Abstract
Background
Physical activity has shown a positive impact on aging and neurodegeneration and represents a possible treatment option in cognitive decline. However, its underlying mechanisms and influences on brain pathology remain unclear. Dementia‐MOVE (Multi‐Objective Validation of Exercise) is a randomized‐controlled pilot trial, including 50 patients with amnestic cognitive impairment associated with Alzheimer's pathology, aiming to analyze the effect of physical activity and fitness on disease progression.
Methods
Dementia‐MOVE is divided into two arms, of either an intervention comprising physical activity, for at least twice a week, combined with a psychoeducational program, or a sole psychoeducational program. Physical activity intervention includes a supervised and unsupervised multimodal concept combining resistance, endurance, coordinative, and aerobic training. The primary outcome is the change of brain metabolism due to physical interventional treatment. Besides metabolic magnetic resonance imaging (MRI) including sodium and phosphorus imaging, resting state functional MRI, T1‐, T2‐weighted and fluid‐attenuated inversion recovery (FLAIR), as well as diffusion‐weighted imaging (DWI) of the brain and whole‐body fat MRI are performed before and after intervention, and will be compared in their sensitivity for the detection of intervention effects. We further assess cognitive performance, neuropsychiatric symptoms, quality of life, fitness, and sleep via questionnaires/interviews and/or fitness trackers, as well as microbiome, under the aspect of Alzheimer's pathology.
Discussion
The aim of Dementia‐MOVE is to investigate the effect of a multimodal exercise program on Alzheimer's pathology under different aspects of the disease. In this context, one of the main aims is the comparison of different MRI methods regarding their responsiveness for the detection of alterations induced by physical activity. As an underlying goal, new treatment and diagnostic options, as well as the exploration of fitness effects on brain structure and metabolism within a whole‐body perspective of Alzheimer's disease are envisaged.
Keywords: 31P MR spectroscopy, Alzheimer's disease, dementia, exercise, fat MRI, fitness, intervention, MRI, neuroimaging, phosphorus, physical activity, sodium imaging, 23Na MRI
1. BACKGROUND
Exercise represents a viable low‐cost, low‐risk, individualized, and widely available effective non‐pharmacological treatment option in cognitive decline: So far, studies focusing on animal models and in healthy and cognitively impaired humans have shown a positive effect of physical activity and cardiorespiratory fitness on cognition, behavior, and brain structure, 1 , 2 , 3 even indicating a preventive effect of physical activity for the risk of incident dementia. 4 , 5 In this context, studies in mild cognitive impairment (MCI), as well as in Alzheimer's disease (AD), show effectiveness of sports programs with a duration of at least 3 months and a frequency of at least twice a week. 1 , 6 Especially multimodal intervention concepts, combining aerobic, resistance, and coordinative training, even in combination with cognitive intervention, seem to be advantageous in improving not only several functional domains affected by cognitive decline, but also cognition and brain structure. 1 , 7 Despite several intervention studies on physical activity in the aging population, changes in clinical as well as structural and metabolic parameters induced by physical activity during the process of neurodegeneration are still poorly understood. Our recent review pointed to an influence of physical activity on brain regions sensitive to neurodegeneration, using several structural and functional magnetic resonance imaging (MRI) methods. 8 Following such evidence, metabolic MRI, by providing information on tissue viability, such as homeostasis, cell integrity, and energy metabolism preceding structural changes in neurodegeneration, may serve as a more sensitive tool to detect early changes in comparison to standard structural MRI methods. Sodium MRI has shown high sensitivity to cell degeneration 9 , 10 and may provide a deeper insight into the pathophysiological mechanisms of tissue decline in neurodegenerative disorders. 11 , 12 Another technique with a focus on energy metabolism is 31P MR spectroscopy (MRS). Because phosphorus is present in tissues in the forms of high‐energy phosphates (ATP, PCr), it allows insights into the alteration of mitochondrial energy supply capabilities in the neuronal energy metabolism. 13 However, to go beyond alterations on cerebral level, we aim for a holistic understanding of the influence of physical activity on AD on different aspects. For example, studies show that environmental and circulatory factors influence cognition and brain aging. 14 , 15 Similarly, alterations on other organs and body parts in AD have been reported, 16 , 17 which renders the study of exercise as a possible non‐pharmacological treatment beyond cerebral changes even more important.
We here present the protocol of a randomized, controlled clinical pilot trial (Multi‐Objective Validation of Exercise in Dementia—Dementia‐MOVE) among patients with AD or Alzheimer's pathological change (Alzheimer's continuum 18 ) comparing the effect of a multicomponent exercise program with a psychoeducational program on several clinical and imaging parameters. Assessment methods will include cerebral and body fat MRI, neuropsychological testing, fitness assessment, serology, and questionnaires/interviews to assess sleep and quality of life. The goal of this study is to evaluate the responsiveness of novel MRI methods in the detection of exercise‐induced brain changes in metabolism and to achieve a better understanding of the influence of a multicomponent program on the progression of AD, assuming a holistic perspective of the disease.
2. METHODS/DESIGN
2.1. Study design
Dementia‐MOVE is a monocentric randomized controlled clinical trial with two arms, one including an intervention group (IG), participating in a multicomponent physical exercise and psychoeducation program, and a second arm including a psychoeducational group (PG), as a control condition. The intervention, with a total duration of 6 months, takes place at least two times per week, comprising participation in a supervised exercise group and unsupervised home‐exercises, as well as in an additional psychoeducational program. Outcome assessment will take place at baseline (T1), after 3 months (T2), and postinterventional after 6 months (T3) at the RWTH Aachen University Hospital (see Figure 1). The physical exercise intervention takes place at one of two facilities (department of ambulatory physiotherapy of the RWTH Aachen University Hospital or in an ambulatory physiotherapy practice [MedAix in Simmerath]). The psychoeducational program also takes place in one of the two sites. Home activity and unsupervised physical activity are monitored via written diaries, which are completed by the participants and with support of their relatives. Diaries are checked regularly for protocol adherence and are used for comparison with fitness tracker results. Subject recruitment is mainly secured via the RWTH Aachen University Hospital Neurology Department's memory clinic, neighboring hospitals, as well as ambulatory neurology and general practitioners (GP) service providers and advertisement. The study is conducted according to the Declaration of Helsinki and approved by the local ethics committee (EK 306/18). The trial is registered at ClinicalTrials.gov (NCT03939286).
FIGURE 1.
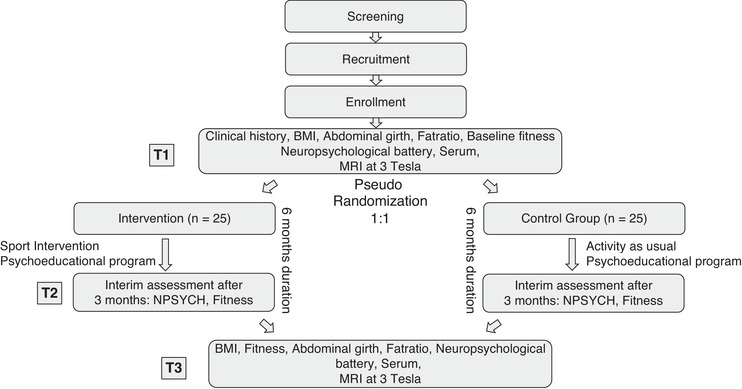
Flow diagram of the study with two intervention branches: (1) intervention with physical activity combined with psychoeducational program and (2) control group with psychoeducational program to stabilize for social group effects and activity as usual, assessed via diaries. T1 represents the timepoint at baseline, T2 after 3 months, and T3 after 6 months
RESEARCH IN CONTEXT
Systematic review: Physical activity is a non‐pharmacological treatment option in cognitive decline for which several systematically reviewed studies (eg, Haeger et al. 8 ) pointed to an advantageous effect. However, its induced effects during disease still need to be explored in detail and more studies are needed for optimizing future therapeutics and diagnostics.
Interpretation: So far, multimodal exercise concepts have been identified as most beneficial, which we translated in our intervention protocol Dementia‐MOVE in Alzheimer's disease (AD). In this context, metabolic magnetic resonance imaging comprising sodium and phosphorus imaging can offer a novel way for the detection of intervention effects on a more metabolic‐associated level.
Future directions: This clinical pilot study protocol proposes an intervention, allowing researchers to analyze the effect of physical activity on AD from different perspectives on a holistic level, with a focus on metabolic imaging. This will allow a deeper understanding of AD and facilitate planning of future treatment.
2.2. Study participants and inclusion/exclusion criteria
Patients with amnestic‐cognitive impairment and/or cerebrospinal fluid (CSF) constellation matching Alzheimer's continuum according to the criteria defined by Jack et al. 18 will be included. An overview of the inclusion and exclusion criteria is given in Table 1.
TABLE 1.
Inclusion and exclusion criteria for study participants
| Inclusion criteria | Exclusion criteria |
|---|---|
|
|
Abbreviations: MMSE, Mini‐Mental State Examination; MRI, magnetic resonance imaging
In short, participants should be between 50 and 80 years old, be able to consent, present either with MCI or mild dementia, without a medical condition hindering participation in a sports program. Medical history is collected by interview and medical reports review. If needed, GPs are contacted to confirm aptitude for participation in a regular sports program.
Study participation excludes simultaneous participation in other intervention trials. Written consent to study protocol and storage of blood samplings within the RWTH Biobank project will be obtained. Participants will profit from accident insurance during participation of the trial.
2.3. Randomization, allocation, concealment, and blinding
Random stratified allocation of subjects is performed blinded and 1:1 to each study arm using WINPEPI software (http://www.brixtonhealth.com/). Stratification follows age and fitness level, as determined via VO2 max, or in case of non‐availability, the result of the 6‐minute walk test (6MWT). 19 Site of residency is further taken into consideration when allocating between Aachen and Simmerath. In case of unequal numbers of participants, allocation is performed in favor of the intervention group, but balanced distribution is envisaged. After randomization, participants are informed about their group allocation.
2.4. Individual pre‐interventional assessment
Pre‐interventional assessment is done to gather information about the participant's general health, activity level, and fitness status. Participants and caregivers are interviewed about cardiovascular, musculoskeletal, respiratory, neurological, psychiatric conditions, as well as about any current acute illnesses. Possible physical limitations of the participants are taken into consideration for the physical exercise planning. One of the main goals of the pre‐interventional assessments is to access fitness levels and to set an adjusted individual exercise intensity. A submaximal ergometer test on a Corival cpet ‐ Lode is performed to estimate VO2 max via a modified Astrand‐protocol. 20 Afterward, grip force and postural stability are estimated via a hand dynamometer (RFM) and the MFT S3‐Check. The 6MWT, which is well applicable and adapted to AD patients, 19 is performed with standardized instructions and phrases of encouragement. The distance walked in 6 minutes is measured while the participant also wears a heart rate monitor, recording the resting and average heart rates. The Borg Scale of perceived exertion (RPE), adapted for use in AD patients in scales of 10, 21 is used to assess exercise intensity. Visual indicators such as sweating, skin color, and breathing rate are also considered for evaluation of intensity. Heart rate (HR) is also monitored on the circle ergometer as an indicator of exertion, but not taken as first indicator due to the presumable high prevalence of beta blockers and drugs influencing heart rate in this population.
2.5. Physical exercise program
Exercise intervention is developed on a multicomponent concept including endurance, resistance, and coordination training. The exercise program comprises 5 to 10 minutes of warm‐up, 5 to 10 minutes of coordinative training on an equilibrium circle, 30 minutes of resistance training, and 10 to 15 minutes of endurance and aerobic fitness training, leading to a total session duration of about 60 minutes. Participants further train unsupervised at home, for at least 30 minutes (e.g., via participation in a community sports group), with moderate exercise according to the Borg RPE‐scale adapted to patients with dementia, 22 which is documented in the written diary. We focus on moderate training, as studies point to the largest effects in comparison to light or intensive exercise. 23 , 24 Furthermore, subjects receive training cards available from the Federal Centre for Health Education (https://www.aelter-werden-in-balance.de/bewegungspackung/mein-uebungsprogramm/) with stretch and toning exercises, which they should perform twice a week for 15 minutes.
The target aerobic intensity rate is estimated according to Luxton et al., 25 calculating the peak work rate for ergometer from the 6MWT. For resistance training, the whole circle consisting of 12 training devices is divided into subgroups for training upper extremity, lower extremity, and trunk. Two devices from each subgroup are chosen for each session. Training intensity with one repetition maximum (RM) is estimated according to the submaximal RM and the Epley formula. 26
2.6. Psychoeducational program
The psychoeducational program consists of monthly presentations with information about cognitive impairment, treatment options, cognitive training, and lifestyle, which have shown positive influence on cognition. 27 These presentations are open to the general public. There is one additional separate session for participants and their relatives about nutrition. Furthermore, information material in the form of brochures is distributed to the participants to keep them informed. Both the IG and PG participate in the psychoeducational program, to control for the social effect of group interventions.
2.7. Outcome assessment
All outcome measures will be measured at time points T1 (baseline) and T3 (6 months). Neuropsychological testing and fitness assessment will also be performed at T2 (3 months). Study process and outcome are reported according to the SPIRIT (Standard Protocol Items: Recommendations for Interventional Trials) criteria and illustrated in Table 2.
TABLE 2.
SPIRIT diagram outlining enrolment, intervention, and assessment of study participants during the course of the study
| Study period | |||||||
|---|---|---|---|---|---|---|---|
| Enrolment | Allocation | Post‐allocation | Time‐out | ||||
| Timepoint | T1 | 0 | T2 | T3 | |||
| Enrolment | Enrolment | x | |||||
| Eligibility screening | x | ||||||
| Informed consent | x | ||||||
| Fitness assessment | x | ||||||
| Allocation | x | ||||||
| Interventions | Multimodal exercise intervention |

|
|||||
| Psychoeducational program |

|
||||||
| Assessment | MRI (sodium, phosphorus, T1, T2, FLAIR, DWI, fat, resting state) | x | x | ||||
| Neuropsychological assessment | x | x | x | ||||
| VO2max | x | x | x | ||||
| Posturography | x | x | x | ||||
| Grip strength | x | x | x | ||||
| 6MWT | x | x | x | ||||
| PAQ50+ | x | x | x | ||||
| PSQI | x | x | x | ||||
| QoL | x | x | x | ||||
| APOE | x | ||||||
| Serology | x | x | |||||
| Stool sample | x | x | |||||
| Body fat scale | x | x | x | ||||
| Fitness tracker | x | ||||||
Note: Duration of intervention is 6 months.
Abbreviations: 6MWT, 6‐minute walk test; APOE, Apoliprotein E; DWI, diffusion‐weighted imaging; FLAIR, fluid‐attenuated inversion recovery; MRI, magnetic resonance imaging; PSQI, Pittsburgh Sleep Quality Index; QoL, Quality of Life; SPIRIT, Standard Protocol Items: Recommendations for Interventional Trials
2.7.1. Primary outcomes
Changes detected via metabolic MRI: The primary objective of the study is to evaluate the effect of 6 months of physical intervention compared to a psychoeducational program on cerebral metabolism assessed by metabolic MRI. In more detail, the primary outcome measures include change in cerebral metabolism via sodium and/or phosphorus MRI, under the hypothesis that energy metabolism is a sensitive parameter for the evaluation of intervention effects: for cell homeostasis, sodium gradient between extracellular and intracellular space is essential because it supports maintaining the cellular resting potential and is, in terms of consumed energy, very demanding: up to 23% of the adenosine triphosphate (ATP) available to the cell is used by the sodium pump to sustain this function. Another technique with a main focus on energy metabolism is in vivo 31P MRS. Phosphorus is present in tissues in the forms of high‐energy phosphates (ATP, PCr) and in other phosphorylated biomolecules partially involved in cell membrane maintenance or redox‐state equilibrium. It therefore allows insights into the alteration of mitochondrial energy supply. 13 Recent results point to increases in tissue sodium concentration (TSC) in neurodegenerative diseases, even in preclinical states. 28 , 29 However, there have not been any previous data on alteration in TSC and phosphorus induced by an intervention with physical activity of 6‐months duration so far. We hypothesize that TSC, especially in brain regions affected by neurodegeneration such as the hippocampus and other regions from the temporal lobe, precuneus, anterior and posterior cingulate, will show lower increases of TSC as a marker of decreased neurodegeneration compared to the non‐interventional control condition. Postinterventional cerebral regional metabolic changes will be compared to alterations in volume and cortical thickness detected via structural MRI methods to investigate the sensitivity of different MRI methods in detection of intervention effects.
2.7.2. Secondary outcomes
Structural and functional MRI alterations: Change in structure and function of brain regions associated with fitness will be measured using different MR sequences including isotropic high‐resolution T1‐weighted‐sequences, diffusion‐weighted imaging (DWI), and resting state functional MRI. Increase or protected preservation of volumetrics and cortical thickness especially in regions of interest (ROIs) of the temporal lobe are expected in the intervention group. T1‐weighted images will be segmented into different brain tissues as white matter, gray matter, and CSF and ROIs via neuroimaging analysis tools such as ANTs (advanced normalization tools), Avants et al. 2011. Additionally, T2‐weighted‐ and fluid‐attenuated inversion recovery (FLAIR)‐ sequences are planned to identify white matter lesions with VolBrain toolbox. 30 A whole‐body fat MRI sequence from peak of diaphragm to patella will be added, via usage of additional body coils, to measure subcutaneous and visceral fat distribution. The ratio between different fat distributions will be assessed via segmentation of the different compartments with differentiation between muscles, subcutaneous and visceral fat, and later compared to fat and muscle distributions assessed via a fat distribution scale (see below). Change in fat ratio distribution is expected in the exercise group compared to the solely educational group.
Changes in cognitive performance: In addition to the Montreal Cognitive Assessment (MoCA) 31 , 32 for global cognitive screening, the neuropsychological assessment battery focuses on three major cognitive domains—attention, memory, and executive functions—applying standardized and commonly used tests in clinical and research settings. Detection of alterations in cognitive performance induced by physical activity is the aim. Verbal memory is examined using the California Verbal Learning Test (CVLT), 33 a word list recall task. Attention is assessed through computer‐based (Test of Attentional Performance, TAP) cued and non‐cued reaction time (phasic and intrinsic alertness) tasks 34 and digit span forwards. 35 Processing speed and executive functions are assessed using the Trail Making Test (TMT) forms A and B, 36 phonemic and semantic word fluency, 37 digit span backwards, 35 and the interference task of the Stroop test. 38 To avoid practice effects, alternate forms of the tests are used for the majority of instruments, with the exception of alertness and digit span tasks.
Fitness assessment and their changes during the intervention: Physical activity will be evaluated with the German PAQ50+ questionnaire, which is a questionnaire adapted for elderly subjects considering the altered focus of physical activity of persons over the age of 50 years. 39 Furthermore, wearable fitness‐tracker (Fitbit Charge 2) are worn by the participants for a duration of about 2 weeks for evaluation of physical activity. Data provided by the fitness tracker will include steps; undergone distance; sitting; light, moderate, and intensive activity in minutes; burnt calories and sleep in minutes; frequency of awakening during night; time spent in bed; REM‐sleep; light and deep sleep phases. Results from fitness trackers will be associated with results from patients’ diaries keeping track of their weekly activity. As mentioned before, VO2 max, postural instability, hand grip, and 6MWT are further assessed as fitness parameters to evaluate change in these parameters in the exercise group. Aim is the detection of improvement in fitness, force, and stability as well as sleep via our multimodal intervention program.
Body fat distributions: Body fat distribution with percentage of visceral and subcutaneous fat, muscle mass and body mass index (BMI) will be assessed via Omron fat distribution scale (Omron B511). The aim is a comparison with fat and muscle distribution ratios resulting from our whole‐body‐fat MRI.
Neuropsychiatric symptoms and sleep: The Neuropsychiatric Inventory Questionnaire (NPI‐Q), 40 measuring 12 neuropsychiatric symptoms in patients with dementia, as well as informant distress, is applied. Self‐report of anxiety and depression are assessed using the Hospital Anxiety and Depression Scale (HADS‐D). 41 The Quality of Life in AD (QoL‐AD) 42 is a 13‐item measure developed for individuals with cognitive impairment and a study partner. Sleep quality is assessed with the Pittsburgh Sleep Quality Index (PSQI) 43 comprising 18 items assessing sleep quality, disturbances, duration, and times. Sleep is further registered by the wearable fitness‐tracker (Fitbit Charge 2). We expect an increase in life quality, but also in possible depressive symptoms and sleep quality.
Serology, neurodegenerative markers, genetics, and microbiome: Fat metabolism including levels of cholesterol and leptin are assessed in serological examinations. Furthermore, Apolipoprotein E (APOE) status is assessed. Analyses will be performed considering neurodegenerative markers (amyloid‐beta 1‐42, amyloid‐beta 1‐40, amyloid‐ratio, total‐tau, and phosphor‐tau concentrations) in CSF, available from the diagnosis workout prior to inclusion. Furthermore, stool samples are collected for microbiome analysis at T1 and T3.
2.8. Safety and withdrawal of participants
All serious adverse events (SAE)/adverse events (AE) will be recorded and discussed. Adherence to protocol will be supported by regular interviews, encouragements, and problem assessment (e.g., occurrence of health problems and their treatment). Participants who wish to withdraw from the study (i.e., drop‐outs) will be asked to undergo further outcome assessment. Drop‐outs will be included in final analyses. Missing data will be handled with appropriate statistical methods as via multiple imputation.
2.9. Statistical analysis and sample size
The outcomes will be compared between both groups. The primary analysis of this study will be comparison of metabolic and standard structural and functional MRI techniques regarding their responsiveness to detect intervention effects and to perform exploratory analysis of TSC changes induced by the therapeutic intervention condition. This will be performed on a global cerebral level, as well as with ROI analyses (see primary outcomes section).
Furthermore, cognitive functioning within‐ and between‐group over the three outcome assessment timepoints will be analyzed using mixed‐model statistics. For statistical analysis, results from the different cognitive domains as well as a composite score will be calculated.
Changes in physical fitness will be assessed primarily via pVO2 and results from the 6MWT. 19 , 44 Absolute scores of questionnaires of PAQ50+, quality of life, and sleep quality will be compared between and within groups. Fitness trackers (Fitbit) will be used to assess physical activity, divided into low, mild, and high activity in daily routine, compared to physical activity according to diaries and correlated via Pearson and Spearman's rank correlations.
A P‐value of <0.05 and Cohen's d for effect sizes will be used to assess statistical significance. Analyses within and between groups will be performed with analyses of variance (ANOVA). For end points comprising three data points (i.e., T1, T2, and T3), time point curves for neuropsychological and fitness assessment for both groups will be compared.
Because there are no previous studies applying sodium and phosphorus imaging in intervention studies with physical activity, the decision on our sample size of 50 patients (25 per group) was made primarily based on two aspects: (1) sample sizes reported from previous intervention studies in cognitive impairment 8 , 45 , 46 , 47 , 48 and (2) preliminary data from an observational study on hippocampal TSC in AD from our work group. Here, for sample size estimation, mean TSC of left and right hippocampus from AAL‐atlas 49 was compared between two AD subgroups (total n = 53 patients) created via median split of normalized hippocampal volume, finally leading to a sample size estimation of 22 subjects per group (α = 0.5; β = 0.2; power = 0.8). Our further data on TSC in neurodegenerative diseases so far under ultra‐high‐field conditions 8 , 29 and previous studies on31P MR spectroscopy 50 point to sufficient sample size power for this exploratory intervention.
3. DISCUSSION
We present the study protocol of a multimodal exercise intervention in Alzheimer's disease. As discussed, several studies have already pointed to a positive effect of different exercise interventions on prevention, as well as progression, of cognitive impairment. Alterations on cerebral level detected via MRI have recently been discussed in a review from our research group. 8 Our study aims at combining positive results from research of physical activity on AD progression and we therefore conceptualized our program according to these findings. We followed recommendations and so far positive results 8 for study duration, session frequency, and intensity. For maximal adherence of participants, we only perform one session of supervised physical activity per week. Otherwise, the participants perform their additional exercise at home based in a self‐efficacy concept (either other sports groups, single sports, etc.) which is checked via participants’ diary, interviews, and validated via fitness trackers. We plan on examining cerebral changes on the metabolic, energy, structural, and functional level induced by physical activity and its impact on neurodegeneration. 51 Beyond previous studies that mainly focused on cerebral structural alteration, we present a holistic approach, also including body fat MRI with subcutaneous and visceral fat distribution and examining effects on quality of life, neuropsychiatric symptoms, and sleep, which have been shown to be altered in AD. 52 , 53 , 54 Analyses will be performed considering genetic risk (APOE‐status) and CSF‐based neurodegenerative markers. Cardiovascular fitness and mobility, coordination, as well as strength will be measured and examined as a moderator of the exercise–brain–cognition relationship. Because the number of falls is increased in MCI and is also a possible risk factor for further cognitive decline, 55 reduction of instability and muscle loss are also underlying goals of our program.
There are current limitations of this protocol which are, e.g., the decision of our sample size based on previous studies on sodium and phosphorus imaging and also based on other intervention studies applying physical activity so far. Because there are no previous studies applying metabolic imaging with a 6‐month intervention of physical activity, the exploratory approach of our study on metabolic imaging has to be underlined in this context.
Altogether, Dementia‐MOVE is a randomized controlled pilot trial on physical activity with several goals: we envisage to examine physical activity and its influence on the progress of AD in many aspects, not only on brain pathology, but also on whole body metabolism, under the influence of genetics and neurodegeneration. A better understanding of the influence of physical activity and fitness on AD pathology could therefore support the opening of avenues for possible new diagnostics and therapeutic treatments.
FUNDING
The research project is funded by RWTH‐Startup grant (StUpPD_329‐18). A. Haeger received a rotation and START stipend (121/18) of RWTH Aachen University.
CONFLICTS OF INTEREST
The authors declare that they have no competing interests.
ACKNOWLEDGMENTS
We thank the physiotherapy team, especially Rudolph Schifflers, for their support in designing the intervention and MedAix, especially Gökhan Giray, the team of the memory clinic for their support in the psychoeducational program, the Alzheimer Gesellschaft Simmerath, especially Dr. Ulrich Albert, for their support in this study; the BMB biobank project of RWTH Aachen University Hospital; Prof. Eggermann from the department of human genetics for support in genetic analyses; Shahram Mirzazade for his support in data acquisition; and the Center for Translational & Clinical Research (CTC‐A) for their support.
Haeger A, Costa AS, Romanzetti S, et al. Effect of a multicomponent exercise intervention on brain metabolism: A randomized controlled trial on Alzheimer's pathology (Dementia‐MOVE). Alzheimer's Dement. 2020;6:e12032 10.1002/trc2.12032
REFERENCES
- 1. Pitkälä K, Savikko N, Poysti M, Strandberg T, Laakkonen M‐L. Efficacy of physical exercise intervention on mobility and physical functioning in older people with dementia: a systematic review. Exp Gerontol. 2013;48(1):85‐93. 10.1016/j.exger.2012.08.008 [DOI] [PubMed] [Google Scholar]
- 2. Lautenschlager NT, Cox K, Cyarto EV. The influence of exercise on brain aging and dementia. Biochim Biophys Act. 2012;1822(3):474‐481. 10.1016/j.bbadis.2011.07.010 [DOI] [PubMed] [Google Scholar]
- 3. Schultz SA, Boots EA, Almeida RP, etal. Cardiorespiratory fitness attenuates the influence of amyloid on cognition. J Int Neuropsychol Soc. 2015;21(10):841‐850. 10.1017/S1355617715000843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ngandu T, Lehtisalo J, Solomon A, etal. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at‐risk elderly people (FINGER): A randomised controlled trial. Lancet North Am Ed. 2015;385(9984):2255‐2263. 10.1016/S0140-6736(15)60461-5 [DOI] [PubMed] [Google Scholar]
- 5. Sabia S, Fayosse A, Dumurgier J, etal. Association of ideal cardiovascular health at age 50 with incidence of dementia: 25 year follow‐up of Whitehall II cohort study. BMJ. 2019;36:l4414 10.1136/bmj.l4414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lautenschlager NT, Cox KL, Flicker L, etal. Effect of physical activity on cognitive function in older adults at risk for Alzheimer Disease. JAMA. 2008;300(9):1027 10.1001/jama.300.9.1027 [DOI] [PubMed] [Google Scholar]
- 7. Blankevoort CG, Van Heuvelen MJG, Boersma F, Luning H, De Jong J, Scherder EJA. Review of effects of physical activity on strength, balance, mobility and ADL performance in elderly subjects with dementia. Dement Geriatr Cogn Disord. 2010;30(5):392‐402. 10.1159/000321357 [DOI] [PubMed] [Google Scholar]
- 8. Haeger A, Costa AS, Schulz JB, Reetz K. Cerebral changes improved by physical activity during cognitive decline: a systematic review on MRI studies. Neuroimage Clin. 2019;23:101933 10.1016/j.nicl.2019.101933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mellon EA, Pilkinton DT, Clark CM, etal. Sodium MR imaging detection of mild Alzheimer disease: preliminary Study. Am J Neuroradiol. 2009;30(5):978‐984. 10.3174/ajnr.A1495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Madelin G, Lee J‐S, Regatte RR, Jerschow A. Sodium MRI: methods and applications. Prog Nucl Magn Reson Spectrosc. 2014;79:14‐47. 10.1016/j.pnmrs.2014.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Reetz K, Romanzetti S, Dogan I, etal. Increased brain tissue sodium concentration in Huntington's disease — a sodium imaging study at 4T. Neuroimage. 2012;63(1):517‐524. 10.1016/j.neuroimage.2012.07.009 [DOI] [PubMed] [Google Scholar]
- 12. Huhn K, Engelhorn T, Linker RA, Nagel AM. Potential of sodium MRI as a biomarker for neurodegeneration and neuroinflammation in multiple sclerosis. Front Neurol. 2019;10:84 10.3389/fneur.2019.00084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hugg JW, Matson GB, Twieg DB, Maudsley AA, Sappey‐Marinier D, Weiner MW. Phosphorus‐31 MR spectroscopic imaging (MRSI) of normal and pathological human brains. Magn Reson Imaging. 1992;10(2):227‐243. [DOI] [PubMed] [Google Scholar]
- 14. Wyss‐Coray T. Ageing, neurodegeneration and brain rejuvenation. Nature. 2016;539(7628):180‐186. 10.1038/nature20411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Villeda SA, Luo J, Mosher KI, etal. The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature. 2011;477(7362):90‐94. 10.1038/nature10357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Diehl‐Wiesenecker E, Von Armin CAF, Dupuis L, Müller HP, Ludolph AC, Kassubek J. Adipose tissue distribution in patients with Alzheimer's disease: a whole body MRI case‐control study. J Alzheimers Dis. 2015;48(3):825‐832. 10.3233/JAD-150426 [DOI] [PubMed] [Google Scholar]
- 17. Kowalski K, Mulak A. Brain‐gut‐microbiota axis in Alzheimer's disease. J Neurogastroenterol Motil. 2019;25(1):48‐60. 10.5056/jnm18087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jack CR, Bennett DA, Blennow K, etal. NIA‐AA research framework: toward a biological definition of Alzheimer's disease. Alzheimers Dement.. 2018;14(4):535‐562. 10.1016/j.jalz.2018.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ries JD, Echternach JL, Nof L, Gagnon Blodgett M. Test‐retest reliability and minimal detectable change scores for the timed “Up & Go” Test, the Six‐Minute Walk Test, and Gait Speed in people with Alzheimer disease. Phys Ther. 2009;89(6):569‐579. 10.2522/ptj.20080258 [DOI] [PubMed] [Google Scholar]
- 20. Åstrand P‐O, Ryhming I. A nomogram for calculation of aerobic capacity (Physical Fitness) from pulse rate during submaximal work. J Appl Physiol. 1954;7(2):218‐221. 10.1152/jappl.1954.7.2.218 [DOI] [PubMed] [Google Scholar]
- 21. Yu F, Kolanowski A. Facilitating aerobic exercise training in older adults with Alzheimer's disease. Geriatr Nurs. 2009;30(4):250‐259. 10.1016/j.gerinurse.2008.11.001 [DOI] [PubMed] [Google Scholar]
- 22. Yu F. Improving recruitment, retention, and adherence to 6‐month cycling in Alzheimer's disease. Geriatr Nurs. 2013;34(3):181‐186. 10.1016/j.gerinurse.2012.12.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Geda YE, Roberts RO, Knopman DS, etal. Physical exercise, aging, and mild cognitive impairment a population‐based study. Arch Neurol. 2010;67(1):80‐86. 10.1001/archneurol.2009.297.Physical [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Makizako H, Liu‐Ambrose T, Shimada H, etal. Moderate‐intensity physical activity, hippocampal volume, and memory in older adults with mild cognitive impairment. J Gerontol A Biol Sci Med Sci. 2015;70(4):480‐486. 10.1093/gerona/glu136 [DOI] [PubMed] [Google Scholar]
- 25. Luxton N, Alison JA, Wu J, Mackey MG. Relationship between field walking tests and incremental cycle ergometry in COPD. Respirology. 2008;13(6):856‐862. 10.1111/j.1440-1843.2008.01355.x [DOI] [PubMed] [Google Scholar]
- 26. Epley B. Poundage chart. In: Boyd Epley Workout. Lincoln, NE: Body Enterprises, 2985; 1985:86. [Google Scholar]
- 27. Pentikäinen H, Ngandu T, Liu Y, etal. Cardiorespiratory fitness and brain volumes in men and women in the FINGER study. Age Ageing. 2017;46(2):310‐314. 10.1093/ageing/afw191 [DOI] [PubMed] [Google Scholar]
- 28. Haeger A, Coste A, Lerman‐Rabrait C, etal. Quantitative sodium imaging using ultra‐high field magnetic resonance imaging in patients with Alzheimer`s disease. AAIC Alzheimer's and Dementia, accepted. 2020. [Google Scholar]
- 29. Reetz K, Romanzetti S, Dogan I, etal. Increased brain tissue sodium concentration in Huntington's Disease ‐ a sodium imaging study at 4T. Neuroimage. 2012;63(1):517‐524. 10.1016/j.neuroimage.2012.07.009 [DOI] [PubMed] [Google Scholar]
- 30. Manjón J V, Coupé P. volBrain: an online MRI brain volumetry system. Front Neuroinform. 2016;10:30 10.3389/fninf.2016.00030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Costa AS, Fimm B, Friesen P, etal. Alternate‐form reliability of the Montreal cognitive assessment screening test in a clinical setting. Dement Geriatr Cogn Disord. 2012;33(6):379‐384. 10.1159/000340006 [DOI] [PubMed] [Google Scholar]
- 32. Nasreddine ZS, Phillips NA, Bédirian V, etal. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695‐699. 10.1111/j.1532-5415.2005.53221.x [DOI] [PubMed] [Google Scholar]
- 33. Niemann H, Sturm W, Thöne‐Otto AIT, Willmes K. CVLT California Verbal Learning Test. German adaptation. Manual. Frankfurt: Pearson Assessment; 2008.
- 34. Zimmermann P, Fimm B. Testbatterie zur Aufmerksamkeitsprüfung (TAP) Version 1.7. Herzogenrath: PSYTEST Verlag; 2002. [Google Scholar]
- 35. Aste M, Neubauer A, Horn R. Wechsler Intelligenztest Fuer Erwachsene (WIE). Deutschsprachige Bearbeitung Und Adaptation Des WAIS‐ III von David Wechsler. Frankfurt: Harcourt Test Services; 2006. [Google Scholar]
- 36. Wagner S, Helmreich I, Dahmen N, Lieb K, Tadic A. Reliability of three alternate forms of the trail making tests A and B. Arch Clin Neuropsychol. 2011;26(4):314‐321. 10.1093/arclin/acr024 [DOI] [PubMed] [Google Scholar]
- 37. Aschenbrenner S, Tucha O, Lange K. Regensburg Word Fluency Test [Regensburger Wortflüssigkeits‐Test (RWT)]. Göttingen: Hogrefe; 2000. [Google Scholar]
- 38. Bäumler G. Farbe‐Wort‐Interferenztest (FWIT) nach J. R. Stroop. Verlag für Psychologie Hogrefe; 1985. [Google Scholar]
- 39. Huy C, Schneider S. Instrument für die Erfassung der physischen Aktivität bei Personen im mittleren und höheren Erwachsenenalter. Zeitschrift für Gerontologie und Geriatrie. 2008;41(3):208‐216. 10.1007/s00391-007-0474-y [DOI] [PubMed] [Google Scholar]
- 40. Cummings JL, Mega M, Gray K, Rosenberg‐Thompson S, Carusi DA, Gornbein J. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology. 1994;44(12):2308‐2314. 10.1212/wnl.44.12.2308 [DOI] [PubMed] [Google Scholar]
- 41. Herrmann‐Lingen C, Buss U, Snaith RP. Hospital Anxiety and Depression Scale: HADS‐D ; Deutsche Version ; Ein Fragebogen Zur Erfassung von Angst Und Depressivität in Der Somatischen Medizin ; Testdokumentation Und Handanweisung . Huber; 2005.
- 42. Logsdon RG, Gibbons LE, McCurry SM, Teri L. Assessing quality of life in older adults with cognitive impairment. Psychosom Med. 64(3):510‐519. [DOI] [PubMed] [Google Scholar]
- 43. Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193‐213. 10.1016/0165-1781(89)90047-4 [DOI] [PubMed] [Google Scholar]
- 44. Carter R, Holiday DB, Nwasuruba C, Stocks J, Grothues C, Tiep B. 6‐Minute walk work for assessment of functional capacity in patients with COPD. Chest. 2003;123(5):1408‐1415. 10.1378/chest.123.5.1408 [DOI] [PubMed] [Google Scholar]
- 45. Frederiksen KS, Larsen CT, Hasselbalch SG, etal. A 16‐week aerobic exercise intervention does not affect hippocampal volume and cortical thickness in mild to moderate Alzheimer's disease. Front Aging Neurosci. 2018;10(September):1‐10. 10.3389/fnagi.2018.00293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Anderson‐Hanley C, Barcelos NM, Zimmerman EA, etal. The Aerobic and Cognitive Exercise Study (ACES) for community‐dwelling older adults with or at‐risk for mild cognitive impairment (MCI): neuropsychological, neurobiological and neuroimaging outcomes of a randomized clinical trial. Front Aging Neurosci. 2018;10(MAY):76. 10.3389/fnagi.2018.00076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Chirles TJ, Reiter K, Weiss LR, Alfini AJ, Nielson KA, Smith JC. Exercise training and functional connectivity changes in mild cognitive impairment and healthy elders. J Alzheimers Dis. 2017;57(3):845‐856. 10.3233/JAD-161151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Reiter K, Nielson KA, Smith TJ, Weiss LR, Alfini AJ, Carson Smith J. Improved cardiorespiratory fitness is associated with increased cortical thickness in mild cognitive impairment. J Int Neuropsychol Soc. 2015;21(10):757‐767. 10.1017/S135561771500079X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Tzourio‐Mazoyer N, Landeau B, Papathanassiou D, etal. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single‐subject brain. Neuroimage. 2002;15(1):273‐289. 10.1006/nimg.2001.0978 [DOI] [PubMed] [Google Scholar]
- 50. Rijpma A, van der Graaf M, Meulenbroek O, Olde Rikkert MGM, Heerschap A. Altered brain high‐energy phosphate metabolism in mild Alzheimer's disease: a 3‐dimensional 31 P MR spectroscopic imaging study. Neuroimage Clin. 2018;18:254‐261. 10.1016/j.nicl.2018.01.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Frederiksen KS, Gjerum L, Waldemar G, Hasselbalch SG. Physical activity as a moderator of Alzheimer Pathology: a systematic review of observational studies. Curr Alzheimer Res. 2019;16(4):362‐378. 10.2174/1567205016666190315095151 [DOI] [PubMed] [Google Scholar]
- 52. Lucey BP, McCullough A, Landsness EC, etal. Reduced non–rapid eye movement sleep is associated with tau pathology in early Alzheimer's disease. Sci Transl Med. 2019;11(474):eaau6550 10.1126/SCITRANSLMED.AAU6550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Yulug B, Hanoglu L, Kilic E. Does sleep disturbance affect the amyloid clearance mechanisms in Alzheimer's disease? Psychiatry Clin Neurosci. 2017;71(10):673‐677. 10.1111/pcn.12539 [DOI] [PubMed] [Google Scholar]
- 54. Peter‐Derex L, Yammine P, Bastuji H, Croisile B. Sleep and Alzheimer's disease. Sleep Med Rev. 2015;19:29‐38. 10.1016/j.smrv.2014.03.007 [DOI] [PubMed] [Google Scholar]
- 55. Robertson DA, Savva GM, Kenny RA. Frailty and cognitive impairment—a review of the evidence and causal mechanisms. Ageing Res Rev. 2013;12(4):840‐851. 10.1016/j.arr.2013.06.004 [DOI] [PubMed] [Google Scholar]


