ABSTRACT
We report an instructive case of acute myeloid leukemia with histiocytic differentiation (acute histiocytic leukemia) arising in a patient, a 52-year-old woman with a history of follicular lymphoma. The results of genetic studies proved a clonal relationship between the lymphoma and the leukemic cells. To our knowledge, this is the first report of leukemic transdifferentiation of follicular lymphoma into modified base 5-methylcytosine (M5c)–like acute histiocytic leukemia and the first reported karyotype on a transdifferentiated neoplasm.
Keywords: transdifferentiation, lymphoma, leukemia, histiocytic, sarcoma, B cell
Transdifferentiation (the shift from one hematopoietic cell lineage to another) has been reported in the literature with B-cell lymphomas such as follicular lymphoma and chronic lymphoid leukemia/small lymphocytic leukemia (CLL/SLL), in which a subsequent histiocytic sarcoma shares molecular/genetic features with the lymphoid neoplasm.1‐5 Herein, we present an instructive case of leukemic transdifferentiation of a follicular lymphoma.
Case Study
A 52-year-old Caucasian woman had presented with stage IV grade 1 follicular lymphoma 2 years ago and was treated at that time with bendamustine and rituximab, which yielded a partial response, followed by maintenance rituximab. Follow-up computed tomography (CT) scans showed no evidence of disease. The patient recently sought treatment for pancytopenia, bruising, dyspnea, and coagulopathy that resembled disseminated intravascular coagulation (DIC).
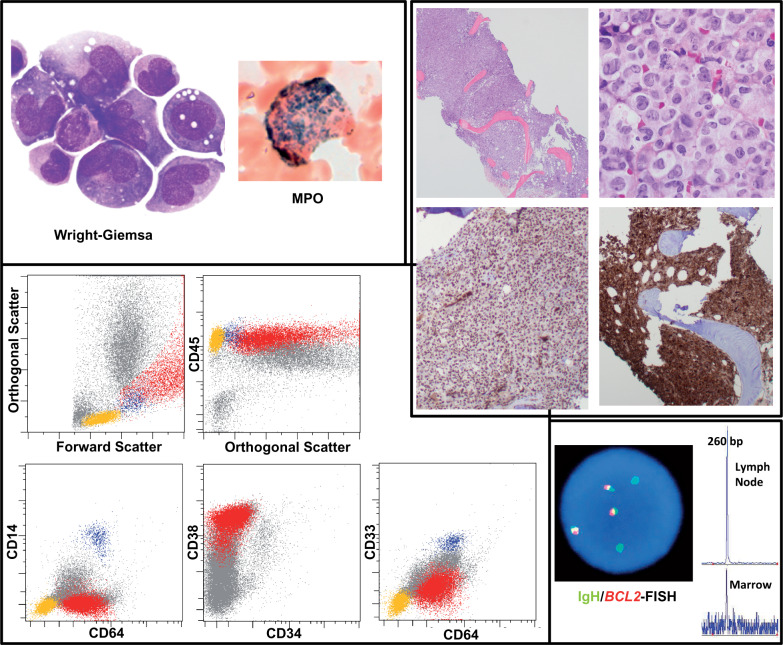
Blood and bone marrow smear results revealed extremely large cells with folded nuclei that had delicate chromatin and abundant basophilic cytoplasm containing vacuoles and scattered granules, which stained strongly with myeloperoxidase. These findings raised suspicion for acute promyelocytic leukemia (Figure 1). Flow cytometry revealed cluster of differentiation (CD)45(moderate+), CD64+, CD38+, CD15(partial +), and CD71(partial +) cells that tested negative for other myeloid and lymphoid markers, including CD14, CD33, CD34, and human leukocyte antigen–antigen D related (Figure 1 and data not shown). The core biopsy results revealed 100% cellular marrow entirely replaced by tumor cells (Figure 1). The results of immunohistochemical studies revealed the cells to have tested positive for CD4, PU.1, and lysozyme and dim CD31, whereas those cells did not express S100, CD79a, PAX5, BOB1, OCT2, BCL2, BCL6, CD68, or CD163 (Figure 1 and data not shown).
Figure 1.
Morphologic, immunohistochemical, flow cytometric, and genetic findings on bone-marrow specimen from our patient, a 52-year-old woman. Images at the top left depict tumor-cell morphologic characteristics on Wright-Giemsa–stained bone marrow smear preparations, with cytochemical staining for myeloperoxidase (MPO). The cluster of images at the top right shows hematoxylin-eosin (H&E)–stained sections of the bone-marrow core (showing 100% cellularity), with positive staining for PU.1 and lysozyme by immunohistochemistry. Dot plots on the bottom left demonstrate flow-cytometric findings (red: neoplastic population; blue: normal background monocytes, yellow: normal lymphocytes). Images at the bottom right depict the relatedness of the follicular lymphoma and myeloid leukemia: fluorescence in situ hybridization (FISH) positivity of tumor cells for the t(14;18) translocation involvingBCL2 (red: BCL2; green: immunoglobulin [Ig]H); and (2) presence of identical (260-bp) rearranged framework region 2 (FR2) IgH clonal peak in marrow and lymph-node specimens.CD indicates cluster of differentiation.
The results of fluorescence in situ hybridization (FISH) studies for t(15;17) were negative. Karyotypic evaluation revealed complex cytogenetic abnormalities [42-51, XX, +X[10], +X[5], +1[9], add(1)(p10)[10], -4[9],+6[7], del(6)(q23)[9], +12[9], t(14;18)(q32;q21)[11], -15[8], and +1∼2mar[10][cp11]/46, XX[9]. The results of additional FISH studies confirmed that the t(14;18) translocation involved theBCL2 gene and was associated with an extra fusion signals and extra immunoglobulin (Ig)H with loss ofBCL2 (Figure 1). FISH analysis of the previous follicular lymphoma of the patient also revealed t(14,18) involving theBCL2 locus but no evidence of trisomy 12 (data not shown). We evaluated DNA extracts from a recent bone marrow specimen and the follicular lymphoma from the patient for Ig heavy-chain framework region 2 rearrangements; both showed a peak at 260 bp (Figure 1), indicative of a clonal relationship.
Repeat CT scans showed widespread new lymphadenopathy (which had not been present 2 months previously), suspicious for involvement by acute myeloid leukemia. There was no evidence of a dominant mass lesion suggestive of sarcoma.
Discussion
To our knowledge, this is the first report of leukemic transdifferentiation of follicular lymphoma into M5c-like acute histiocytic leukemia and the first reported karyotype of a transdifferentiated neoplasm. Similar to the sarcomatous lesions described previously,1‐5 the leukemic transdifferentiated tumor also followed a monocytic/histiocytic differentiation. A previous study of sporadic histiocytic sarcomas in patients with no known history of lymphoma revealed clonal IgH rearrangements, clonal light-chain rearrangements, and also a t(14;18) translocation in the sporadic tumors.6 However, the finding of the t(14;18) translocation and the same-sized IgH rearrangements in the current case strongly suggests transdifferentiation instead of a coincidental sporadic leukemia.
This finding was also associated with loss ofPAX5 expression and gain of PU.1 expression, similar to that described in sarcomatous lesions. Thus, although there is evidence of lineage plasticity among these tumors, there appears to be a clear preference toward this specific differentiation pathway, which suggests a stepwise reprogramming of lymphomatous B cells into leukemic macrophages.7,8 This case also suggests that therapy-related acute myeloid leukemias, many of which are monocytic in nature, may have an underrecognized subset of transdifferentiated tumors that need further elucidation.
Glossary
Abbreviations
- CLL/SLL
chronic lymphoid leukemia/small lymphocytic leukemia
- CT
computed tomography
- DIC
disseminated intravascular coagulation
- CD
cluster of differentiation
- FISH
fluorescence in situ hybridization
- Ig
immunoglobulin
- MPO
myeloperoxidase
- H&E
hematoxylin-eosin
- FR2
framework region 2
References
- 1. Feldman AL,Arber DA,Pittaluga S,et al. Clonally related follicular lymphomas and histiocytic/dendritic cell sarcomas: evidence for transdifferentiation of the follicular lymphoma clone.Blood.2008;111(12):5433–5439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Shao H,Xi L,Raffeld M,et al. Clonally related histiocytic/dendritic cell sarcoma and chronic lymphocytic leukemia/small lymphocytic lymphoma: a study of seven cases.Mod Pathol. 2011;24(11):1421–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wang E,Hutchinson CB,Huang Q,et al. Histiocytic sarcoma arising in indolent small B-cell lymphoma: report of two cases with molecular/genetic evidence suggestive of a 'transdifferentiation' during the clonal evolution.Leuk Lymphoma. 2010;51(5):802–812. [DOI] [PubMed] [Google Scholar]
- 4. Zhang D,McGuirk J,Ganguly S,Persons DL.. Histiocytic/dendritic cell sarcoma arising from follicular lymphoma involving the bone: a case report and review of literature.Int J Hematol. 2009;89(4):529–532. [DOI] [PubMed] [Google Scholar]
- 5. Wang E,Papalas J,Hutchinson CB,et al. Sequential development of histiocytic sarcoma and diffuse large B-cell lymphoma in a patient with a remote history of follicular lymphoma with genotypic evidence of a clonal relationship: a divergent (bilineal) neoplastic transformation of an indolent B-cell lymphoma in a single individual.Am J Surg Pathol.2011;35(3):457–463. [DOI] [PubMed] [Google Scholar]
- 6. Chen W,Lau SK,Fong D,Wang J,Wang E,Arber DA.. High frequency of clonal immunoglobulin receptor gene rearrangements in sporadic histiocytic/dendritic cell sarcomas.Am J Surg Pathol. 2009;33(6):863–873. [DOI] [PubMed] [Google Scholar]
- 7. Xie H,Ye M,Feng R,Graf T.. Stepwise reprogramming of B cells into macrophages.Cell. 2004;117(5):663–676. [DOI] [PubMed] [Google Scholar]
- 8. Tagoh H,Schebesta A,Lefevre P,et al. Epigenetic silencing of thec-fms locus during B-lymphopoiesis occurs in discrete steps and is reversible.EMBO J.2004;23(21):4127–4364. [DOI] [PMC free article] [PubMed] [Google Scholar]