Abstract
Objective
Universal Newborn Hearing Screening (UNHS) uses otoacoustic emissions testing (OAE) and auditory brainstem response testing (ABR) to screen all newborn infants for hearing loss (HL), but may not identify infants with mild HL at birth or delayed onset HL. The purpose of this review is to examine the role of genetic screening to diagnose children with pre-lingual HL that is not detected at birth by determining the rate of children who pass UNHS but have a positive genetic screening. This includes a summary of the current UNHS and its limitations, and a review of genetic mutations and screening technologies used to detect patients with an increased risk of undiagnosed pre-lingual HL.
Design
Literature review of studies that compare UNHS with concurrent genetic screening
Study Sample
Infants and children with hearing loss
Results
Sixteen studies were included encompassing 137,895 infants. Pathogenic mutations were detected in 8.66% of patients. In total, 545 patients passed the UNHS but had a positive genetic screening. The average percentage of patients who passed UNHS but had a positive genetic screening was 1.4%.
Conclusion
This review demonstrates the positive impact of concurrent genetic screening with UNHS to identify patients with pre-lingual HL.
Keywords: congenital hearing loss, pre-lingual hearing loss, newborn hearing screen, genetic screen, hereditary hearing loss, non-congenital pre-lingual hearing loss
Introduction
Hearing loss (HL) is the most common sensory disorder, affecting 32 million children worldwide [1]. In the United States, the incidence of hearing loss at birth is estimated at 1.5 per 1000 newborns, and rises in school-aged children [2], corresponding to approximately 7 million US children with hearing loss [3]. The etiology of childhood HL is variable, but up to 50% of pre-lingual HL in developed countries is thought to be genetic [4, 5]. Non-syndromic hearing loss accounts for the majority of genetic cases, and over 100 mutations in at least 44 genes have been identified to increase the risk for HL [5].
Early detection of hearing loss in infancy and childhood can prevent and avoid devastating effects on speech and language development. Stimulation of the auditory cortex before 12 months of age is essential for language development [6]. Universal Newborn Hearing Screening (UNHS) was developed nearly two decades ago to screen infants born in U.S. hospitals for congenital HL [7]. Infants typically undergo otoacoustic emissions testing (OAE), auditory brainstem response testing (ABR), or both prior to discharge. Infants who fail the initial screening or have a recognized risk factor for later onset HL are referred for further testing. Since its inception in 1998, the UNHS is considered a public health success, reducing the average age of diagnosis for most children with congenital or early-onset HL [7]. However, the current UNHS is not as effective at identifying patients with mild HL or with delayed onset pre-lingual HL. Furthermore, the current UNHS does not determine the etiology of HL.
Continued research in genetic technology has created opportunities to improve the current screening model. The development of HL-specific genetic screening panels have allowed researchers to screen for multiple HL mutations in less time and for less cost than was previously possible with direct Sanger sequencing [5, 6]. The literature is expanding with several studies that have examined the addition of genetic screening to UNHS on both a small and a large scale.
This paper includes a review of current UNHS in the Unites States and its limitations for identifying all cases of pre-lingual HL. A brief discussion of current genetic screening technologies, including NGS, is also included, as well as a summary of the most common mutations associated with pre-lingual HL. A literature review was conducted on the addition of genetic screening to UNHS, and we include a discussion of the implication of genetic testing on the future of UNHS.
Methods
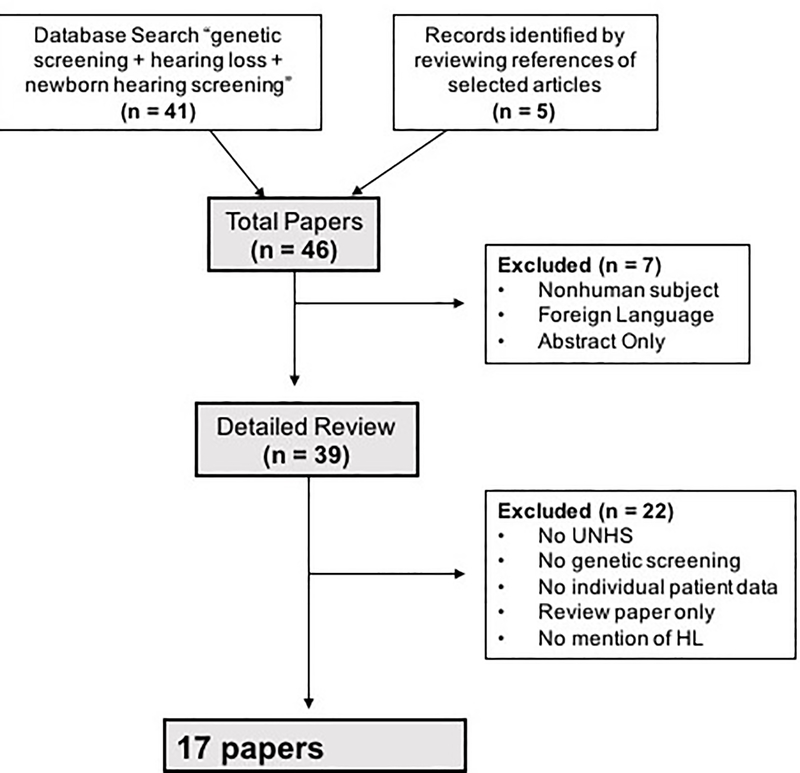
A literature review was conducted to identify papers discussing the application of genetic testing in addition to UNHS. A PubMed search was carried out using the search headings “genetic testing hearing loss” and “newborn hearing screening” from 1980 to the present. The search was later expanded to include key words for genetic testing and delayed HL. Additional papers were culled from the references of relevant search results when they discussed the genetic etiology of delayed onset HL or NGS technology. Exclusion criteria included foreign language papers, non-human subject research, and review papers without patient data. Figure 1 describes the process of selecting papers for analysis in this review. For the purposes of this review, the authors define undiagnosed pre-lingual HL as infants that pass screening with UNHS but later develop HL. In the included studies, this concept is represented by patients who passed the UNHS but had a positive genetic screening. When applicable, median, mean, and weighted averages were calculated using Microsoft Excel.
Figure 1: Study Selection.
The flowchart represents how studies were selected for inclusion and review in this paper.
To clarify terminology used in this review, patients who do not have HL identified on UNHS will be referred to as “UNHS pass”. Patients who are referred for audiology or otolaryngology follow-up on UNHS are hereby referred to as “UNHS refer”. Patients who do not have genetic mutations for HL identified on genetic screening are “negative genetic screening”, and patients who do have a mutation identified are described as “positive genetic screening”.
Results
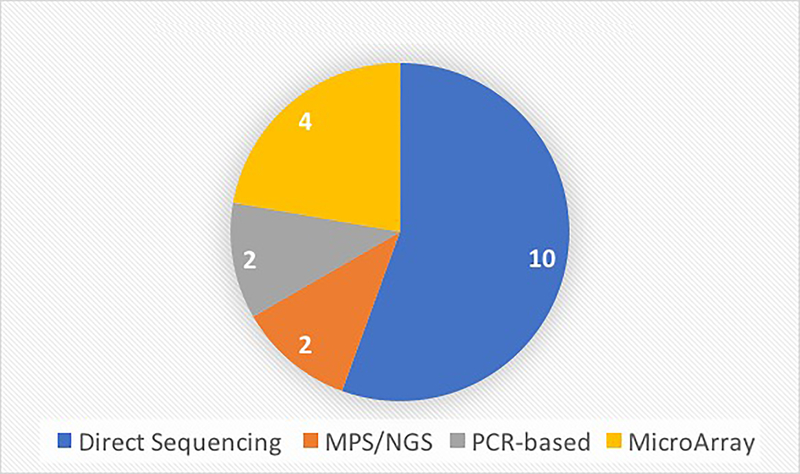
This review includes 16 relevant studies involving genetic testing in children or infants that had undergone UNHS. These studies are summarized in Table 1. The majority of the studies originated from Asia, with 6 from China alone. Three studies included were from the United States. The majority of studies (10) used direct sequencing to analyze results, although in many cases direct sequencing was used as the secondary method to verify results. Microarray screening and NGS were also commonly used, with 4 and 2 studies respectively incorporating these technologies (Figure 2). The median number of patients per study was 4,427, and in total 137,895 patients were studied. One study was a single case report. Eleven studies described the pass/refer rate of UNHS. The weighted average UNHS pass rate was 91.01% and the weighted average refer rate was 6.21%. Twelve studies reported a genetic testing mutation detection rate which ranged from 0.70% to 41% depending on the population and the testing method. The weighted average genetic mutation detection rate (“positive genetic screening”) among all applicable studies was 8.66%. Using the weighted average UNHS refer rate and the weighted average positive genetic screening rate to determine the utility of the addition of genetic screening, the authors calculated the absolute risk reduction and number needed to treat to be 0.238 and 42, respectively. Thus, the addition of genetic screening to the UNHS may detect an additional at-risk infant for every 42 infants screened. However, please note that the data aggregated to calculate these numbers come from various studies with different genetic screening techniques.
Table 1: Studies with UNHS and Concurrent Genetic Screening.
This table summarizes the 16 studies included in this review. After an extensive literature review, these studies analyzed the findings of infants who underwent UNHS and concurrent genetic screening.
| Title | Author | Year | Country of Origin | Number of Patients | Type of genetic testing | Genes Screened (Number of mutations) | UNHS Results (Pass/Fail) | Genetic Fail | Genetic Screen Results | UNHS pass/genetic fail % |
|---|---|---|---|---|---|---|---|---|---|---|
| Prevalence of A1555G mitochondrial mutation in Chinese newborns and the correlation with neonatal hearing screening | Chen et al. | 2011 | China | 865 | Direct sequencing | mtDNA 12s rRNA (mt1555G>A) | 84.5% (n=731)/15.5% (n=134) | 0.7% (n=6) | All homoplasmic mutations | 0.69 |
| GJB2-associated hearing loss undetected by hearing screening of newborns | Minami et al. | 2013 | Japan | 924 | Direct sequencing | GJB2 | 22.1% (204/924) with pathologic biallelic mutations. 18 different mutations identified | 1.5 | ||
| Newborn Genetic Screening for Hearing Impairment: A Preliminary Study at a Tertiary Center | Wu et al. | 2011 | Taiwan | 1017 | Next Generation Sequencing | GJB2 (2), SLC26A4 (1), mtDNA 12S rRNA (1) | 96.2%(n=979)/3.8% (n=38) | 19.6% (n=199) | 11 homozygous for GJB2 p.V37I, 6 compound heterozygous for GJB2 p.V37I and c.235delC, 1 homoplasmic for m.1555A.G. | 0.88 |
| Incidence of the 35delG/GJB2 mutation in low-risk newborns | Zaputovic et al. | 2008 | Croatia | 1048 | Direct sequencing | GJB2 (c.35delG mutation) | 99.7% (n=1045)/0.3% (n=3) | 1.3% (n=14) with mutation; 13/14 biallelic, 1/14 mono-allelic | 1.24 | |
| Evaluation of newborn screening bloodspot-based genetic testing as second tier screen for bedside newborn hearing screening | Schimmenti et al. | 2011 | United States | 2354 | Direct sequencing | GJB2 (c.35delG, c.167delT, c.235delC, and p.V37I mutations) | Case-control study with 50%/50% selected | 10.88% (n=139) | 0.98% (n=23) of all infants had bi-allelic GJB2 mutations, 9.9% (n=116) of all infants had mono-allelic mutations (carrier, not affected) | 0.04 |
| Newborn genetic screening for hearing impairment: a population-based longitudinal study | Wu et al. | 2016 | Taiwan | 5173 | Next Generation Sequencing | GJB2 (2), SLC26A4 (1), mtDNA 12S rRNA (1) | 94% (n=5008)/6% (n=165) | 16.2% (n=839 ) | 736 heterozygous for GJB2 p.V37I, 50 heterozygous for GJB2 c.235delC, 53 heterozygous for SLC26A4 c.919–2A>G). 82 (1.6%) babies with causative genotypes, 62 GJB2 p.V37I/p.V37I, 16 GJB2 p.V37I/c.235delC, 4 m.1555A>G | 0.89 |
| SNaPshot reveals high mutation and carrier frequencies of 15 common hearing loss mutants in a Chinese newborn cohort | Chen et al. | 2015 | China | 5800 | SNaPshot Multiplex System; Sanger sequencing | GJB2 (5), SLC26A4 (5), mtDNA 12s rRNA (5) | 15.9% (n=923) | GJB2 mutants accounted for up to 12.7% (735/5800). In addition, 2.17% (126/5800) had at least one mutant allele of SLC26A4, and 1.07% (62/5800) were carriers of a mitochondrial mutant allele. | 0.36 | |
| Newborn hearing screening and genetic testing in 8974 Brazilian neonates | Nivoloni et al. | 2010 | Brazil | 8974 | Direct sequencing | GJB2 (1), mtDNA 12s rRNA (2) | 99.8%(n=8957)/0.2%(n=17) | 84/8974 (0.94%) heterozygous for 35delG, 4/8974 (0.04%) homozygous for this mutation, no other GJB2 mutations found. Among 17 newborns who failed in TOAE, 4/17 were 35delG homozygous, confirming genetic etiology deafness (23.5%). 3/17 possessed 827A>G mtDNA mutation | ||
| Concurrent Genetic and Standard Screening for Hearing Impairment in 9317 Southern Chinese Newborns | Peng et al. | 2016 | China | 9317 | MALDI-TOF-MS PCR based array | GJB2 (5), GJB3 (2), SLC26A4 (11), mtDNA 12S rRNA (2) | 88.45% (n=8241)/11.55% (n=1076) | 3.74% of all infants (n=348) with at least 1 mutation. Only 0.36% (n=34) with causal mutation. | 310 heterozygous, 10 compound heterozygous, 28 homozygous. 30 mtDNA 12S rRNA, 1 GJB2 c.235delC homozyous, 3 SLC26A4 compound heterozygous. | 0.311 |
| Auditory screening concurrent deafness predisposing genes screening in 10,043 neonates in Gansu province, China | Zhang et al. | 2012 | China | 10043 | Restriction endonuclease; Direct sequencing | mtDNA 12sRNA, SLC26A4, GJB2 | 85.6%/14.4% | 2.29% (230/10,043) with at least 1 mutation | 21/230 genetically referred (18 mtDNA 12S rRNA mutation, 2 GJB2 c. [235delC] + [235- delC] homozygotes, 1 SLC26A4 c.[919–2A>G] + 12S rRNA 1555A>G compound type) | 1.91 |
| Newborn hearing concurrent gene screening can improve care for hearing loss: A study on 14,913 Chinese newborns | Wang et al. | 2011 | China | 14913 | Restriction endonuclease; Direct sequencing | mtDNA 12sRNA, SLC26A4, GJB2 | 86.1% (n=12,837)/13.9% (n=2073) | 2.1% (n=306) with at least 1 mutation | 26/306 were considered genetic refers (18 12S rRNA 1555A>G mutation, 5 GJB2 c.[235delC] + [235delC] homozygotes, 1 GJB2 c.[235delC] + [299delAT] compound heterozygote, 1 SLC26A4 c.[919–2A>G] + c.[2168 A>G] compound type, 1 compound type GJB2 c.[235delC] + SLC26A4 c.[919–2A>G]) | 0.13 |
| Government-funded universal newborn hearing screening and genetic analyses of deafness predisposing genes in Taiwan | Chu et al. | 2015 | Taiwan | 15345 | Direct sequencing | GJB2, GJB4, GJA1P1, GJB6, GJB3, GJA1, GJB1, GJC3, SLC26A4 | 97.32%(n=14934)/2.68%(n=411) | Only 26/32 with diagnosed hearing loss underwent genetic testing. Mutation detected in 38.5% (10/26). | 1 GJB3 c.580G>A, 1 GJB3 c.520G>A, 1 GJB2 c.235delC homozygote, 1 GJA1P1 c.929delC, 1 GJA1P1 novel variant c.1081C>T, 1 GJB4 c.302G>A, 1 GJA1 novel variant c.1–33C>G, 1 SLC26A4 c.919–2A>G + novel variant c.164+1G>C, 1 SLC26A4 c.919–2A>G, 1 SLC26A4 novel variant c.818C>T. | |
| Newborn hearing concurrent genetic screening for hearing impairment—A clinical practice in 58,397 neonates in Tianjin, China | Zhang et al. | 2013 | China | 58397 | MALDI-TOF-MS PCR based array | GJB2 (5), GJB3 (2), SLC26A4 (11), mtDNA 12s rRNA (2) | 89.1%(n=52020)/10.9%(n=6377) | 5.52% (n=3225) with at least one mutated allele | 0.25% (n=143) were referred (biallelic non carrier mutation) due to mitochondrial, homozygous GJB2, or homozygous SLC26A4 mutations. 5.28% (n=3082) infants were genetic mutation carriers. | 0.19 |
| Prenatal Diagnosis of SLC26A4 Mutation and Delayed Onset of Hearing Loss | Iwata et al. | 2012 | United States | 1 HL | Direct sequencing | SLC26A4 mutations G316X and 918–2A>G | Passed | Positive for both SLC26A4 mutations G316X and 918–2A>G | 100 | |
| Utility of Genetic Testing for the Detection of Late-Onset Hearing Loss in Neonates | Lim et al. | 2013 | United States | 3681 NSHL | SoundGene Panel | GJB6 deletion, GJB2 (4), SLC26A4 (4), mtDNA 12s rRNA (5); CMV PCR | 99.6% (n=3667)/0.14% (n=14) | 7.0% of all infants (n=259) with a mutation. Only 0.95% (n=35) with positive SoundGene panel | 1 GJB2, 16 mtDNA 12s rRNA, 10 SLC26A4, 3 compound heterozygote, 5 CMV. | 0.9 |
| Limitations of hearing screening in newborns with PDS mutations | Kim et al. | 2012 | Korea | 43 SNHL | Direct sequencing | SLC26A4 | All neonates with PDS pendrin mutation | 28.5 |
Figure 2: Type of Genetic Screening Technology Used.
The number of each major genetic screening technology used by the studies included in this review is represented here. The major technologies include direct sanger sequencing, next generation sequencing, microarray sequencing and PCR-based screening.
Table 2 describes results from the 16 studies that included data on patients that passed UNHS but had a positive genetic screening. In total, 545 patients met these criteria. The rate of UNHS pass/positive genetic screening ranged from 0.04% to 100% (the single case report), depending on population studied. The weighted average proportion across the studies of patients that passed UNHS but had a positive genetic screening was 1.4%.
Table 2: Screening Rates.
UNHS and genetic screening rates were calculated for the 16 studies included in this review.
| Genes & Mutations | Range of genes screened per study | 1 to 21 |
| Range of mutations screened per study | 1 to 82 | |
| Mean number of genes screened per study | 3.95 | |
| Mean number of mutations screened per study | 12.93 | |
| UNHS Results | Average UNHS “pass” | 92.76% |
| Average UNHS “refer” | 6.32% | |
| Weighted average UNHS “pass” | 91.01% | |
| Weighted average UNHS “refer” | 6.21% | |
| Mutation Detection | Range of mutation detection rate per study | 0.70% to 41% |
| Average mutation detection rate | 11.13% | |
| Weighted average mutation detection rate | 8.66% | |
| UNHS Pass/Genetic Negative | Range of UNHS pass/positive genetic screening detection rate | 0.04% to 100% |
| Average UNHS pass/positive genetic screening detection rate | 9.20% | |
| Weighted average UNHS pass/positive genetic screening detection rate | 1.40% | |
| Total number of UNHS pass/positive genetic screening patients | 545 |
This review also identified genes and mutations associated with pre-lingual HL, summarized in detail in Table 3. Currently, 73 mutations in 41 genes have been associated with pre-lingual HL of delayed diagnosis and have been documented in the literature. The genes most commonly associated with this phenotype are GJB2, SLC26A4, and 12s mitochondrial rRNA.
Table 3: Genes Associated with Pre-lingual Deafness of Delayed Diagnosis.
This comprehensive table summarizes genes associated with pre-lingual HL, which have been published in the literature.
| Gene | Gene Function | Common Mutation(s) | Inheritance | Ethnic Group | Description of Phenotype (onset) | Carrier Frequency or Prevalence | Reference |
|---|---|---|---|---|---|---|---|
| GJB2 | Gap junction in cochlea involved in ion recycling | c.109G>A (p.V37I) | AR | Chinese, Japanese | Mild to severe-profound, bilateral. Onset in first decade of life. | Control allele frequency 6.2% | Chen et al. 2014; Li et al. 2012; Minami et al. 2013 |
| c.235delC | AR | Japanese | Mild to severe-profound. Onset between 4 months and 6 years | Minami et al. 2013 | |||
| c.512ins4 | AR | Japanese | Profound-severe. Onset before 1 year. | Minami et al. 2013 | |||
| p.Y136X-p.G45E | AR | Japanese | Moderate-severe. Onset before 2 years | Minami et al. 2013 | |||
| c.176–191del16 | AR | Japanese | Moderate. Onset at 3 years. | Minami et al. 2013 | |||
| p.R143W | AR | Japanese | Moderate-profound. Onset before 2 years. | Minami et al. 2013 | |||
| SLC17A8 | Encodes a vesicular glutamate transporter in inner hair cells | c.616dupA (p.M206Nfs*4) | AD | Korean | Bilateral, severe | Novel mutation | Ryu et al. 2016 |
| c.632C>T (p.A211V) | AD | American, Czech and German descent | Progressive. Onset beginning at age 20. | Not found in controls | Ruel et al. 2008 | ||
| SLC26A4 | Transports chloride and iodine to maintain pH of endolymph | p.1652insT | AR | Japanese | Age of onset 1–2 years. | Miyagawa et al. 2014 | |
| p.H723R | AR | Japanese | Age of onset 1–10 years. | Miyagawa et al. 2014 | |||
| p.T527P | AR | Japanese | Age of onset 2–3 years. | Miyagawa et al. 2014 | |||
| p. M147V | AR | Japanese | Age of onset 2–3 years. | Miyagawa et al. 2014 | |||
| p.919–2A>G | AR | Japanese | Age of onset 1–14 years. | Miyagawa et al. 2014 | |||
| p.1707+5G>A | AR | Japanese | Age of onset 1–4 years. | Miyagawa et al. 2014 | |||
| KCNQ4 | Voltage gated potassium channel | c.211delC (P.Q71fs) | AD | Japanese | Mild-severe, high frequency, progressive. Patients presented at 7 and 13 years | Sakuma et al. | |
| c.664_681del | AD | Korean | Bilateral, symmetric, mild to profound, progressive. Onset beginning at 4 years. | Novel mutation | Baek et al. 2011 | ||
| MYO15A | Unconventional motor molecule | c.3756+1G>A; c.4660G>A (p.A1554T) | AR | Japanese | Profound, progressive. Presented at age 5. | Sakuma et al. | |
| c.9478C>T; 1179_1185insC | AR | Japanese | Progressive. Detected on NBHS | Miyagawa et al. 2013 | |||
| LOXHD1 | c.G3076G>T (p.V1026F); c.4375+1G>T | Sporadic | Japanese | Profound, downsloping, stable. Presented at age 3. | Sakuma et al. | ||
| USH2A | c.57_58del (p.M14fs); c.9079G4T (p.E3027X) | Sporadic | Japanese | Moderate, downsloping, progressive. Presented at age 10. | Sakuma et al. | ||
| WSF1 | Membrane glycoprotein that localizes to endoplasmic reticulum | c.2508G>T (p.K836N) | AD | Japanese | Moderate, U-shape, stable. Presented at age 5. | Sakuma et al. | |
| STRC | Stereocilia protein | CNV | AR | Japanese | Mild-moderate, progressive. Detected in early childhood | 1.1%−2.5% | Moteki et al. |
| ADCY1 | Adenylate cyclase 1 of hair cells | c.3112C>T (p.Arg1038*) | AR | Pakistani | Highly variable: Bilateral symmetric mild-moderate; asymmetric profound; mixed. | Novel mutation | Santos-cortez et al. 2014 |
| TBC1D24 | GTPase-activating protein expressed in the cochlea | c.208G>T (p.Asp70Tyr); c.878G>C (p.Arg293Pro) | AR | Pakistani | Prelingual; Severe-profound, downsloping or flat | Not found in controls | Rehman et al. 2014 |
| c.533C>T (p.Ser178Leu) | AD | European, Chinese | Progressive, downsloping. Age of onset 3rd decade | Not found in controls | Azaiez et al. 2014; Zhang et al. 2014 | ||
| DCDC2 | Kinocilium of sensory hair cells and cilia of supporting cells | c.1271A>C, p.Gln424Pro | AR | Tunisian | Congenital; Severe-profound | Not found in controls | Grati et al. 2015 |
| NARS2 | Mitochondrial asparaginyl-tRNAsynthetase | c.637G>T; p.Val213Phe | AR | Pakistani | Bilateral, severe-profound | Not found in controls | Simon et al. 2015 |
| MET | Receptor tyrosine kinase | c.2521T>G (p.F841V) | AR | Pakistani | Severe-profound. Hearing loss at or before 2 years of age | Not found in controls | Mujtaba et al. 2015 |
| TMEM132E | Mechanotransduction of hair cells | c.1259G>A, p.Arg420Gln | AR | Chinese | Pre-lingual; Bilateral, Severe-profound | Not found in controls | Li et al. 2015 |
| GRXCR2 | Devlopment of stereocilia bundles | c.714dupT (p.G239WfsX74) | AR | Pakistani | Moderate to severe, progressive. Onset at 2 years of age. | Not found in controls | Imtiaz et al. 2014 |
| EPS8 | EGFR pathway substrate 8, expressed in hair bundle in cochlea | c.88C > T (p.Gln30*) | AR | Algerian | Congenital; Profound; | Not found in controls | Behlouli et al. 2014 |
| CLIC5 | Vestibular function | c.96T4A (p.(Cys32Ter) | AR | Turkish | Pre-lingual; Progrssive from mild to severe before second decade, profound. Onset within first 2 years. | Not found in controls | Seco et al. 2014 |
| FAM65B | Plasma membrane associated protein of hair cell stereocilia | c.102–1G>A | AR | Turkish | Pre-lingual; Symmetric, profound, stable | Not found in controls | Diaz-Horta et al. 2014 |
| OSBPL2 | Intracellular lipid receptor | c.153_154delC (p.Gln53Argfs*100) | AD | Chinese | Bilateral, progressive. Age of onset 5–32 years. | Not found in controls | Xing et al. 2014 |
| c.141_142delTG (p.Arg50Alafs*103) | AD | German | Bilateral, mild to profound, progressive. Onset betwen 10–30 years. | Not found in controls | Thoenes et al. 2015 | ||
| HOMER2 | Intracellular calcium homeostasis and cytoskletal organization | p.Arg185Pro | AD | European | Normal downsloping to moderate-severe. Post lingual, progressive | Azaiez et al. 2015 | |
| COL4A6 | Collagen type IV | c.1771G>A, p.Gly591Ser | X linked | Hungarian | Congenital; severe in males, mild-moderate in female carriers | Rost et al. 2014 | |
| MT-TL1 | m.3243A>G | Mitochondrial | Japanese | Onset in childhood, or 3rd and 4th decade. | 6.57–16.3 per 100000 | Kato et al. 2010 | |
| 12s rRNA | m.1555A>G | Mitochondrial | Polish | Onset within first 2 decades. | 0.40% | Rydzanicz et al. 2010 | |
| m.669T>C | Mitochondrial | Polish | Onset between 5–54 years. | 0.20% | Rydzanicz et al. 2010 | ||
| m.827A>G | Mitochondrial | Polish | Onset at less than 1 year. | 0.20% | Rydzanicz et al. 2010 | ||
| m.961 delT+C(n) | Mitochondrial | Polish | Onset at 20 years. | 0% | Rydzanicz et al. 2010 | ||
| m.988G>A | Mitochondrial | Polish | Onset within first 3 years of life. One outlier at 41 years. | 0.20% | Rydzanicz et al. 2010 | ||
| m.1453A>G | Mitochondrial | Polish | Onset in early childhood. | 0% | Rydzanicz et al. 2010 | ||
| COCH | Extracellular matrix protein | c.1196_1213del | AR | Bilateral, progressive late onset with tinnitus.Onset in 20s. | Gallant et al.2013 | ||
| TECTA | Structural component of tectorial membrane | c.1471C>T (p.R491C); c.596delT | AR | Japanese | Progressive hearing loss. Detected on NBHS | Miyagawa et al. 2013 | |
| 9.6kb deletion (p.984X) | AR | Iranian | Pre-lingual; Moderate-severe | Meyer et al. 2007 | |||
| 266delT (p.122X) | AR | Iranian | Pre-lingual; Moderate-severe | Meyer et al. 2007 | |||
| c.5211C > A (p.Y1737X) | AR | Iranian | Pre-lingual; Moderate-severe | Meyer et al. 2007 | |||
| c.6203–6218del (p.2110X) | AR | Iranian | Pre-lingual; Moderate-severe | Alasti et al.2008 | |||
| BDP1 | Subunit of RNA polymerase III transcription initiation factor IIIB | c.7873 T>G (p.*2625Gluext*11) | AR | Qatari | Pre-lingual; Moderate-severe, downsloping, progressive. Onset between 2 and 4 years old. | Girotto et al. 2013 | |
| CABP2 | Calcium binding protein 2 | c.637+1G>T | AR | Iranian | Pre-lingual; Moderate-severe, flat to shallow U shaped audiogram | Schrauwen et al. 2012 | |
| ELMOD3 | Engulfment and cell motility family | c.794T>C; p.Leu265Ser | AR | Pakistani | Pre-lingual; Severe-profound, mixed | Not found in controls | Jaworek et al. 2013 |
| GPSM2 | G-protein signaling modulator 2 | p.R127X | AR | Palastinian | Pre-lingual; Severe-profound, down sloping | Not found in controls | Walsh et al. 2010 |
| c.1684C>T (p.Q562X) | AR | Turkish | Pre-lingual; Bilateral, Severe-profound | Not found in controls | Yariz et al. 2012 | ||
| KARS | Lysyl-tRNA synthetase | c.1129G>A (p.Asp377Asn) | AR | Pakistani | Pre-lingual; Bilateral, symmetric severe moderate-severe or severe-profound, stable | Not found in controls | Basit et al. 2011; Santos-Cortez et al. 2013. |
| OTOGL | Otogelin-like protein in innr ear | c.1430 delT (p.Val477Glufs∗25) | AR | Turkish | Pre-lingual; symmetric, moderate, stable | Yariz et al. 2012 | |
| c.547C>T (p.Arg183∗); c.5238+5G>A | AR | Dutch | Pre-lingual with diagnosis at age 3, or congenital. Moderate, stable. | Yariz et al. 2012 | |||
| c.1558C>T (p.Gln520*); c.2773C>T (p.Arg925*) | AR | French | Mild. Diagnosed at 5 years. | Bonnet et al. 2013 | |||
| TPRN | Taperin; possible role in actin dynamics | c.1347delG (p.Thr450LeufsX32) | AR | Dutch | Pre-lingual; Severe-profound, downsloping or flat; diagnosis within first 4 years. | Not found in controls | Li et al. 2010 |
| c.42_52del (p.Gly15AlafsX150) | AR | Moroccan; Pakistani | Congenital or pre-lingual; Severe-profound, progressive | Not found in controls | Li et al. 2010; Rehman et al. 2010 | ||
| CEACAM16 | Carcinoembryonic antigen related cell adhesion molecule in outer hair cells | c.418A>C (p.T140P) | AD | American | Bilateral, moderate, progressive. Onsetin early adolescence | Not found in controls | Zheng et al. 2011 |
| c.505G4A (p.G169R) | AD | Chinese | Severe-profound, progressive Onset in first 3 decades. | Not found in controls | Wang et al. 2015 | ||
| P2RX2 | Ligand-gated ion channel, purinergic receptor | c.178G>T (p.V60L) | AD | Chinese | Bilateral, symmetric, moderate-severe, progressive. Onset between 12 to 20 years. | <0.001 in Chinese, <0.0001 in mix-ed ancestry | Yan et al.2013 |
| c.1057G>C (p.Gly353Arg) | AD | Italian | Bilateral, mild to profound, progressive. Age of onset 2nd decade. | Not found in controls | Faletra et al.2014 | ||
| TNC | Tenacin-C in extracellular matrix, basilar membrane, and cochlea | c.5317G>A (p.V1773M) | AD | Chinese | Bilateral, symmetric, severe, progressive. Onset from 8 to 30 years. | <0.0005 | Zhao et al.2013 |
| TSPEAR | Thrombospondin-type lamin G domain and EAR repeats | c.1726G>T1c.1728delC (p.V576LfsX37) | AR | Iranian | Congenital; Bilateral, profound | Not found in controls | Delmaghani et al. 2012 |
| SMPX | Small muscle protein | c.214G>T (p.Glu72X) | X linked | Dutch | Bilateral, progressive. Onset in males 2–10 years, and in females 3–48 years. | Not found in controls | Schraders et al. 2011 |
| c.109G>T (p.Glu37X) | X linked | German | Moderate, progressive. Onset in males 3–7 years, and in females 10–15 years | Huebner et al. 2011 | |||
| c.175G>T (p.Gly59X) | X linked | Spanish | Bilateral, symmetric, severe-profound, progressive. Onset in males 5–7 years, and in females 4th decade. | Huebner et al. 2011 | |||
| TMPRSS3 | Type II transmembrane serine protease | c.607C>T (p.Q203X); c1159G>A (p.A387T) | AR | Japanese | Progressive. Onset in childhood. | Miyagawa et al. 2013 | |
| ACTG1 | Actin isoform in auditory hair cells | c.895C.G (p.L299V) | AD | Japanese | Bilateral, progressive. Onset at age 20. | Miyagawa et al. 2013 | |
| CLDN14 | Component of tight junctions | c.488C>T (p.Ala163Val) | AR | Canadian | Pre-lingual, onset at 3 years.Bilateral, symmetrical, severe, progressive. | 1% | Pater et al. 2017 |
| DIAPH1 | Regulation of actin polymerization in hair cells of inner ear | Onset 0–20 years. |
Review of Current UNHS
In 1993, the NIH’s Consensus Development Conference on Early Identification of Hearing Loss determined that all newborns should have hearing screening, ideally before discharge from the hospital. This was translated into law after Congress passed the Newborn and Infant Hearing Screening and Intervention Act in 1999 [8]. Currently, the US Preventive Services Task Force recommends that all newborn infants be screened for hearing loss by 1 month of age. Although actual screening requirements differ by state, the current UNHS protocol usually utilizes a two-step screening process consisting of OAE and ABR. According to the Joint Commission on Infant Hearing, at least one screening method must be performed on all infants before hospital discharge, and an ABR is recommended when the infant is hospitalized in the neonatal intensive care unit for over 5 days [7].
Since the initiation of UNHS, the average age of diagnosis of HL has decreased from more than 2 years of age to between 2 and 5 months of age [7]. Because earlier identification allows for fitting of hearing aids and other interventions prior to the critical period of language development, these children are able to develop speech and language skills at levels similar to their normal hearing peers [7, 9].
Review of Delayed Onset HL
UNHS was designed to identify infants with HL present at birth. However, a significant cohort of infants exists with pre-lingual HL that is not identified by the traditional UNHS protocol. The critical period of language development in children is from 0 to 3 years [7], and auditory stimulation is essential to develop this pathway. If HL is not identified and treated before this critical period has elapsed, permanent ramifications to speech and language acquisition are inevitable. In a landmark study by Yoshinaga-Itano, et al., hearing impaired children who were diagnosed by 6 months of age had significantly higher receptive and expressive language scores than their peers diagnosed later, regardless of sex, socioeconomic status, or degree of hearing loss [10]. A growing body of literature demonstrates that children with HL identified by 6 months of age perform 20–40% higher on measures such as vocabulary, articulation, intelligibility, social adjustment, and behavior [7]. Furthermore, children who are enrolled in early intervention programs before 1 year of age demonstrate language skills within the normal range of development by 5 years of age [7].
Although prevalent, the exact estimate of children with pre-lingual HL whose diagnosis is delayed is difficult to quantify. One review found that among deaf children less than 9 years old, 22% had a non-congenital impairment [11]. Another Canadian review demonstrated that among a cohort of deaf children, 35.8% passed the UNHS [12]. In a study of 1,300 Danish children with permanent hearing loss, 43.9% of children demonstrated a progressive hearing loss before 4 years of age, with genetic factors being the predominant etiology [13]. In the UK, the prevalence of late onset hearing loss (defined as identification after birth in the publication) is estimated at 0.25 per 1,000 births [14]. Estimates in the literature for non-congenital HL have ranged from 11% of deaf children under 15 years [15], to 30–50% of deaf children under 9 years [16]. Regardless of the estimate, a portion of children with pre-lingual HL are not identified by current UNHS and risk the sequelae of a late diagnosis.
Limitations of Current UNHS
Although generally considered a public health success, current UNHS is not without significant limitations. First, in its current manifestation, UNHS has a high loss to follow up rate, with the literature citing anywhere from 10 to 45% of infants with no follow-up after initial testing in hospital [18, 19]. National data from the CDC also indicates that in 2014, the overall rate of loss to follow-up of UNHS ranged from 3–85%, depending on the state [4]. Second, while the current protocol may identify symptomatic hearing loss, it does not determine the etiology of the hearing loss. This may discourage further healthcare follow-up. Immediate understanding of the molecular etiology of the newborn’s hearing loss may encourage timely follow-up and treatment. Furthermore, etiologic diagnosis of hearing loss using genetic testing may impact an infant’s management [6]. For example, infants with identified mitochondrial mutations would be encouraged to avoid aminoglycosides.
Most relevant to this review, current UNHS is unable to identify patients with delayed onset or progressive HL, even if that HL develops in infancy. Furthermore, current UNHS is not 100% sensitive and as a result, thousands of children with congenital HL may not be identified by the current protocol. Some studies suggest this is because OAE measurements and ABR screening protocols are not sensitive enough to detect mild hearing loss. While the sensitivity for profound hearing loss is 98% for OAE and 90% for ABR, the sensitivities for mild hearing loss are lower at 80% and 84%, respectively [20, 21]. False negative results may also be the case for certain auditory neuropathies, depending on the assessment tool. A number of these patients may have normal outer hair cell response as evaluated by OAE, but would be found to have abnormal brainstem response if the newborn were screened using ABR testing [22]. However, if such infants have a short hospital stay and are not immediately sent for ABR testing, HL detection could be missed in this cohort of patients.
Many of the limitations seen in current UNHS could be improved by the addition of genetic screening. Concurrent genetic screening would identify the etiology of many cases congenital HL, and identify some patients at risk for delayed onset and progressive HL. Furthermore, the addition of concurrent genetic screening may improve follow-up rates in UNHS refer infants, and would positively impact management of infants in which the etiology is identified. Although genetic screening would not be able to identify all cases of pre-lingual hearing loss, it has the potential to greatly improve the sensitivity of the protocol.
Genetic Screening Technologies
This review identified several different technologies used for genetic screening. Direct Sanger Sequencing remains the gold standard of genetic testing and was used as the primary or confirmatory method in 10 studies. However, advancements in NGS and the development of microarray chips have allowed for rapid, cost-effective screening in population that had previously been unthinkable.
The majority of studies included in this review screen for common mutations in the unique populations studied, including GJB2, SLC26A4, and mitochondrial RNA mutations including mt1555G>A. Further details about specific genes screened and in the included studies and other genes associated with pre-lingual HL are described in Table 3.
Direct Sequencing
Direct sequencing is used to determine the exact order of nucleotide bases in a given gene or region of interest, typically 1000 base pairs in length [23]. The most widely used method is Sanger Sequencing. The advantage of this method is that it is able to identify almost all mutations present in a sequence, including novel mutations, and is considered the most accurate. However, this method is the most time consuming, labor intensive, and expensive. Thus, this method is now typically used to identify novel mutations or to verify results from an experimental screening technology. This review identified several papers that used direct sequencing as the primary screening mechanism, but these studies generally screened large populations for 1–5 mutations in only 1–3 genes [24–30].
Microarrays
Microarrays, also known as mutation chips, offer a way to screen for multiple mutations at one time. Mutation chips are easily customizable and can be adjusted based on the mutation frequencies in a given population. They are also less expensive and faster than direct sequencing since multiple genes can be screened simultaneously. However, this method is only able to screen for the mutations included on the chip and cannot detect novel mutations in a gene. Furthermore, although several mutations can be screened at once, there is a limit as to how many mutations can be included without significantly increasing cost and time [23]. Currently available mutation chips can identify anywhere from 15 to 300 mutations in 4 to 31 of the most common genes associated with hearing loss [23, 31]. This review identified 7 studies that used microarray sequencing to identify known genetic mutations in specific populations. In the majority of these cases, large populations were screened [32–37].
Next Generation Sequencing
In our review, 5 studies incorporated NGS, considered the cutting edge of genetic sequencing technology. As with Sanger sequencing, NGS, also known as massively parallel sequencing, directly sequences DNA samples. However, unlike Sanger sequencing, NGS sequences millions of DNA fragments in parallel rather than a single gene in a serial fashion. This technology is most useful for resequencing many selected parts of a genome, such as all exons from a particular set of genes [38]. Several NGS-based gene panels for comprehensive genetic testing for hearing loss are available on the market, and many more are in development. Available panels screen for between 80 to 180 known deafness causing genes and can take between 4 weeks to 3 months to complete [39].
The critical difference between Sanger sequencing or single mutation testing and next generation sequencing (NGS) is sequencing volume. While the Sanger method only sequences a single DNA fragment at a time, NGS is massively parallel, sequencing millions of fragments simultaneously per run. This high-throughput process translates into sequencing hundreds of genes at one time. NGS offers greater discovery power to detect novel or rare variants with deep sequencing. The benefits of Sanger sequencing include fast, cost-effective sequencing for low numbers of targets (1–20 targets) whereas NGS has higher sequencing depth enabling higher sensitivity (down to 1%), faster turnaround time for high sample volumes, comprehensive genomic coverage, higher throughput with sample multiplexing, higher mutation resolution, more data produced with the same amount of input DNA. It is not without its drawbacks, however. These include shorter reads, difficulty in detection certain types of variation (e.g., repeat expansions), and some areas of the genome which are resistant to NGS.
Population Applications of Widespread Genetic Screening
In the 16 studies in this review that incorporated genetic screening for HL with UNHS results, 91.01% of infants passed UNHS and genetic mutations were detected in 8.59%. In total, 545 infants were identified that passed UNHS but had a positive genetic screening, with an average detection rate of 1.4%. It is important to note, however, that different patient populations and different genetic screening methods were used in every study and the number of genes and mutations screened was variable between studies. Furthermore, identifying a genetic mutation does not always indicate that the individual will have or develop HL and the included studies did not stratify prevalence of mutations by hearing ability. Among studies that screened small cohorts with known HL, the UNHS pass/positive genetic screening rate varied from 0.9 to 28.5% [28, 29, 32, 33]. In studies where large populations were screened, the UNHS pass/positive genetic screening rate ranged from 0.04 to 1.91% [24–26, 37, 40–46].
Should genetic testing be incorporated into UNHS, large population analyses provide the most relevant data. Wang et al. performed UNHS in combination with genetic testing in 14,913 Chinese newborns prior to discharge from the hospital [26]. DNA for genetic testing was obtained from umbilical blood spot and stored on universal genetic screening cards that could potentially last up to 16 years. Genetic testing of three common HL genes identified 306 newborns with at least one mutation and, in 25 of those patients, it was a causative mutation. In the 86.1% (n=12,837) of newborns who passed the UNHS protocol, several mutations were identified, including 17 cases of m.1555A>G and one case with both a GJB2 and an SLC26A4 mutation. In those newborns that were UNHS refer (n=2,076), genetic testing demonstrated 18 patients with m.1555A>G and 5 GJB2 c.235delC homozygotes. Thus, mutations were identified in both UNHS pass patients and UNHS refer patients, indicating a role for genetic testing to complement UNHS in diagnosing HL.
Zhang et al. (2013) performed another large-scale study on newborn infants in Tianjin, China [42]. 58,397 infants were recruited to undergo standard UNHS and genetic screening. Microarrays were used to screen for 20 common mutations on 4 genes. Mutations were identified in 5.52% (n=3,225) of infants, and overall, 89.1% (n=52,020) of infants passed UNHS. However, of the over 3,000 children with a mutation identified, only 0.25% (n=143) were bi-allelic and thus considered genetic positive, while the vast majority were found to be carriers. However, of the positive genetic screening group, 76.2% (n=109) had originally passed UNHS. Overall, 0.19% (n=143) of infants enrolled in the study were considered UNHS pass/positive genetic screening, indicating that they would not have been identified had they not undergone genetic testing.
No studies of similar scale have demonstrated the utility of genetic testing as a complement to the current UNHS in Caucasian or European populations. However, Schimmenti, et al. (2011) used this concept and took infant bloodspots from the Minnesota Department of Health in order to identify a cohort of patients who were UNHS refer [25]. These were matched to infants who had passed UNHS during the same time period. 2,354 bloodspots were analyzed for common alleles of GJB2 mutations. Twenty-three of the 1,177 bloodspots of infants who were UNHS refer had a biallelic GJB2 mutation, a prevalence of 1 in 50. Furthermore, a biallelic GJB2 mutation was also identified in an infant that had passed the UNHS, suggesting a missed diagnosis and failure to identify potential HL in the traditional screening method. However, the authors did not comment on the hearing loss phenotype found in this patient. Although this represents a missed diagnosis in only 1 sample of 2,354, only certain alleles for GJB2 mutations were analyzed. If expanded to incorporate more mutations on different genes unique to the population, more infants would likely be identified.
On the basis of these studies, this review showed that the overall weighted UNHS pass/positive genetic screening rate was 1.4%, totaling 545 infants. These patients would not have been detected by conventional UNHS had genetic testing not been incorporated. When this rate is applied to the general U.S. population, thousands of additional infants with pre-lingual HL could be identified annually. Furthermore, this review does not account for patients who are UNHS refer and have a positive genetic screening and later developed hearing loss. The addition of genetic screening for these patients would potentially establish the etiology of their HL. These patients would likely experience benefits with increased audiology follow-up, understanding of disease progression, and improved management. Finally, incorporating genetic screening into UNHS would also identify HL gene carriers, may help with early identification for siblings and other close family members, and may be useful for family planning.
Cost of Genetic Screening
On average, the economic burden of severe to profound hearing loss in the United States is estimated to be $297,000 over the lifetime of an individual [4]. This number can exceed $1 million in children with pre-lingual HL. Medical expenses for deaf individuals contributes very little to the economic burden when compared to societal costs. Hearing impaired individuals require special educational and social resources during childhood and adolescence with costs that can amount to over a half million dollars during the course of their education [4]. As these children mature to adults, they lack economic productivity when compared to their hearing peers. One study found adults with HL earn nearly 25% less than their hearing counterparts, after adjusting for other factors [47]. Emmett et al. found adults with HL had 1.58 higher odds of earning less than $20,000 per year and 1.98 higher odds of being unemployed or underemployed when compared with their hearing peers [48]. The economic burden of HL in the U.S. is estimated to be between $1.8 billion and $194 billion annually, depending on the population studied [49].
For many, the primary argument against the incorporation of genetic screening into UNHS is the additional cost. Accurate estimates of cost are challenging, as there are few rigorous studies comparing NGS to other forms of testing, testing prices continue to fall, and the diagnostic yield of testing increases [50]. For example, the estimated cost of a routine CBC blood test in the UK is 5.60 pounds ($7.38 US), while a targeted genetic sequencing panel could reach up to 530 pounds ($698.38 US) [50]. Abou-Tayoun, et al.(2016), in a comprehensive study of the OtoGenome2 NGS panel for hearing loss, estimated the technological cost to be $8 per amplicon [51]. They also factored in the time and cost needed for analysis. They estimated a genetic counselor would take 22–102 minutes to review the panel at $35/hour, followed by a review by a certified geneticist at $56/hour. They found that by excluding genes with only weak associations to HL, they could potentially save $26.82 per eliminated gene per sample.
One should expect costs of sequencing tests to fall as technology advances. When genetic screening was first developed, the cost of analysis of a single gene ranged from $1,000 to $3,000. Today, the cost of commercially available genetic panels screening between 29 and 129 genes ranges from $596 to $3,800, representing a 10,000% decrease in cost per gene [6].
Genetic screening for congenital disorders in infants is not a novel concept in the U.S. In most states, genetic testing is already provided for rare disorders identified by the US Department of Health and Human Services Advisory Committee on Heritable Disorders in Newborns and Children. Twenty-nine hereditary disorders have been mandated by the American College of Medical Genetics, and individual states may add additional newborn screening for rare disorders based on their unique populations. None of the mandated disorders are present with the frequency of congenital HL. Although screening for these hereditary disorders does not use the same genetic testing technology that would be used if the current UNHS was expanded to include genetic screening, the concept of screening for genetic disorders is not unique and an expansion of the scope of hereditary screening would be both feasible and worthwhile.
The authors argue that any potential cost associated with genetic testing is outweighed by the potential public health and economic benefit ensured by identifying thousands of additional pre-lingual HL patients. As an example, if the estimated lifetime cost of a missed diagnosis in an infant with pre-lingual HL is $1,000,000 [4], while the cost of our in-house developed CapitalBioMiamiOto micro array panel is $30 per patient [5], over 33,000 infants could be screened using the microarray panel for the same cost. CapitalBioMiamiOtoArray that allows simultaneous analysis of the nine most common mutations in patients of European descent in five genes. It was developed using a universal array approach termed multiplex allele-specific PCR-based universal array (ASPUA) and the amplification refractory mutation system (ARMS) with the detection power of microarray hybridization. The combination of the ASPUA and ARMS technologies have been found to be specific, sensitive and has high resolution (Li et al 2008). Identifying these individuals early could potentially offset the significant economic burden associated with undetected pre-lingual HL.
Limitations of Genetic Screening
Despite its potential to revolutionize UNHS and improve the diagnosis of pre-lingual HL, several authors have noted significant challenges that would be associated incorporating widespread use of genetic deafness screening. Foremost, the heterogeneity of genetic hearing loss, the hundreds of associated genes, and the variable penetrance associated with many mutations may make the interpretation of results difficult. As Vona, et al., elegantly pens, “the $1,000 genome, the $100,000 analysis” [52]. While screening large scale populations may be technically and financially feasible, the additional cost associated with statistical analysis, interpretation, and counselling is difficult to estimate and may be burdensome. Additionally, novel mutations are routinely discovered, making pre-programmed microarray panels potentially obsolete [23].
Furthermore, as the studies reviewed above indicate, genetic screening more frequently identifies mono-allelic mutations than bi-allelic. Due to the variable penetrance of many deafness causing genes, the impact and associated financial cost of ensuring follow-up for these patients is unclear. Widespread genetic screening would thus identify millions of carriers of deafness-causing genes, the impact of which cannot be underestimated. In some patients, deafness causing genes may be identified before hearing loss manifests. This puts an additional burden on providers to monitor patients without clearly established guidelines in place. Conversely, patients without genetic risk factors identified may be less likely to follow-up, regardless of the traditional UNHS results. Extensive family counseling would be vital to ensure the success of the program. Finally, not all cases of undiagnosed pre-lingual HL at birth would have a genetic cause. Although a large portion of additional patients may be identified, the patients with environmental pre-lingual HL or mutations in novel genes would be missed. As of yet, no screening method is 100% sensitive to identify all patients with pre-lingual HL. Despite these significant hurdles, the authors believe that the addition of widespread genetic screening to current UNHS would be ultimately advantageous, by identifying thousands of additional cases of pre-lingual HL and ultimately reducing healthcare and economic costs.
Limitations of this Review
The authors felt it was important to review the current literature on the addition of genetic screening to the UNHS, but this review is not without limitations. Foremost, the 16 papers included in the formal review consist of varied patient populations; some only include patients with hearing loss, while others describe screening of the general population. Furthermore, the type of genetic testing and UNHS protocol used varies between studies. Thus, UNHS pass and positive genetic screening rates are expected to vary widely between these groups. Furthermore, as noted above, the UNHS and genetic screening are only intended to identify infants who are at risk for hearing loss, and are not conclusive in their diagnosis. Just as a UNHS refer infant does not conclusively have HL, an infant with a positive genetic screening result does not guarantee a diagnosis of HL. Thus, the rate of undiagnosed pre-lingual HL is likely to be lower than is estimated in this review, as patients identified as UNHS refer or with a positive genetic screening will not necessarily have hearing loss. Please also note that many of the studies included “variants of uncertain significance” when testing for genetic mutations and not just mutations confirmed to be associated with hearing loss. These variants are included in the “positive genetic screening group” and this may further dilute the results.
Conclusion
The primary aim in this review was to determine the impact of the addition of genetic screening on the current UNHS protocol, specifically on the identification of undiagnosed pre-lingual HL. Secondarily, the authors reviewed the latest advancements in genetic screening technology and their impact on the future of newborn hearing screening. Overall, the authors argue that the addition of genetic screening was successful in identifying patients with pre-lingual HL, with the overall detection rate of UNHS pass/positive genetic screening being 1.4%. The authors believe that genetic screening could identify an additional infant with HL for every 42 infants screened. This represents the identification of thousands of additional infants when applied to large populations. By creating population-specific microarray or NGS-based gene detection panels, the most common mutations in different communities could be screened cost-effectively. This screening should be applied as an adjunct to UNHS nationwide, with the goal of screening all infants born in U.S. hospitals. However, further research on cost analysis, genetic interpretation, and the societal implications of widespread genetic screening is warranted. Despite these limitations, the authors believe the addition of widespread genetic screening to UNHS will soon be both financially and technically feasible, and necessary for the future of HL detection and management.
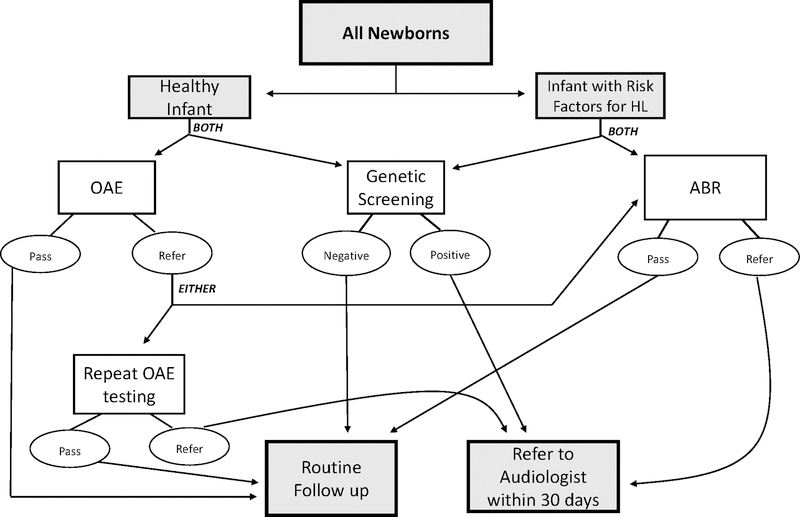
Figure 3: Suggested Newborn Hearing Screening Flowchart.
The authors propose adding genetic screening to the UNHS, as depicted by this flowchart. Infants with risk factors for hearing loss include those with a NICU stay after birth, prolonged inpatient use of antibiotics after birth, and mechanical ventilation, or as determined by the UNHS legislation enacted in their respective state.
Acknowledgements
Dr. Liu’s Laboratory is supported by R01 DC005575, R01DC017264, T32 DC015995, and R01 DC012115 from the National Institutes of Health/National Institute on Deafness and Other Communication Disorders
References
- 1.Organization, W.H. Deafness & Hearing Loss. WHO Fact Sheet 2015. [cited 2017; Available from: http://www.who.int/en/news-room/fact-sheets/detail/deafness-and-hearing-loss.
- 2.Boulet SL, Boyle CA, and Schieve LA, Health care use and health and functional impact of developmental disabilities among US children, 1997–2005. Arch Pediatr Adolesc Med, 2009. 163(1): p. 19–26. [DOI] [PubMed] [Google Scholar]
- 3.Niskar AS, et al. , Prevalence of hearing loss among children 6 to 19 years of age: the Third National Health and Nutrition Examination Survey. JAMA, 1998. 279(14): p. 1071–5. [DOI] [PubMed] [Google Scholar]
- 4.Prevention, C.f.D.C.a. Data & Statistics. Hearing Loss in Children. 2017. July 21, 2017 [cited 2017; Available from: https://www.cdc.gov/ncbddd/hearingloss/data.html. [Google Scholar]
- 5.Liu XZS, Kun; Jing Qing; Jing Cheng; Denise Yan, Non-Syndrome Hearing Loss and High-Throughput Strategies to Decipher Its Genetic Heterogeneity. Journal of Otology, 2013. 8(1): p. 6–24. [Google Scholar]
- 6.Jasper KM, Jamshidi A, and Reilly BK, Pediatric otolaryngology, molecular diagnosis of hereditary hearing loss: next-generation sequencing approach. Curr Opin Otolaryngol Head Neck Surg, 2015. 23(6): p. 480–4. [DOI] [PubMed] [Google Scholar]
- 7.American Academy of Pediatrics, J.C.o.I.H., Year 2007 position statement: Principles and guidelines for early hearing detection and intervention programs. Pediatrics, 2007. 120(4): p. 898–921. [DOI] [PubMed] [Google Scholar]
- 8.Shen JM, CC, Next-Generation Newborn Hearing Screening in Genetics of Deafness, H.T. Vona B, Editor. 2016, Monogr Hum Genet: Basel, Karger; p. 30–39. [Google Scholar]
- 9.Sininger YS, et al. , Newborn hearing screening speeds diagnosis and access to intervention by 20–25 months. J Am Acad Audiol, 2009. 20(1): p. 49–57. [DOI] [PubMed] [Google Scholar]
- 10.Yoshinaga-Itano C, et al. , Language of early- and later-identified children with hearing loss. Pediatrics, 1998. 102(5): p. 1161–71. [DOI] [PubMed] [Google Scholar]
- 11.Weichbold V, Nekahm-Heis D, and Welzl-Mueller K, Universal newborn hearing screening and postnatal hearing loss. Pediatrics, 2006. 117(4): p. e631–6. [DOI] [PubMed] [Google Scholar]
- 12.Barreira-Nielsen C, et al. , Progressive Hearing Loss in Early Childhood. Ear Hear, 2016. 37(5): p. e311–21. [DOI] [PubMed] [Google Scholar]
- 13.Johansen IR, et al. , Longitudinal study of hearing impairment in children. Int J Pediatr Otorhinolaryngol, 2004. 68(9): p. 1157–65. [DOI] [PubMed] [Google Scholar]
- 14.Watkin P and Baldwin M, The longitudinal follow up of a universal neonatal hearing screen: the implications for confirming deafness in childhood. Int J Audiol, 2012. 51(7): p. 519–28. [DOI] [PubMed] [Google Scholar]
- 15.MacAndie C, Kubba H, and McFarlane M, Epidemiology of permanent childhood hearing loss in Glasgow, 1985–1994. Scott Med J, 2003. 48(4): p. 117–9. [DOI] [PubMed] [Google Scholar]
- 16.Fortnum HM, et al. , Prevalence of permanent childhood hearing impairment in the United Kingdom and implications for universal neonatal hearing screening: questionnaire based ascertainment study. BMJ, 2001. 323(7312): p. 536–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kawada J, et al. , Viral load in children with congenital cytomegalovirus infection identified on newborn hearing screening. J Clin Virol, 2015. 65: p. 41–5. [DOI] [PubMed] [Google Scholar]
- 18.Holte L, et al. , Factors influencing follow-up to newborn hearing screening for infants who are hard of hearing. Am J Audiol, 2012. 21(2): p. 163–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ye XT, Tri; Smith, Jo Mary; Jeanette Webb; Terri Mohren; Melinda Peat, Improvement in Loss to Follow-up of Newborn Hearing Screening: A lesson from Lousiana Early Hearing Detection and Intervention Program. Online J Public Health Inform, 2014. 6(1): p. e47. [Google Scholar]
- 20.Norton SJ, et al. , Identification of neonatal hearing impairment: evaluation of transient evoked otoacoustic emission, distortion product otoacoustic emission, and auditory brain stem response test performance. Ear Hear, 2000. 21(5): p. 508–28. [DOI] [PubMed] [Google Scholar]
- 21.Thompson DC, et al. , Universal newborn hearing screening: summary of evidence. JAMA, 2001. 286(16): p. 2000–10. [DOI] [PubMed] [Google Scholar]
- 22.Morton CC and Nance WE, Newborn hearing screening--a silent revolution. N Engl J Med, 2006. 354(20): p. 2151–64. [DOI] [PubMed] [Google Scholar]
- 23.Linden Phillips L, et al. , The future role of genetic screening to detect newborns at risk of childhood-onset hearing loss. Int J Audiol, 2013. 52(2): p. 124–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zaputovic S, et al. , Incidence of the 35delG/GJB2 mutation in low-risk newborns. J Matern Fetal Neonatal Med, 2008. 21(7): p. 463–8. [DOI] [PubMed] [Google Scholar]
- 25.Schimmenti LA, et al. , Evaluation of newborn screening bloodspot-based genetic testing as second tier screen for bedside newborn hearing screening. Genet Med, 2011. 13(12): p. 1006–10. [DOI] [PubMed] [Google Scholar]
- 26.Wang QJ, et al. , Newborn hearing concurrent gene screening can improve care for hearing loss: a study on 14,913 Chinese newborns. Int J Pediatr Otorhinolaryngol, 2011. 75(4): p. 535–42. [DOI] [PubMed] [Google Scholar]
- 27.Iwata AJ, et al. , Prenatal diagnosis of SLC26A4 mutation and delayed onset of hearing loss. Otolaryngol Head Neck Surg, 2013. 148(4): p. 705–6. [DOI] [PubMed] [Google Scholar]
- 28.Minami SB, et al. , GJB2-associated hearing loss undetected by hearing screening of newborns. Gene, 2013. 532(1): p. 41–5. [DOI] [PubMed] [Google Scholar]
- 29.Kim BG, et al. , Limitations of hearing screening in newborns with PDS mutations. Int J Pediatr Otorhinolaryngol, 2013. 77(5): p. 833–7. [DOI] [PubMed] [Google Scholar]
- 30.Dai ZY, et al. , Correlation analysis of phenotype and genotype of GJB2 in patients with non-syndromic hearing loss in China. Gene, 2015. 570(2): p. 272–6. [DOI] [PubMed] [Google Scholar]
- 31.Hilgert N, Smith RJ, and Van Camp G, Forty-six genes causing nonsyndromic hearing impairment: which ones should be analyzed in DNA diagnostics? Mutat Res, 2009. 681(2–3): p. 189–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen G, Wang X, and Fu S, Prevalence of A1555G mitochondrial mutation in Chinese newborns and the correlation with neonatal hearing screening. Int J Pediatr Otorhinolaryngol, 2011. 75(4): p. 532–4. [DOI] [PubMed] [Google Scholar]
- 33.Lim BG, et al. , Utility of genetic testing for the detection of late-onset hearing loss in neonates. Am J Audiol, 2013. 22(2): p. 209–15. [DOI] [PubMed] [Google Scholar]
- 34.Zhang J, et al. , Newborn hearing concurrent genetic screening for hearing impairment-a clinical practice in 58,397 neonates in Tianjin, China. Int J Pediatr Otorhinolaryngol, 2013. 77(12): p. 1929–35. [DOI] [PubMed] [Google Scholar]
- 35.Jiang H, Liu Q, and Chen L, Screening and analysis of mutation hot-spots in deafness-associated genes among adolescents with hearing loss. Mol Med Rep, 2015. 12(6): p. 8179–84. [DOI] [PubMed] [Google Scholar]
- 36.Svidnicki MC, et al. , Screening of genetic alterations related to non-syndromic hearing loss using MassARRAY iPLEX(R) technology. BMC Med Genet, 2015. 16: p. 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peng Q, et al. , Concurrent Genetic and Standard Screening for Hearing Impairment in 9317 Southern Chinese Newborns. Genet Test Mol Biomarkers, 2016. 20(10): p. 603–608. [DOI] [PubMed] [Google Scholar]
- 38.Yan D, et al. , Next-generation sequencing in genetic hearing loss. Genet Test Mol Biomarkers, 2013. 17(8): p. 581–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yan D, et al. , Screening of deafness-causing DNA variants that are common in patients of European ancestry using a microarray-based approach. PLoS One, 2017. 12(3): p. e0169219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nivoloni Kde A, et al. , Newborn hearing screening and genetic testing in 8974 Brazilian neonates. Int J Pediatr Otorhinolaryngol, 2010. 74(8): p. 926–9. [DOI] [PubMed] [Google Scholar]
- 41.Wu CC, et al. , Newborn genetic screening for hearing impairment: a preliminary study at a tertiary center. PLoS One, 2011. 6(7): p. e22314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang J and Du G, The chemistry, pharmacology and clinical therapeutic effects of danshen. Ying Quan ban wen; ban di 1. 2013, Beijing: Hua xue gong ye chu ban she; 270 pages. [Google Scholar]
- 43.Barkai G, et al. , Universal neonatal cytomegalovirus screening using saliva - report of clinical experience. J Clin Virol, 2014. 60(4): p. 361–6. [DOI] [PubMed] [Google Scholar]
- 44.Chen Y, et al. , SNaPshot reveals high mutation and carrier frequencies of 15 common hearing loss mutants in a Chinese newborn cohort. Clin Genet, 2015. 87(5): p. 467–72. [DOI] [PubMed] [Google Scholar]
- 45.Chu CW, et al. , Government-funded universal newborn hearing screening and genetic analyses of deafness predisposing genes in Taiwan. Int J Pediatr Otorhinolaryngol, 2015. 79(4): p. 584–90. [DOI] [PubMed] [Google Scholar]
- 46.Wu CC, et al. , Newborn genetic screening for hearing impairment: a population-based longitudinal study. Genet Med, 2017. 19(1): p. 6–12. [DOI] [PubMed] [Google Scholar]
- 47.Jung D and Bhattacharyya N, Association of hearing loss with decreased employment and income among adults in the United States. Ann Otol Rhinol Laryngol, 2012. 121(12): p. 771–5. [DOI] [PubMed] [Google Scholar]
- 48.Emmett SD and Francis HW, The socioeconomic impact of hearing loss in U.S. adults. Otol Neurotol, 2015. 36(3): p. 545–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huddle MG, et al. , The Economic Impact of Adult Hearing Loss: A Systematic Review. JAMA Otolaryngol Head Neck Surg, 2017. 143(10): p. 1040–1048. [DOI] [PubMed] [Google Scholar]
- 50.Beale S, et al. , A scoping study to explore the cost-effectiveness of next-generation sequencing compared with traditional genetic testing for the diagnosis of learning disabilities in children. Health Technol Assess, 2015. 19(46): p. 1–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Abou Tayoun AN, et al. , Improving hearing loss gene testing: a systematic review of gene evidence toward more efficient next-generation sequencing-based diagnostic testing and interpretation. Genet Med, 2016. 18(6): p. 545–53. [DOI] [PubMed] [Google Scholar]
- 52.Vona B, et al. , Non-syndromic hearing loss gene identification: A brief history and glimpse into the future. Mol Cell Probes, 2015. 29(5): p. 260–70. [DOI] [PubMed] [Google Scholar]