; Sleiman et al., 2011; Zhang et al., 2012). Nevertheless, the drug suppresses growth of two different ES xenografts including a marked regression of the TC32 xenograft. Finally, after identifying the compound, we found precedence of mithramycin use in Ewing sarcoma with two CRs reported out of 5 patients treated in the 1960s (Kofman et al., 1973).
We now have a phase I/II clinical trial open to again evaluate the activity of mithramycin in Ewing sarcoma. In our study, we are mandating that patients are EWS-FLI1 positive. In addition, we are requiring pre and post treatment biopsies to evaluate the effect of mithramycin treatment on EWS-FLI1 activity. Finally, we are working in the preclinical setting to evaluate a series of mithramycin analogs that appear to be more potent and specific than the parent compound (unpublished data).
An alternative approach to functional drug screening is to characterize critical mediators of EWS-FLI1 activity and screen for compounds that interfere with this interaction. Phage display was used to identify an interaction between EWS-FLI1 and RNA helicase A. This interaction was shown to enhance the activity of the ID2 promoter in Ewing sarcoma cells and facilitate soft agar colony formation in Mouse embryonic fibroblasts transformed with EWS-FLI1 (Toretsky et al., 2006). Subsequent to this study, the interaction was further defined and an ex vivo approach was used to identify a small molecule that disrupts this protein-protein interaction to interfere with ES growth in vitro and in vivo (Erkizan et al., 2009). Work is ongoing to develop a drug directed against RNA helicase A in the clinic.
Finally, a more recent screen evaluated the expression of 5 targets of EWS-FLI1 utilizing standard qPCR to screen a library of small molecules to identify the kinase inhibitor, midostaurin as an inhibitor of EWS-FLI1 (Boro et al., 2012). This compound reversed expression of these targets and induced apoptosis of Ewing sarcoma cells both in vitro and in vivo using two different xenograft studies. It is notable that this study also identified camptothecin and doxorubicin as both active agents in the clinic. Doxorubicin as a mainstay of Ewing sarcoma therapy, was previously independently shown to reverse a gene signature of EWS-FLI1 (Stegmaier et al., 2007). Therefore, the contribution of this activity to the mechanism of these drugs in Ewing sarcoma patients is not clear.
In summary, the direct targeting of EWS-FLI1 with small molecules offers the hope of directly targeting the biology the tumor depends on for survival. However, significant challenges remain likely secondary to the complexity of eukaryotic transcription. It is interesting that several compounds previously characterized to be active in the clinic such as doxorubicin, irinotecan and mithramycin show variable effects on EWS-FLI1 activity. Nevertheless, lessons learned from the clinical translation of cytarabine as well as clinical trials involving ET-743 suggest the need for strong clinical pharmacodynamic markers of EWS-FLI1 activity as well as the pre-selection of patients that are EWS-FLI1 positive.
8. Conclusions
Improving the outcomes for patient with Ewing sarcoma will require the concerted effort of preclinical and clinical investigators. The success of which will likely come from a thorough understanding of the activities of promising agents and the translation and verification of these activities in targeting biologically relevant drivers of Ewing sarcoma growth in patients. In this review, we focused on a number of approaches to target specific pathways thought to be important for Ewing sarcoma growth in patients and attempted to highlight some of the remaining challenges of each individual agent.
Targeting the EWS-FLI1 transcription factor directly is an approach that faces the challenge of the complexity of eukaryotic transcription. From a practical standpoint, the development of inhibitors identified in preclinical investigations including mithramycin and ET-743, will require the development of pharmacodynamic assays to correlate drug exposure with EWS-FLI1 blockade. In addition, these agents will need to be tested in EWS-FLI1 positive tumors that have not been heavily pretreated with chemotherapy.
Alternatively, inhibiting important pathways associated with EWS-FLI1 such as the IGF pathway, epigenetic pathways or pathways associated with the DNA damage response such as PARP, will require a more thorough understanding of the most effective way to utilize these agents. In the case of IGF, this may require combination therapies that directly target mechanisms of resistance or the pre-selection of patients likely to respond to these therapies. The clinical targeting of the epigenome in Ewing sarcoma is still in its early stages, therefore a number of strategies may achieve success with this approach including but not limited to combination therapies that exploit specific epigenetic changes or even more specific targeted agents as they become available. Finally, an understanding of the altered DNA damage response in Ewing sarcoma will likely lead to novel therapeutics targeting these pathways.
Finally, continued systematic clinical investigation that integrates active agents such as the camptothecins into Ewing sarcoma therapy will likely lead to improved outcomes or salvage regimens. These methods will undoubtedly evolve and integrate preclinical investigations that identify predictors of sensitivity such as SLFN11 (Barretina et al., 2012). These investigations will be important to the realization of the success of all these agents and hopefully improve survival for patients with Ewing sarcoma.
Acknowledgments
Research Support: PJG is a Turner-Hazinski Scholar and also receives research support from the Sarcoma Alliance for Research through Collaboration (SARC), St. Baldrick’s and Hyundai Hope on Wheels. LJH receives research support from the intramural program at the Center for Cancer Research, NCI.
Abbreviations:
- COG
Children’s Oncology Group
- ES
Ewing sarcoma
- IC50
Concentration of Half-Maximal Inhibition
- IGF
Insulin-Like Growth Factor
- PARP
poly ADP ribose polymerase
- PPTP
Pediatric Preclinical Testing Program
References
- Aich P, & Dasgupta D (1995). Role of magnesium ion in mithramycin-DNA interaction: binding of mithramycin-Mg2+ complexes with DNA. Biochemistry 34, 1376–1385. [DOI] [PubMed] [Google Scholar]
- Asmane I, Watkin E, Alberti L, Duc A, Marec-Berard P, Ray-Coquard I, et al. (2012). Insulin-like growth factor type 1 receptor (IGF-1R) exclusive nuclear staining: a predictive biomarker for IGF-1R monoclonal antibody (Ab) therapy in sarcomas. Eur J Cancer 48(16), 3027–3035. [DOI] [PubMed] [Google Scholar]
- Aune GJ, Takagi K, Sordet O, Guirouilh-Barbat J, Antony S, Bohr VA, et al. (2008). Von Hippel-Lindau-coupled and transcription-coupled nucleotide excision repair-dependent degradation of RNA polymerase II in response to trabectedin. Clin Cancer Res 14, 6449–6455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awad O, Yustein JT, Shah P, Gul N, Katuri V, O’Neill A, et al. (2010). High ALDH activity identifies chemotherapy-resistant Ewing’s sarcoma stem cells that retain sensitivity to EWS-FLI1 inhibition. PLoS One 5, e13943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ban J, Bennani-Baiti IM, Kauer M, Schaefer KL, Poremba C, Jug G, et al. (2008). EWS-FLI1 suppresses NOTCH-activated p53 in Ewing’s sarcoma. Cancer Res 68, 7100–7109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banville DL, Keniry MA, & Shafer RH (1990). NMR investigation of mithramycin A binding to d(ATGCAT)2: a comparative study with chromomycin A3. Biochemistry 29, 9294–9304. [DOI] [PubMed] [Google Scholar]
- Barretina J, Caponigro G, Stransky N, Venkatesan K, Margolin AA, Kim S, et al. (2012). The Cancer Cell Line Encyclopedia enables predictive modelling of anticancer drug sensitivity. Nature 483, 603–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltran PJ, Chung YA, Moody G, Mitchell P, Cajulis E, Vonderfecht S, et al. (2011). Efficacy of ganitumab (AMG 479), alone and in combination with rapamycin, in Ewing’s and osteogenic sarcoma models. J Pharmacol Exp Ther 337, 644–654. [DOI] [PubMed] [Google Scholar]
- Benini S, Manara MC, Baldini N, Cerisano V, Massimo S, Mercuri M, et al. (2001). Inhibition of insulin-like growth factor I receptor increases the antitumor activity of doxorubicin and vincristine against Ewing’s sarcoma cells. Clin Cancer Res 7, 1790–1797. [PubMed] [Google Scholar]
- Bernstein ML, Devidas M, Lafreniere D, Souid AK, Meyers PA, Gebhardt M, et al. (2006). Intensive therapy with growth factor support for patients with Ewing tumor metastatic at diagnosis: Pediatric Oncology Group/Children’s Cancer Group Phase II Study 9457—a report from the Children’s Oncology Group. J Clin Oncol 24, 152–159. [DOI] [PubMed] [Google Scholar]
- Bertolotti A, Lutz Y, Heard DJ, Chambon P, & Tora L (1996). hTAF(II)68, a novel RNA/ssDNA-binding protein with homology to the pro-oncoproteins TLS/FUS and EWS is associated with both TFIID and RNA polymerase II. EMBO J 15, 5022–5031. [PMC free article] [PubMed] [Google Scholar]
- Bid HK, Zhan J, Phelps DA, Kurmasheva RT, & Houghton PJ (2012). Potent inhibition of angiogenesis by the IGF-1 receptor-targeting antibody SCH717454 is reversed by IGF-2. Mol Cancer Ther 11, 649–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisogno G, Riccardi R, Ruggiero A, Arcamone G, Prete A, Surico G, et al. (2006). Phase II study of a protracted irinotecan schedule in children with refractory or recurrent soft tissue sarcoma. Cancer 106, 703–707. [DOI] [PubMed] [Google Scholar]
- Blaney SM, Needle MN, Gillespie A, Sato JK, Reaman GH, Berg SL, et al. (1998). Phase II trial of topotecan administered as 72-hour continuous infusion in children with refractory solid tumors: a collaborative Pediatric Branch, National Cancer Institute, and Children’s Cancer Group Study. Clin Cancer Res 4, 357–360. [PubMed] [Google Scholar]
- Blume SW, Snyder RC, Ray R, Thomas S, Koller CA, & Miller DM (1991). Mithramycin inhibits SP1 binding and selectively inhibits transcriptional activity of the dihydrofolate reductase gene in vitro and in vivo. J Clin Invest 88, 1613–1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borinstein SC, Barkauskas DA, Krailo M, Scher D, Scher L, Schlottmann S, et al. (2011). Investigation of the insulin-like growth factor-1 signaling pathway in localized Ewing sarcoma: a report from the Children’s Oncology Group. Cancer 117, 4966–4976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boro A, Pretre K, Rechfeld F, Thalhammer V, Oesch S, Wachtel M, et al. (2012). Small-molecule screen identifies modulators of EWS/FLI1 target gene expression and cell survival in Ewing’s sarcoma. Int J Cancer 13(9), 2153–2164. [DOI] [PubMed] [Google Scholar]
- Brenner JC, Feng FY, Han S, Patel S, Goyal SV, Bou-Maroun LM, et al. (2012). PARP-1 inhibition as a targeted strategy to treat Ewing’s sarcoma. Cancer Res 72, 1608–1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao L, Yu Y, Darko I, Currier D, Mayeenuddin LH, Wan X, et al. (2008). Addiction to elevated insulin-like growth factor I receptor and initial modulation of the AKT pathway define the responsiveness of rhabdomyosarcoma to the targeting antibody. Cancer Res 68, 8039–8048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carol H, Houghton PJ, Morton CL, Kolb EA, Gorlick R, Reynolds CP, et al. (2010). Initial testing of topotecan by the pediatric preclinical testing program. Pediatr Blood Cancer 54, 707–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey DA, Wexler LH, Merchant MS, Chou AJ, Merola PR, Price AP, et al. (2009). Irinotecan and temozolomide for Ewing sarcoma: the Memorial Sloan-Kettering experience. Pediatr Blood Cancer 53, 1029–1034. [DOI] [PubMed] [Google Scholar]
- Cesne AL, Cresta S, Maki RG, Blay JY, Verweij J, Poveda A, et al. (2012). A retrospective analysis of antitumour activity with trabectedin in translocation-related sarcomas. Eur J Cancer 48(16), 3036–3044. [DOI] [PubMed] [Google Scholar]
- Charytonowicz E, Terry M, Coakley K, Telis L, Remotti F, Cordon-Cardo C, et al. (2012). PPARgamma agonists enhance ET-743-induced adipogenic differentiation in a transgenic mouse model of myxoid round cell liposarcoma. J Clin Invest 122, 886–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee S, Zaman K, Ryu H, Conforto A, & Ratan RR (2001). Sequence-selective DNA binding drugs mithramycin A and chromomycin A3 are potent inhibitors of neuronal apoptosis induced by oxidative stress and DNA damage in cortical neurons. Ann Neurol 49, 345–354. [PubMed] [Google Scholar]
- Chuk MK, Aikin A, Whitcomb T, Widemann BC, Zannikos P, Bayever E, et al. (2012). A phase I trial and pharmacokinetic study of a 24-hour infusion of trabectedin (Yondelis(R), ET-743) in children and adolescents with relapsed or refractory solid tumors. Pediatr Blood Cancer 59, 865–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuk MK, Balis FM, & Fox E (2009). Trabectedin. Oncologist 14, 794–799. [DOI] [PubMed] [Google Scholar]
- Cironi L, Riggi N, Provero P, Wolf N, Suva ML, Suva D, et al. (2008). IGF1 is a common target gene of Ewing’s sarcoma fusion proteins in mesenchymal progenitor cells. PLoS One 3, e2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delattre O, Zucman J, Plougastel B, Desmaze C, Melot T, Peter M, et al. (1992). Gene fusion with an ETS DNA-binding domain caused by chromosome translocation in human tumours. Nature 359, 162–165. [DOI] [PubMed] [Google Scholar]
- Dileo P, Grosso F, Casanova M, Jimeno J, Marsoni S, Sanfilippo R, et al. (2007). Trabectedin in metastatic Ewing’s family tumors patients progressing after standard chemotherapy. J Clin Oncol 25, 10040. [Google Scholar]
- DuBois SG, Krailo MD, Lessnick SL, Smith R, Chen Z, Marina N, et al. (2009). Phase II study of intermediate-dose cytarabine in patients with relapsed or refractory Ewing sarcoma: a report from the Children’s Oncology Group. Pediatr Blood Cancer 52, 324–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erkizan HV, Kong Y, Merchant M, Schlottmann S, Barber-Rotenberg JS, Yuan L, et al. (2009). A small molecule blocking oncogenic protein EWS-FLI1 interaction with RNA helicase A inhibits growth of Ewing’s sarcoma. Nat Med 15, 750–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esiashvili N, Goodman M, & Marcus RB Jr. (2008). Changes in incidence and survival of Ewing sarcoma patients over the past 3 decades: Surveillance Epidemiology and End Results data. J Pediatr Hematol Oncol 30, 425–430. [DOI] [PubMed] [Google Scholar]
- Fleuren ED, Versleijen-Jonkers YM, van de Luijtgaarden AC, Molkenboer-Kuenen JD, Heskamp S, Roeffen MH, et al. (2011). Predicting IGF-1R therapy response in bone sarcomas: immuno-SPECT imaging with radiolabeled R1507. Clin Cancer Res 17, 7693–7703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forni C, Minuzzo M, Virdis E, Tamborini E, Simone M, Tavecchio M, et al. (2009). Trabectedin (ET-743) promotes differentiation in myxoid liposarcoma tumors. Mol Cancer Ther 8, 449–457. [DOI] [PubMed] [Google Scholar]
- Fouladi M, Park JR, Stewart CF, Gilbertson RJ, Schaiquevich P, Sun J, et al. (2010). Pediatric phase I trial and pharmacokinetic study of vorinostat: a Children’s Oncology Group phase I consortium report. J Clin Oncol 28, 3623–3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frapolli R, Tamborini E, Virdis E, Bello E, Tarantino E, Marchini S, et al. (2010). Novel models of myxoid liposarcoma xenografts mimicking the biological and pharmacologic features of human tumors. Clin Cancer Res 16, 4958–4967. [DOI] [PubMed] [Google Scholar]
- Furman WL, Stewart CF, Poquette CA, Pratt CB, Santana VM, Zamboni WC, et al. (1999). Direct translation of a protracted irinotecan schedule from a xenograft model to a phase I trial in children. J Clin Oncol 17, 1815–1824. [DOI] [PubMed] [Google Scholar]
- Garnett MJ, Edelman EJ, Heidorn SJ, Greenman CD, Dastur A, Lau KW, et al. (2012). Systematic identification of genomic markers of drug sensitivity in cancer cells. Nature 483, 570–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grier HE, Krailo MD, Tarbell NJ, Link MP, Fryer CJ, Pritchard DJ, et al. (2003). Addition of ifosfamide and etoposide to standard chemotherapy for Ewing’s sarcoma and primitive neuroectodermal tumor of bone. N Engl J Med 348, 694–701. [DOI] [PubMed] [Google Scholar]
- Grohar PJ, Griffin LB, Yeung C, Chen QR, Pommier Y, Khanna C, et al. (2011). Ecteinascidin 743 interferes with the activity of EWS-FLI1 in Ewing sarcoma cells. Neoplasia 13, 145–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grohar PJ, Woldemichael GM, Griffin LB, Mendoza A, Chen QR, Yeung C, et al. (2011). Identification of an inhibitor of the EWS-FLI1 oncogenic transcription factor by high-throughput screening. J Natl Cancer Inst 103, 962–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosso F, Sanfilippo R, Virdis E, Piovesan C, Collini P, Dileo P, et al. (2009). Trabectedin in myxoid liposarcomas (MLS): a long-term analysis of a single-institution series. Ann Oncol 20, 1439–1444. [DOI] [PubMed] [Google Scholar]
- Guirouilh-Barbat J, Redon C, & Pommier Y (2008). Transcription-coupled DNA double-strand breaks are mediated via the nucleotide excision repair and the Mre11-Rad50-Nbs1 complex. Mol Biol Cell 19, 3969–3981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahm KB, Cho K, Lee C, Im YH, Chang J, Choi SG, et al. (1999). Repression of the gene encoding the TGF-beta type II receptor is a major target of the EWS-FLI1 oncoprotein. Nat Genet 23, 222–227. [DOI] [PubMed] [Google Scholar]
- Hawkins DS, Bolling T, DuBois S, Hogendoorn PCW, Jurgens H, Paulussen M, et al. (2011). Ewing Sarcoma (6th ed.). Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins. [Google Scholar]
- Hawkins DS, Bradfield S, Whitlock JA, Krailo M, Franklin J, Blaney SM, et al. (2006). Topotecan by 21-day continuous infusion in children with relapsed or refractory solid tumors: a Children’s Oncology Group study. Pediatr Blood Cancer 47, 790–794. [DOI] [PubMed] [Google Scholar]
- Herrero-Martin D, Osuna D, Ordonez JL, Sevillano V, Martins AS, Mackintosh C, et al. (2009). Stable interference of EWS-FLI1 in an Ewing sarcoma cell line impairs IGF-1/IGF-1R signalling and reveals TOPK as a new target. Br J Cancer 101, 80–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofbauer S, Hamilton G, Theyer G, Wollmann K, & Gabor F (1993). Insulin-like growth factor-I-dependent growth and in vitro chemosensitivity of Ewing’s sarcoma and peripheral primitive neuroectodermal tumour cell lines. Eur J Cancer 29A, 241–245. [DOI] [PubMed] [Google Scholar]
- Houghton PJ, Cheshire PJ, Hallman JD II, Lutz L, Friedman HS, Danks MK, et al. (1995). Efficacy of topoisomerase I inhibitors, topotecan and irinotecan, administered at low dose levels in protracted schedules to mice bearing xenografts of human tumors. Cancer Chemother Pharmacol 36, 393–403. [DOI] [PubMed] [Google Scholar]
- Houghton PJ, Morton CL, Gorlick R, Kolb EA, Keir ST, Reynolds CP, et al. (2010). Initial testing of a monoclonal antibody (IMC-A12) against IGF-1R by the Pediatric Preclinical Testing Program. Pediatr Blood Cancer 54, 921–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunold A, Weddeling N, Paulussen M, Ranft A, Liebscher C, & Jurgens H (2006). Topotecan and cyclophosphamide in patients with refractory or relapsed Ewing tumors. Pediatr Blood Cancer 47, 795–800. [DOI] [PubMed] [Google Scholar]
- Jaboin J, Wild J, Hamidi H, Khanna C, Kim CJ, Robey R, et al. (2002). MS-27-275, an inhibitor of histone deacetylase, has marked in vitro and in vivo antitumor activity against pediatric solid tumors. Cancer Res 62, 6108–6115. [PubMed] [Google Scholar]
- Kang HG, Jenabi JM, Liu XF, Reynolds CP, Triche TJ, & Sorensen PH (2010). Inhibition of the insulin-like growth factor I receptor by epigallocatechin gallate blocks proliferation and induces the death of Ewing tumor cells. Mol Cancer Ther 9, 1396–1407. [DOI] [PubMed] [Google Scholar]
- Kauer M, Ban J, Kofler R, Walker B, Davis S, Meltzer P, et al. (2009). A molecular function map of Ewing’s sarcoma. PLoS One 4, e5415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsey M, Smith R, Iyer AK, McCabe ER, & Lessnick SL (2009). EWS/FLI and its downstream target NR0B1 interact directly to modulate transcription and oncogenesis in Ewing’s sarcoma. Cancer Res 69, 9047–9055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kofman S, Perlia CP, & Economou SG (1973). Mithramycin in the treatment of metastatic Ewing’s sarcoma. Cancer 31, 889–893. [DOI] [PubMed] [Google Scholar]
- Kolb EA, Gorlick R, Houghton PJ, Morton CL, Lock R, Carol H, et al. (2008). Initial testing (stage 1) of a monoclonal antibody (SCH 717454) against the IGF-1 receptor by the pediatric preclinical testing program. Pediatr Blood Cancer 50, 1190–1197. [DOI] [PubMed] [Google Scholar]
- Kolb EA, Gorlick R, Lock R, Carol H, Morton CL, Keir ST, et al. (2011). Initial testing (stage 1) of the IGF-1 receptor inhibitor BMS-754807 by the pediatric preclinical testing program. Pediatr Blood Cancer 56, 595–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan K, Bruce B, Hewitt S, Thomas D, Khanna C, & Helman LJ (2006). Ezrin mediates growth and survival in Ewing’s sarcoma through the AKT/mTOR, but not the MAPK, signaling pathway. Clin Exp Metastasis 23, 227–236. [DOI] [PubMed] [Google Scholar]
- Kummar S, Chen A, Ji J, Zhang Y, Reid JM, Ames M, et al. (2011). Phase I study of PARP inhibitor ABT-888 in combination with topotecan in adults with refractory solid tumors and lymphomas. Cancer Res 71, 5626–5634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurmasheva RT, Dudkin L, Billups C, Debelenko LV, Morton CL, & Houghton PJ (2009). The insulin-like growth factor-1 receptor-targeting antibody, CP-751,871, suppresses tumor-derived VEGF and synergizes with rapamycin in models of childhood sarcoma. Cancer Res 69, 7662–7671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau L, Supko JG, Blaney S, Hershon L, Seibel N, Krailo M, et al. (2005). A phase I and pharmacokinetic study of ecteinascidin-743 (Yondelis) in children with refractory solid tumors. A Children’s Oncology Group study. Clin Cancer Res 11, 672–677. [PubMed] [Google Scholar]
- Lawlor ER, & Thiele CJ (2012). Epigenetic changes in pediatric solid tumors: promising new targets. Clin Cancer Res 18, 2768–2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leavey PJ, Mascarenhas L, Marina N, Chen Z, Krailo M, Miser J, et al. (2008). Prognostic factors for patients with Ewing sarcoma (EWS) at first recurrence following multi-modality therapy: a report from the Children’s Oncology Group. Pediatr Blood Cancer 51, 334–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Li X, Fan G, Fukushi J, Matsumoto Y, Iwamoto Y, et al. (2012). Impairment of p53 acetylation by EWS-Fli1 chimeric protein in Ewing family tumors. Cancer Lett 320, 14–22. [DOI] [PubMed] [Google Scholar]
- Liu L, Chen L, Luo Y, Chen W, Zhou H, Xu B, et al. (2010). Rapamycin inhibits IGF-1 stimulated cell motility through PP2A pathway. PLoS One 5, e10578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maksimenko A, & Malvy C (2005). Oncogene-targeted antisense oligonucleotides for the treatment of Ewing sarcoma. Expert Opin Ther Targets 9, 825–830. [DOI] [PubMed] [Google Scholar]
- Malempati S, & Hawkins DS (2012). Rhabdomyosarcoma: review of the Children’s Oncology Group (COG) Soft-Tissue Sarcoma Committee experience and rationale for current COG studies. Pediatr Blood Cancer 59, 5–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malempati S, Weigel B, Ingle AM, Ahern CH, Carroll JM, Roberts CT, et al. (2012). Phase I/II trial and pharmacokinetic study of cixutumumab in pediatric patients with refractory solid tumors and Ewing sarcoma: a report from the Children’s Oncology Group. J Clin Oncol 30, 256–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manara MC, Landuzzi L, Nanni P, Nicoletti G, Zambelli D, Lollini PL, et al. (2007). Preclinical in vivo study of new insulin-like growth factor-I receptor-specific inhibitor in Ewing’s sarcoma. Clin Cancer Res 13, 1322–1330. [DOI] [PubMed] [Google Scholar]
- Manara MC, Perdichizzi S, Serra M, Pierini R, Benini S, Hattinger CM, et al. (2005). The molecular mechanisms responsible for resistance to ET-743 (Trabectidin; Yondelis) in the Ewing’s sarcoma cell line, TC-71. Int J Oncol 27, 1605–1616. [PubMed] [Google Scholar]
- Martins AS, Mackintosh C, Martin DH, Campos M, Hernandez T, Ordonez JL, et al. (2006). Insulin-like growth factor I receptor pathway inhibition by ADW742, alone or in combination with imatinib, doxorubicin, or vincristine, is a novel therapeutic approach in Ewing tumor. Clin Cancer Res 12, 3532–3540. [DOI] [PubMed] [Google Scholar]
- Matsumoto Y, Tanaka K, Nakatani F, Matsunobu T, Matsuda S, & Iwamoto Y (2001). Downregulation and forced expression of EWS-Fli1 fusion gene results in changes in the expression of G(1)regulatory genes. Br J Cancer 84, 768–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May WA, Gishizky ML, Lessnick SL, Lunsford LB, Lewis BC, Delattre O, et al. (1993). Ewing sarcoma 11;22 translocation produces a chimeric transcription factor that requires the DNA-binding domain encoded by FLI1 for transformation. Proc Natl Acad Sci U S A 90, 5752–5756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGregor LM, Stewart CF, Crews KR, Tagen M, Wozniak A, Wu J, et al. (2012). Dose escalation of intravenous irinotecan using oral cefpodoxime: a phase I study in pediatric patients with refractory solid tumors. Pediatr Blood Cancer 58, 372–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinsey EL, Parrish JK, Irwin AE, Niemeyer BF, Kern HB, Birks DK, et al. (2011). A novel oncogenic mechanism in Ewing sarcoma involving IGF pathway targeting by EWS/Fli1-regulated microRNAs. Oncogene 30, 4910–4920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naing A, LoRusso P, Fu S, Hong DS, Anderson P, Benjamin RS, et al. (2012). Insulin growth factor-receptor (IGF-1R) antibody cixutumumab combined with the mTOR inhibitor temsirolimus in patients with refractory Ewing’s sarcoma family tumors. Clin Cancer Res 18, 2625–2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen LA, Kowalewski AA, & Lessnick SL (2008). EWS/FLI mediates transcriptional repression via NKX2.2 during oncogenic transformation in Ewing’s sarcoma. PLoS One 3, e1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappo AS, Patel SR, Crowley J, Reinke DK, Kuenkele KP, Chawla SP, et al. (2011). R1507, a monoclonal antibody to the insulin-like growth factor 1 receptor, in patients with recurrent or refractory Ewing sarcoma family of tumors: results of a phase II Sarcoma Alliance for Research through Collaboration study. J Clin Oncol 29, 4541–4547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel M, Simon JM, Iglesia MD, Wu SB, McFadden AW, Lieb JD, et al. (2012). Tumor-specific retargeting of an oncogenic transcription factor chimera results in dysregulation of chromatin and transcription. Genome Res 22, 259–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulussen M, Craft AW, Lewis I, Hackshaw A, Douglas C, Dunst J, et al. (2008). Results of the EICESS-92 Study: two randomized trials of Ewing’s sarcoma treatment—cyclophosphamide compared with ifosfamide in standard-risk patients and assessment of benefit of etoposide added to standard treatment in high-risk patients. J Clin Oncol 26, 4385–4393. [DOI] [PubMed] [Google Scholar]
- Pommier Y (2006). Topoisomerase I inhibitors: camptothecins and beyond. Nat Rev Cancer 6, 789–802. [DOI] [PubMed] [Google Scholar]
- Prasad SC, Thraves PJ, Bhatia KG, Smulson ME, & Dritschilo A (1990). Enhanced poly(adenosine diphosphate ribose) polymerase activity and gene expression in Ewing’s sarcoma cells. Cancer Res 50, 38–43. [PubMed] [Google Scholar]
- Prieur A, Tirode F, Cohen P, & Delattre O (2004). EWS/FLI-1 silencing and gene profiling of Ewing cells reveal downstream oncogenic pathways and a crucial role for repression of insulin-like growth factor binding protein 3. Mol Cell Biol 24, 7275–7283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray R, Snyder RC, Thomas S, Koller CA, & Miller DM (1989). Mithramycin blocks protein binding and function of the SV40 early promoter. J Clin Invest 83, 2003–2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remsing LL, Bahadori HR, Carbone GM, McGuffie EM, Catapano CV, & Rohr J (2003). Inhibition of c-src transcription by mithramycin: structure-activity relationships of biosynthetically produced mithramycin analogues using the c-src promoter as target. Biochemistry 42, 8313–8324. [DOI] [PubMed] [Google Scholar]
- Richter GH, Plehm S, Fasan A, Rossler S, Unland R, Bennani-Baiti IM, et al. (2009). EZH2 is a mediator of EWS/FLI1 driven tumor growth and metastasis blocking endothelial and neuro-ectodermal differentiation. Proc Natl Acad Sci U S A 106, 5324–5329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggi N, & Stamenkovic I (2007). The biology of Ewing sarcoma. Cancer Lett 254, 1–10. [DOI] [PubMed] [Google Scholar]
- Riggi N, Suva ML, Suva D, Cironi L, Provero P, Tercier S, et al. (2008). EWS-FLI-1 expression triggers a Ewing’s sarcoma initiation program in primary human mesenchymal stem cells. Cancer Res 68, 2176–2185. [DOI] [PubMed] [Google Scholar]
- Sastry M, & Patel DJ (1993). Solution structure of the mithramycin dimer-DNA complex. Biochemistry 32, 6588–6604. [DOI] [PubMed] [Google Scholar]
- Saylors RL III, Stine KC, Sullivan J, Kepner JL, Wall DA, Bernstein ML, et al. (2001). Cyclophosphamide plus topotecan in children with recurrent or refractory solid tumors: a Pediatric Oncology Group phase II study. J Clin Oncol 19, 3463–3469. [DOI] [PubMed] [Google Scholar]
- Scotlandi K, Benini S, Nanni P, Lollini PL, Nicoletti G, Landuzzi L, et al. (1998). Blockage of insulin-like growth factor-I receptor inhibits the growth of Ewing’s sarcoma in athymic mice. Cancer Res 58, 4127–4131. [PubMed] [Google Scholar]
- Scotlandi K, Benini S, Sarti M, Serra M, Lollini PL, Maurici D, et al. (1996). Insulin-like growth factor I receptor-mediated circuit in Ewing’s sarcoma/peripheral neuroectodermal tumor: a possible therapeutic target. Cancer Res 56, 4570–4574. [PubMed] [Google Scholar]
- Scotlandi K, Manara MC, Nicoletti G, Lollini PL, Lukas S, Benini S, et al. (2005). Antitumor activity of the insulin-like growth factor-I receptor kinase inhibitor NVP-AEW541 in musculoskeletal tumors. Cancer Res 65, 3868–3876. [DOI] [PubMed] [Google Scholar]
- Scotlandi K, Perdichizzi S, Manara MC, Serra M, Benini S, Cerisano V, et al. (2002). Effectiveness of Ecteinascidin-743 against drug-sensitive and -resistant bone tumor cells. Clin Cancer Res 8, 3893–3903. [PubMed] [Google Scholar]
- Shi Y, Yan H, Frost P, Gera J, & Lichtenstein A (2005). Mammalian target of rapamycin inhibitors activate the AKT kinase in multiple myeloma cells by up-regulating the insulin-like growth factor receptor/insulin receptor substrate-1/phosphatidylinositol 3-kinase cascade. Mol Cancer Ther 4, 1533–1540. [DOI] [PubMed] [Google Scholar]
- Sleiman SF, Langley BC, Basso M, Berlin J, Xia L, Payappilly JB, et al. (2011). Mithramycin is a gene-selective Sp1 inhibitor that identifies a biological intersection between cancer and neurodegeneration. J Neurosci 31, 6858–6870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soldatenkov VA, Albor A, Patel BK, Dreszer R, Dritschilo A, & Notario V (1999). Regulation of the human poly(ADP-ribose) polymerase promoter by the ETS transcription factor. Oncogene 18, 3954–3962. [DOI] [PubMed] [Google Scholar]
- Soldatenkov VA, Trofimova IN, Rouzaut A, McDermott F, Dritschilo A, & Notario V (2002). Differential regulation of the response to DNA damage in Ewing’s sarcoma cells by ETS1 and EWS/FLI-1. Oncogene 21, 2890–2895. [DOI] [PubMed] [Google Scholar]
- Stegmaier K, Wong JS, Ross KN, Chow KT, Peck D, Wright RD, et al. (2007). Signature-based small molecule screening identifies cytosine arabinoside as an EWS/FLI modulator in Ewing sarcoma. PLoS Med 4, e122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strammiello R, Benini S, Manara MC, Perdichizzi S, Serra M, Spisni E, et al. (2003). Impact of IGF-I/IGF-IR circuit on the angiogenetic properties of Ewing’s sarcoma cells. Horm Metab Res 35, 675–684. [DOI] [PubMed] [Google Scholar]
- Subbiah V, Naing A, Brown RE, Chen H, Doyle L, LoRusso P, et al. (2011). Targeted morphoproteomic profiling of Ewing’s sarcoma treated with insulin-like growth factor 1 receptor (IGF1R) inhibitors: response/resistance signatures. PLoS One 6, e18424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton KA (2010). Trabectedin: the evidence for its place in therapy in the treatment of soft tissue sarcoma. Core Evid 4, 191–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toretsky JA, Erkizan V, Levenson A, Abaan OD, Parvin JD, Cripe TP, et al. (2006). Oncoprotein EWS-FLI1 activity is enhanced by RNA helicase A. Cancer Res 66, 5574–5581. [DOI] [PubMed] [Google Scholar]
- Toretsky JA, Kalebic T, Blakesley V, LeRoith D, & Helman LJ (1997). The insulin-like growth factor-I receptor is required for EWS/FLI-1 transformation of fibroblasts. J Biol Chem 272, 30822–30827. [DOI] [PubMed] [Google Scholar]
- von Levetzow C, Jiang X, Gwye Y, von Levetzow G, Hung L, Cooper A, et al. (2011). Modeling initiation of Ewing sarcoma in human neural crest cells. PLoS One 6, e19305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner L (2011). Camptothecin-based regimens for treatment of Ewing sarcoma: past studies and future directions. Sarcoma 2011, 957957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner LM, Crews KR, Stewart CF, Rodriguez-Galindo C, McNall-Knapp RY, Albritton K, et al. (2008). Reducing irinotecan-associated diarrhea in children. Pediatr Blood Cancer 50, 201–207. [DOI] [PubMed] [Google Scholar]
- Wan X, Harkavy B, Shen N, Grohar P, & Helman LJ (2007). Rapamycin induces feedback activation of Akt signaling through an IGF-1R-dependent mechanism. Oncogene 26, 1932–1940. [DOI] [PubMed] [Google Scholar]
- Wang Z, Wang F, Tang T, & Guo C (2012). The role of PARP1 in the DNA damage response and its application in tumor therapy. Front Med 6, 156–164. [DOI] [PubMed] [Google Scholar]
- Zhang M, Mathur A, Zhang Y, Xi S, Atay S, Hong JA, et al. (2012). Mithramycin represses basal and cigarette smoke-induced expression of ABCG2 and inhibits stem cell signaling in lung and esophageal cancer cells. Cancer Res 72, 4178–4192. [DOI] [PMC free article] [PubMed] [Google Scholar]