Abstract
Oxidatively-induced DNA damage has previously been associated with bipolar disorder. More recently, impairments in DNA repair mechanisms have also been reported. We aimed to investigate oxidatively-induced DNA lesions and expression of DNA glycosylases involved in base excision repair in euthymic patients with bipolar disorder compared to healthy individuals. DNA base lesions including both base and nucleoside modifications were measured using gas chromatography-tandem mass spectrometry and liquid chromatography-tandem mass spectrometry with isotope-dilution in DNA samples isolated from leukocytes of euthymic patients with bipolar disorder (n = 32) and healthy individuals (n = 51). The expression of DNA repair enzymes OGG1 and NEIL1 were measured using quantitative real-time polymerase chain reaction. The levels of malondialdehyde were measured using high performance liquid chromatography. Seven DNA base lesions in DNA of leukocytes of patients and healthy individuals were identified and quantified. Three of them had significantly elevated levels in bipolar patients when compared to healthy individuals. No elevation of lipid peroxidation marker malondialdehyde was observed. The level of OGG1 expression was significantly reduced in bipolar patients compared to healthy individuals, whereas the two groups exhibited similar levels of NEIL1 expression. Our results suggest that oxidatively-induced DNA damage occurs and base excision repair capacity may be decreased in bipolar patients when compared to healthy individuals. Measurement of oxidatively-induced DNA base lesions and the expression of DNA repair enzymes may be of great importance for large scale basic research and clinical studies of bipolar disorder.
Keywords: Bipolar disorder, DNA damage, DNA repair, base excision repair, formamidopyrimidines
1. Introduction
Bipolar disorder (BD) is a chronic, severe and highly disabling psychiatric disorder, which is considered as one of the leading causes of disability amongst all medical and psychiatric conditions [1–3]. BD has previously been associated with increased mortality and morbidity due to general medical conditions such as cardiovascular, metabolic or inflammatory diseases [4–12]. Despite vast uncertainties about the underlying molecular mechanisms, recent evidence has shown that increased oxidatively-induced DNA damage may have a central role in the pathophysiology of BD and increased cellular aging and comorbidity in BD [13–15]. Oxygen-derived free radicals are constantly generated as by-products of aerobic metabolism. Oxidative stress occurs when enzymatic and non-enzymatic antioxidant defense systems are overwhelmed by elevated levels of oxygen-derived free radicals [16]. Oxidative stress damages biological molecules such as DNA, proteins and lipids, causing multiple forms of DNA damage including base and sugar modifications, strand breaks and DNA-protein cross-links [17]. Oxidatively-induced damage to DNA can initiate mutagenic processes and early aging [18]. This type of DNA damage has been shown to play a role in the pathophysiology of cardiovascular diseases, diabetes mellitus, various cancers and psychiatric disorders including BD [18–20]. Previous studies focusing on antioxidant enzymes and oxidatively-induced damage to proteins and lipids in BD reported consistent and significant alterations in antioxidant enzymes, lipid peroxidation and nitric oxide levels [21–23]. Increased levels of DNA single- or double-strand breaks have been shown in both postmortem brain tissues [24–26] and lymphocytes of patients with BD [27]. Moreover, levels of 8-hydroxy-2’-deoxyguanosine (8-OH-dG) have been reported to be increased in blood [28, 29] and urine samples of patients with BD [30, 31]. Despite a plethora of known oxidatively-induced DNA base lesions, previous research in psychiatric disorders focused on 8-OH-dG only [20]. Therefore, there are no data on the alterations of the levels of DNA base lesions other than that of 8-OH-dG in BD.
Various DNA repair mechanisms exist to repair oxidatively-induced DNA base damage. The base excision repair (BER) is the major mechanism for the repair of this type of DNA damage. It recognizes and removes modified DNA bases by DNA glycosylases, followed by the activity of other enzymes to complete DNA repair [32–34]. In BER, OGG1 is a specific enzyme for the excision of 8-OH-Gua and 2,6-diamino-4-hydroxy-5-formamidopyrimidine (FapyGua), whereas 4,6-diamino-5-formamidopyrimidine (FapyAde) and FapyGua are removed by NEIL1 and NEIL3, but not 8-OH-Gua [34]. Two studies showed that increases in expression of OGG1 were associated with depressive symptoms in cancer patients [35,36]. A decrease in BER capacity in recurrent depressive disorder [37], and down regulated OGG1 levels in rapid-cycling BD [38] have also been reported.
The objective of the present study was to investigate a more extensive set of markers of oxidatively-induced DNA damage and DNA repair enzymes in DNA samples isolated from leukocytes of euthymic patients with BP in comparison to healthy individuals.
2. Materials and Methods
2.1. Participants
Patients with BD (n = 32) and healthy individuals (n = 51) were included in this study. The patients who had been euthymic for at least 6 months were recruited from the Bipolar Disorders Outpatient Unit, Department of Psychiatry, Dokuz Eylul University, Izmir, Turkey. Diagnoses were confirmed using the Structured Clinical Interview for the Diagnostic Manual of Mental Disorders [39] and clinical variables were recorded by experienced clinicians of the research team. Patients with neurological disorders, history of head trauma, chronic medical condition (e.g., hypertension, diabetes mellitus) and substance use were excluded. Other exclusion criteria included comorbid Axis I psychiatric diagnosis, neurodegenerative diseases, epilepsy or previous brain surgery, auditory or visual impairment, and being pregnant or breastfeeding. Symptomatic severity was assessed using Young Mania Rating Scale (YMRS) [40], Hamilton Depression Scale-17 (HAM-D) [41], Clinical Global Impression Scale (CGI) [42] and Global Assessment of Functionality (GAF) [43]. Healthy individuals with no known medical problems, no family history of major psychiatric or no neurological disorders, including dementia, mental retardation, cancer, cardiovascular disease or diabetes mellitus in the first-degree relatives or psychiatric history were enrolled in this study. Psychiatric conditions of the healthy individuals were confirmed by the Structural Clinical Interview for DSM-IV interview [38]. The study was approved by Dokuz Eylul University Hospital Ethics Committee (Approval date: 12.07.2012; protocol no: 2012/16–13). All participants provided written informed consent.
2.2. Collection of the blood samples
Each participant provided 10 mL blood sample collected in EDTA-coagulated tubes (for leukocyte, RNA and plasma isolation) by venipuncture. At the day of the venipuncture, leukocytes were isolated from blood samples by density gradient separation using Histopaque-1119 and total RNA was extracted from 500 μL blood samples using GeneJet RNA Purification Kit (Fermentas, MA, USA). Leukocytes were frozen at –80 °C until DNA isolation. The RNA samples were frozen at –80 °C until they were converted to first-strand cDNA with an oligo-2’-deoxythymidine (dT) 18 primer. The RNA samples were converted to first-strand cDNA using the First Strand cDNA Synthesis Kit (Fermentas, MA, USA) and were frozen at –80 °C until quantitative real-time polymerase chain reaction (QRT-PCR) was performed.
2.3. DNA isolation and analysis
DNA was isolated from leukocytes by using salting-out/NaCl method [44]. DNA concentration was measured by recording the UV spectrum of each sample using an absorption spectrophotometer between the wavelengths of 200 nm and 350 nm. The absorbance at 260 nm was used to measure the DNA concentration. Subsequently, 50 μg aliquots of DNA samples were dried in a SpeedVac under vacuum. According to a Material Transfer Agreement between Dokuz Eylul University, Izmir, Turkey and National Institute of Standards and Technology (NIST), Gaithersburg, MD, USA, DNA samples were sent to NIST for analysis by gas chromatography-tandem mass spectrometry (GC-MS/MS) and liquid chromatography-tandem mass spectrometry (LC-MS/MS).
2.4. Gas chromatography-tandem mass spectrometry
GC-MS/MS with isotope dilution was used to identify and quantify FapyAde, FapyGua, 8-OH-Gua, thymine glycol (ThyGly), 5-hydroxycytosine (5-OH-Cyt) and 5-hydroxy-5-methylhydantoin (5-OH-5-MeHyd). Aliquots (50 μg) of DNA samples were supplemented with aliquots of internal standards FapyAde-13C,15N2, FapyGua-13C,15N2, 8-OH-Gua-15N5, ThyGly-2H4, 5-OH-Cyt-13C,15N2 and 5-OH-5-MeHyd-13C,15N2. DNA samples were dissolved in 50 μL of an incubation buffer consisting of 50 mM phosphate buffer (pH 7.4), 100 mM KCl, 1 mM EDTA, and 0.1 mM dithiothreitol, and then incubated with 2 μg of E. coli Fpg and 2 μg of E. coli Nth for 1 h at 37 °C to release DNA base lesions from DNA. Subsequently, 100 μL ethanol were added to precipitate DNA. After centrifugation, supernatant fractions were separated, lyophilized and trimethylsilylated. Derivatized samples were analyzed by GC-MS/MS as described previously [45].
2.5. Liquid chromatography-tandem mass spectrometry
LC-MS/MS with isotope dilution was used to measure the levels of (5’S)-8,5’-cyclo-2’-deoxyadenosine (S-cdA) and 8-OH-dG, which is the 2’-deoxynucleoside form of 8-OH-Gua. Aliquots of S-cdA-15N5 and 8-OH-dG-15N5 as internal standards were added to an aliquot of 50 μg of DNA samples, which were then dried in a SpeedVac. Subsequently, DNA samples were hydrolyzed with a mixture of nuclease P1, phosphodiesterase I and alkaline phosphatase according to a published procedure [45]. All samples were filtered using Millipore Microcon Ultracel YM-3 ultrafiltration membranes (Millipore, Bedford, MA) with molecular mass cutoff of 3 kDa by centrifugation at 12000×g for 30 min. LC-MS/MS analyses were performed using a Thermo-Scientific Finnigan TSQ Quantum Ultra AM triple quadrupole MS/MS system with an installed heated electrospray-ionization source, as described previously [46]. Hydrolyzed DNA samples (20 μL injection volume, no waste mode) were analyzed using a Zorbax SB-Aq rapid resolution narrow-bore LC column (2.1 mm x 150 mm, 3.5 μm particle size) (Agilent Technologies, Wilmington, DE) with an attached Agilent Eclipse XDB-C8 guard column (2.1 mm x 12.5 mm, 5 μm particle size). In all instances, the autosampler and column temperature were kept at 5 °C and 40 °C, respectively. Mobile phases A and B were water and acetonitrile, respectively, both containing 0.1 % formic acid (v/v). A gradient analysis of 3% (v/v) of B/min starting from 98 % A/2 % B (v/v) was used. After 6 min, B was increased to 60 % in 0.1 min and kept at this level for 1 min and then another 13 min at 2 % to equilibrate the column. The flow rate was 0.5 mL/min and the total analysis time was 20 min. Analysis by LC-MS/MS was performed using selected-reaction monitoring mode with the mass transitions m/z 284 → m/z 168 and m/z 289 →m/z 173 for 8-OH-dG and 8-OH-dG-15N5, respectively, and with the mass transitions m/z 250 → m/z 164 and m/z 255 → m/z 169 for S-cdA and S-cdA-15N5 respectively.
2.6. Measurement of expression levels of DNA repair enzymes
The mRNA expression levels of human OGG1 and human NEIL1 were determined in samples of BD patients (n = 17) and healthy individuals (n = 19). Expressions of NEIL1 and OGG1 were measured by QRT-PCR using Maxima Sybr Green qPCR Master Mix (2x) (Fermentas, MA, USA) Kit. β-Actin was used as housekeeping gene. The amplification was performed in Light Cycler 1.5 (Roche Applied Science, Penzberg, Germany). Three independently prepared samples were used for each data point. The difference of cycle of threshold (Ct) between reference and target gene locus was observed by normalizing using housekeeping gene and calculating ΔΔCt ratio (ΔΔCt = ΔCt sample – ΔCt reference). Gene expression levels were calculated using the formula 2 – ΔΔCt [47, 48].
2.7. Measurement of malondialdehyde
Malondialdehyde was extracted and analyzed according to a previously described method with slight modifications [49]. Briefly 40 μL plasma was diluted with 100 μL of H2O and mixed with 20 μL of 2.8 mmol/L BHT in 95 % ethanol, 40 μL of 81 g/L sodium dodecyl sulfate, and 600 μL of thiobarbituric acid (TBA) reagent consisting of 8 g/L TBA diluted 1:1 with 200 mL/L acetic acid adjusted to pH 3.5 with NaOH. The mixture was immediately incubated in a 90 °C water bath for 60 min and cooled on ice; 200 μL of H2O and 1 mL of butanol-pyridine (15:1 by vol.) were then added. After vigorous mixing, the organic layer was separated by centrifugation (10 min at 10000 rpm). An aliquot (10 μL) was directly injected onto the high-performance liquid chromatography (HPLC). Calibration curves were constructed using 1,1,3,3-tetraethoxypropane (0.75 μmol/L – 40 μmol/L). The separation of the extracts was performed on an automated Shimadzu HPLC system (VP Series, Kyoto, Japan). The analytical column was a reverse phase silica based C18 column (GL Sciences/Inertsil ODS-3), with column dimensions of 150×4.6 mm, 5 μm. The mobile phase consisted of 70 % 10 mM KH2PO4, pH 7.0 and 30 % MeOH. The sample run was 5 min, with a flow rate of 0.8 mL/min, and fluorescence detection at 515 nm (excitation) and 553 nm (emission).
2.8. Statistical analyses
The IBM SPSS Statistics 23.0 (Chicago IL, USA) for Windows was used for data analysis. The Shapiro-Wilk’s test was used to confirm normal distribution for continues variables. Where necessary, logarithmic transformations were applied in order to improve normality. Subsequently, transformed data were reassessed for normality. Group differences on continuous variables regarding demographic and clinical variables were evaluated with independent samples t-test. Chi-Square test was used to examine categorical data.
The statistical analysis of the GC-MS/MS and LC-MS/MS data was performed using the GraphPad Prism 7.01 software (La Jolla, CA, USA) and the unpaired, two-tailed nonparametric Mann Whitney test with Gaussian approximation and confidence level of 95 % to 99 %. A p-value < 0.05 was assumed to correspond to statistically significant difference between medians.
3. Results and Discussion
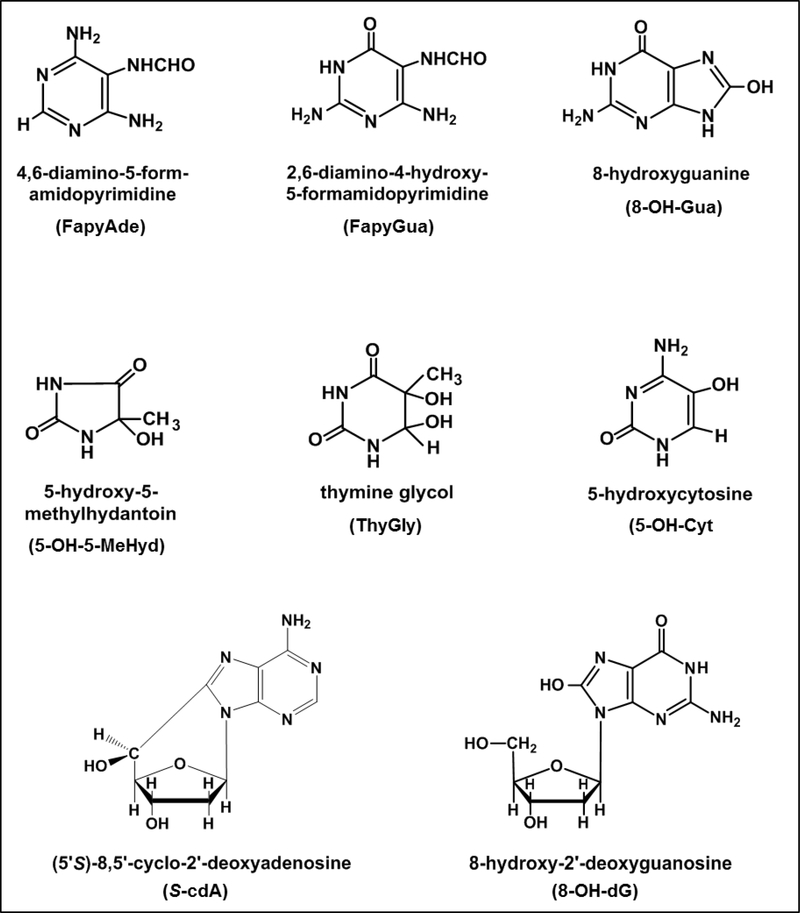
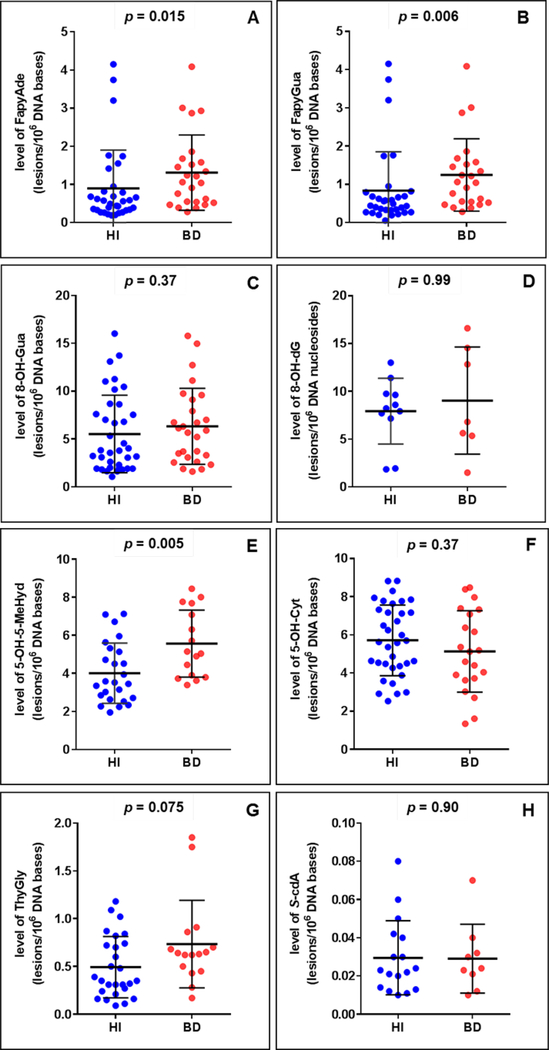
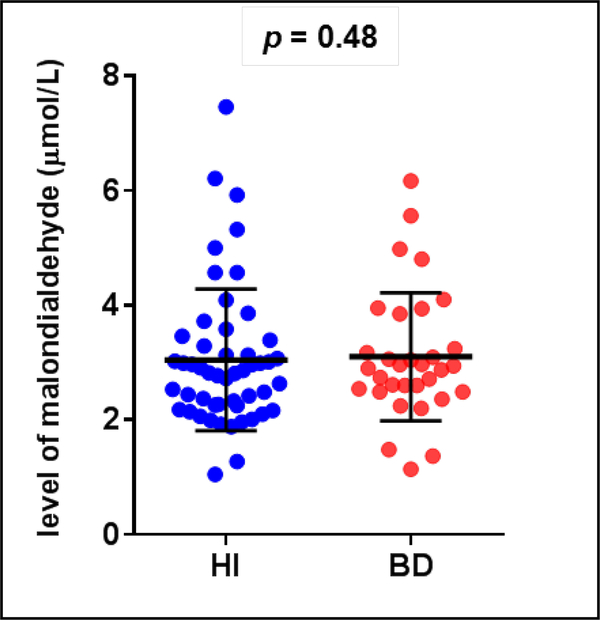
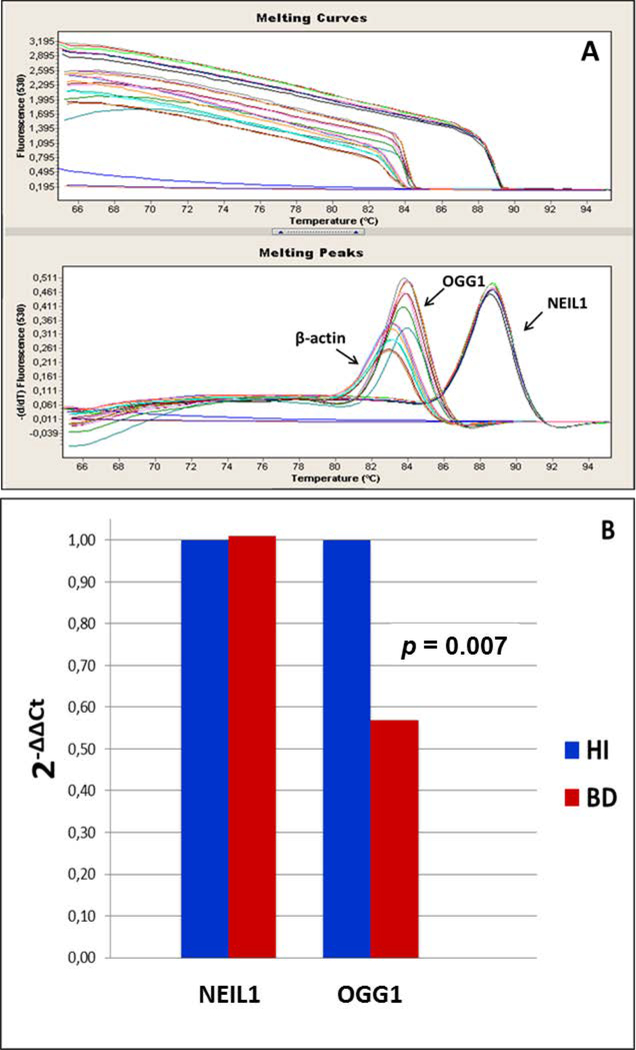
Sociodemographic and clinical characteristics of the BD patients and healthy individuals are described in Table 1. The groups did not differ from each other with regard to gender, age or smoking. One of the patients was drug-free, 9 patients were on mood-stabilizers as monotherapy (lithium or valproate), 19 patients were receiving a mood-stabilizer in combination with a second generation-antipsychotic, and one patient was receiving a mood-stabilizer in combination with an antidepressant. We identified and quantified six DNA base lesions by GC-MS/MS and two modified 2’-deoxynucleosides by LC-MS/MS in DNA samples from both BD patients and healthy individuals. The structures of these lesions are given in Fig. 1. It should be noted that 8-OH-dG is the 2’-deoxynucleoside form of 8-OH-Gua. Figures 2A-H show the levels of the lesions shown in Figure 1. A large group of samples was used for the measurements by GC-MS/MS. S-cdA and 8-OH-dG were measured in the remaining samples by LC-MS/MS. In various samples, some lesions could not be quantified with certainty. Therefore, the number of patient samples and that of healthy individual samples in the figures somewhat differ from lesion to lesion. The number of samples in each case is given in the legends of the figures. The levels of FapyAde, FapyGua and 5-OH-5-MeHyd in BD patients were significantly greater than those in healthy individuals. The confidence level was even 99 % for the latter two lesions. No significant difference between the levels of other lesions was observed when both groups were compared. Similarly, patient and healthy individual groups did not differ significantly with regard to the levels of malondialdehyde (Figure 3). Figure 4 illustrates the expression levels of OGG1 and NEIL1. The expression level of OGG1 in BD patients was 43 % lower than that in healthy individuals and the difference between the two groups was significant after adjusting for age, gender and smoking (F = 3.278, df = 4, p = 0.007). On the other hand, no significant difference was observed between the expression levels of NEIL1 in both groups (p = 0.49) (Figure 4).
Table 1.
Demographic and clinical characteristics of the participants.
| Gender (number of females; their percentage)a | 20; 62.5 % | 30; 58.8 % | 0.74 |
| Ageb | 37.63 ± 9.96 | 36.28 ± 11.45 | 0.58 |
| Smoking status (number of smokers; their percentage)a | 15; 46.9 % | 16; 31.4 % | 0.16 |
| Age of onset of illnessb | 26.83 ± 10.21 | ||
| Duration of illness (years)b | 10.63 ± 8.72 | ||
| Total number of episodesb | 5.63 ± 4.34 | ||
| Number of manic episodesb | 3.20 ± 2.59 | ||
| Number of depressive episodesb | 2.20 ± 2.46 | ||
| Clinical Global Impressions scaleb | 1.23 ± 0.43 | ||
| Global assessment of functionalityb | 86 ± 6.21 | ||
| Hamilton Depression Scaleb | 1.17 ± 1.49 | ||
| Young Mani Rating Scaleb | 0.47 ± 0.97 |
Chi-Square
independent samples t test.
Fig. 1.
The structures of the DNA lesions measured in this work.
Fig. 2.
The levels of DNA lesions. A: FapyAde in healthy individuals (n = 33) and BD patients (n = 25); B: FapyGua in healthy individuals (n = 32) and BD patients (n = 24); C: 8-OH-Gua in healthy individuals (n = 36) and BD patients (n = 27); D: 8-OH-dG in healthy individuals (n = 11) and BD patients (n = 7); E: 5-OH-5-MeHyd in healthy individuals (n = 25) and BD patients (n = 16); F: 5-OH-Cyt in healthy individuals (n = 35) and BD patients (n = 21); G: ThyGly in healthy individuals (n = 28) and BD patients (n = 16); H: S-cdA in healthy individuals (n = 17) and BD patients (n = 9).
Fig. 3.
The level of malondialdehyde in healthy individuals (n = 51) and BD patients (n = 31).
Fig. 4.
A: Analysis of the melting curves of the OGG1, NEIL1 and β-actin (housekeeping) genes: The coefficient of variations (% CV) for the expressions of the genes NEIL1, OGG1 and β-actin were 0.6, 1.2 and 1.5, respectively. B: Expression profiles of OGG1 and NEIL1: OGG1 (2-ΔΔCt) 0.43 (↓) (p = 0.007) and NEIL1(2-ΔΔCt) 0.01 (↑) (p = 0.489). The results are adjusted by age, gender, smoking status using lineer regression. 2-ΔΔCt (fold change) is computed using α = 0.016. Ct: cycle of threshold; ΔCt: the difference of Ct between target gene and β-actin (housekeeping) gene.
Our results show that oxidatively-induced DNA damage occurs in DNA of BD patients compared to healthy individuals. Three out of the 8 DNA lesions measured in the present work exhibited significantly greater levels in BD patients than those in healthy individuals. To the best of our knowledge, this is the first study assessing different types of DNA lesions, representing oxidatively-induced damage to all four DNA bases in BD patients. The current knowledge on DNA base damage in BD has been based on the levels of 8-OH-dG only, which has been mostly measured as “the most prominent DNA base lesion” in biological samples because of the limitations of the methodologies used. Therefore, there were no available data on the lesions derived from adenine, cytosine and thymine, and one other important lesion of guanine, i.e., FapyGua. To this end, it is well known that hydroxyl radical attack on Gua produces both 8-OH-Gua and FapyGua by oxidation and reduction of the same Gua–OH-adduct radical, respectively. Moreover, the yields of these products depend on the reaction conditions [50].
In the present work, 8-OH-Gua and 8-OH-dG were measured by two different techniques. It is important to note that GC-MS/MS and LC-MS/MS yielded almost identical levels of these compounds. In both cases, the levels 8-OH-Gua and 8-OH-dG did not differ between BD patients and healthy individuals. In contrast, the level of FapyGua was found to be significantly greater in BD patients than in healthy individuals (p = 0.006 with the confidence level of 99 %). Thus, our results clearly show that the measurement of one DNA lesion such as 8-OH-dG (or 8-OH-Gua) only does not necessarily prove whether DNA damage in a given biological system occurs or not. Past published data on 8-OH-dG in BD patients differed among the studies. For example, a meta-analysis of the existing data [51] and more recent studies [29, 30] showed greater levels of 8-OH-dG in BD patients than in healthy individuals, whereas several other studies reported unchanged levels of 8-OH-dG in in both cases [28, 30, 52]. The discrepancy among these findings may be due to the methodological differences between the studies, and to the differences between clinical features of the study populations including illness state, course of illness, medications, smoking status, etc.
We also measured the expression levels of OGG1 and NEIL1 in both BD patients and healthy individuals. The expression level of OGG1 was found to be lower in BD patients than in healthy individuals. This is on a par with the previously reported down-regulated OGG1 expression in a rapid-cycling group of BD patients [38]. On the other hand, no elevation of NEIL1 expression was observed in BD patients. OGG1 and NEIL1 are bifunctional DNA glycosylases that are involved in the first step of the BER pathway to remove modified DNA bases from damaged DNA [32–34]. Their specificities differ from each other in that OGG1 removes FapyGua and 8-OH-Gua with similar excision kinetics, whereas NEIL1 is mainly specific for FapyAde and FapyGua, and to a lesser extent for 5-OH-5-MeHyd and ThyGly, but not for 8-OH-Gua [34]. In general, DNA glycosylases possess broad specificities for removal of DNA base lesions. For example, besides NEIL1, NTH1 and NEIL3 are specific for FapyAde removal in mammalian cells. The latter also removes FapyGua. NTH1 is the major DNA glycosylase that acts on ThyGly and 5-OH-Cyt in mammalian cells [33, 34]. Therefore, the correlation of the levels of DNA base lesions with the expression levels of DNA glycosylases is quite complex, and not well understood. Low expression level of OGG1 in BD patients may be one of the factors leading to the greater level of FapyGua. On the other hand, similar levels of NEIL1 in both groups did not seem to affect the significant accumulation of FapyAde and 5-OH-5-MeHyd in BD patients. To this end, it is well known that various polymorphic variants of NEIL1 exist in human population such as NEIL1-Ser82Cys, NEIL1-Gly83Asp, NEIL1-Cys136Arg, NEIL1-Asp252Asn and NEIL1-Pro208Ser (reviewed in [33, 34]). Among these variants, NEIL1-Gly83Asp and NEIL1-Cys136Arg have been shown to be completely devoid of glycosylase activity. Furthermore, NEIL1-Gly83Asp and NEIL1-Cys136Arg had significantly reduced activity. Such polymorphic variants may affect their binding, catalytic activity or protein-protein interaction with other DNA repair proteins such as PARP1, XRCC1 and CSB. Such effects may cause the accumulation of typical substrates of NEIL1 such as FapyAde, FapyGua and 5-OH-5-MeHyd, as was found in this work. Future studies might include exomic sequencing of the NEIL1 gene to examine for such polymorphisms in BD patients.
The increased levels of oxidatively induced DNA lesions observed might be explained by increased oxidative stress in BD. Previous studies reported several alterations in oxidative markers including lipid peroxidation markers, antioxidant enzymes and nitric oxide levels in BD [53, 54]. Malondialdehyde has been one of the most consistent lipid peroxidation marker that was found to be elevated in BD [55]. However, our results demonstrated no significant alterations in malondialdehyde levels, implying unchanged levels of oxidative stress load in the patient population compared to healthy individuals.
Several studies suggest that BD is associated with increased incidence for several medical comorbidities including cardiovascular, endocrine, inflammatory diseases [4–12], as well as various types of cancers [56–59]. DNA damage and reduced DNA repair capacity have been suggested to be one of the key mechanisms that underlie high clinical comorbidity, vulnerability to several cancers, neurocognitive decline and early aging in BD patients [13–15]. Some of the DNA base lesions identified in this work are strongly mutagenic and thus may contribute to those symptoms and others in BD patients. Thus, 8-OH-Gua and FapyGua pair with non-cognate Ade and lead to G → T transversion mutations [60–64]. The level of 8-OH-Gua was not increased in BD patients; however, 8-OH-Gua is readily oxidized, leading to the formation of spiroiminohydantoin (Sp) and 5-guanidinohydantoin (Gh), which exhibit mutagenic effects as well as cytotoxic effects [65]. Sp and Gh were not measured in the present work. Facile oxidation of 8-OH-Gua may prevent its accurate measurement in vivo. FapyAde leads to A → T transversions and is mutagenic, albeit to a lesser extent than FapyGua [66]. 5-OH-5-MeHyd can be a lethal or mutagenic lesion, because it constitutes a replication block for some DNA polymerases or is by-passed by low fidelity polymerases [67–70] (for more information on the mutagenic effects of oxidatively-induced DNA base lesions identified in this work, see reviews [33] and [71]).
Clinical characteristics of the patient population of this study needs consideration while interpreting our results. It is important to note that our study population consisted of only euthymic patients with BD. Previously, manic or depressive patients were shown to have higher levels of 8-OH-dG lesions than euthymic patients [28,31]. Further studies are needed to identify different types of DNA lesions presenting oxidatively-induced damage to all DNA bases measured in this study and DNA repair enzyme profiles across different states of BD (mania, depression and euthymia). Smoking status of participants might affect the levels of DNA damage/repair [72, 73]. In this work, however, double comparisons between smokers and non-smokers did not show any significant difference with respect to DNA lesions and DNA glycosylases (Table 2).
Table 2.
Comparisons of markers between smokers and non-smokers.
| Non-smoker participants (n = 31) | Smoker participants (n = 52) | p-valuea | |
|---|---|---|---|
| DNA lesion (lesions/106 DNA bases) | |||
| FapyAde | 1.06±1.11 | 1.35±1.26 | 0.249 |
| FapyGua | 0.90±0.80 | 1.22±1.10 | 0.234 |
| 8-OH-Gua | 6.30±4.44 | 5.15±3.12 | 0.484 |
| 8-OH-dG | 8.38±3.77 | 8.32±5.59 | 1.000 |
| 5-OH-5-MeHyd | 4.38±1.75 | 5.27±1.94 | 0.140 |
| 5-OH-Cyt | 5.25±2.03 | 5.85±1.84 | 0.360 |
| ThyGly | 0.57±0.39 | 0.67±0.47 | 0.788 |
| S-cdA | 0.03±0.02 | 0.03±0.02 | 0.357 |
| Malondialdehyde (μmol/L) | 3.06±1.25 | 3.08±1.11 | 0.843 |
| DNA glycosylases | |||
| OGG1 (ΔCt) | 6.31±0.83 | 6.46±0.89 | 0.566 |
| NEIL1 (ΔCt) | 2.76±0.47 | 2.77±0.51 | 0.986 |
Mann-Whitney U test.
Ct: cycle of threshold; ΔCt: the difference of Ct between target gene and β-actin (housekeeping) gene.
Medication effect is the other parameter that requires attention while studying DNA damage in any patient population. Our patient population was predominantly on mood stabilizing medications (i.e., lithium or valproate). Previous evidence suggested that lithium and valproate treatments may have antioxidant properties [74–79], and may decrease DNA damage [24, 74–79]. However, some studies showed similarly higher 8-OH-dG levels in both unmedicated [29] and medicated patient populations [27, 28, 30, 31], leaving the effect of psychotropic medications on DNA damage equivocal. Future prospective studies specifically designed to understand the effects of mood stabilizing treatments on DNA damage/repair processes are needed.
In conclusion, our results show enhanced levels of several oxidatively-induced DNA base lesions and reduced levels of OGG1 in leukocytes of patients with BD when compared with healthy individuals. These findings suggest a defect in base excision repair in BD. Measurement of oxidatively-induced DNA base lesions and expression levels of DNA repair enzymes may be of great importance for large scale basic research and clinical studies of BD, contributing to a comprehensive understanding of the DNA damage/repair mechanisms in BD.
Acknowledgements
DNA samples were transferred to NIST from Dokuz Eylul University and analyzed at NIST pursuant to a Material Transfer Agreement between NIST and Dokuz Eylul University. Certain commercial equipment or materials are identified in this paper in order to specify the experimental procedure adequately. Such identification does not imply recommendation or endorsement by the National Institute of Standards and Technology, nor does it imply that the materials or equipment identified are necessarily the best available for the purpose. The Psychiatric Association of Turkey awarded an Encouragement Prize to this research project.
Abbreviations
- BD
bipolar disorder
- HI
healthy individuals
- FapyAde
4,6-diamino-5-formamidopyrimidine
- FapyGua
2,6-diamino-4-hydroxy-5-formamidopyrimidine
- 8-OH-Gua
8-hydroxyguanine
- 8-OH-dG
8-hydroxy-2’-deoxyguanosine
- 5-OH-5-MeHyd
5-hydroxy-5-methylhydantoin
- 5-OH-Cyt
5-hydroxycytosine
- ThyGly
thymine glycol
- S-cdA
(5’S)-8,5’-cyclo-2’-deoxyadenosine
- RT-PCR
real-time polymerase chain reaction
- GC-MS/MS
gas chromatography/tandem mass spectrometry
- LC-MS/MS
liquid chromatography/tandem mass spectrometry
Footnotes
Conflict of Interest Statement
The authors declare that there are no conflicts of interest.
References
- [1].Belmaker R, Bipolar disorder N Engl. J. Med. 351 (2004) 476–486. [DOI] [PubMed] [Google Scholar]
- [2].Üstün T, Ayuso-Mateos JL, Chatterji S, Mathers C, Murray CJ, Global burden of depressive disorders in the year 2000, Br. J. Psychiatry 184 (2004) 386–392. [DOI] [PubMed] [Google Scholar]
- [3].Murray C, Lopez AD, The utility of DALYs for public health policy and research: a reply, Bulletin of the World Health Organization 75 (1997) 377. [PMC free article] [PubMed] [Google Scholar]
- [4].Sylvia LG, Shelton RC, Kemp DE, Bernstein EE, Friedman ES, Brody BD, McElroy SL, Singh V, Tohen M, Bowden CL, Ketter TA, Deckersbach T, Thase ME, Reilly-Harrington NA, Nierenberg AA, Rabideau DJ, Kinrys G, Kocsis JH, Bobo WV, Kamali M, McInnis MG, Calabrese JR, Medical burden in bipolar disorder: findings from the Clinical and Health Outcomes Initiative in Comparative Effectiveness for Bipolar Disorder study (Bipolar CHOICE), Bipolar Disord. 17 (2015) 212–223. [DOI] [PubMed] [Google Scholar]
- [5].Kessing LV, Andersen PK, Does the risk of developing dementia increase with the number of episodes in patients with depressive disorder and in patients with bipolar disorder? J. Neurol. Neurosurg. Psychiatry 75 (2004) 1662–1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Kilbourne AM, Perron BE, Mezuk B, Welsh D, Ilgen M, Bauer MS, Co-occurring conditions and health-related quality of life in patients with bipolar disorder, Psychosom. Med. 71 (2009) 894–900. [DOI] [PubMed] [Google Scholar]
- [7].McIntyre RS, Konarski JZ, Soczynska JK, Wilkins K, Panjwani G, Bouffard B, Bottas A, Kennedy SH, Medical comorbidity in bipolar disorder: implications for functional outcomes and health service utilization, Psychiatr. Serv. 57 (2006) 1140–1144. [DOI] [PubMed] [Google Scholar]
- [8].Thompson WK, Kupfer DJ, Fagiolini A, Scott JA, Frank E, Prevalence and clinical correlates of medical comorbidities in patients with bipolar I disorder: analysis of acute-phase data from a randomized controlled trial, J. Clin. Psychiatry 67 (2006) 783–788. [DOI] [PubMed] [Google Scholar]
- [9].Crump C, Sundquist K, Winkleby MA, Sundquist J, Comorbidities and mortality in bipolar disorder: a Swedish national cohort study, JAMA Psychiatry 70 (2013) 931–939. [DOI] [PubMed] [Google Scholar]
- [10].van Winkel R, De Hert M, Van Eyck D, Hanssens L, Wampers M, Scheen A, Peuskens J, Prevalence of diabetes and the metabolic syndrome in a sample of patients with bipolar disorder, Bipolar Disord. 10 (2008) 342–348. [DOI] [PubMed] [Google Scholar]
- [11].Goldstein BI, Fagiolini A, Houck P, Kupfer DJ, Cardiovascular disease and hypertension among adults with bipolar I disorder in the United States, Bipolar Disord. 11 (2009) 657–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Kupfer DJ, The increasing medical burden in bipolar disorder, JAMA 293 (2005) 2528–2530. [DOI] [PubMed] [Google Scholar]
- [13].Berk M, Kapczinski F, Andreazza AC, Dean OM, Giorlando F, Maes M, Yücel M, Gama CS, Dodd S, Dean B, Magalhães PV, Amminger P, McGorry P, Malhi GS, Pathways underlying neuroprogression in bipolar disorder: focus on inflammation, oxidative stress and neurotrophic factors, Neurosci. Biobehav. Rev. 35 (2011) 804–817. [DOI] [PubMed] [Google Scholar]
- [14].Rizzo LB, Costa LG, Mansur RB, Swardfager W, Belangero SI, Grassi-Oliveira R, McIntyre RS, Bauer ME, Brietzke E, The theory of bipolar disorder as an illness of accelerated aging: implications for clinical care and research, Neurosci. Biobehav. Rev. 42 (2014) 157–169. [DOI] [PubMed] [Google Scholar]
- [15].McGorry P, Keshavan M, Goldstone S, Amminger P, Allott K, Berk M, Lavoie S, Pantelis C, Yung A, Wood S, Hickie I, Biomarkers and clinical staging in psychiatry, World Psychiatry 13 (2014) 211–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Halliwell B, Gutteridge JMC, Free Radicals in Biology and Medicine, Fifth Ed. Oxford University Press, 2015. [Google Scholar]
- [17].Dizdaroglu M, Jaruga P, Birincioglu M, Rodriguez H, Free radical-induced damage to DNA: mechanisms and measurement, Free Radic. Biol. & Med. 32 (2002) 1102–1115. [DOI] [PubMed] [Google Scholar]
- [18].Maynard S, Schurman SH, Harboe C, de Souza-Pinto NC, Bohr VA, Base excision repair of oxidative DNA damage and association with cancer and aging, Carcinogenesis 30(1) (2009) 2–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Raza MU, Tufan T, Wang Y, Hill C, Zhu MY, DNA Damage in Major Psychiatric Diseases. Neurotox. Res. 30 (2016) 251–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Dizdaroglu M, Oxidatively induced DNA damage: mechanisms, repair and disease, Cancer Lett. 327 (2012) 26–47. [DOI] [PubMed] [Google Scholar]
- [21].Andreazza AC, Kauer-Sant’anna M, Frey BN, Bond DJ, Kapczinski F, Young LT, Yatham LN, Oxidative stress markers in bipolar disorder: a meta-analysis, J. Affect. Disord. 111 (2008) 135–144. [DOI] [PubMed] [Google Scholar]
- [22].Bengesser SA, Lackner N, Birner A, Fellendorf FT, Platzer M, Mitteregger A, Unterweger R, Reininghaus B, Mangge H, Wallner-Liebmann SJ, Zelzer S, Fuchs D, McIntyre RS, Kapfhammer HP, Reininghaus EZ, Peripheral markers of oxidative stress and antioxidative defense in euthymia of bipolar disorder--Gender and obesity effects, J. Affect. Disord. 172 (2015) 367–374. [DOI] [PubMed] [Google Scholar]
- [23].Siwek M, Sowa-Kućma M, Dudek D, Styczeń K, Szewczyk B, Kotarska K, Misztakk P, Pilc A, Wolak M, Nowak G, Oxidative stress markers in affective disorders, Pharmacol. Rep. 65 (2013) 1558–1571. [DOI] [PubMed] [Google Scholar]
- [24].Buttner N, Bhattacharyya S, Walsh J, Benes FM, DNA fragmentation is increased in non-GABAergic neurons in bipolar disorder but not in schizophrenia, Schizophr. Res. 93 (2007) 33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Mustak MS, Hegde ML, Dinesh A, Britton GB, Berrocal R, Subba Rao K, Shamasundar NM, Rao KS, Sathyanarayana Rao TS, Evidence of altered DNA integrity in the brain regions of suicidal victims of Bipolar Depression, Indian J. Psychiatry 52 (2010) 220–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Che Y, Wang JF, Shao L, Young T, Oxidative damage to RNA but not DNA in the hippocampus of patients with major mental illness, J. Psychiatry Neurosci. 35 (2010) 296–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Andreazza AC, Frey BN, Erdtmann B, Salvador M, Rombaldi F, Santin A, Gonçalves CA, Kapczinski F, DNA damage in bipolar disorder, Psychiatry Res. 153 (2007) 27–32. [DOI] [PubMed] [Google Scholar]
- [28].Ceylan D, Scola G, Tunca Z, Isaacs-Trepanier C, Can G, Andreazza AC, Young LT, Özerdem A, DNA redox modulations and global DNA methylation in Bipolar Disorder: Effects of sex, smoking and illness state, J. Psychiatr. Res. 261 (2018) 589–596. [DOI] [PubMed] [Google Scholar]
- [29].Soeiro-de-Souza MG, Andreazza AC, Carvalho AF, Machado-Vieira R, Young LT, Moreno RA, Number of manic episodes is associated with elevated DNA oxidation in bipolar I disorder, Int. J. Neuropsychopharmacol. 16 (2013) 1505–1512. [DOI] [PubMed] [Google Scholar]
- [30].Munkholm K, Poulsen HE, Kessing LV, Vinberg M, Elevated levels of urinary markers of oxidatively generated DNA and RNA damage in bipolar disorder, Bipolar Disord. 17 (2015) 257–268. [DOI] [PubMed] [Google Scholar]
- [31].Jacoby AS, Vinberg M, Poulsen HE, Kessing LV, Munkholm K, Increased DNA and RNA damage by oxidation in patients with bipolar I disorder, Transl. Psychiatry 6 (2016) e867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Friedberg EC, Walker GC, Siede W, Wood RD, Schultz RA, Ellenberger T, DNA Repair and Mutagenesis, ASM Press, Washington, D.C., 2006. [Google Scholar]
- [33].Wallace SS, Murphy DL, Sweasy JB, Base excision repair and cancer, Cancer Lett. 327 (2012) 73–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Dizdaroglu M, Coskun E, Jaruga P, Repair of oxidatively induced DNA damage by DNA glycosylases: Mechanisms of action, substrate specificities and excision kinetics, Mutat. Res./Reviews Mutat. Res. 771 (2017) 99–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Zhou F, Zhang W, Wei Y, Zhou D, Su Z, Meng X, Hui L, Tian W W, The changes of oxidative stress and human 8-hydroxyguanine glycosylase1 gene expression in depressive patients with acute leukemia, Leuk. Res. 31 (2007) 387–393. [DOI] [PubMed] [Google Scholar]
- [36].Wei YC, Zhou FL, He DL, Bai JR, Ding H, Wang XY, Nan KJ, Oxidative stress in depressive patients with gastric adenocarcinoma, Int. J. Neuropsychopharmacol. 12 (2009) 1089–1096. [DOI] [PubMed] [Google Scholar]
- [37].Czarny P, Kwiatkowski D, Kacperska D, Kawczyńska D, Talarowska M, Orzechowska A, Bielecka-Kowalska A, Szemraj J, Gałecki P, Śliwiński T, Elevated level of DNA damage and impaired repair of oxidative DNA damage in patients with recurrent depressive disorder, Med. Sci. Monit. 21 (2015) 412–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Munkholm K, Peijs L, Vinberg M, Kessing LV, A composite peripheral blood gene expression measure as a potential diagnostic biomarker in bipolar disorder, Transl. Psychiatry 5 (2015) e614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].First MB, Spitzer RL, Gibbon M, Williams JB, User’s guide for the Structured clinical interview for DSM-IV axis I disorders SCID-I: Clinician Version, American Psychiatric Pub. (1997). [Google Scholar]
- [40].Young R, Biggs J, Ziegler V, Meyer D D, A rating scale for mania: reliability, validity and sensitivity, Br. J. Psychiatry. 133 (1978) 429–435. [DOI] [PubMed] [Google Scholar]
- [41].Hamilton M, A rating scale for depression, J. Neurol. Neurosurg. Psychiatry 23 (1960) 56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Bussner J, Targum SD, The clinical global impressions scale: applying a research tool in clinical practice, Psychiatry (Edgmont). 4 (2007) 28–37. [PMC free article] [PubMed] [Google Scholar]
- [43].Patterson DA, Lee MS, Field trial of the global assessment of functioning scale-modified, Am. J. Psychiatry 15 (1995) 1386. [DOI] [PubMed] [Google Scholar]
- [44].Miller S, Dykes D, Polesky H,. A simple salting out procedure for extracting DNA from human nucleated cells, Nucleic Acids Res. 16 (1988) 1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Jaruga P, Coskun E, Kimbrough K, Jacob A, Johnson WE, Dizdaroglu M, Biomarkers of oxidatively induced DNA damage in dreissenid mussels: A genotoxicity assessment tool for the Laurentian Great Lakes, Environ. Toxicol 32 (2017) 2144–2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Kirkali G, Jaruga P, Reddy PT, Tona A, Nelson BC, Li M, Wilson DM III, Dizdaroglu M, Identification and quantification of DNA repair protein apurinic/apyrimidinic endonuclease 1 (APE1) in human cells by liquid chromatography/isotope-dilution tandem mass spectrometry, PLoSOne. 8 (2013) e69894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Livak KJ, Schmittgen TD, Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCt, Methods 25 (2001) 402–408. [DOI] [PubMed] [Google Scholar]
- [48].Fleige S, Walf V, Huch S, Prgomet C, Sehm J, Pfaffl MW, Comparison of relative mRNA quantification models and the impact of RNA integrity in quantitative real-time RT-PCR, Biotechnol. Lett. 28 (2006) 1601–1613. [DOI] [PubMed] [Google Scholar]
- [49].Lykkesfeldt J, Determination of malondialdehyde as dithiobarbituric acid adduct in biological samples by HPLC with fluorescence detection: comparison with ultraviolet-visible spectrophotometry, Clin. Chem.47 (2001) 1725–1727. [PubMed] [Google Scholar]
- [50].Dizdaroglu M, Coskun E, Jaruga P, Measurement of oxidatively induced DNA damage and its repair, by mass spectrometric techniques, Free Radic. Res. 49 (2015) 525–548. [DOI] [PubMed] [Google Scholar]
- [51].Brown NC, Andreazza AC, Young LT, An updated meta-analysis of oxidative stress markers in bipolar disorder, Psychiatry Res. 218 (2014) 61–68. [DOI] [PubMed] [Google Scholar]
- [52].Tsai MC, Huang TL, Thiobarbituric acid reactive substances (TBARS) is a state biomarker of oxidative stress in bipolar patients in a manic phase, J. Affect. Disord. 173 (2015) 22–26. [DOI] [PubMed] [Google Scholar]
- [53].Andreazza AC, Kauer-Sant’anna M, Frey BN, Bond DJ, Kapczinski F, Young LT, Yatham LN, Oxidative stress markers in bipolar disorder: a meta-analysis, J. Affect. Disord.111(2008),135–144. [DOI] [PubMed] [Google Scholar]
- [54].Brown NC, Andreazza AC, Young LT, An updated meta-analysis of oxidative stress markers in bipolar disorder, Psychiatry Res. 218(2014), 61–68. [DOI] [PubMed] [Google Scholar]
- [55].Kunz M, Gama CS, Andreazza AC, Salvador M, Ceresér KM, Gomes FA., Belmonte-de-Abreu PS, Berk M, Kapczinski F Elevated serum superoxide dismutase and thiobarbituric acid reactive substances in different phases of bipolar disorder and in schizophrenia, Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 32 (2008), 1677–1681. [DOI] [PubMed] [Google Scholar]
- [56].BarChana M, Levav I, Lipshitz I, Pugachova I, Kohn R, Weizman A, Grinshpoon A, Enhanced cancer risk among patients with bipolar disorder, J. Affect. Disord. 108 (2008) 43–48. [DOI] [PubMed] [Google Scholar]
- [57].McGinty EE, Zhang Y, Guallar E, Ford DE, Steinwachs D, Dixon LB, Keating NL, Daumit GL, Cancer incidence in a sample of Maryland residents with serious mental illness, Psychiatr. Serv. 63 (2012) 714–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Lin GM, Chen YJ, Kuo DJ, Jaiteh LE, Wu YC, Lo TS, Li YH, Cancer incidence in patients with schizophrenia or bipolar disorder: a nationwide population-based study in Taiwan, 1997–2009. Schizophr. Bull. 39 (2013) 407–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Hung YN, Yang SY, Huang MC, Lung FW, Lin SK, Chen KY, Kuo CJ, Chen YY, Cancer incidence in people with affective disorder: nationwide cohort study in Taiwan, 1997–2010, Br. J. Psychiatry 205 (2014) 183–188. [DOI] [PubMed] [Google Scholar]
- [60].Kuchino Y, Mori F, Kasai H, Inoue H, Iwai S, Miura K, Ohtsuka E, Nishimura S, Misreading of DNA templates containing 8-hydroxydeoxyguanosine at the modified base and at adjacent residues, Nature 327 (1987) 77–79. [DOI] [PubMed] [Google Scholar]
- [61].Wood ML, Dizdaroglu M, Gajewski E, Essigmann JM, Biochemistry (1990) 7024–7032. [DOI] [PubMed] [Google Scholar]
- [62].Wiederholt CJ, Greenberg MM, Fapy.dG instructs Klenow exo(−) to misincorporate deoxyadenosine, J. Am. Chem. Soc, 124 (2002) 7278–7279. [DOI] [PubMed] [Google Scholar]
- [63].Greenberg MM, In vitro and in vivo effects of oxidative damage to deoxyguanosine, Biochem. Soc. Trans. 32 (2004) 46–50. [DOI] [PubMed] [Google Scholar]
- [64].Pande P, Haraguchi K, Jiang YL, Greenberg MM, Basu AK, Unlike catalyzing error-free bypass of 8-oxodGuo, DNA polymerase λ is responsible for a significant part of Fapy·dG-induced G → T mutations in human cells, Biochemistry. 54 (2015)1859–1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Neeley WL, Essigmann JM, Mechanisms of formation, genotoxicity, and mutation of guanine oxidation products, Chem. Res. Toxicol. 19 (2006) 491–505. [DOI] [PubMed] [Google Scholar]
- [66].Delaney MO, Wiederholt CJ, Greenberg MM, Fapy.dA induces nucleotide misincorporation translesionally by a DNA polymerase, Angew. Chem. Int. Ed. Engl. 41 (2002) 771–773. [DOI] [PubMed] [Google Scholar]
- [67].Gasparutto D, Ait-Abbas M, Jaquinod M, Boiteux S, Cadet J, Repair and coding properties of 5-hydroxy-5-methylhydantoin nucleosides inserted into DNA oligomers, Chem. Res. Toxicol. 13 (2000) 575–584. [DOI] [PubMed] [Google Scholar]
- [68].Gasparutto D, Muller E, Boiteux S, Cadet J, Excision of the oxidatively formed 5-hydroxyhydantoin and 5-hydroxy-5-methylhydantoin pyrimidine lesions by Escherichia coli and Saccharomyces cerevisiae DNA N-glycosylases, Biochim. Biophys. Acta. 1790 (2009) 16–24. [DOI] [PubMed] [Google Scholar]
- [69].McDonald JP, Hall A, Gasparutto D, Cadet J, Ballantyne J, Woodgate R, Novel thermostable Y-family polymerases: applications for the PCR amplification of damaged or ancient DNAs, Nucleic Acids Res. 34 (2006) 1102–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].d’Abbadie M, Hofreiter M, Vaisman A, Loakes D, Gasparutto D, Cadet J, Woodgate R, Pääbo S, Holliger P, Molecular breeding of polymerases for amplification of ancient DNA. Nat. Biotechnol. 25 (2007) 939–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Dizdaroglu M, Oxidatively induced DNA damage and its repair in cancer, Mutat. Res./Reviews Mutat. Res. 763 (2015) 212–245. [DOI] [PubMed] [Google Scholar]
- [72].K Ellegaard P, E Poulsen H, Tobacco smoking and oxidative stress to DNA: a meta-analysis of studies using chromatographic and immunological methods, Scand. J. Clin. Lab. Invest. 76(2016), 151–158. [DOI] [PubMed] [Google Scholar]
- [73].Valavanidis A, Vlachogianni T, Fiotakis C, 8-hydroxy-2’-deoxyguanosine (8-OHdG): A critical biomarker of oxidative stress and carcinogenesis, J. Environ. Sci. Health. C. Environ. Carcinog. Ecotoxicol. Rev 27(2009),120–139 [DOI] [PubMed] [Google Scholar]
- [74].Cui J, Shao L, Young LT, Wang JF, Role of glutathione in neuroprotective effects of mood stabilizing drugs lithium and valproate, Neuroscience 144 (2007)1447–1453. [DOI] [PubMed] [Google Scholar]
- [75].Andreazza AC, Kauer-Sant’Anna M, Frey BN, Stertz L, Zanotto C, Ribeiro L, Giasson K, Valvassori SS, Réus GZ, Salvador M, Quevedo J, Gonçalves CA, Kapczinski F, Effects of mood stabilizers on DNA damage in an animal model of mania, J. Psychiatry Neurosci. 33 (2008) 516. [PMC free article] [PubMed] [Google Scholar]
- [76].Shao L, Young LT, Wang JF, Chronic treatment with mood stabilizers lithium and valproate prevents excitotoxicity by inhibiting oxidative stress in rat cerebral cortical cells, Biol. Psychiatry 58 (2005) 879–884. [DOI] [PubMed] [Google Scholar]
- [77].Valvassori SS, Resende WR, Lopes-Borges J, Mariot E, Dal-Pont GC, Vitto MF, Luz G, de Souza CT, Quevedo J, Effects of mood stabilizers on oxidative stress-induced cell death signaling pathways in the brains of rats subjected to the ouabain-induced animal model of mania: Mood stabilizers exert protective effects against ouabain-induced activation of the cell death pathway, J. Psychiatr. Res. 65 (2015) 63–70. [DOI] [PubMed] [Google Scholar]
- [78].Banerjee U, Dasgupta A, Rout JK, Singh OP, Effects of lithium therapy on Na+–K+-ATPase activity and lipid peroxidation in bipolar disorder, Prog. Neuropsychopharmacol. Biol. Psychiatry. 37 (2012) 56–61. [DOI] [PubMed] [Google Scholar]
- [79].Frey BN, Valvassori SS, Réus GZ, Martins MR, Petronilho FC, Bardini K, Dal-Pizzol F, Kapczinski F, Quevedo J, Effects of lithium and valproate on amphetamine-induced oxidative stress generation in an animal model of mania, J. Psychiatry and Neurosci. 31 (2006) 326. [PMC free article] [PubMed] [Google Scholar]