Abstract
Purpose
To describe results of the Amyloid, Tau, Neurodegeneration (ATN) research framework classification in the Argentine‐Alzheimer's Disease Neuroimaging Initiative (arg‐ADNI) cohort.
Methods
Twenty‐three patients with mild cognitive impairment (MCI), 12 dementia of Alzheimer's type (DAT), and 14 normal controls were studied following the ADNI2 protocol. Patients were categorized according to presence or absence of the biomarkers for amyloid beta (Aβ; A: amyloid positron emission tomography [PET] scan or cerebrospinal fluid [CSF] Aβ42), tau (T: CSF phosphorylated‐tau), and neurodegeneration (N: CSF total‐tau, fluorodeoxyglucose [FDG]‐PET scan, or structural magnetic resonance imaging [MRI] scan).
Results
A+T+N+ biomarker profile was identified at baseline in 91% of mild dementia patients, 20% of early MCI patients, 46% of late MCI patients, and 14% of control subjects. Suspected non‐AD pathophysiology (SNAP, A‐T‐N+) was found in 8% of mild dementia, 20% of early MCI, 15% of late MCI, and 7% of control subjects. Conversion rates to dementia after 5‐year follow‐up were 85% in A+T+N+ MCI patients and 50% in A‐T‐N+ patients.
Conclusions
We present initial 5‐year follow‐up results of a regional ADNI based on AD biomarkers and the ATN classification.
Keywords: Alzheimer's disease, biomarkers, amyloid, tau, neurodegeneration, classification
1. INTRODUCTION
Aggregation of amyloid beta (Aβ) and abnormal phosphorylated tau deposits in neurofibrillary tangles represent the hallmarks of Alzheimer's disease (AD). Recent scientific advances now allow Aβ1‐42, as well as total (t) and hyper phosphorylated (p) tau levels to be assayed in cerebrospinal fluid (CSF), 1 and amyloid deposits to be detected on positron emission tomography (PET) scans. 2 The National Institute on Aging (NIA) and the Alzheimer's Association (AA) put together a task force and applying these new technologies, developed recommendations for AD diagnosis. 3 , 4
Jack et al. proposed a new biomarker classification based on the ATN (amyloid, tau, neurodegeneration) system with the goal of providing both more accurate AD characterization and an improved understanding of the sequence of events leading to AD. 5 , 6 The National Institute of Aging and Alzheimer's Association (NIA‐AA) has since launched a classification as framework for AD research, both observational and intervention‐related, but not for everyday clinical practice. 7 It is based on AD biomarker profiles divided in binary categories (positive or negative) in which “A” refers to Aβ biomarkers (amyloid PET or CSF Aβ42), “T” to tau pathology biomarker (CSF phosphorylated‐tau or tau PET), and “N” a quantitative or topographic biomarker of neurodegeneration (CSF total‐tau, fluorodeoxyglucose [FDG]‐PET, or structural magnetic resonance imaging [MRI]). 7 , 8
The objective of this study was to evaluate the ATN research framework classification for AD in the Argentine ADNI cohort and examine its prognostic value predicting transformation rate to dementia during 5 years of prospective follow‐up.
2. MATERIAL AND METHODS
Fifty‐six individuals were prospectively enrolled into the Argentine Alzheimer Disease Neuroimaging Initiative (Arg‐ADNI) study cohort. 2 Patients were referred from the Memory & Aging Center at FLENI Institute for Neurological Research in Buenos Aires, Argentina, and normal controls recruited from the community. Methods used for data collection as well as study design have already been described in detail elsewhere. 2 Initial patient workup included a structured interview, laboratory tests, and anatomical and molecular brain imaging. Thirteen subjects were excluded during the follow‐up (one due to cancer, one due to psychiatric disease, four due to institutionalization, four due to refusal to continue, and three for lack of compliance). Forty‐nine participants were included for initial assessment and 43 for the follow‐up analysis.
The study was approved by the institutional ethics committee (IRB no.: 07/11) and written informed consent obtained either from participant, and/or their legal representative.
2.1. Neuropsychological assessment
Subjects were evaluated using locally adapted validated versions of the following tests, translated into Spanish: Mini Mental State Examination (MMSE), 8 Wechsler's Logical Memory Scale, 9 Rey Auditory Verbal Learning Test (RAVLT), 10 Boston Naming Test, 11 , 12 Animal Fluency and Letter Fluency, 13 Trail Making Test A and B (TMT‐A, TMT‐B), 14 Clinical Dementia Rating (CDR), 15 Neuropsychiatric Inventory Q (NPI‐Q), 16 Geriatric Depression Scale (GDS), 17 and Functional Assessment Questionnaire (FAQ). 18
RESEARCH IN CONTEXT
Systematic review: The authors reviewed the literature using traditional sources (e.g., PubMed, Scopus, Latindex) for the recommendation for diagnosis of pre symptomatic Alzheimer's disease (AD) and mild cognitive impairment (MCI) using AD biomarkers, but not all were considered for prediction of dementia. Jack et al. (2016) proposed a new classification based on amyloid (A), tau (T), and neurodegeneration (N).
Interpretation: Our objective was to describe the A/T/N classification in the Argentine Alzheimer's Disease Neuroimaging Initiaive (ADNI) cohort and the prognosis of conversion to dementia. Our findings suggest that MCI patients A+/T+/N+ (due to AD) or MCI patients A−/T−/N+ (with neurodegeneration) must be recognized for their high prognosis of dementia in a short time.
Future Directions: The possibility of having local results in Latin America will allow us to add and compare what has been obtained in developed countries and to better characterize this pathology in a more generalized way.
2.2. Structural MRI analyses
All subjects underwent a 3‐Tesla magnetic resonance imaging (MRI) scan of the brain. Presence or absence of hippocampal atrophy was classified based on the visual inspection by the radiologist (medial temporal atrophy) 19 and hippocampal reconstruction performed using FreeSurfer v 4.3 automated volumetric output (http://surfer.nmr. mgh.harvard.edu/).
2.3. Metabolic and amyloid PET scan (FDG and PiB)
PET with FDG and 11C‐Pittsburgh compound‐B (PiB) was performed in every subject. The FDG PET acquisition protocol was used to evaluate parieto‐temporal AD hypometabolism and the Pittsburgh compound B PET acquisition protocol, to look for amyloid deposits in the brain. Synthesis of 11C‐PiB was synthetized in a GE TRACER lab FXC PRO module, and lineal color scales used to detect metabolism as well as presence and spatial distribution of cortical amyloid.
2.4. Apolipoprotein E genotyping
Apolipoprotein E (APOE) genotyping was performed in all participants by polymerase chain reaction (PCR) following the Hixson and Vernier protocol as previously described, 2 and the test considered positive if one or more ε4 allele was detected (ε4+).
2.5. CSF AD biomarkers
CSF samples were obtained by lumbar puncture. The FLENI biomolecular laboratory runs CSF biomarkers using INNOTEST (Fujirebio) enzyme‐linked immunosorbent assay kits. Cutoff values are: Aβ42 less than 534 pg/mL, total tau (t‐tau) higher than 343.9 pg/mL, and phosfo tau (p‐tau) higher than 42.4 pg/mL.
2.6. Patient classification based on clinical data
ADNI‐2 procedure manual definitions were applied for: normal controls (NC), early MCI (e‐MCI), late MCI (l‐MCI), and dementia of Alzheimer's type (DAT) patients.
Cognitively normal subjects were normal subjects with baseline MMSE 8 scores between 24 and 30 points, a CDR 15 score of 0. Normal memory function was documented for score above educational‐adjusted cutoffs, on the delayed recall of one paragraph from Wechsler Memory Scale Logical Memory II. 9
Early MCI subjects (e‐MCI) were individuals with MMSE 8 scores between 24 and 30, a CDR 15 of 0.5, an objective memory loss score adjusted for age and education (≥16 years: 9–11; 8–15 years: 5–9; 0–7 years: 3–6) on Wechsler Memory Scale (WMS) Logical Memory II, 9 and preserved activities of daily living.
Late MCI subjects (l‐MCI) had MMSE 8 scores between 24 and 30 (inclusive), a CDR 15 of 0.5, an objective memory loss score (≥16 years: ≤8; 8–15 years: ≤4; 0–7 years: ≤2) on the Wechsler Memory Scale Logical Memory II, 9 and preserved activities of daily living.
Mild dementia of Alzheimer's type (DAT) subjects: MMSE 8 scores between 20 and 26 (inclusive), a CDR 15 of 1.0, meeting NINCDS/ADRDA criteria for probable AD. 20
2.7. The ATN biomarker classification
Patients were categorized according to six AD biomarkers available at baseline in FLENI. Although every biomarker exists on a continuous scale, normal versus abnormal cutoff points exist for most disease categories. To apply the ATN classification, a biomarker dichotomy was used. For CSF biomarkers, cutoff values validated at FLENI were applied in each case. For neuroimaging biomarkers, visual inspection by two neuroradiologists, blinded to patient clinical data was applied to establish presence or absence of Aβ deposits on PET with PiB and presence or absence of parieto‐temporal hypometabolism on FDG‐PET. Hippocampal atrophy was considered present on MRI if medial temporal atrophy index was equal to or greater than 1. 19 Thus “A” indicated Aβ biomarker presence defined as a high ligand retention on amyloid PET with PiB or/and Aβ 42 in CSF levels below the specified cut‐off point. “T” corresponded to abnormal tau defined as p‐tau in CSF above established cutoff point. “N” for neurodegeneration was defined as either elevated CSF t‐tau levels or, imaging biomarker presence as either bilaterally parieto‐temporal hypo metabolism on FDG‐PET or medial temporal region atrophy on structural MRI. 19
2.8. Follow‐up
Of the initial cohort totaling 56 participants, 43 completed longitudinal follow‐up (ie, baseline‐, 12‐, 30‐, and 60‐month visits). Conversion to dementia was reported in MCI patients and control individuals when the CDR score changed to 1. Kaplan–Meier plots were used to estimate survival and outcome was defined as a transition from control state (CDR 0) or MCI (CDR 0.5) to dementia (CDR 1) during the follow‐up (dichotomous outcome). Time in this analysis corresponded to duration of follow‐up (months).
2.9. Data analysis
Statistical analyses were performed with the SPSS v19.0 (USA). Categorial variables were expressed as frequency (%) and continuous variables as the mean (SD). Normality was tested using the Shapiro–Wilk test. The ANOVA test was used for comparisons between continuous variables and the χ2 test for categorical variables. Kruskal–Wallis one‐way analysis of variance test was used if samples were not distributed normally. For multiple comparisons, Bonferroni's post hoc tests were applied. Conversion proportion was estimated by Survival Kaplan–Meier analysis. Differences between the means were considered statistically significant at P < 0.05.
3. RESULTS
3.1. Baseline characteristics of study subjects
Demographic, clinical, cognitive, and biomarker information at baseline is summarized in Table 1 for each group. All four groups (normal controls, e‐MCI, l‐MCI, and DAT patients) shared similar demographic data (education level, age, and sex) and all subjects were white (predominantly European descent) native Spanish speakers. Globally, all four populations were similar regarding percentage of APOE 4 carriers, though slightly lower numbers were found in controls.
Table 1.
Demographic, clinical, cognitive, and cerebrospinal fluid biomarker variables at baseline
| Controls | e‐MCI | l‐MCI | DAT | F (p) | * P (<0.05) | |
|---|---|---|---|---|---|---|
| Demographics | ||||||
| Number | 14 | 10 | 13 | 12 | ||
| Age (years) | 70.1(8.2) | 73.0(8.8) | 74.8(6.3) | 77.9(5.5) | 2.562 (ns) | |
| Sex (male/female) | 4/10 | 6/4 | 5/8 | 5/7 | ||
| Education (years) | 14.1 (2.8) | 12.8 (5.3) | 14.3(4.2) | 12.3 (4.0) | 0.702 (ns) | |
| APOE carrier ɛ4, % | 35.7% | 40% | 53.8% | 50% | **ns | |
| Clinical | ||||||
| CDR | 0 | 0.5 | 0.5 | 1 | – | |
| MMSE | 29.6 (0.8) | 29.3 (0.8) | 28.1 (1.4) | 22.5 (3.3) | 34.470 (<0.000) | c, e, f |
| NPI‐Q | 0.4 (1.0) | 3.8 (4.7) | 5.7(7.9) | 5.9 (4.7) | 2.222 (ns) | |
| GDS | 1.2 (1.1) | 2.3 (2.1) | 2.7(2.5) | 1.8 (1.7) | 0.850 (ns) | |
| FAQ | 0.0 (0.0) | 3.0 (3.6) | 3.1(3.0) | 8.3 (6.7) | 6.119 (<0.005) | c |
| Cognitive | ||||||
| RAVLT delay recall | 8.3 (2.7) | 3.6 (1.8) | 2.0 (2.3) | 0.4 (0.8) | 29.781(<0.000) | a, b, c, d, f |
| RAVLT recognition | 13.3 (1.2) | 11.3 (2.7) | 8.1 (4.1) | 6.0 (3.6) | 11.310(<0.000) | b, c, f |
| BNT | 28.5 (2.1) | 26.9 (3.2) | 24.6 (4.9) | 20.0 (6.2) | 7.047(<0.001) | c, f |
| SVF (animals) | 22.0 (3.2) | 18.5 (2.8) | 17.1 (3.4) | 11.8 (4.0) | 17.420 (<0.000) | b, c, f |
| FVF (“p”) | 19.7 (4.4) | 14.4 (6.8) | 16.5 (4.0) | 13.8 (5.4) | 2.861 (<0.05) | |
| Span direct | 5.6 (1.2) | 5.5 (1.1) | 5.3 (1.0) | 5.3 (1.0) | ns | |
| TMT A (seconds) | 32.5 (9.7) | 47.6 (20.1) | 47.7 (20.8) | 131.9 (121.3) | 5.614 (<0.002) | |
| TMT B (seconds) | 69.1 (23.1) | 150.0(125.6) | 129.2(61.1) | 332.1 (134.5) | 17.497 (<0.000) | c, f |
| AD Biomarkers (% positive) | ||||||
| A (amyloid) | ||||||
| Aβ1‐42 ‐ CSF | 30% | 42% | 64% | 88% | ** <0.05 | |
| Amyoid PET (PiB) | 14% | 30% | 53% | 83% | ** <0.05 | |
| T (tau) | ||||||
| Phosfo‐tau CSF | 20% | 42% | 64% | 86% | ** <0.05 | |
| N (neurodegeneration) | ||||||
| Left hippocampus‐MRI | 0% | 22% | 50% | 56% | ** <0.05 | |
| t‐tau CSF | 10% | 29% | 55% | 88% | ** <0.01 | |
| FDG PET | 7% | 40% | 54% | 100% | ** <0.00 | |
eMCI, early mild cognitive impairment; lMCI, late mild cognitive impairment; DAT, dementia of Alzheimer's type; CDR, clinical dementia rating; MMSE, Mini Mental State Exam; NPI‐Q, Neuropsychiatric Inventory; GDS, Geriatric Depression Scale; FAQ, Functional Activities Questionnaire; RAVLT, Rey Auditory Verbal Learning Test; BNT, Boston Naming Test; SVF, Semantic Verbal Fluency (animals); PVF, Phonologic Verbal Fluency (p); TMT A and B, Trail Making Test A and B; Rey fig, Figure of Rey.
Mean (SD); P (ANOVA) and * Bonferroni post hoc; a, controls versus eMCI; b, controls versus lMCI; c, controls versus DAT; d, eMCI versus LMCI; e, eMCI versus DAT; f, lMCI versus DAT. ** χ2.
As expected, statistically significant difference was observed on baseline MMSE, 8 and on FAQ 18 test results between normal controls and the DAT group. No differences however were found in behavioral symptoms (NPI‐Q 16 and GDS 17 ). Mean scores on neuropsychological assessment differed significantly among groups, with a typical continuum in cognitive performance, as previously described. 2
3.2. AD biomarker results
The ATN framework in our sample provides multiples options for defining A and N. We consider the biomarker present when at least one method gives a positive result.
Amyloid biomarkers (A). Amyloid PET scan was positive (A+) at baseline in 2/14 (14%) normal controls; 3/10 subjects with e‐MCI (30%); 7/13 subjects with l‐MCI (53%); and 10/12 subjects with mild DAT (83%), with significant difference across diagnostic groups (P < 0.05). Correlation between amyloid PET scan and Aβ1‐42 (A) was globally 0.8 and 0.9 in DAT subjects; 4/19 (21%) subjects (two normal controls and two MCI subjects) were positive in Aβ1‐42 and negative on the amyloid PET scan.
Tau biomarker (T). Phosphorylated tau in CSF was positive (T+) in 20% of normal controls and 86% of DAT subjects.
Neurodegeneration biomarkers (N) studied by volumetric MRI analysis of the hippocampus, FDG PET scan, and total tau in CSF showed significant differences among patient groups (MCI and DAT) and normal controls (P < 0.05) but global correlation between hippocampus volume and FDG PET scan was 0.4 and with total tau 0.2.
Correlation between total and phosphorylated tau was 0.8 in all populations.
Subjects were classified at baseline according to ATN classification (Figure 1) as follows:
-
‐
Group A+T+N+: 14% (2/14) were controls, 20% (2/10) were early‐MCI, 46% (6/13) were late‐MCI, and 92% (11/12) were mild DAT patients.
-
‐
Group A‐T‐N+ (SNAP): 7% (1/14) were controls, 20% (2/10) were early‐MCI, 15% (2/13) were late‐MCI, and 8% (1/12) were mild DAT patients.
-
‐
Group A‐T‐N‐: 57% (8/14) were normal controls, 40% (4/10) were early‐MCI, 23% (3/13) were late‐MCI patients, but we found no cases of mild DAT.
FIGURE 1.
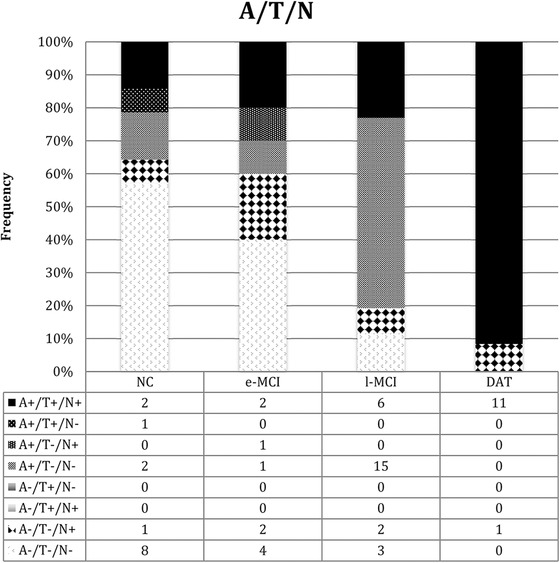
Frequency of ATN in the controls, early mild cognitive impairment, late mild cognitive impairment, and dementia of Alzheimer's type
3.3. Follow‐up subjects
Subjects were followed longitudinally (baseline, and at 12, 30, and 60 months).
Of the normal control group at baseline, 1/14 (7%) and 3/14 (21%) were diagnosed as MCI at 30 and 60 months, respectively. No normal subject became demented.
Survival was analyzed (Kaplan–Meier) in MCI according to the ATN classification, in which the outcome was defined as transition from MCI to dementia during the follow‐up (dichotomous outcome; see Figure 2). Conversion to dementia was observed after 60‐month follow‐up in 85% (7/8) of A+T+N+, and 50% (2/4) A‐T‐N+ patients. No cases of conversion were identified in A+T‐N‐ or A‐T‐N‐ patients.
FIGURE 2.
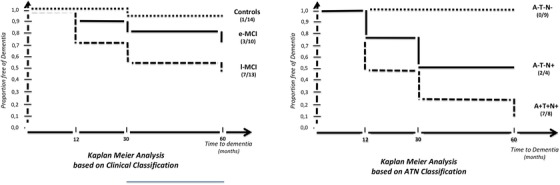
Survival analysis of mild cognitive impairment by clinical (left side) versus biomarkers ATN (right side) classifications
4. DISCUSSION
The goal of our study was to apply the ATN classification at baseline in an Argentine ADNI cohort and study risk of progression to dementia based on biomarker results. The Memory and Aging Center from the Instituto de Investigaciones Neurologicas FLENI, in Buenos Aires, Argentina, was the first center in Latin America to routinely use AD biomarkers. 22 , 23 , 24 No significant differences were found between MCI patient groups in cognitive functions, except for episodic memory (l‐MCI patients scored lower on delayed recall of the RAVLT), behavioral symptoms (evaluated using NPI‐Q and GDS), and functional activities (FAQ). As previously mentioned, atrophy of the hippocampus showed progression along the classic continuum, correlating with degree of memory loss observed in patients, from controls to e‐MCI, to l‐MCI, to DAT. 25
In relation to AD biomarkers, we found a statistically significant difference in results between controls and DAT patients. PiB‐PET scans show greater disease activity levels in DAT than in either MCI group. In agreement with published literature, 14% of normal controls presented evidence of brain amyloid deposits on PiB PET. 3
For this study, we used the biomarker‐based ATN classification system, 2 , 3 which provides a common research framework for centers worldwide to describe AD based on neuropathological changes, rather than on a clinical syndrome, generating a biological rather than syndromic definition of AD. 7 In work previously published we applied the new ATN classification, 3 and found normal biomarker results (A‐T‐N‐) in 58% of clinically normal controls, 40% of e‐MCI cases, and 23% of l‐MCI cases, but in no cases of mild DAT. Positivity of all three biomarkers (A+T+N+), the hallmark for AD, was found in this same study in 14% of normal controls, 20% of e‐MCI, 47% of l‐MCI, and 91% of mild DAT cases. Presence of neurodegeneration without amyloid (A‐T‐N+) indicates non‐Alzheimer's pathology, also defined as SNAP (suspected non‐Alzheimer's pathophysiology). 5 In this study SNAP was detected in 7% of normal controls, 20% of e‐MCI, 15% of l‐MCI, and 9% of mild DAT cases, similar to results from other ADNI study centers worldwide, in which SNAP incidence was 17% of MCI cases and 7% of DAT cases. 26
When we studied rate of progression to dementia based on clinical classification, 10% of normal controls, 20% of e‐MCI, and 46% of l‐MCI patients progressed after 2 years follow‐up. 24 In the late MCI group, percentage of annual progression in our series was higher than the classical 5% to 10% per year described by Petersen et al. in their original cohort. 27 We therefore decided to apply the ATN classification system that is independent of cognitive status to the MCI group as a whole and observed that 75% of A+T+N+ patients progressed to dementia at 30 months and 85% at 60 months. This rate was 50% in A‐T‐N+. Our findings suggest that A+T+N+ MCI patients have worse prognosis, but A‐T‐N+ MCI patients (neurodegeneration only) also present risk of progression. We had only four A‐T‐N+ MCI patients, two of which developed dementia. One was ultimately diagnosed with hippocampal sclerosis probably due to TDP43 proteinopathy; in the second case, although dementia of hippocampal profile was diagnosed, neuropathological findings were inconclusive. Finally, two other patients with amyloidosis but no signs of neurodegeneration (A+T‐N‐) have shown no progression in 5 years of follow‐up.
The new ATN classification framework used for research is important because globally, it not only identifies patients with positive or negative amyloid, but also others with variable combinations AD biomarkers. Current results are more promising because MCI prognosis and progression to dementia are better characterized than in the classic clinical classification. Measuring relevant pathophysiology in living subjects using biomarkers provides a novel approach to the interpretation of the continuum from proteinopathy to AD as well as of other degenerative dementias.
Diagnosis of dementia of Alzheimer's type used to be based on clinical criteria exclusively, 19 but this has recently been modified to include biological criteria through application of AD biomarker results. 21 The fact that the typical syndrome is neither sensitive nor specific enough to characterize neuropathological changes of AD suggests that relying only on cognitive symptoms is not an ideal way to define AD. 7 To better understand the pathophysiology and to discover a treatment for AD, we need to move toward a biological classification of neurodegenerative disease. 7 The AD profile is characterized by abnormal patterns in structural (MRI) and functional (FDG‐PET scan) imaging, as well as by a signature CSF abnormality, namely abnormal levels of Aβ1‐42, total tau, and phosphorylated‐tau, 28 as well as by increased amyloid uptake on PiB‐PET. In recent years, neurodegenerative diseases have undergone a conceptual change from being distinct clinical syndromes to becoming diseases related to accumulation of proteins.
In symptomatic individuals (MCI and dementia), biomarkers have been used to refine etiological diagnosis 4 and during preclinical stages they have been used to define the AD construct. 3 Petersen et al. described mild cognitive impairment as a dementia risk construct. 27 MCI can result from diseases other than AD (cerebrovascular disease, fronto‐temporal dementia, etc.). Prototypical multidomain amnestic dementia used to clinically define AD dementia is present in 10% to 30% of individuals with normal amyloid PET scan and/or normal CSF Aβ1‐42 levels, and must be therefore produced by other diseases. 29 In late aging it is most commonly associated with multiple pathologies. 30 Disease variability or final MCI prognosis risk still generate confusion in research. 31 , 32 Major criteria for MCI diagnosis have been proposed by the International Working Group (IWG) 33 on one hand, and by the National Aging Institute and Alzheimer Association (NIA‐AA) 4 on the other. Clinical diagnosis of MCI requires one positive AD biomarker and is called prodromal AD by the IWG. 33 Whereas one amyloid plus one degeneration biomarker need to be present to diagnose MCI due to AD according to the NIA‐AA 4 AD, biomarkers determine more precise diagnoses and better treatment monitoring. 32 In a previous study, we evaluated MCI patients 23 and found an AD profile present in 18% of controls, 64% of MCI, and 92% of DAT. SNAP was present in 11% of controls, 6% of MCI, and 8% of DAT patients. MCI with or without detectable amyloid can progress to dementia. At 2‐year follow‐up, 45% of patients with amyloid positive MCI and 20% of MCI with SNAP had progressed to dementia. 23 Constellations with conflicting CSF biomarker findings (eg, neurodegeneration without amyloid—SNAP) are not currently considered by IWG or NIA‐AA classifications. 34 In some patients presenting the typical multidomain amnesic phenotype used to defined probable AD, no AD tissue pathology was found. 22 , 29 Conversely, non‐amnesic syndromes may also be atypical forms of AD, with some patients presenting fronto‐temporal dementia phenotype due to AD. 35 Analyzing degree of concordance between 11C‐PiB‐PET findings and clinical diagnosis in patients from our memory clinic we observed 72% of agreement between positive amyloid and typical clinical AD diagnosis. 22 Twenty‐five percent of patients presenting fronto‐temporal phenotype had positive amyloid on PET. 22 Also, 10% of patients in this cohort with low CSF Aβ1‐42 had a negative amyloid PiB‐PET scan. 22 In the Arg‐ADNI sample 21% were only positive for CSF Aβ1‐42 levels, with 0.8 of correlation with amyloid PET scan. This mismatch between PiB PET amyloid detection and Aβ1‐42 level in CSF has been explained as representing different stages of amyloid plaque formation, because CSF Aβ1‐42 reduction has systematically been described as presenting first, and amyloid binding on PET later. 36 Correlation between neurodegeneration biomarkers was very low, ranging from 0.2 to 0.4. This result could contribute to a better understanding of the utility of each option. The most significant (P < 0.00) difference between the populations was observed for FDG‐PET scan results, 7% positive in normal controls, 40% to 54% in MCI, and 100% in DAT.
Limitations to this study include small initial sample size (56 subjects), small sample size at 60 months (42 patients), short follow‐up (60 months), and lack of autopsy validation. Our results cannot therefore be generalized to all MCI patients, because the ADNI cohort was limited to amnesic MCI phenotypes to enrich the sample with pre‐dementia AD cases. Effects of other brain pathologies associated with increased p‐tau or t‐tau levels could thus have been minimized in the ADNI cohort. Another difficulty could be the artificial borderline biomarker value range used to define positive or negative results.
Nevertheless, despite the limitations described above, the results are significant because FLENI is the only ADNI center in South America applying rigorous systematic evaluation of biomarkers for AD. The vast majority of published data is on PET and CSF results from patients recruited from selected centers in developed countries. 37 The possibility of generating results from Latin America even though participants in this cohort were predominantly of European ancestry, will contribute valuable information for comparison purposes with results from developed countries, and help characterize the condition in a more generalized manner. Some authors believe biomarker‐based research from low and middle incomes countries in developing world regions might not be reliable, a concept our efforts attempt to refute. 38
5. CONCLUSION
The present study provides evidence on application of ATN criteria in a developing country from South America, currently used only in the framework of research on AD worldwide. To the best of our knowledge, this is the first AD biomarker‐based report from this region with 60 months follow‐up, applying the new ATN classification. Our findings suggest both A+T+N+ MCI patients (due to AD) and A‐T‐N+ MCI patients (SNAP) should be diagnosed early, because of their increased risk of dementia transformation in a relatively short time period. Biomarker results will provide more details on AD physiopathology and probably represent the step forward needed to resolve pharmacological treatment.
CONFLICTS OF INTEREST
Authors reports no conflicts of interest to report in relation to this study.
AUTHOR CONTRIBUTIONS
Ricardo F. Allegri and Gustavo Sevlever designed the study, and supervised data collection. Ricardo F. Allegri, Patricio Chrem Méndez, and Gabriela Cohen wrote the paper. Ismael Calandri was responsible for statistical analysis. Gabriela Cohen, Patricio Chrem Méndez, María Julieta Russo, Lucia Pertierra, Fernanda Tapajóz, María Florencia Clarens, Jorge Campos, Federico E. Nahas, Ezequiel Surace, and Silvia Vázquez collect the data and assisted with writing article.
ACKNOWLEDGMENTS
Data collection and sharing for this project were funded by FLENI, by CONICET (Consejo Nacional de Investigaciones Cientificas y Tecnologicas ‐ PICT 2110‐2015), and by CIS (Consejo de Investigación GCBA), Argentina.
Allegri RF, Méndez PC, Calandri I, et al. Prognostic value of ATN Alzheimer biomarkers: 60‐month follow‐up results from the Argentine‐Alzheimer's Disease Neuroimaging Initiative. Alzheimer's Dement. 2020;12:e12026 10.1002/dad2.12026
REFERENCES
- 1. Bloom GS. Amyloid‐β and tau: the trigger and bullet in Alzheimer disease pathogenesis. JAMA Neurol. 2014;71(4):505‐508. [DOI] [PubMed] [Google Scholar]
- 2. Russo MJ, Gustafson S, Vazquez S, et al. Argentina‐Alzheimer's disease neuroimaging initiative. Creation of the Argentina—Alzheimer Disease Neuroimaging Initiative. Alzheimers Dement. 2014;10(1 suppl):S84‐S87. [DOI] [PubMed] [Google Scholar]
- 3. Sperling RA, Aisen PS, Beckett LA, et al. Toward defining the preclinical stages of Alzheimer's disease: recommendations from the National Institute on Aging‐Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7(3):280‐292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging‐Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7(3):270‐279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jack CR Jr, Knopman DS, Weigand SD, et al. An operational approach to National Institute on Aging–Alzheimer's Association criteria for preclinical Alzheimer disease. Ann Neurol. 2012;71(6):765–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jack CR Jr, Bennett DA, Blennow K, et al. ATN: an unbiased descriptive classification scheme for Alzheimer disease biomarkers. Neurology. 2016;87(5):539‐547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jack CR Jr, Bennett DA, Blennow K, et al. NIA‐AA Research Framework: toward a biological definition of Alzheimer's disease. Alzheimers Dement. 2018;14(4):535‐562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Allegri RF, Ollari JA, Mangone CA, Arizaga RL, De Pascale A, Pellegrini M. Mini‐Mental State Examination in Argentina: instruction for assessment. Rev Neurol Argent. 1999;24(1):31‐35. [Google Scholar]
- 9. Wechsler D. Wechsler Memory Scale 3rd ed Psychol Corp, San Antonio: TX, 1997. [Google Scholar]
- 10. Burin D, Ramenzoni VA. Neuropsychological assessment in elderly: norms by age and educational level. Rev Neurol Argent. 2003;28(3):149‐152. [Google Scholar]
- 11. Kaplan EF, Goodglass H, Weintraub S. Boston Naming Test. Philadelphia, PA: Lea & Febiger; 1983. [Google Scholar]
- 12. Serrano C, Allegri RF, Drake M, et al. A shortened form of the Spanish Boston naming test: a useful tool for the diagnosis of Alzheimer's disease. Rev Neurol. 2001;33(7):624‐647. [PubMed] [Google Scholar]
- 13. Butman J, Allegri RF, Harris PD. Spanish verbal fluency. Normative data in Argentina. Medicina. 2000;60(5 pt1):561‐564. [PubMed] [Google Scholar]
- 14. Arango‐Lasprilla JC, Rivera D, Aguayo A, et al. Trail Making Test: normative data for the Latin American Spanish speaking adult population. NeuroRehabilitation. 2015;37(4):639‐661. [DOI] [PubMed] [Google Scholar]
- 15. Berg L. Clinical Dementia Rating (CDR). Psychopharmacol Bull 1988;24(4):637‐639. [PubMed] [Google Scholar]
- 16. Kaufer DI, Cummings JL, Ketchel P, et al. Validation of the NPI‐Q, a brief clinical form of the Neuropsychiatric Inventory. J Neuropsychiatry Clin Neurosci. 2000;12(2):233‐239. [DOI] [PubMed] [Google Scholar]
- 17. Sheikh JI, Yesavage JA. Geriatric Depression Scale (GDS): recent evidence and development of a shorter version Clinical Gerontology: A Guide to Assessment and Intervention. New York: Haworth Press; 1986: 165‐173. [Google Scholar]
- 18. Pfeffer RI, Kurosaki TT, Jr Harrah CH, Chance JM, Filos S. Measurement of functional activities in older adults in the community. J Gerontol.1982;37(3):323‐329. [DOI] [PubMed] [Google Scholar]
- 19. Wahlund LO, Julin P, Johansson SE, Scheltens P. Visual rating and volumetry of the medial temporal lobe on magnetic resonance imaging in dementia: a comparative study. J Neurol Neurosurg Psychiatry. 2000;69(5):630‐635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS ADRDA work group under the auspices of Department of Health and Human Services Task Force on Alzheimer's disease. Neurology. 1984;34(7):939‐944. [DOI] [PubMed] [Google Scholar]
- 21. McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging and the Alzheimer's Association Workgroup. Alzheimers Dement. 2011;7(3):263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chrem P, Cohen G, Russo JM, et al. Concordance between 11C‐PIB‐PET and clinical diagnosis in a memory clinic. Am J Alzheimers Dis Other Dement. 2015;30(6):599‐606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Allegri RF, Chrem Mendez P, Russo MJ, et al. Biomarkers of Alzheimer's disease in mild cognitive impairment: experience in a memory clinic from Latin America. Neurologia, 2018. pii: S0213‐4853(18)30028‐8. 10.1016/j.nrl.2017.12.011. [DOI] [PubMed] [Google Scholar]
- 24. Allegri RF, Pertierra L, Cohen G, et al. A biological classification for Alzheimer's disease—Amyloid, Tau and Neurodegeneration (A/T/N): results from the Argentine‐Alzheimer's Disease Neuroimaging Initiative. Int Psychogeriatr. 2019;31(12):1837‐1838. 10.1017/S1041610219000085. [DOI] [PubMed] [Google Scholar]
- 25. Russo MJ, Cohen G, Chrem Mendez P. Predicting episodic memory performance using different biomarkers: results from Argentina‐Alzheimer's Disease Neuroimaging Initiative. Neuropsychiatr Dis Treat. 2016;12:2199‐2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lowe VJ, Peller PJ, Weigand SD, et al. Application of the National Institute on Aging‐Alzheimer's Association AD criteria to ADNI. Neurology. 2013;80(23):2130‐2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Petersen RC, Aisen P, Boeve BF, et al. Criteria for mild cognitive impairment due to Alzheimer's disease in the community. Ann Neurol. 2013;74(2):199‐208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Scheltens P, Blennow K, Breteler MM, et al. Alzheimer's disease. Lancet. 2016;388(10043):505‐517. [DOI] [PubMed] [Google Scholar]
- 29. Serrano‐Pozo A, Qian J, Monsell SE, Betensky RA, Hyman BT. Mild to moderate Alzheimer dementia with insufficient neuropathological changes. Ann Neurol. 2014;75(4):597‐601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Schneider JA, Arvanitakis Z, Leurgans SE, Bennett DA. The neuropathology of probable Alzheimer disease and mild cognitive impairment. Ann Neurol. 2009;66(2):200‐208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. De Carli Ch. Mild cognitive impairment: Prevalence, prognosis, aetiology and treatment. Lancet Neurol. 2003;2:15‐21 [DOI] [PubMed] [Google Scholar]
- 32. Allegri RF, Glaser FB, Taragano FE, Buschke H. Mild cognitive impairment: believe it or not?. Int Rev Psychiatry. 2008;20(4):357‐363. [DOI] [PubMed] [Google Scholar]
- 33. Dubois B, Feldman HH, Jacova C, et al. Research criteria for the diagnosis of Alzheimer's disease: revising the NINCDS‐ADRDA criteria. Lancet Neurol. 2007;6(8):734‐746. [DOI] [PubMed] [Google Scholar]
- 34. Alexopoulos P, Werle L, Roesler J, et al. Conflicting cerebrospinal fluid biomarkers and progression to dementia due to Alzheimer's disease. Alzheimers Res Ther. 2016;8(1):51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Riudavets MA, Bartoloni L, Troncoso JC, et al. Familial dementia with frontotemporal features associated with M146V presenilin‐1 mutation. Brain Pathol. 2013;23(5):595‐600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Palmqvist S, Mattsson N. Hansson O and for the Alzheimer Disease Neuroimaging Initiative . Cerebrospinal fluid analysis detects cerebral amyloid‐B accumulation earlier than positron emission tomography. Brain 2016;139(pt 4):1226‐1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Petersen RC, Aisen PS, Beckett LA, et al. Alzheimer's Disease Neuroimaging Initiative (ADNI): clinical characterization. Neurology 2010;74(3):201‐209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cece Y, Shifu X. Are the revised diagnostic criteria for Alzheimer's disease useful in low‐ and middle‐income countries? Shanghai Arch Psychiatry 2015;27(2):119‐123. [DOI] [PMC free article] [PubMed] [Google Scholar]


