Abstract
A novel library of amide functionality having 1,2,4-thiadiazole-1,2,4-triazole (8a–j) analogs was designed, synthesized, and structures were characterized by 1H NMR, 13C NMR, and mass (ESI–MS) spectral data. Further, all compounds were evaluated for their anticancer activities against four different cancer cell lines including breast cancer (MCF-7, MDA MB-231), lung cancer (A549), and prostate cancer (DU-145) by MTT reduction assay method, and etoposide acts as a standard drug. The results confirmed that majority of the synthesized compounds showed moderate to potent anticancer activities aligned with four cell lines. Among the synthesized compounds, 8b, 8c, 8d, 8e, 8g and 8i displayed more potent activity along with inhibitory concentration values ranging from 0.10 ± 0.084 to 11.5 ± 6.49 µM than the standard IC50 values, which ranges from 1.91 ± 0.84 to 3.08 ± 0.135 µM, respectively.
Electronic supplementary material
The online version of this article (10.1007/s13369-020-04626-z) contains supplementary material, which is available to authorized users.
Keywords: Letrozole; 3,5-Bis(pyridin-3-yl)-1,2,4-thiadiazole; 1,2,4-Triazole; 1,2,4-Thiadiazole; Anticancer activity
Introduction
Cancer is very dangerous disease with uncontrolled growth and rapid spreading of abnormal cells [1]. Several external and internal factors are caused to abnormal growth of cell lines and induced the different cancers [2–7]. Currently, three types of treatment are available for cancer disease including chemotherapy, radiotherapy, and surgery [8]. The standard treatment for cancer patients is chemotherapy, in which different chemotherapeutic agents are used to kill the cancer cells without any harmful effective on normal kidney cells [9–13].
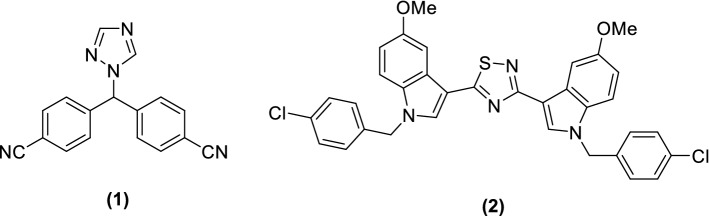
Nitrogen atoms contain heterocyclic ring moieties that are present both in natural products and in synthetic derivatives and exhibited potent anticancer activities against different human cancer cell lines [14–32]. Three nitrogen atoms containing heterocyclic ring such as 1,2,4-triazoles play an important critical role in the structural elucidation of various natural products [33] and are able to form hydrogen bonding with suitable targets leading to improving of pharmacokinetics, pharmacological, and toxicological properties [34, 35]. These 1,2,4-triazole derivatives are associated with different pharmaceutical activities such as anticancer [36], antibacterial [37], antitubercular [38], antifungal [39], antiviral [40], analgesic [41], anti-inflammatory [42], and tubulin inhibitors [43]. Letrozole (1, Fig. 1) [44, 45] is a triazole structural unit containing aromatase inhibitor and is used for cancer treatment.
Fig. 1.
Structures of letrozole (1) and 3,5-bis(pyridine-3-yl)-1,2,4-thiadiazole (2)
Similarly, 1,2,4-thiadiazoles are considered as most significant subclass of bioactive five-membered organic compounds for medicinal chemistry [46] and showed a remarkable biological activities such as cyclooxygenase inhibitors [47], human leukemia [48], antibacterial [49], antiulcerative [50], antihypertensive [51], cathepsin B inhibitors [52], anticonvulsant [53], antidiabetic [54], anti-inflammatory [47], and allosteric modulators [55]. One of the anticancer drug scaffolds like 3,5-bis(pyridin-3-yl)-1,2,4-thiadiazole (2) is inhibitor of aromatase and used for treatment of various types of cancers [56, 57].
Previously, we reported the synthesis of a library of novel 2-(4-arylsubstituted-1H-1,2,3-triazol-1-yl)-N-{4-[2-(thiazol-2-yl)benzo[d]thiazol-6-yl]phenyl}acetamide derivatives and screened their anticancer activities against MCF-7, A549, Colo-205, and A2780 cell lines with etoposide as standard drug. The anticancer target compounds reported by us and the literature reveal hazardous solvent usage, harsh reaction conditions, and longer reaction sequences. To overcome the above drawbacks and inspired by special features of both 1,2,4-triazole and 1,2,4-oxadiaxole, we have to design and synthesize new amide functionality bearing 1,2,4-thiadiazole-1,2,4-triazole derivatives (8a–j). These derivatives were examined for their anticancer activities against four different human cancer cell lines like breast cancer (MCF-7, MDA MB-231), lung cancer (A549), and prostate cancer (DU-145). These derivatives may act as drug lead molecules in cancer chemotherapy.
Experimental
General Conditions
All the solvents, salts, reagents, and fine chemicals were purchased from Sigma-Aldrich and Alfa Aesar companies. These chemical items were used without further purification. 1H and 13C NMR spectra were recorded with 400 MHz and 300 MHz frequency Gemini Varian-VXR-unity instruments. Chemical shifts (δ) were noted in ppm toward downfield with respect to tetramethylsilane as internal standard. ESI spectra were recorded at 3.98 kV capillary voltages with micro-mass, Quattro LC instrument using ESI + software. Melting points were noted with the help of electrothermal melting point apparatus.
Synthesis
5-(3,4,5-Trimethoxyphenyl)-3-p-Tolyl-1H-1,2,4-Triazole (5)
To a dried 250-mL round-bottom flask were added 4-methylbenzonitrile (4) (4.7 mL, 0.039 mol), 3,4,5-trimethoxy benzamidine (3) (14 g, 0.066 mol), Cs2CO3 (43 g, 0.316 mol), CuBr (312 mg, 0.00217 mol, 5 mol%), and DMSO (80 mL). The reaction mixture was stirred under atmospheric air at 120 °C for 24 h. After cooling to atmospheric temperature, the reaction mixture was extracted with ethyl acetate solvent (3 × 15 = 45 mL) and successively washed with 5% aqueous NaHCO3 (3 × 10 = 30 mL) and brine (10 mL). The organic layer was dried on MgSO4 and concentrated under reduced pressure. The crude residue was purified with silica gel through column chromatography by using 2:8 ratio ethyl acetate/hexane solvent mixture to afford pure yellow color compound 5 as 13.8 g with 64% yield. Mp: 166–168 °C. 1HNMR (400 MHz, DMSO-d6): δ 2.43 (s, 3H, –CH3), 3.72 (s, 3H, –OCH3), 3.89 (s, 6H, 2-OCH3), 7.73 (d, 2H, J = 8.2 Hz), 7.89 (s, 2H), 8.29 (d, 2H, J = 8.2 Hz), 10.32 (brs, 1H, –NH); 13C NMR (75 MHz, DMSO-d6): δ 22.8, 56.2, 60.9, 107.3, 124.5, 126.9, 128.6, 133.3, 141.6, 141.9, 155.8, 162.4, 169.1; MS (ESI): m/z 326 [M + H]+.
5-(3,4,5-Trimethoxyphenyl)-3-(4-(3-(3,4,5-Trimethoxyphenyl)-1,2,4-Thiadiazol-5-yl)Phenyl)-1H-1,2,4-Triazole (6)
A mixture of 5-(3,4,5-trimethoxyphenyl)-3-p-tolyl-1H-1,2,4-triazole (5) (12 g, 0.037 mol), 3,4,5-trimethoxy benzamidine (3) (15.5 g, 0.0738 mol), sulfur S (5.9 g, 0.185 mol), potassium phosphate tribasic trihydrate (29.5 g, 0.111 mol), and DMSO (100 mL) was discharged in 200 mL of dried round-bottom flask under air. The reaction vessel was stirred at 130 °C for 12 h. After cooling to 27 °C, the solvent was removed under reduced pressure. The crude residue was purified on silica gel through column chromatography with 3:7 ratio of ethyl acetate/hexane solvent mixture to afford the desired yellow color product 6 as 14.6 g with 71% yield. Mp: 196–198 °C; 1HNMR (400 MHz, DMSO-d6): δ 3.72 (s, 3H, –OCH3), 3.79 (s, 6H, 2-OCH3), 3.89 (s, 6H, 2-OCH3), 3.92 (s, 3H, –OCH3), 7.78 (s, 2H), 7.89 (d, 2H, J = 8.4 Hz), 8.10 (s, 2H), 8.49 (d, 2H, J = 8.4 Hz), 10.34 (brs, 1H, –NH); 13C NMR (75 MHz, DMSO-d6): δ 56.3, 56.6, 60.8, 106.6, 108.7, 124.2, 124.6, 128.9, 130.1, 133.6, 136.5, 142.8, 144.1, 154.2, 155.1, 162.4, 168.9, 171.4, 184.2; MS (ESI): m/z 562 [M + H]+.
(5-(3,4,5-Trimethoxyphenyl)-3-(4-(3-(3,4,5-Trimethoxyphenyl)-1,2,4-Thiadiazol-5-yl)Phenyl)-1H-1,2,4-Triazol-1-yl)(Phenyl)Methanone (8a)
The compound 5-(3,4,5-trimethoxyphenyl)-3-(4-(3-(3,4,5-trimethoxyphenyl)-1,2,4-thiadiazol-5-yl)phenyl)-1H-1,2,4-triazole (6) (500 mg, 0.891 mol) was dissolved in 40 mL of dry acetonitrile, followed by addition of benzoyl chloride (7a) (0.1 mL, 0.891 mmol) and Cs2CO3 (580 mg, 1.78 mol). The reaction mixture allowed for stirring at room temperature over a time period of 12 h. After completion of reaction, the reaction mass was washed with 3 mL water and diluted with dichloromethane (3 × 3 = 9 mL). It was dried on anhydrous Na2SO4. The crude residue was purified with silica gel through column chromatography by using 1:1 ratio ethyl acetate/hexane solvent mixture and then afforded pure yellow color compound 8a in 310.8 mg, 53% yield. Mp: 178–180 °C, 1H NMR (300 MHz, DMSO-d6): δ 3.72 (s, 3H, –OCH3), 3.79 (s, 6H, 2-OCH3), 3.89 (s, 6H, 2-OCH3), 3.92 (s, 3H, –OCH3), 7.58–7.62 (m, 1H), 7.67–7.73 (m, 2H), 7.79 (s, 2H), 7.93 (d, 2H, J = 8.5 Hz), 8.15 (d, 2H, J = 7.6 Hz), 8.48 (s, 2H), 8.66 (d, 2H, J = 8.5 Hz); 13C NMR (75 MHz, DMSO-d6): δ 57.6, 58.5, 61.4, 61.8, 106.7, 109.5, 116.4, 125.7, 129.3, 129.7, 132.2, 132.5, 134.3, 135.7, 136.3, 137.6, 142.4, 144.5, 153.4, 154.6, 155.4, 164.5, 168.7, 169.4, 176.7; MS (ESI): m/z 666 [M + H]+.
(3,4,5-Trimethoxyphenyl)(5-(3,4,5-Trimethoxyphenyl)-3-(4-(3-(3,4,5-Trimethoxyphenyl)-1,2,4-Thiadiazol-5-yl)Phenyl)-1H-1,2,4-Triazol-1-yl)Methanone (8b)
This compound 8b was synthesized by the same method involved in the synthesis of 8a, employing 6 (500 mg, 0.891 mol) with 3,4,5-trimethoxybenzoyl chloride (7b) (206 mg, 0.891 mmol), Cs2CO3 (580 mg, 1.78 mol), and the crude residue was purified with silica gel through column chromatography by using 1:1 ratio ethyl acetate/hexane solvent mixture and then afforded pure yellow color compound 8b, 338.4 mg in 50% yield. Mp: 200–202 °C, 1H NMR (300 MHz, DMSO-d6): δ 3.65 (s, 3H, –OCH3), 3.72 (s, 3H, –OCH3), 3.80 (s, 6H, 2-OCH3), 3.89 (s, 6H, 2-OCH3), 3.92 (s, 3H, –OCH3), 3.95 (s, 6H, 2-OCH3), 7.68 (s, 2H), 7.79 (s, 2H), 7.92 (d, 2H, J = 8.4 Hz), 8.47 (s, 2H), 8.67 (d, 2H, J = 8.4 Hz); 13C NMR (75 MHz, DMSO-d6): δ 57.4, 57.8, 58.5, 61.4, 61.7, 62.4, 106.7, 107.8, 109.3, 116.5, 125.6, 131.5, 132.4, 133.7, 134.5, 137.2, 142.9, 144.7, 145.8, 153.6, 154.3, 155.8, 157.9, 162.5, 168.5, 169.4, 176.8; MS (ESI): m/z 756 [M + H]+.
(3,5-Dimethoxyphenyl)(5-(3,4,5-Trimethoxyphenyl)-3-(4-(3-(3,4,5-Trimethoxyphenyl)-1,2,4-Thiadiazol-5-yl)Phenyl)-1H-1,2,4-Triazol-1-yl)Methanone (8c)
This compound 8c was synthesized by the same method involved in the synthesis of 8a, employing 6 (500 mg, 0.891 mol) with 3,5-dimethoxybenzoyl chloride (7c) (179 mg, 0.891 mol), Cs2CO3 (580 mg, 1.78 mol), and the crude residue was purified with silica gel through column chromatography by using 1:1 ratio ethyl acetate/hexane solvent mixture and then afforded pure yellow color compound 8c, 320.6 mg in 50% yield. Mp: 190–192 °C, 1H NMR (300 MHz, DMSO-d6): δ 3.67 (s, 3H, –OCH3), 3.72 (s, 3H, –OCH3), 3.80 (s, 6H, 2-OCH3), 3.89 (s, 6H, 2-OCH3), 3.92 (s, 3H, –OCH3), 7.24 (s, 1H), 7.34 (s, 2H), 7.79 (s, 2H), 7.93 (d, 2H, J = 8.5 Hz), 8.47 (s, 2H), 8.66 (d, 2H, J = 8.5 Hz); 13C NMR (75 MHz, DMSO-d6): δ 56.7, 57.8, 58.5, 61.5, 62.4, 106.5, 108.2, 109.7, 116.4, 120.5, 125.5, 132.4, 133.6, 133.9, 134.6, 137.4, 142.3, 144.8, 153.2, 154.6, 155.8, 162.3, 166.8, 168.2, 169.6, 176.8; MS (ESI): m/z 726 [M + H]+.
(4-Methoxyphenyl)(5-(3,4,5-Trimethoxyphenyl)-3-(4-(3-(3,4,5-Trimethoxyphenyl)-1,2,4-Thiadiazol-5-yl)Phenyl)-1H-1,2,4-Triazol-1-yl)Methanone (8d)
This compound 8d was prepared following the method described for the preparation of the compound 8a, employing 6 (500 mg, 0.891 mol) with 4-methoxybenzoyl chloride (7d) (0.12 mL, 0.891 mol), Cs2CO3 (580 mg, 1.78 mol), and the crude residue was purified with silica gel through column chromatography by using 1:1 ratio ethyl acetate/hexane solvent mixture and then afforded afford pure yellow color compound 8d, 312.8 mg in 51% yield. Mp: 195–197 °C, 1H NMR (300 MHz, DMSO-d6): δ 3.72 (s, 3H, –OCH3), 3.76 (s, 3H, –OCH3), 3.80 (s, 6H, 2-OCH3), 3.89 (s, 6H, 2-OCH3), 3.92 (s, 3H, –OCH3), 7.79 (s, 2H), 7.91–8.07 (m, 4H), 8.17 (d, 2H, J = 7.9 Hz), 8.47 (s, 2H), 8.67 (d, 2H, J = 8.6 Hz); 13C NMR (75 MHz, DMSO-d6): δ 56.7, 57.6, 58.7, 61.4, 62.5, 106.5, 109.2, 114.7, 116.8, 125.4, 130.2, 131.4, 132.6, 133.8, 134.6, 137.4, 142.3, 144.6, 153.7, 154.5, 155.8, 164.2, 166.8, 168.4, 169.7, 176.7; MS (ESI): m/z 696 [M + H]+.
(5-(3,4,5-Trimethoxyphenyl)-3-(4-(3-(3,4,5-Trimethoxyphenyl)-1,2,4-Thiadiazol-5-yl)Phenyl)-1H-1,2,4-Triazol-1-yl)(4-Nitrophenyl)Methanone (8e)
This compound 8e was synthesized by the same method involved in the synthesis of 8a, employing 6 (500 mg, 0.891 mol) with 4-nitrobenzoyl chloride (7e) (165 mg, 0.891 mol), Cs2CO3 (580 mg, 1.78 mol), and the crude residue was purified with silica gel through column chromatography by using 1:1 ratio ethyl acetate/hexane solvent mixture and then afforded pure yellow color compound 8e, 385.4 mg in 61% yield. Mp: 230–232 °C, 1H NMR (300 MHz, DMSO-d6): δ 3.72 (s, 3H, –OCH3), 3.83 (s, 6H, 2-OCH3), 3.89 (s, 6H, 2-OCH3), 3.93 (s, 3H, –OCH3), 7.80 (s, 2H), 7.94 (d, 2H, J = 8.7 Hz), 8.30 (d, 2H, J = 8.1 Hz), 8.40 (d, 2H, J = 8.1 Hz), 8.49 (s, 2H), 8.68 (d, 2H, J = 8.7 Hz); 13C NMR (75 MHz, DMSO-d6): δ 57.6, 58.7, 61.4, 62.7, 106.4, 109.7, 116.8, 125.3, 126.5, 131.2, 132.6, 133.5, 134.8, 137.6, 141.3, 142.6, 44.5, 153.4, 154.6, 154.9, 155.6, 164.5, 168.4, 169.7, 176.8; MS (ESI): m/z 711 [M + H]+.
(5-(3,4,5-Trimethoxyphenyl)-3-(4-(3-(3,4,5-Trimethoxyphenyl)-1,2,4-Thiadiazol-5-yl)Phenyl)-1H-1,2,4-Triazol-1-yl)(3,5-Dinitrophenyl)Methanone (8f)
This compound 8f was synthesized by the same method involved in the synthesis of 8a, employing 6 (500 mg, 0.891 mol) with 3,5-dinitrobenzoyl chloride (7f) (205 mg, 0.891 mol), Cs2CO3 (580 mg, 1.78 mol), and the crude residue was purified with silica gel through column chromatography by using 1:1 ratio ethyl acetate/hexane solvent mixture and then afforded pure yellow color compound 8f, 410.5 mg in 61% yield. Mp: 254–256 °C, 1H NMR (300 MHz, DMSO-d6): δ 3.73 (s, 3H, –OCH3), 3.84 (s, 6H, 2-OCH3), 3.89 (s, 6H, 2-OCH3), 3.95 (s, 3H, –OCH3), 7.80 (s, 2H), 7.94 (d, 2H, J = 8.8 Hz), 8.52 (s, 2H), 8.68 (d, 2H, J = 8.8 Hz), 8.92 (s, 2H), 9.14 (s, 1H); 13C NMR (75 MHz, DMSO-d6): δ 57.6, 58.4, 61.5, 62.7, 106.4, 109.7, 116.6, 125.4, 126.7, 128.5, 132.4, 133.6, 134.5, 135.7, 137.4, 142.3, 144.5, 148.6, 153.4, 154.6, 155.7, 157.6, 168.4, 169.7, 176.8; MS (ESI): m/z 756 [M + H]+.
(4-Chlorophenyl)(5-(3,4,5-Trimethoxyphenyl)-3-(4-(3-(3,4,5-Trimethoxyphenyl)-1,2,4-Thiadiazol-5-yl)Phenyl)-1H-1,2,4-Triazol-1-yl)Methanone (8g)
This compound 8g was synthesized by the same method involved in the synthesis of 8a, employing 6 (500 mg, 0.891 mol) with 4-chlorobenzoyl chloride (7 g) (0.11 mL, 0.891 mol), Cs2CO3 (580 mg, 1.78 mol), and the crude residue was purified with silica gel through column chromatography by using 1:1 ratio ethyl acetate/hexane solvent mixture and then afforded pure yellow color compound 8g, 348.7 mg in 56% yield. Mp: 233–235 °C, 1H NMR (300 MHz, DMSO-d6): δ 3.73 (s, 3H, –OCH3), 3.82 (s, 6H, 2-OCH3), 3.89 (s, 6H, 2-OCH3), 3.93 (s, 3H, –OCH3), 7.73 (d, 2H, J = 8.02 Hz), 7.80 (s, 2H), 7.94 (d, 2H, J = 8.7 Hz), 8.19 (d, 2H, J = 8.02 Hz), 8.50 (s, 2H), 8.68 (d, 2H, J = 8.7 Hz); 13C NMR (75 MHz, DMSO-d6): δ 57.6, 58.7, 61.5, 62.8, 106.5, 109.8, 116.5, 125.4, 130.5, 132.5, 133.2, 134.7, 135.2, 135.7, 137.5, 142.3, 142.6, 144.5, 153.4, 154.6, 155.8, 164.3, 168.3, 169.7, 176.8; MS (ESI): m/z 700 [M + H]+.
(4-Bromophenyl)(5-(3,4,5-Trimethoxyphenyl)-3-(4-(3-(3,4,5-Trimethoxyphenyl)-1,2,4-Thiadiazol-5-yl)Phenyl)-1H-1,2,4-Triazol-1-yl)Methanone (8h)
This compound 8h was synthesized by the same method involved in the synthesis of 8a, employing 6 (500 mg, 0.891 mol) with 4-bromobenzoyl chloride (7h) (196 mg, 0.891 mol), Cs2CO3 (580 mg, 1.78 mol), and the crude residue was purified with silica gel through column chromatography by using 1:1 ratio ethyl acetate/hexane solvent mixture and then afforded pure yellow color compound 8h, 336.2 mg in 51% yield. Mp: 241–243 °C, 1H NMR (300 MHz, DMSO-d6): δ 3.73 (s, 3H, –OCH3), 3.82 (s, 6H, 2-OCH3), 3.89 (s, 6H, 2-OCH3), 3.93 (s, 3H, –OCH3), 7.78 (d, 2H, J = 8.00 Hz), 7.80 (s, 2H), 7.94 (d, 2H, J = 8.6 Hz), 8.15 (d, 2H, J = 8.00 Hz), 8.51 (s, 2H), 8.68 (d, 2H, J = 8.6 Hz); 13C NMR (100 MHz, DMSO-d6): δ 57.5, 58.7, 61.4, 62.7, 106.8, 109.8, 116.4, 125.6, 126.3, 130.2, 132.4, 133.2, 134.5, 134.7, 135.6, 137.6, 142.4, 144.5, 153.2, 154.6, 155.8, 164.5, 168.4, 169.8, 177.1; MS (ESI): m/z 746 [M + H]+.
4-[(5-(3,4,5-Trimethoxyphenyl)-3-4-[3-(3,4,5-Trimethoxyphenyl)-1,2,4-Thiadiazol-5-yl]Phenyl-1H-1,2,4-Triazol-1-yl)Carbonyl]Benzonitrile (8i)
This compound 8i was synthesized by the same method involved in the synthesis of 8a, employing 6 (500 mg, 0.891 mol) with 4-cyanobenzoyl chloride (7i) (148 mg, 0.891 mol), Cs2CO3 (580 mg, 1.78 mol), and the crude residue was purified with silica gel through column chromatography by using 1:1 ratio ethyl acetate/hexane solvent mixture and then afforded pure yellow color compound 8i, 156.8 mg in 26% yield. Mp: 247–249 °C, 1H NMR (300 MHz, DMSO-d6): δ 3.73 (s, 3H, –OCH3), 3.82 (s, 6H, 2-OCH3), 3.89 (s, 6H, 2-OCH3), 3.93 (s, 3H, –OCH3), 7.81 (s, 2H), 7.94 (d, 2H, J = 8.6 Hz), 8.21 (d, 2H, J = 8.04 Hz), 8.39 (d, 2H, J = 8.04 Hz), 8.53 (s, 2H), 8.68 (d, 2H, J = 8.6 Hz); 13C NMR (75 MHz, DMSO-d6): δ 57.4, 58.6, 61.6, 62.7, 106.4, 109.7, 114.6, 116.7, 119.4, 125.4, 131.2, 132.5, 133.6, 134.2, 135.8, 137.3, 138.6, 142.5, 144.7, 153.4, 154.7, 155.6, 164.2, 168.6, 169.7, 177.3; MS (ESI): m/z 691 [M + H]+.
(5-(3,4,5-Trimethoxyphenyl)-3-(4-(3-(3,4,5-Trimethoxyphenyl)-1,2,4-Thiadiazol-5-yl)Phenyl)-1H-1,2,4-Triazol-1-yl)(p-Tolyl)Methanone (8j)
This compound 8j was synthesized by the same method involved in the synthesis of 8a, employing 6 (500 mg, 0.891 mol) with 4-methylbenzoyl chloride (7j) (0.8 mL, 0.891 mmol), Cs2CO3 (580 mg, 1.78 mmol), and the crude residue was purified with silica gel through column chromatography by using 1:1 ratio ethyl acetate/hexane solvent mixture and then afforded pure yellow color compound 8j, 362.4 mg in 60% yield. Mp: 186–188 °C, 1H NMR (300 MHz, DMSO-d6): δ 2.45 (s, 3H, –CH3), 3.73 (s, 3H, –OCH3), 3.81 (s, 6H, 2-OCH3), 3.89 (s, 6H, 2-OCH3), 3.92 (s, 3H, –OCH3), 7.68 (d, 2H, J = 7.9 Hz), 7.81 (s, 2H), 7.94 (d, 2H, J = 8.6 Hz), 8.18 (d, 2H, J = 7.9 Hz), 8.49 (s, 2H), 8.68 (d, 2H, J = 8.6 Hz); 13C NMR (75 MHz, DMSO-d6): δ 24.8, 57.6, 58.3, 61.4, 62.6, 106.7, 109.5, 116.3, 125.7, 129.4, 130.2, 132.5, 133.6, 134.4, 135.3, 137.5, 142.4, 144.7, 146.6, 153.2, 154.6, 155.8, 164.5, 168.4, 169.7, 176.9; MS (ESI): m/z 680 [M + H]+.
MTT Assay
Individual wells microtiter plate from a 96-well tissue culture was inoculated with 100 µL of complete medium containing 1 × 104 cells. These microtiter plates were incubated at a temperature of 37 °C in 5% CO2-humidified incubator over a time period of 18 h prior to the experiment. After the removal of medium, a fresh medium of 100 µL containing both the test compounds and standard drug and etoposide at a variable concentrations of 0.5, 1, 2, and 4 µM was added to each well and incubated over 24-h time period at 37 °C temperature. Now, this medium was removed and replaced by 10 µL MTT assay dye. Again, the plates were allowed for incubation at a temperature of 37 °C over 2-h time period. The obtained formazan crystals were dissolved in 100 µL extraction buffer. The OD value was read with multimode Varioskan Instrument, Themo Scientific microplate reader at 570 nm. The % of DMSO-d6 in the medium should not exceed 0.25% at any time. Each of the data of the IC50 values were represented as mean of ± SD values that means each experiment was performed three times.
Results and Discussion
Chemistry
The synthesis of 1,2,4-thiadiazole-1,2,4-triazole derivatives bearing amide functionality (8a–j) is shown in Scheme 1. Starting material 3,4,5-trimethoxybenzamidine (3) undergoes cyclization reaction with 4-methylbenzonitrile (4) in the presence of CuBr catalyst, Cs2CO3 base in DMSO solvent at 120 °C temperature over 24 h to afford triazole intermediate 5. The ESI–MS peak at m/z 326 [M + H]+ confirmed the structure of compound 5. The triazole compound 5 reacted with 3,4,5-trimethoxybenzamidine (3) in the presence of potassium phosphate tribasic trihydrate (K3PO4·3H2O) base, and sulfur in DMSO solvent was heated at 130 °C for 12 h to afford pure 1,2,4-thiadiazole intermediate 6. The ESI–MS peak at m/z 562 [M + H]+ confirmed the structure of compound 6. Then, this intermediate 5 was coupled with substituted aromatic acid chlorides (7a–j) in the presence of Cs2CO3 base in anhydrous acetonitrile solvent at room temperature for 12 h to afford the 1,2,4-thiadiazole-1,2,4-triazole derivatives 8a–j. The ESI–MS peak at m/z 666 [M + H]+ confirmed the structure of compound 8a.
Scheme 1.
Synthesis of amide functionality bearing 1,2,4-thiadiazole-1,2,4-triazole derivatives
The new library of 1,2,4-thiadiazole-1,2,4-triazole derivatives having amide functionality (8a–j).
Biological Evaluation
In Vitro Cytotoxicity
The new library of 1,2,4-thiadiazole-1,2,4-triazole derivatives having amide functionality (8a–j), was examined for their anticancer activity toward a pane of four different human cancer cell lines such as breast cancer (MCF-7, MDA MB-231), lung cancer (A549), and prostate cancer (DU-145) by MTT assay and compared with the standard reference etoposide. The obtained results were presented as IC50 (µM) values in Table 1. The results indicated that most of the synthesized compounds exhibited moderate to excellent anticancer activity aligned with four cell lines. Among the library of examined compounds, compounds 8b, 8c, 8d, 8e, 8g, and 8i displayed more potent activity with IC50 values ranging from 0.10 ± 0.084 to 11.5 ± 6.49 µM and standard showed IC50 value range as 1.91 ± 0.84 µM to 3.08 ± 0.135 µM. Further, all these compounds were investigated for structure–activity relationship (SARs) studies. Compound 8b with electron-donating group (3,4,5-trimethoxy) showed highest anticancer activity toward MCF-7, A549, DU-145, and MDA MB-231 with IC50 values of 0.10 ± 0.084 µM, 0.17 ± 0.032 µM, 0.83 ± 0.091 µM, and 0.28 ± 0.017 µM, respectively, where slight decrease in activity was observed for 8c and 8d with IC50 values of MCF-7 = 1.12 ± 0.64 µM; A549 = 1.79 ± 0.59 µM; DU145 = 1.98 ± 0.22 µM, MDA MB-231 = 2.33 ± 1.52 µM, and MCF-7 = 1.44 ± 0.17 µM; A549 = 2.10 ± 1.44 µM; DU145 = 2.76 ± 1.88 µM, MDA MB-231 = 2.35 ± 1.51 µM, when compared with 8b compound. The replacement of 4-methoxy group with 4-nitro (8e) showed improved anticancer activity against four cell lines (MCF-7 = 0.23 ± 0.014 µM; A549 = 1.64 ± 0.53 µM; DU145 = 0.19 ± 0.011 µM, MDA MB-231 = 1.55 ± 0.63 µM) compared with 8d. Replacement of 4-nitro substituent with 4-chloro and then the compound 8g showed acceptable activity (MCF-7 = 1.02 ± 0.65 µM; A549 = 1.69 ± 0.13 µM; DU145 = 2.13 ± 1.98 µM, MDA MB-231 = 2.15 ± 1.08 µM). When 3,5-dinitro group was introduced, 4-bromo substituents on the phenyl ring resulted compounds, namely 8f and 8h, were displayed very poor activity on all cell lines. Interestingly, compound 8i with 4-cyano electron-withdrawing group showed better anticancer activity (MCF-7 = 1.27 ± 0.92 µM; A549 = 1.90 ± 0.46 µM; DU145 = 0.60 ± 0.014 µM, MDA MB-231 = 1.59 ± 0.37 µM) than 8g. Compound 8j with weak electron-donating group on the phenyl ring demonstrated moderate activity.
Table 1.
In vitro cytotoxicity of newly target compounds 8a–j with IC50 in µM
| Compound | MCF-7 | A549 | DU-145 | MDA MB-231 |
|---|---|---|---|---|
| 8a | 3.57 ± 2.81 | 2.98 ± 1.76 | ND | 4.11 ± 2.30 |
| 8b | 0.10 ± 0.084 | 0.17 ± 0.032 | 0.83 ± 0.091 | 0.28 ± 0.017 |
| 8c | 1.12 ± 0.64 | 1.79 ± 0.59 | 1.98 ± 0.22 | 2.33 ± 1.52 |
| 8d | 1.44 ± 0.17 | 2.10 ± 1.44 | 2.76 ± 1.88 | 2.35 ± 1.51 |
| 8e | 0.23 ± 0.014 | 1.64 ± 0.53 | 0.19 ± 0.011 | 1.55 ± 0.63 |
| 8f | 5.66 ± 2.38 | ND | 7.23 ± 4.52 | ND |
| 8g | 1.02 ± 0.65 | 1.69 ± 0.13 | 2.13 ± 1.98 | 2.15 ± 1.08 |
| 8h | 7.28 ± 3.67 | ND | 8.22 ± 4.33 | 10.7 ± 5.26 |
| 8i | 1.27 ± 0.92 | 1.90 ± 0.46 | 0.60 ± 0.014 | 1.59 ± 0.37 |
| 8j | 5.94 ± 3.26 | 11.5 ± 6.49 | 9.37 ± 6.21 | ND |
| Etoposide | 2.11 ± 0.024 | 3.08 ± 0.135 | 1.97 ± 0.45 | 1.91 ± 0.84 |
ND Not determined
MCF-7: human breast cancer cell line. A549: human lung cancer cell line. DU-145: human prostate cancer cell line. MDA MB-231: human breast cancer cell line
From the structure–activity relationship studies, it can be concluded that the presence of three electron-donating –OCH3 group at 3,4,5 positions on phenyl ring displayed excellent potent anticancer activities against four specified cancer cell lines. The decrease in anticancer activity would be observed with two –OCH3 groups at 3,5 positions and one –OCH3 group at 4th position. The presence of strong-withdrawing group –NO2 at 3, 5 positions on phenyl ring displayed very less anticancer activity against specified cancer cell lines, when compared to one –NO2 group at 4th position. In this series, cytotoxicity effect decreases from the electron-donating group to electron-withdrawing group derivatives.
Conclusion
The new library of 1,2,4-thiadiazole-1,2,4-triazole derivatives having amide functionality (8a–j) was designed, synthesized, and examined for their anticancer activities against four different human cancer cell lines including breast cancer (MCF-7, MDA MB-231), lung cancer (A549), and prostate cancer (DU-145) by making use of MTT assay. Here, Etoposide acts as standard drug, and the obtained results were presented as IC50 (µM) values. The results indicated that most of the synthesized compounds exhibited moderate to excellent anticancer activity aligned with four cell lines. Among them, compounds 8b, 8c, 8d, 8e, 8g, and 8i displayed more potent activity with IC50 values ranging from 0.10 ± 0.084 to 11.5 ± 6.49 µM and standard showed IC50 value ranges from 1.91 ± 0.84 µM to 3.08 ± 0.135 µM. These derivatives may act as drug lead molecules in cancer chemotherapy.
Electronic supplementary material
Below is the link to the electronic supplementary material.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J. Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Park SK, Cho LY, Yang JJ, Park B, Chang SH, Lee KS, Kim H, Yoo KY, Lee CT. Lung cancer risk and cigarette smoking, lung tuberculosis according to histologic type and gender in a population based case-control study. Lung Cancer. 2010;68:20–26. doi: 10.1016/j.lungcan.2009.05.017. [DOI] [PubMed] [Google Scholar]
- 3.Meffert MK, Chang JM, Wiltgen BJ, Fanselow MS, Baltimore D. NF-kappa B functions in synaptic signalling and behaviour. Nat. Neurosci. 2003;6:1072–1078. doi: 10.1038/nn1110. [DOI] [PubMed] [Google Scholar]
- 4.Clemens MR. Free radicals in chemical carcinogenesis. Klin. Wochenschr. 1991;69:1123–1134. doi: 10.1007/BF01645172. [DOI] [PubMed] [Google Scholar]
- 5.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 6.Clayton PE, Banerjee I, Murray PG, Renehan AG. Growth hormone, the insulin-like growth factor axis, insulin and cancer risk. Nat. Rev. Endocrinol. 2011;7:11–24. doi: 10.1038/nrendo.2010.171. [DOI] [PubMed] [Google Scholar]
- 7.Porta C, Riboldi E, Sica A. Mechanisms linking pathogens-associated inflammation and cancer. Cancer Lett. 2011;305:250–262. doi: 10.1016/j.canlet.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 8.Khan FA, Akhtar SS, Sheikh MK. Cancer treatment—objectives and quality of life issues. Malays. J. Med. Sci. 2005;12:3–5. [PMC free article] [PubMed] [Google Scholar]
- 9.Menta E, Palumbo M. Novel antineoplastic agents. Exp. Opin. Ther. Patents. 1997;7:1401–1426. doi: 10.1517/13543776.7.12.1401. [DOI] [Google Scholar]
- 10.Nussbaumer S, Bonnabry P, Veuthey J-L, Fleury-Souverain S. Analysis of anticancer drugs: a review. Talanta. 2011;85:2265–2289. doi: 10.1016/j.talanta.2011.08.034. [DOI] [PubMed] [Google Scholar]
- 11.Rebucci M, Michiels C. Molecular aspects of cancer cell resistance to chemotherapy. Biochem. Pharmacol. 2013;85:1219–1226. doi: 10.1016/j.bcp.2013.02.017. [DOI] [PubMed] [Google Scholar]
- 12.Holohan C, Van Schaeybroeck S, Longley DB, Johnston PG. Cancer drug resistance: an evolving paradigm. Nat. Rev. Cancer. 2013;13:714–726. doi: 10.1038/nrc3599. [DOI] [PubMed] [Google Scholar]
- 13.Rosa R, Monteleone F, Zambrano N, Bianco R. In vitro and in vivo models for analysis of resistance to anticancer molecular molecular therapies. Curr. Med. Chem. 2014;21:1595–1606. doi: 10.2174/09298673113209990226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reddy NB, Burra VR, Ravindranath LK, Sreenivasulu R, Kumar VN. Synthesis and biological evaluation of benzoxazole fused combretastatin derivatives as anticancer agents. Monatsh. Chem. 2016;147:593–598. doi: 10.1007/s00706-016-1685-y. [DOI] [Google Scholar]
- 15.Reddy NB, Burra VR, Ravindranath LK, Kumar VN, Sreenivasulu R, Sadanandam P. Synthesis and biological evaluation of benzimidazole fused ellipticine derivatives as anticancer agents. Monatsh. Chem. 2016;147:599–604. doi: 10.1007/s00706-016-1684-z. [DOI] [Google Scholar]
- 16.Hatti I, Sreenivasulu R, Jadav SS, Jayaprakash V, Kumar CG, Raju RR. Synthesis, cytotoxic activity and docking studies of new 4-aza podophyllotoxin derivatives. Med. Chem. Res. 2015;24:3305–3313. doi: 10.1007/s00044-015-1375-z. [DOI] [Google Scholar]
- 17.Sreenivasulu R, Reddy KT, Sujitha P, Ganesh C, Raju RR. Synthesis, antiproliferative and apoptosis induction potential activities of novel bis(indolyl)hydrazide-hydrazone derivatives. Bioorg. Med. Chem. 2019;27:1043–1055. doi: 10.1016/j.bmc.2019.02.002. [DOI] [PubMed] [Google Scholar]
- 18.Agarwal M, Singh V, Sharma SC, Sharma P, Ansari MdY, Jadav SS, Yasmin S, Sreenivasulu R, Hassan MdH. Design and synthesis of new 2,5-disubstituted 1,3,4-oxadiazole analogues as anticancer agents. Med. Chem. Res. 2016;25:2289–2303. doi: 10.1007/s00044-016-1672-1. [DOI] [Google Scholar]
- 19.Spandana Z, Sreenivasulu R, Rekha TM, Rao MVB. Novel 1,3,4-oxadiazole fused thiadiazole derivatives: synthesis and study of anticancer activities. Lett. Drug Des. Discov. 2019;16:656–662. doi: 10.2174/1570180816666181031125946. [DOI] [Google Scholar]
- 20.Madhavi S, Sreenivasulu R, Raju RR. Synthesis and biological evaluation of oxadiazole incorporated ellipticine derivatives as anticancer agents. Monatsh. Chem. 2017;148:933–938. doi: 10.1007/s00706-016-1790-y. [DOI] [Google Scholar]
- 21.Sreenivasulu R, Durgesh R, Jadav SS, Sujitha P, Kumar CG, Raju RR. Synthesis, anticancer evaluation and molecular docking studies of bis(indolyl)triazinones, nortopsentin analogues. Chem. Pap. 2018;72:1369–1378. doi: 10.1007/s11696-017-0372-8. [DOI] [Google Scholar]
- 22.Subramanyam M, Sreenivasulu R, Rambabu G, Rao MVB, Rao KP. Synthesis, biological evaluation and docking studies of 1,3,4-oxadiazole fused benzothiazole derivatives for anticancer drugs. Lett. Drug Des. Discov. 2018;15:1299–1307. doi: 10.2174/1570180815666180219165119. [DOI] [Google Scholar]
- 23.Ahsan MJ, Choudhary K, Jadav SS, Yasmin S, Ansari MY, Sreenivasulu R. Synthesis, antiproliferative activity and molecular docking studies of curcumin analogues bearing pyrazole ring. Med. Chem. Res. 2015;24:4166–4180. doi: 10.1007/s00044-015-1457-y. [DOI] [Google Scholar]
- 24.Madhavi S, Sreenivasulu R, Jyotsna Y, Raju RR. Synthesis of chalcone incorporated quinazoline derivatives as anticancer agents. Saudi Pharm. J. 2017;25:275–279. doi: 10.1016/j.jsps.2016.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hatti I, Sreenivasulu R, Jadav SS, Ahsan MJ, Raju RR. Synthesis and biological evaluation of 1,3,4-oxadiazole linked bis indole derivatives as anticancer agents. Monatsh. Chem. 2015;146:1699–1705. doi: 10.1007/s00706-015-1448-1. [DOI] [Google Scholar]
- 26.Durgesh R, Sreenivasulu R, Pinapati SR, Raju RR. Synthesis and anticancer evaluation of indazole-aryl hydrazide-hydrazone derivatives. J. Ind. Chem. Soc. 2018;95:433–438. [Google Scholar]
- 27.Spandana Z, Sreenivasulu R, Rao MVB. Design, synthesis and anticancer evaluation of carbazole fused aminopyrimidine derivatives. Lett. Org. Chem. 2019;16:662–667. doi: 10.2174/1570178616666181211094526. [DOI] [Google Scholar]
- 28.Madhavi S, Sreenivasulu R, Ansari MdY, Ahsan MJ, Raju RR. Synthesis, biological evaluation and molecular docking studies of pyridine incorporated chalcone derivatives as anticancer agents. Lett. Org. Chem. 2016;13:682–692. doi: 10.2174/1570178613666161021105317. [DOI] [Google Scholar]
- 29.Sreenivasulu R, Sujitha P, Jadav SS, Ahsan MJ, Kumar CG, Raju RR. Synthesis, antitumor evaluation and molecular docking studies of indole–indazolyl hydrazide–hydrazone derivatives. Monatsh. Chem. 2017;148:305–314. doi: 10.1007/s00706-016-1750-6. [DOI] [Google Scholar]
- 30.Reddy KT, Sreenivasulu R, Raju RR. Synthesis and biological evaluation of 1,2,4-oxadiazole linked imidazopyrazine derivatives as anticancer agents. J. Ind. Chem. Soc. 2019;96:1085–1090. [Google Scholar]
- 31.Murthy IS, Sreenivasulu R, Alluraiah G, Raju RR. Design, synthesis and anticancer evaluation of 1,2,3-triazole linked 1,2-isoxazole-imidazo[4,5-b]pyridine derivatives. Russ. J. Gen. Chem. 2019;89:1718–1723. doi: 10.1134/S1070363219080279. [DOI] [Google Scholar]
- 32.Kala P, Sharif SK, Krishna CHM, Ramachandran D. Design, synthesis and anticancer evaluation of 1,2,4-oxadiazole functionalized quinolone derivatives. Med. Chem. Res. 2019 doi: 10.1007/s00044-019-02467-6. [DOI] [Google Scholar]
- 33.Asami T, Min YK, Nagata N, Amagishi KY, Takatsuto S, Fujioka S, Murofushi N, Yamaguchi I, Yoshida S. Characterization of brassinazole, a triazole-type brassinosteroid biosynthesis inhibitor. Plant Physiol. 2000;123:93–100. doi: 10.1104/pp.123.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kaur P, Chawla A. Hepatoprotective activity of Inula cappa DC. Aqueous extract against carbon tetrachloride induced hepatotoxicity in Wistar rats. Int. Res. J. Pharm. 2017;8:14–19. doi: 10.7897/2230-8407.08013. [DOI] [Google Scholar]
- 35.Kapron B, Luszczki JJ, Plaziska A, Siwek A, Karcz T, Grybos A, Bowak G, Makuch-Kocka A, Walczak K, Langner E, Szalast K, Marciniak S, Paczkowska M, Cielecka J, Ciesla LM, Plech T. Development of the 1,2,4-triazole-based anticonvulsant drug candidates acting on the voltage-gated sodium channels. Insights from in vivo, in vitro, and in silico studies. Eur. J. Pharm. Sci. 2019;129:42–57. doi: 10.1016/j.ejps.2018.12.018. [DOI] [PubMed] [Google Scholar]
- 36.Holla BS, Poojary KN, Rao BS, Shivananda M. New bis-aminomercapto triazoles and bis-triazolothiadiazoles as possible anticancer agents. Eur. J. Med. Chem. 2002;37:511–517. doi: 10.1016/S0223-5234(02)01358-2. [DOI] [PubMed] [Google Scholar]
- 37.Eswaran S, Adhikari AV, Shetty NS. Synthesis and antimicrobial activities of novel quinoline derivatives carrying 1,2,4-triazole moiety. Eur. J. Med. Chem. 2009;44:4637–4647. doi: 10.1016/j.ejmech.2009.06.031. [DOI] [PubMed] [Google Scholar]
- 38.Zhang S, Xu Z, Gao C, Ren QC, Chang L, Lv ZS, Feng LS. Triazole derivatives and their anti-tubercular activity. Eur. J. Med. Chem. 2017;138:501–513. doi: 10.1016/j.ejmech.2017.06.051. [DOI] [PubMed] [Google Scholar]
- 39.Jin RY, Zeng CY, Liang XH, Sun XH, Liu YF, Wang YY, Zhou S. Design, synthesis, biological activities and DFT calculation of novel 1,2,4-triazole Schiff base derivatives. Bioorg. Chem. 2018;80:253–260. doi: 10.1016/j.bioorg.2018.06.030. [DOI] [PubMed] [Google Scholar]
- 40.Wittine K, Babic MS, Makuc D, Plavec J, Pavelic S, Sedic M, Pavelic K, Leyssen P, Neyts J, Balzarini J, Mintas M. Novel 1,2,4-triazole and imidazole derivatives of l-ascorbic and imino-ascorbic acid: synthesis, anti-HCV and antitumor activity evaluations. Bioorg. Med. Chem. 2012;20:3675–3685. doi: 10.1016/j.bmc.2012.01.054. [DOI] [PubMed] [Google Scholar]
- 41.Mathew V, Keshavayya J, Vaidya VP, Giles D. Studies on synthesis and pharmacological activities of 3,6-disubstituted-1,2,4-triazolo[3,4-b]-1,3,4-thiadiazoles and their dihydro analogues. Eur. J. Med. Chem. 2007;42:823–840. doi: 10.1016/j.ejmech.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 42.El Shehry MF, Abu-Hashem AA, El-Telbani EM. Synthesis of 3-((2,4-dichlorophenoxy)methyl)-1,2,4-triazolo(thiadiazoles and thiadiazines) as anti-inflammatory and molluscicidal agents. Eur. J. Med. Chem. 2010;45:1906–1911. doi: 10.1016/j.ejmech.2010.01.030. [DOI] [PubMed] [Google Scholar]
- 43.Zhang Q, Peng Y, Wang XI, Keenan SM, Arora S, Welsh WJ. Highly potent triazole-based tubulin polymerization inhibitors. J. Med. Chem. 2007;50:749–754. doi: 10.1021/jm061142s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maiti A, Reddy PVN, Sturdy M, Marler L, Pegan SD, Mesecar AD, Pezzuto JM, Cushman M. Synthesis of casimiroin and optimization of its quinone reductase 2 and aromatase inhibitory activities. J. Med. Chem. 2009;52:1873–1884. doi: 10.1021/jm801335z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stresser DM, Turner SD, McNamara J, Stocker P, Miller VP, Crespi CL, Patten CJ. A high-throughput screen to identify inhibitors of aromatase (CYP19) Anal. Biochem. 2000;284:427–430. doi: 10.1006/abio.2000.4729. [DOI] [PubMed] [Google Scholar]
- 46.Kumar D, Kumar N-M, Chang K-H, Shah K. Synthesis and anticancer activity of 5-(3-indolyl)-1,3,4-thiadiazoles. Eur. J. Med. Chem. 2010;45:4664–4668. doi: 10.1016/j.ejmech.2010.07.023. [DOI] [PubMed] [Google Scholar]
- 47.Unangst PC, Shrum GP, Connor DT, Dyer RD, Schrier DJ. Novel 1,2,4-oxadiazoles and 1,2,4-thiadiazoles as dual 5-lipoxygenase and cyclooxygenase inhibitors. J. Med. Chem. 1992;35:3691–3698. doi: 10.1021/jm00098a015. [DOI] [PubMed] [Google Scholar]
- 48.Romagnoli R, Baraldi PG, Carrion MD, Cruz-Lopez O, Preti D, Tabrizi MA, Fruttarolo F, Heilmann F, Bermejo J, Estevez F. Hybrid molecules containing benzo[4,5]imidazo[1,2-d][1,2,4]thiadiazole and α-bromoacryloyl moieties as potent apoptosis inducers on human myeloid leukaemia cells. Bioorg. Med. Chem. Lett. 2007;17:2844–2848. doi: 10.1016/j.bmcl.2007.02.048. [DOI] [PubMed] [Google Scholar]
- 49.Harai R, Sakamoto K, Hisamichi H, Nagano N. Structure–activity relationships of cephalosporins having a (dimethyl isoxazolidi nio) vinyl moiety at their 3-position. J. Antibiot. 1996;49:1162–1171. doi: 10.7164/antibiotics.49.1162. [DOI] [PubMed] [Google Scholar]
- 50.Kharimian, K.; Tam, T.F.; Leung-Toung, R.C.; Li, W.: Thiadiazole compounds useful as inhibitors of hb/kb atpase. PCT Int. Appl. WO9951584A1 (1999).
- 51.Kohara Y, Kubo K, Imamiya E, Wada T, Inada Y, Naka T. Synthesis and angiotensin II receptor antagonistic activities of benzimidazole derivatives bearing acidic heterocycles as novel tetrazole bioisosteres. J. Med. Chem. 1996;39:5228–5235. doi: 10.1021/jm960547h. [DOI] [PubMed] [Google Scholar]
- 52.Leung-Toung R, Wodzinska J, Li W, Lowrie J, Kukreja R, Desilets D, Karimian K, Tam TF. 1,2,4-thiadiazole: a novel cathepsin B inhibitor. Bioorg. Med. Chem. 2003;11:5529–5537. doi: 10.1016/j.bmc.2003.09.040. [DOI] [PubMed] [Google Scholar]
- 53.Castro A, Castano T, Encinas A, Porcal W, Gil C. Advances in the synthesis and recent therapeutic applications of 1,2,4-thiadiazole heterocycles. Bioorg. Med. Chem. 2006;14:1644–1652. doi: 10.1016/j.bmc.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 54.Johnstone, C.; Mckerrecher, D.; Pike, K.G.; Waring, M.J.: Heteroaryl benzamide derivatives for use as Glk activators in the treatment of diabetes. PCT Int. Appl. WO2005121110A1 (2005).
- 55.van den Nieuwendijk AMCH, Pietra D, Heitman L, Goblyos A, Ijzerman AP. Synthesis and biological evaluation of 2,3,5-substituted [1,2,4]thiadiazoles as allosteric modulators of adenosine receptors. J. Med. Chem. 2004;47:663–672. doi: 10.1021/jm030863d. [DOI] [PubMed] [Google Scholar]
- 56.Mayhoub AS, Marler L, Kondratyuk TP, Park EJ, Pezzuto JM, Cushman MC. Optimizing thiadiazole analogues of resveratrol versus three chemopreventive targets. Bioorg. Med. Chem. 2012;20:510–520. doi: 10.1016/j.bmc.2011.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kumar D, Kumar NM, Chang KH, Gupta R, Shah K. Synthesis and in vitro anticancer activity of 3,5-bis(indolyl)-1,2,4-thiadiazoles. Bioorg. Med. Chem. Lett. 2011;21:5897–5900. doi: 10.1016/j.bmcl.2011.07.089. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.