Abstract
Introduction:
In the phase 3 PACIFIC study of patients with unresectable stage III NSCLC without progression after chemoradiotherapy, durvalumab demonstrated significant improvements versus placebo in the primary end points of progression-free survival (hazard ratio [HR] = 0.52, 95% confidence interval [CI]: 0.42–65, p < 0.0001) and overall survival (OS) (HR = 0.68, 95% CI: 0.53–0.87, p = 0.00251), with manageable safety and no detrimental effect on patient-reported outcomes. Here, we report 3-year OS rates for all patients randomized in the PACIFIC study.
Methods:
Patients, stratified by age, sex, and smoking history, were randomized (2:1) to receive durvalumab, 10 mg/kg intravenously every 2 weeks, or placebo for up to 12 months. OS was analyzed by using a stratified log-rank test in the intention-to-treat population. Medians and rates at 12, 24, and 36 months were estimated by the Kaplan-Meier method.
Results:
As of January 31, 2019, 48.2% of patients had died (44.1% and 56.5% in the durvalumab and placebo groups, respectively). The median duration of follow-up was 33.3 months. The updated OS remained consistent with that previously reported (stratified HR = 0.69 [95% CI: 0.55– 0.86]); the median OS was not reached with durvalumab but was 29.1 months with placebo. The 12-, 24- and 36- month OS rates with durvalumab and placebo were 83.1% versus 74.6%, 66.3% versus 55.3%, and 57.0% versus 43.5%, respectively. All secondary outcomes examined showed improvements consistent with previous analyses.
Conclusions:
Updated OS data from PACIFIC, including 3-year survival rates, demonstrate the long-term clinical benefit with durvalumab after chemoradiotherapy and further establish the PACIFIC regimen as the standard of care in this population.
Keywords: Durvalumab, NSCLC, Overall survival, PACIFIC, Three-year update
Introduction
NSCLC is one of the leading causes of mortality worldwide; in approximately 30% of patients, it is diagnosed when they already have stage III disease, which is often unresectable.1–3 The historical standard of care for patients with unresectable stage III NSCLC was platinum-based doublet chemotherapy concurrent with radiotherapy (chemoradiotherapy [CRT]) with curative intent; however, patient prognosis with this treatment was poor, with 5-year survival rates of approximately 15 to 30%.4,5 In 2017, management of stage III NSCLC changed with publication of results from the PACIFIC trial and subsequent worldwide health authority approvals of durvalumab in this setting.
Durvalumab is a selective, high-affinity, human immunoglobulin G1 monoclonal antibody that blocks programmed cell death ligand 1 (PD-L1) from binding to programmed death 1 and CD80, resulting in antitumor T-cell activity.6 In the phase III PACIFIC study (NCT02125461) of durvalumab versus placebo in patients with unresectable stage III NSCLC who did not progress while undergoing CRT, durvalumab demonstrated significant improvements in the primary end points of progression-free survival (PFS) (hazard ratio [HR] = 0.52, 95% confidence interval [CI]: 0.42–0.65, p < 0.0001) and overall survival (OS) (HR 0.68, 95% CI: 0.53–0.87, p = 0.00251).7–9 With immune-mediated adverse events occurring in 24.2% and 8.1% of patients in the durvalumab and placebo groups, respectively, but with similar rates of grade 3 or 4 immune-mediated adverse events (3.4% and 2.6%), safety was manageable with durvalumab,7 and durvalumab had no detrimental effect on patient-reported outcomes.10 These results have led to the approval of durvalumab for patients with unresectable stage III NSCLC who have not progressed while undergoing CRT9,11 and use of the PACIFIC regimen (CRT followed by durvalumab) as the new standard of care in this setting.
Here, we report updated OS outcomes from PACIFIC, approximately 3 years after the last patient was randomized to this trial, to provide insight into the durability of the effect of durvalumab.
Methods
Study Design
The PACIFIC study design, eligibility criteria, and assessments have been fully described previously.7,8 Eligible patients had histologically and/or cytologically documented stage III unresectable NSCLC, with a WHO performance score of 0 or 1. Patients had to have received at least two cycles of platinum-based chemotherapy concurrently with definitive radiation therapy without progression, and the last radiation dose was administered 1 to 42 days before randomization. Tumor tissue collection was not a prerequisite for inclusion in PACIFIC and enrollment was not restricted to any threshold levels for PD-L1 expression. Patients were randomized 2:1 to receive durvalumab, 10 mg/kg intravenously, or placebo every 2 weeks for up to 12 months or until confirmed disease progression, initiation of alternative cancer therapy, unacceptable toxicity, or consent withdrawal. Randomization was stratified by age of the patient (<65 years versus ≥65 years), sex, and smoking history (current or former smoker versus never smoked).
End Points and Assessments
In this post hoc, exploratory analysis, we report data from up to January 31, 2019, the data cutoff (approximately 3 years after the last patient was randomized), including an update of the primary end point OS (defined as the time from randomization until death from any cause); the OS rates at the landmarks of 12, 24, and 36 months; the time to first subsequent therapy or death and time to second subsequent therapy or death; and the types of postdiscontinuation disease-related anticancer therapies administered. In addition, analyses of OS by PD-L1 expression levels on tumor cells (TCs) (based on PD-L1 testing of pre-CRT archived tumor tissue by using the Ventana SP263 immunohistochemistry assay) was performed with use of prespecified (25%) and exploratory post hoc (1%) PD-L1 cutoffs. Safety data were not collected at this data cutoff.
Statistical Analysis
This post hoc analysis of efficacy end points included all patients who underwent randomization according to the intention-to-treat principle. For OS, the effect of durvalumab as compared with that of placebo was estimated and the HR and corresponding 95% CI were reported. Between-group comparisons were performed by using a stratified log-rank test, with the stratification factors consistent with those used for randomization (age, sex, and smoking history). For all planned analyses of OS in the prespecified and post hoc subgroups, an unstratified Cox regression model was used to calculate HR and 95% CI. No adjustment for multiple comparisons was performed for these subgroup analyses. The medians and percentages of patients alive (OS rates) at 12, 24, and 36 months were estimated by the Kaplan-Meier method.
Results
A total of 713 patients were randomized, of whom 709 received treatment (durvalumab [n = 473] or placebo [n = 236]); the last patient had completed the protocol-defined 12 months of study treatment in May 2017. Baseline characteristics were well balanced in the two treatment groups, as previously reported.7,8
As of January 31, 2019 (data cutoff), 48.2% of patients had died (44.1% and 56.5% in the durvalumab and placebo groups, respectively [see Supplementary Fig. 1 for patient disposition]). The median duration of follow-up was 33.3 months (range 0.2–51.3). In total, 45 new OS events had been reported since the primary analysis of OS (data cutoff March 22, 2018). The updated OS benefit with durvalumab compared with placebo was consistent with the primary analysis result,8 with a 31% reduction in the risk of death (HR = 0.69 [95% CI: 0.55– 0.86] [Fig. 1]). The Kaplan-Meier estimate of the median OS was 29.1 months in the placebo group, whereas it was still not reached in the durvalumab group (see Fig. 1). The 12-, 24-, and 36-month OS rates with durvalumab and placebo were 83.1% versus 74.6%, 66.3% versus 55.3%, and 57.0% versus 43.5%, respectively. In addition, the updated subgroup analysis of OS, including by PD-L1 status (Fig. 2), was consistent with that reported at the time of the primary OS analysis.8
Figure 1.
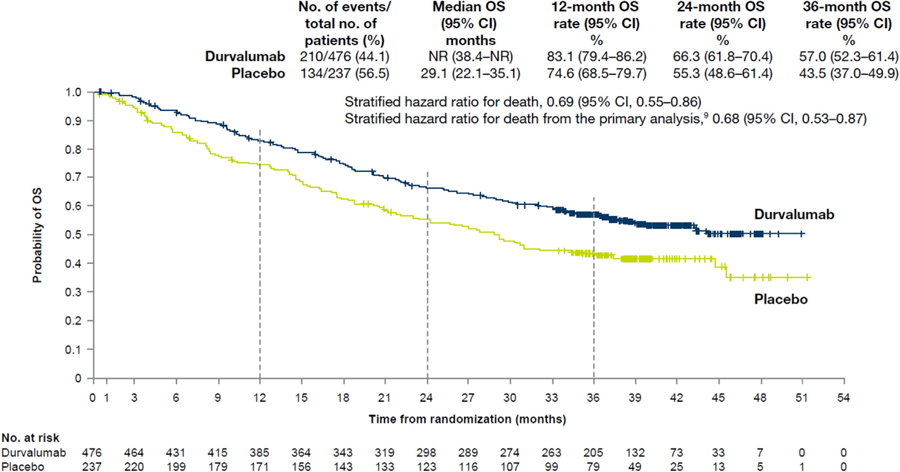
Updated analysis of overall survival (OS) in the intention-to-treat population. Shown are Kaplan-Meier curves for OS. The tick marks indicate censored data, and the dashed vertical lines indicate the times of landmark analyses of OS. The intention-to-treat population included all the patients who underwent randomization. CI, confidence interval; NR, not reached.
Figure 2.
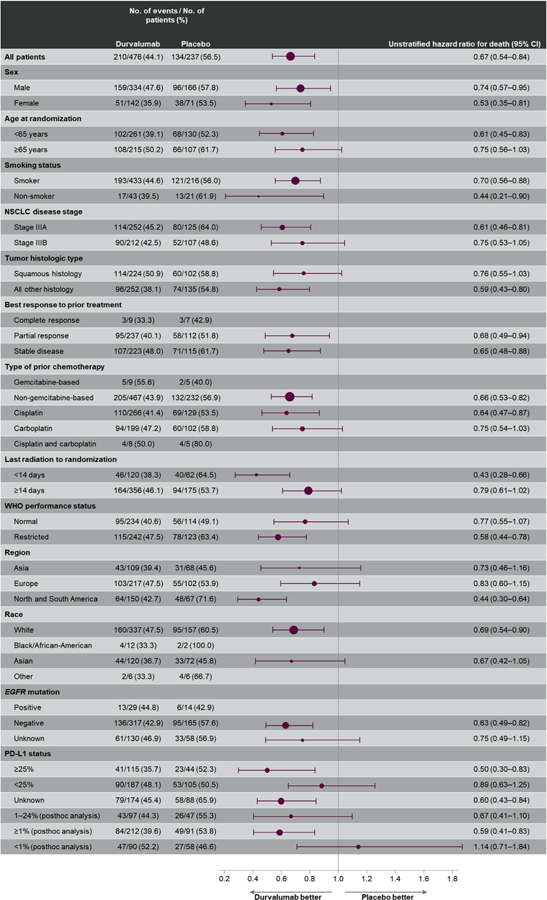
Updated overall survival by prespecified and post hoc exploratory subgroups in the intention-to-treat population.*
(*Hazard ratio and 95% confidence interval [CI] were not calculated if the subgroup had <20 events.) PD-L1, programmed death ligand 1.
After discontinuation of the study treatment, 43.3% and 57.8% in the durvalumab and placebo groups, respectively, received subsequent anticancer therapy (Table 1); in total, 9.7% and 26.6%, respectively, subsequently received immunotherapy (primarily, nivolumab or pembrolizumab). Consistent with the results reported at the time of the primary OS analysis, time to first subsequent therapy or death was markedly longer with durvalumab than with placebo (HR = 0.58 [95% CI: 0.47–0.71] [Supplementary Fig. 2A]), as was time to second subsequent therapy or death (HR = 0.61 [95% CI: 0.49–0.75] [Supplementary Fig. 2B]).
Table 1.
Postdiscontinuation Disease-Related Anticancer Therapy (Intention-to-Treat Population)
| Therapy | Patients, n (%) | |
|---|---|---|
| Receiving Durvalumab (n = 476) | Receiving Placebo (n = 237) | |
| Any therapy | 206 (43.3) | 137 (57.8) |
| Radiotherapy | 89 (18.7) | 60 (25.3) |
| Immunotherapya | 46 (9.7) | 63 (26.6) |
| Cytotoxic chemotherapy | 138 (29.0) | 81 (34.2) |
| Other systemic therapyb | 50 (10.5) | 34 (14.3) |
| Oher | 1 (0.2) | 0 |
Primarily, nivolumab (durvalumab [n = 33] and placebo [n = 52]) or pembrolizumab (durvalumab [n = 10] and placebo [n = 8]).
Including tyrosine kinase inhibitors, among other treatments.
Discussion
The updated results from PACIFIC (approximately 3 years from randomization of the last patient) demonstrate that the clinical benefits of durvalumab, in terms of OS and time to first and second subsequent therapy or death, are maintained over the longer term. Importantly, more than 50% of patients receiving durvalumab were alive at 36 months (specifically, 57.0% versus 43.5% receiving placebo). Improvement in OS with durvalumab versus placebo was observed across most patient subgroups, including those based on disease stage, tumor histologic type, and smoking status, supporting the use of durvalumab in this patient setting. However, some subgroups were small and not powered to assess efficacy, nor were they necessarily balanced with respect to other baseline characteristics. Therefore, future investigation of potential biomarkers may be warranted.
Improved OS with durvalumab was broadly observed irrespective of PD-L1 expression, which is consistent with findings from prespecified and post hoc analyses carried out at the time of the primary OS analysis.8 This includes patients with unknown PD-L1 expression status, for whom median OS was improved by more than 20 months (versus placebo) at this update (data not shown, manuscript in preparation). An exception was the PD-L1 less than 1% subgroup (post hoc analysis), which numerically favored placebo at this update (HR = 1.14 [95% CI: 0.71–1.84]) and at the time of the primary OS analysis (HR = 1.36 [95% CI: 0.79–2.34]). Previous analysis of the placebo arm in the PD-L1 TC less than 1% subgroup that suggested overperformance was confirmed in this analysis (data not shown, manuscript in preparation). The relatively small size of the PD-L1 TC less than 1% subgroup (n = 148), the fact that not all patients in PACIFIC could provide a suitable tumor sample for assessment of PD-L1 expression (63% were PD-L1–evaluable7,8), and the fact that PD-L1 data were based on pre-CRT samples, which may not reflect changes in expression potentially incurred by CRT, should also be taken into consideration when drawing definitive conclusions. PACIFIC was not designed to evaluate the efficacy of durvalumab based on PD-L1 status.
The observation that proportionally fewer patients in the durvalumab arm received subsequent anticancer treatment is likely underpinned by fewer progression events with durvalumab than with placebo. Notably, among patients who received subsequent anticancer therapy, a greater proportion of patients in the placebo arm received subsequent immunotherapy. Otherwise, as reported elsewhere,12 first subsequent treatment type was generally similar between the treatment arms; platinum-doublet chemotherapy was the most common, in part because the study was conducted in the pre–immune checkpoint inhibitor era. Baseline patient and disease characteristics were broadly similar irrespective of treatment arm and use of a first subsequent anticancer treatment.12
Overall, the findings of this analysis underscore the long-term survival benefit with durvalumab after CRT and further establish the PACIFIC regimen as the standard of care in patients with unresectable stage III NSCLC who do not progress while undergoing CRT.
Supplementary Material
Acknowledgments
The authors would like to thank the participating patients, their families, and their caregivers, as well as the PACIFIC investigators and the clinical trial personnel at the 235 centers in 26 countries. This study (NCT02125461) was sponsored by AstraZeneca. Medical writing support, which was in accordance with Good Publication Practice guidelines, was provided by Andrew Gannon, MS, MA, and Hashem Dbouk, PhD, of Cirrus Communications (New York, NY), an Ashfield company, and was funded by AstraZeneca.
Footnotes
Disclosure: Dr. Gray has received research funding, honoraria, and advisory fees from AstraZeneca. Dr. Villegas has received honoraria from AstraZeneca, Gilead, and Seattle Genetics. Dr. Daniel has received institutional research funding from E.R. Squibb and Sons, AstraZeneca, Boehringer Ingelheim, Genentech, Eli Lilly and Company, Novartis Pharmaceuticals, Pfizer, Celgene, and Roche. Dr. Hui has received advisory fees and honoraria from AstraZeneca, Merck Sharp and Dohme, Novartis, Roche, Bristol-Myers Squibb, and Eli Lilly and Company. Dr. Kurata has received research funding and honoraria from AstraZeneca. Dr. Chiappori has received speaker bureau funding from Genentech, Merck, Takeda, Novartis, Boehringer Ingelheim, and Celgene and research funding from Novartis and Bristol-Myers Squibb. Dr. Planchard has received advisory or lecture fees from AstraZeneca, Bristol-Myers Squibb, Boehringer Ingelheim, Celgene, Daiichi Sankyo, Eli Lilly, Merck, MedImmune, Novartis, Pfizer, prIME Oncology, Peer CME, and Roche; honoraria from AstraZeneca, Bristol-Myers Squibb, Boehringer Ingelheim, Celgene, Eli Lilly and Company, Merck, Novartis, Pfizer, prIME Oncology, Peer CME, and Roche; institutional research funding from AstraZeneca, Bristol-Myers Squibb, AbbVie, Boehringer Ingelheim, Eli Lilly and Company, Merck, Novartis, Pfizer, Roche, Medimmune, Sanofi-Aventis, Taiho Pharma, Novocure, and Daiichi Sankyo; and travel, accommodations, or expenses from AstraZeneca, Bristol-Myers Squibb, Boehringer Ingelheim, Roche, Merck, Novartis, prIME Oncology, and Pfizer. Dr. Paz-Ares is a board member of Genomica and has received honoraria from Roche/Genentech, Eli Lilly and Company, Pfizer, Boehringer Ingelheim, Bristol-Myers Squibb, Merck Sharp and Dohme, AstraZeneca, Merck Serono, Pharmamar, Novartis, Celgene, Sysmex, Amgen, and Incyte, as well as travel, accommodations, or expenses from Roche, AstraZeneca, AstraZeneca Spain, Merck Sharp and Dohme, Bristol-Myers Squibb, Eli Lilly and Company, and Pfizer. Dr. Faivre-Finn has received research funding and travel support from Merck, AstraZeneca, and Elekta and travel support only from Pfizer. Dr. Vansteenkiste has received institutional research funding from Merck Sharp and Dohme; advisory fees from Apotex, AstraZeneca, Boehringer Ingelheim, Merck Sharp and Dohme, Novartis, and Roche; and honoraria from AstraZeneca, Bristol-Myers Squibb, Merck Sharp and Dohme, and Roche. Ms. Taboada and Dr. Dennis are full-time employees of AstraZeneca with stock ownership. Ms. Wadsworth was a full-time employee of AstraZeneca when the work was conducted.
The remaining authors declare no conflict of interest.
Trial Registration: A Global Study to Assess the Effects of MEDI4736 following Concurrent Chemoradiation in Patients with Stage III Unresectable Non–Small Cell Lung Cancer. NCT02125461
Supplementary Data
Note: To access the supplementary material accompanying this article, visit the online version of the Journal of Thoracic Oncology at www.jto.org and at https://doi.org/10.1016/j.jtho.2019.10.002.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- 2.Walters S, Maringe C, Coleman MP, et al. Lung cancer survival and stage at diagnosis in Australia, Canada, Denmark, Norway, Sweden and the UK: a population-based study, 2004‒2007. Thorax 2013;68:551–564. [DOI] [PubMed] [Google Scholar]
- 3.Quint LE. Lung cancer: assessing resectability. Cancer Imaging 2003;4:15–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yoon SM, Shaikh T, Hallman M. Therapeutic management options for stage III non-small cell lung cancer. World J Clin Oncol 2017;8:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bradley JD, Hu C, Komaki RU, et al. Long-term results of RTOG 0617: a randomized phase 3 comparison of standard dose versus high dose conformal chemoradiation therapy ± cetuximab for stage III NSCLC. Int J Radiat Oncol Biol Phys 2017;99(suppl):S105. [Google Scholar]
- 6.Stewart R, Morrow M, Hammond SA, et al. Identification and characterization of MEDI4736, an antagonistic anti-PD-L1 monoclonal antibody. Cancer Immunol Res 2015;3:1052–1062. [DOI] [PubMed] [Google Scholar]
- 7.Antonia SJ, Villegas A, Daniel D, et al. Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N Engl J Med 2017;377:1919–1929. [DOI] [PubMed] [Google Scholar]
- 8.Antonia SJ, Villegas A, Daniel D, et al. Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N Engl J Med 2018;379:2342–2350. [DOI] [PubMed] [Google Scholar]
- 9.European Medicines Agency. Durvalumab (Imfinzi). Summary of product characteristics 2018. https://www.ema.europa.eu/en/documents/product-information/imfizi-epar-product-information_en.pdf. Accessed April 22, 2019.
- 10.Hui R, Özgüroğlu M, Villegas A, et al. Patient-reported outcomes with durvalumab after chemoradiotherapy in stage III, locally advanced, unresectable non-small cell lung cancer: results from the randomised phase 3 PACIFIC study. Lancet, in press. [DOI] [PubMed]
- 11.U.S. Food and Drug Safety Administration. IMFINZI (durvalumab) label 2018. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/761069s002lbl.pdf. Accessed April 22, 2019.
- 12.Planchard D, Cho BC, Gray JE, et al. First subsequent treatment after discontinuation of durvalumab in unresectable, stage III NSCLC patients from PACIFIC [abstract]. Poster presented at: American Society of Clinical Oncology Annual Meeting. May 31‒June 4, 2019; Chicago, IL. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


