Abstract
Background:
Excessive alcohol intake is a serious but preventable public health problem in the United States and worldwide. Alcohol and other substance use disorders occur co-morbid with more generalized reward deficiency disorders, characterized by a reduction in dopamine (DA) signaling within the reward pathway, and classically associated with increased impulsivity, risk taking and subsequent drug seeking behavior. It is postulated that increasing dopamine availability and thus restoring DA homeostasis in the mesocorticolimbic system could reduce the motivation to seek and consume ethanol. Here, we treated animals with a neuro-nutrient, KB220Z also known as Synaptamine, designed to augment DA signaling.
Method:
KB220Z was supplied to genetically alcohol-preferring (P) adult male and female rats by oral gavage (PO), intraperioneally (IP), or subcutaneously (SQ) for 4 consecutive days at a 3.4mL/Kg rat equivalent dose and compared to saline (SQ, IP) or water (PO) controls. Subsequent to treatment, lever pressing and consumption of 10% ethanol, or control 3% sucrose during operant responding was assessed using a drinking in the dark multiple scheduled access (DIDMSA) binge drinking protocol. Locomotor and elevated zero maze activity, and DRD2 mRNA expression via in situ hybridization (ISH) were assessed independently following 4 days of a SQ regimen of KB220Z.
Results:
KB220Z markedly and immediately reduced binge drinking of 10% ethanol in both male and female rats via IP and SC administration whereas P.O. took at least 3 days to decrease lever pressing for ethanol in both male and female rats. There was no effect of SQ KB220Z on 3% sucrose drinking. Elevated activity in the open field was significantly decreased, and time spent in the open arm of the EZM was moderately reduced. The regimen of SQ KB220Z did not impact the number of DRD2 punctae in neurons of the NAc, but NAc-shell expressed more DRD2 mRNA/cell than NAc-core independent of KB220Z.
Conclusions:
KB220Z attenuates drinking and other RDS behaviors in P rats possibly by acting on the dopaminergic system, but not by effecting an increase in NAc DRD2 mRNA expression.
Keywords: Reward Deficiency, Nucleus Accumbens, Locomoter Activity, Elevated Zero Maze, Operant responding, binge drinking, alcohol use disorder, hyperactivity
Graphical Abstract

1.0. Introduction
According to the Centers for Disease Control and Prevention (CDC), binge drinking is the most common and deadly pattern of excessive alcohol use in the United States [1]. Binge drinking is associated with a wide variety of serious behavior health risks and medical conditions including but not limited to, violence [2], suicide, sexually transmitted diseases [3], fetal alcohol spectrum disorders [4], cardiovascular disease [5], hepatic disorder [6], cancer, and alcohol dependence [7]. It has been hypothesized that excessive alcohol drinking and abuse of other drugs may occur in adults from negative experiential imprints during early infancy or childhood [8–13].
Substance use disorders occur comorbid with more generalized reward deficiency syndrome (RDS), a term that refers to a chronic hypodopaminergic trait/state and encompasses multiple behaviors resulting from reduced reward inclusive of negative affect as well as addictive, compulsive and impulsive behaviors [14, 15]. Mechanistically, alcohol use disorder (AUD) and RDS share a common characteristic: reduced levels of dopamine leading to impaired dopamine homeostasis in the reward circuitry [16–18]. Growing evidence suggests that decreased DA levels in the mesocorticolimbic brain regions are associated with increased cravings and/or drug seeking behavior [18–21]. Naltrexone which blocks opioid receptors involved in rewarding effects of alcohol, Acomprosate which purported helps already abstinent individuals, Disulfiram believed to induce aversive effects by interfering with degradation of alcohol, and off-label use of topiramate which is thought to increase GABA and reduce glutamate neurotransmission, are among recent drugs of choice for the treatment of AUD [22, 23]. However, in some cases they are associated with side effects like increased anxiety, depression and even addiction liability [24–26], relapse, and non-compliance in the case of Disulfiram. Therefore, novel replacement or adjunctive therapeutic interventions with potentially higher efficacy and, fewer side effects are necessary to improve treatment outcomes [27–30].
Animal models serve as invaluable tools for exploring physiological and pathophysiological functions of the brain and behavior. Through selective breeding, several high alcohol-consuming rat lines have been developed [31, 32], including alcohol-preferring (P) and high-alcohol-drinking (HAD) lines [33, 34]. P rats, used in this study, satisfy all the original criteria proposed for a suitable animal model of alcoholism [35, 36]. Coincidentally, their behaviors also overlap with definitions of RDS, in that they express compulsive drug taking behaviors, depressive-like behaviors, anxiety, impulsivity, hyperactivity [37, 38] all of which are manifested in the Reward Deficiency Syndrome spectrum [39] and in other models of reduced reward such as early life stress [9, 10].
Because binge drinking shares characteristics with the RDS phenotype, we use here a well-described rodent model of excessive alcohol intake and binge drinking, i.e., P rats, [33, 34, 40–42] to obtain greater insight into RDS associated behavioral deficits. Similar to RDS, P rats express decreased DA and DA metabolites [41, 43] in the nucleus accumbens (Acb) and anterior striatum, key brain regions of the reward circuitry, and display decreased dopaminergic neuronal projections from ventral tegmental area (VTA) to the Acb [44]. Additionally, a reduction of dopamine D2 receptors (DRD2) in both the VTA and Acb [45] is present in P rats, and when rescued with DRD2 gene transfer into the Acb core, alcohol drinking was attenuated [46]. Furthermore, other neurotransmitters such as serotonin were reduced in P rat brain, whereas GABAergic terminals and GABAA receptors are increased within the reward circuitry of P rats and rodent stress models. These biochemical alterations are associated with alcohol seeking behavior [37, 42, 47] and increased alcohol tolerance as well as withdrawal symptoms [48], respectively. Serotonin and GABAA deficits also alter dopaminergic function and the potential for dopamine release. Likewise, in genetically predisposed alcohol-preferring C57BL/6J mice, a negative correlation exists between the amount of 10% alcohol consumed and endogenous opioid peptide levels. [49]. While these observations are interesting, an important question has emerged as to whether RDS associated behavioral deficits in P rats are associated with impaired mesocorticolimbic circuitry. These behavioral and biochemical observations in the alcohol-preferring brain are of translational relevance and underscores the validity of the rodent model for binge drinking and RDS studies. In addition to operant responding for alcohol, we also test activity, risk taking and anxiogenic/anxiolytic behaviors in the open field and the EZM as additional readouts of RDS behaviors.
Many natural methods such as exercise, yoga-meditation, other forms of environmental enrichment and naturally occurring products are used to boost brain dopamine signaling [50–53]. In this study, we used neuronutrient, KB220Z also known as Synaptamine, a nutraceutical product designed to enhance DA homeostasis, to supply the brain with molecular precursors and neurotransmitter catabolic inhibitors of reward pathway molecules, with the end goal to augment DA signaling and reduce P rat excessive drug intake behavior. KB220Z has been tested in many clinical trials and cases of patients with RDS [54–57] and was administered in rats to investigate its effect on brain dopamine circuits [58]. No pre-clinical study is available to elucidate the effects of KB220Z specifically on binge ethanol drinking. A preliminary study had demonstrated a putative anti-craving/anti-relapse role of KB220Z in a randomized placebo-controlled crossover study in humans. However, the small sample size restricted definitive interpretation of the results and warranted additional rodent and human studies of KB220Z [54]. Therefore, in the present study, using P adult male and female rats, we not only explored the effects of KB220Z on binge drinking, but also, investigated the most efficient route of administration: oral (P.O.), subcutaneous (S.Q.) and intraperitoneal (I.P.). Our hypothesis is that if a reduction in DA causes behavioral deficits associated with reward, interventions that restore the levels of DA in the brain should attenuate these neurobehavioral deficits. This is the first rodent study, to our knowledge, examining the effects of KB220Z on binge drinking in rats.
2.0. Materials and Methods
2.1. Animals
A total of 72 alcohol-preferring (P) adult male and female rats were reared under similar conditions for any given experiment and were age-matched. Animals were used between 3–4 months for drinking data, although operant training began at 60–70 days of age. Females averaged 250g, and males averaged 400 g. Animals were mated upon request and shipped from Indiana University Alcohol Research Center (School of Medicine, Indiana) as adolescents at about P28, and were raised at the Howard University animal facility until they became young adults (approximately 60–70 days old) when they began training on the operant. P rats were housed in standard cages in the climate-controlled rodent housing facility and adapted to a reverse 12 h/12 h light/dark cycle, which began at 7:00 p.m. [lights on] and lasted to 7:00 a.m. [lights off]. Rats were provided with ad libitum food and water until adulthood when the experiments began. Prior to testing, animals were acclimated to multiple episodes of handling/restraint, saline IP/SQ injections, or water gavage for approximately 3 days, to control for the confounding effects on behavior that could be induced by handling and the initial exposure to these procedures. Experiments were conducted in accordance with the NIH guidelines using protocols under Neurobehavioral Determinants of Ethanol Reward, approved by the Howard University Animal Care and Use Committee.
2.2. Treatment with prodopamine regulator, KB220Z
KB220Z was supplied by Geneus Health (San Antonio, Texas) and was stored at room temperature upon receipt. The proprietary composition of KB220Z is Pyridoxal-5-Phosphate USP, L-Tyrosine, L-Glutamine, Rhodiola Rosea Root SE, Rosavins, Griffonia Seed Extract 5-HTP, L-Phenylalanine, Chromium GTF Plus, Passion Flower SE Isovitexin, N-Acetyl-L-Cysteine USP, Glucosamine N-Acetyl, Arabinogalactan Fiber Aid AG99, Aloe Vera FD Powder 200X, White Birch Bark 4:1 Extract, Bosellia Serrata Gum Extract. The relative concentrations are represented in Table 1. The rat equivalent dose (RED) of 3.4mL/Kg was calculated based on the lowest suggested human daily dose of 1.0 oz (29.6mL) for KB220Z, assuming a 60kg average person × 0.7, the Km ratio. The Km ratio is a function of the weight and surface area of the animal (m2). To derive the RED, a Km ratio of 7 based on a 250g rat was used in accordance with dosing between species [59, 60]. The minimum suggested dose of KB220Z is 1.0 oz whereas the maximum is 4.0 oz per day. Rats received the equivalent of the minimum 1oz per day. The experimental design is depicted in Figure 1. Briefly, all rats were subjected to 4 hrs of fasting (8am to noon) prior to the treatment. This was immediately followed by treating the rats with either sterile saline for IP and SQ diluent controls, or tap water for PO controls on Day 0 followed by KB220Z (3.4 mL/kg, rat equivalent dose) for 4 consecutive days as follows: Intraperitoneal (I.P.) administration: sterile saline followed by KB220Z (days 1–4); Subcutaneous (S.Q.) administration: sterile saline followed by KB220Z (days 1–4); Oral Gavage (P.O.) administration: Tap water followed by KB220Z (days 1–4). Each rat served as its own control for NT (no treatment) or saline administration SQ and IP, or during water gavage.
Table 1.
KB220Z (Synaptamine) components
| Item Description | Relative mg Units |
|---|---|
| Pyridoxal 5 Phosphate (B6) | 1 |
| L-Tyrosine | 10 |
| L-Glutamine | 1 |
| Rhodiola Rosea Ext (3% Rosavin, 1% Salidrosade) | 4 |
| Griffonia Seed SE 99% 5-Hydroxytryptophan | 2 |
| DL Phenylalanine | 40 |
| Kosher GTF Chromium Yeast | 0.008 |
| Passion Flower Extract 3.5–4% Vitexins | 6 |
| N Acetyl L-Cysteine | 10 |
| N Acetyl D-Glucosamine | 1 |
| Larch Arabinogalactans | 10 |
| Organic Aloe Vera Gel Powder 200:1 | 0.3 |
| Birch Leaves Powder (Betula Pendula) | 3 |
| Boswellia Serrata Extract 5:1 | 3 |
Figure 1.

A schematic representation of the experimental design. Female and male adult P rats were subjected to single administration of I.P., S.Q., or P.O. sterile saline/tap water before the start of KB220Z treatment and KB220Z (3.4 mL/kg, rat equivalent dose) on days 1 – 4. Operant self-administration behavioral test was conducted on each day. I.P.. – intraperitoneal, S.Q.. – subcutaneous, P.O. – oral gavage.
2.3. Behavioral Assessments
2.3.1. Operant-self administration apparatus
Self-administration sessions were conducted in standard operant conditioning chambers (Coulbourn Instruments Inc., Lehigh Valley, PA, USA). Each operant chamber consists of the following: two levers, two dippers, triple cue lights per lever, and a house light. The dipper cup size was 0.1 mL. The Coulbourn Graphic State 3 operant software was used to record and analyze the data.
2.3.1.1. Drinking in the Dark Multiple Scheduled Access (DIDMSA) Paradigm
To assess excessive “binge” alcohol drinking, we employed a modified version of the well-established drinking-in-the-dark-multiple-scheduled-access (DIDMSA) binge drinking protocol as previously published by us and many others [9, 38, 61]. Briefly, each exposure to operant chambers consisted of three 30 minutes sessions with 45 minutes rest in between.
2.3.1.2. Training Procedure
Animals were first trained to self-administer 10% sucrose on the fixed ratio 1 (FR1) schedule of reinforcement, i.e. each operant response was reinforced with 0.1mL of the solution. Following FR1, rats were trained on the FR4 schedule where four operant responses were required for the reinforcement. Then, all rats were subjected to sucrose fading procedure where the sucrose concentration was gradually reduced from 10% to 0%, and the ethanol concentration was gradually increased from 0% to 10% v/v. During the sucrose fade, they received 2 days each of 75:25, 50:50, 25:75, 0:100 of 10%sucrose:10% ethanol. Once the rats exhibited stable operant responses to 10% v/v ethanol, typically 2–3 weeks post sucrose fade, the actual experiment and data acquisition began. Reservoirs of the operant conditioning chambers were filled with 10% v/v ethanol, prepared fresh daily. To acclimate animals to handling, prior to administration of KB220Z, they were gavaged with water or injected IP or SQ with saline for at least 3 days.
2.3.1.3. Experimental Procedure
On the days of the actual experiment, all P rats were subjected to 4 hours of fasting (8am to noon), where food and water were removed from the home cage. At the end of 4 hours of fasting, animals were treated with I.P./S.Q. saline or P.O. water or KB220Z and allowed to rest for 15 minutes in their home cage. Then, they were placed in the operant conditioning chambers and subjected to a binge drinking protocol: After the 1st 30 minutes session of the DIDMSA procedure, the animals rested in their home cage for 45 minutes. Then, the 2nd 30 minutes operant session was started, followed by another 45 minute rest period. The 3rd and final 30 minutes operant session followed. During the 45 minute rest periods, P rats had no access to food and water. At the end of the DIDMSA paradigm, the rats were returned to their home cage with ad libitum access to food and water until 8am the next experiment day. The 10% v/v ethanol or 3% sucrose consumption was recorded for each rat using calibrated measuring cylinders, and for ethanol, later calculated as g/kg based on the weight of the animal and the specific activity of ethanol. The same procedure was repeated daily on each experiment day (Figure 1).
In the ethanol/sucrose self-administration studies described above, each animal served as its own control. On day 0, animals received I.P./S.Q. saline or P.O. water. On days 1–4, animals received the KB220Z via the specified route. After discontinuation of KB220Z, animals were tested on the operant apparatus under DIDMSA conditions until drinking levels returned to baseline (Figure 1A). They were maintained at baseline prior to beginning a new route of administration. The same animals were used for SQ, IP, and PO administration of KB220Z for ethanol self-administration studies. A different set of animals were used for control studies of saline only injections overtime and KB220Z injections followed by 3% sucrose operant responding. All animals underwent standard operant training described above, and stabilized on EtOH, and prior to KB220Z, they were fasted for 4 hours. This procedure was identical for both ethanol and sucrose responding.
2.3.2. Anxiety/Risk-taking Behavior Test
Another, separate cohort of animals was used for tests of anxiogenic/anxiolytic/risk taking behavior and locomotor activity. These animals were treated with S.Q. saline on Day 0 and tested on the apparati. After 4 days of treatment with SQ KB220Z, the animals were tested again in the open field for elevated zero maze (EZM) and locomotor activity (LCA) tests on day 4. On Days 0 and 4, the animals were subjected to 4 hours of fasting in the morning. This was followed by treatment with either vehicle on day 0 or KB220Z on day 4; they were allowed to rest for 15 minutes in the homecage prior to testing. For behavioral testing, the animals were first subjected to the EZM for 5 minutes followed by OFT for 60 minutes. On Days 1 – 3, the animals were subjected to 4 hours fasting followed by KB220Z treatment but no behavioral tests were performed.
Elevated Zero Maze (EZM) test:
The EZM apparatus (Noldus Information Technology, Leesburg VA) is a circular runway with a circumference of 125.1cm and a track width of 10.2cm. It consists of four areas (two open and two closed), with the closed areas having a wall height of 20.5cm. The runway was mounted 64 cm above the floor (fall height). Rats purchased as adolescents were allowed to reach adulthood at approximately postnatal day 70 (P70). Handling mirroring the SQ treatment days were performed for at least 3 days to acclimatize animals to being handled and restrained. At Day 0, following SQ saline, and at Day 4, following 3.4mL/kg of SQ KB220Z, each rat was allowed to rest for 15 minutes and then placed in the center of the open arm and behavior was scored live using a rodent behavioral tracking system (Figure 1B). This experiment was performed in a dark room, and thus a camera with an infrared filter, fitted over the EZM, and a computer with pre-installed EthoVision XT video tracking software (Noldus Information Technology, Leesburg VA) was used to track the activities of the rats. The number of entries into the closed and open areas were calculated and considered an index of anxiety response and overall exploratory behavior, respectively. The maze was cleaned with 70% ethanol between the animals.
2.3.3. Locomotor Activity Test (LCA)
Open Field Test (OFT):
After testing on the EZM, Each rat was placed for 60 minutes in an OFT apparatus consisting of individual plexiglas cages (30 cm × 42 cm × 42 cm) using a 16-beam infrared OptoVarimex 4 monitoring system (Columbus, Instruments, Columbus, OH). The cage has a homogenous black plastic floor (Columbus Instruments, USA), designed to measure infrared beam interruption. The number of horizontal beam breaks was used to quantify locomotor activity of the animals, as previously reported [8].
2.4. In Situ Hybridization
In Situ Hybridization was conducted as described by Palop et al. (2011), with modifications, using Digoxigenin (DIG)-Labeled DRD2 probes (AumBiotech, Philadelphia, PA). Rats used for ISH were not previously used for any other experiments and thus were alcohol naïve. Alcohol naïve P rats at P70 were subjected to 4 hours of fasting (8am to noon) and then treated with S.Q. saline or KB220Z and allowed to rest for at least 15 minutes and stayed in their home cage until animals were sacrificed and brains removed within an hour. Whole brains were flash frozen in dry ice, an then stored at −80C until ready for use. Fresh frozen brains were cut on a cryostat at 10 microns beginning at anterior-most regions of the NAc to the posterior-most regions, with a periodicity of approximately 650 microns. Sections were slide mounted and frozen at −80C until needed. On the day of the experiment, sections were fixed in 4% paraformaldehyde (PFA) in phosphate buffer saline (PBS) for 10 minutes, washed 3 times for 3 minutes with PBS and digested in Proteinase K for 13 minutes (1μg/ml in 50mM Tris pH8, 5mM EDTA). The sections were then fixed in 4% PFA for 5 minutes, followed by 3 washes with PBS for 3 minutes.
Acetylation was conducted for 10 minutes in a solution of acetic anhydride and triethanolamine. After that, the brain tissues were permeabilized for 30 minutes with PBT (PBS + 1%triton) followed by 3 washes with PBS for 3 minutes. The sections were then transferred to a humidified chamber where they were incubated in pre-hybridization buffer for 4 hours at room temperature (50% formamide, 5× salt sodium citrate (SSC) buffer, pH 7.0, 5× Denhardt’s solution, 0.25 mg/ml salmon sperm DNA, 0.5 mg/ml yeast tRNA). The pre-hybridization buffer was then replaced by the hybridization solution (pre-hybridization buffer + 500ng probe/mL). The slides were coversliped and left in the humidified chamber overnight at 72°C. Afterwards, the coverslips were removed at 72°C in 5x SSC and the sections were washed twice at 0.2x SSC at 72°C, followed by a wash in 0.2x SSC at room temperature for 5 minutes, and in Tris-Saline buffer (TBS) for 5 min. The sections were transferred to a humidified chamber and blocked with TBS and 10% heat inactivated sheep serum for 2 hours. The incubation with 1:5000 anti-digoxigenin antibody in TBS and 3% HISS was done overnight at 4°C. Next, the excess antibody was removed with 6 × 30 min washes in TBS. The sections were then equilibrated for 5 minutes with alkaline phosphatase buffer containing 100mM NaCl, 100mM Tris-Cl, 50mM MgCl2 and 1% Tween 20 (NTMT) and incubated in ready-to-use BCIP (5-Bromo-4-chloro-3-indolyl phosphate)/NBT (nitro blue tetrazolium) (Sigma-Aldrich, St. Louis, MO) until development at room temperature in the dark. After development, the slides were washed twice with PBS/EDTA for 5 minutes, fixed with 4% PFA for 10 minutes and washed twice with PBS for 10 minutes. They were then rinsed with 0.01M Tris pH 7.5 for 5 minutes and counterstained with nuclear fast red.
2.5. Stereological analysis
Unbiased stereology was used to estimate mean total number of neurons in the Nucleus Accumbens Core and Shell, as well as the total number of mRNA on both regions. Data collection was carried out using the Stereologer software (Stereology Resource Center, Inc. FL) as described in Gondré-Lewis et al. (2016). The sampling grid size was set to 150 μm and the counting frames were set to 54.77 μm × 54.77 μm for neuron couting and to 26.46 μm × 26.46 μm for mRNA counting. The guard volume was fixed to1.0 μm on both sides of the section. Six females were used for the stereological analysis (3 KB220Z-treated and 3 controls).
2.6. Statistical Analyses
All values were expressed as mean ± SEM. Significance was determined by two-way ANOVA with repeated measures for the operant lever pressing and drinking analyses, and two-way ANOVA for the EZM, locomotor activity and in situ experiments. Where the F-test showed significance with a p value of < 0.05, a Bonferroni post-hoc correction for multiple comparison error was employed to calculate the t statistics, and the corresponding p values were used to determine significance at 5% alpha level (Type 1 error) or 95% Confidence Interval, two-tailed. For all multiple comparison tests, p values <0.05 were considered significant. All lever pressing analyses consisted of day 0, compared to days 1–4.
3.0. Results
3.1. I.P., S.Q. and P.O. administration of KB220Z led to reduced ethanol reward seeking.
In the operant self-administration behavioral test, a decrease in the lever presses indicates decreased appetitive behavior for 10% v/v ethanol. We used a 2-way ANOVA model with repeated measures to analyze the individual and combinatorial impact of the sex of the P rat and treatment with KB220Z over four days compared to vehicle on day zero, on operant lever pressing for 10% ethanol. Whereas there was no interaction between sex and time regardless of the route of administration (I.P., F(4,85)= 1.035, p=0.394; S.Q., F(4,100)=0.867, p=0.487; P.O. F(4,95)=0.493), there was a main effect of KB220Z on lever pressing: I.P. F(4,85) =10.93, p<0.001, S.Q. F(4,100) =11.31, p<0.001, P.O. F(4,95) =9.52, p<0.001 whereas there was no main impact of sex, I.P.: F(1,85) =1.53, S.Q.: F(1,100) =2.95, P.O.: F(1,95) =0.00, p>0.05 for all. The Bonferroni post hoc test revealed that females and males exhibited a significant and sustained reduction in lever pressing (36% and 51% respectively) on day 2, day 3, and day 4 of I.P. KB220Z treatment as compared to I.P. Saline (p < 0.01 for each sex on each of those days, n=19; 12 females and 7 males; Figure 2A). Similarly, S.Q. KB220Z administration did not reduce lever presses on Day 1, but led to a sustained ~30% and 48% reduction on days 2–4 by P female and male rats, respectively, compared to S.Q. Saline (p<0.05 for each sex, n=22, 12 females and 10 males, (Figure 2B). Administration of KB220Z orally, P.O., had a delayed impact on lever pressing, producing significant average reductions of 27% and 33% for females and males on days 3 and 4 only (p < 0.05; n=21, 12 females and 9 males; Figure 2C). These data suggest immediate marked decrease in appetitive behavior for 10% ethanol by both male and female P rats administered with I.P. and S.Q. KB220Z, whereas P.O. was delayed in producing a reduction in lever pressing. All lever pressing returned to control levels by 3 days post discontinuation of KB220Z.
Figure 2.

Lever Pressing Behavior in P Rats Following KB220Z Treatment.
(A) I.P. administration gradually reduced lever pressing for ethanol reward among female and male P rats beginning on day 2 (*p<0.001, days 2–4). (B) S.Q. administration reduced lever pressing in both male and female with a similar effectiveness as I.P. administration (*p<0.001). (C) P.O. administration of KB220Z nutraceutical significantly reduced lever pressing behavior, but to a lesser extent than S.Q. and I.P. administration (*p<0.05), n=9–12 rats per sex per group. Lever pressing activity went back to control levels by day 3 and maintained at day 4 post discontinuation of KB220Z for all treatment routes. (D) S.Q. administration of KB220Z had no effect on 3% sucrose operant responding, p>0.05. PD= post discontinuation; Bars represent means ± SEM.
Operant testing was done under DIDMSA conditions as detailed in the methods. To test if the observed reduction in lever pressing was specific to the alcohol reward and not due to nonspecific overall decreased drinking, male and female P rats were subjected to binge drinking of 3% sucrose for 4 consecutive days following SQ KB220Z in a parallel experiment to figure 2B, n=8 per sex. A 2-way ANOVA of this control experiment revealed no main effect of KB220Z on lever pressing for sucrose over time (Figure 2D; F (4, 70) = 2.060; p = 0.095) and no main interaction of sex and time (F (4, 70) = 0.997; p = 0.415), albeit there was a main effect of sex on lever pressing for sucrose (F (1, 70) = 10.702; p = 0.002). Finally, to test if continuous handling impacted drinking behavior, P rats received SQ saline injections and were tested on the operant for 5 consecutive days. Saline alone did not impact ethanol responding over time F (4, 70) = 0.943; p>0.05 (data not shown).
3.2. I.P., S.Q. and P.O. administration of KB220Z led to significant decrease in consumption of 10% ethanol in P rats.
We then analyzed the actual amount of 10% ethanol consumed as a result of KB220Z and the operant action performed. Preliminary ANOVA analysis showed that regardless of administration route, there was a main impact of KB220Z treatment (I.P., F(4,85) =6.3 p<0.001, S.Q. F(4,100) =18.4, p<0.001, P.O. F(4,95) =7.7, p<0.001) and sex (I.P. F(1,85) =79.2, S.Q. F(1,100) =112.1, F(1,95) =141.5, p<0.001) on ethanol consumption, but no interaction between sex and treatment. By day 4 of KB220Z administration, female and male P rats significantly decreased consumption of EtOH (g/Kg), respectively averaging 8% and 25% with I.P. KB220Z (Figure 3A; p<0.05), and 17% and 46% with S.Q. KB220Z (Figure 3B; p<0.001) as compared to the saline vehicle administered on day 0. These patterns were similar to the operant data in figure 1, as the significant reduction began at day 2 of KB220Z exposure. When KB220Z was administered by P.O., this led to an average respective 14% and 22% reduction in ethanol drinking by P female and male rats, as compared to P.O. water, p<0.05 (Figure 3C). Overall, it took at least three consecutive days for KB220Z via the P.O. route to exhibit similar effects. This data suggests slower effect of KB220Z following P.O. administration as compared to I.P and S.Q. Interestingly, when we analyzed sex differences in the effects of KB220Z, irrespective of the treatment condition, female P rats consumed an average of 50.9%, 66.7%, 51.6% more g/kg of EtOH by I.P., S.Q., and P.O. respectively, p<0.001 for each day and route (Figure 3). EtOH drinking returned to its elevated level by 3 or 4 days post discontinuation of KB220Z (Fig 3A–3C), for all routes and groups of animals, except females who received SQ KB220Z (Figure 3B, gray bars). When tested at 8 days post KB220Z, SQ treated females had returned to baseline (data not shown). Finally, a separate age-matched cohort of animals were trained to lever press for ethanol on the FR4 schedule as previously described, then they were allowed to lever press for a 3% sucrose reward as a positive control. Over 4 days with SQ KB220Z administration, A 2-way ANOVA revealed no significant changes in sucrose consumption in male or females over time (Figure 3D; F(4, 70) = 0.239; p = 0.916.
Figure 3.

Ethanol Consumption by P Rats Following KB220Z Treatment.
(A) Daily I.P. Administration of KB220Z reduced ethanol consumption primarily in male P rats. Female subjects consistently consumed more ethanol than males regardless of KB220Z treatment (###p<0.001). (B) S.Q. administration of KB220Z reduced ethanol consumption independent of sex and induced a greater effect than any other route of administration at the dose used (***p<0.001). Female P rats continue to consume more grams of ethanol per kg than male counterparts (###p<0.001). (C) P.O. administration of KB220Z drug significantly reduced ethanol consumption in male subjects and to a greater extent, in the females (*p<0.05 **p<0.01). Female P rats consistently consume more grams of ethanol per kg than male counterparts. (###p<0.001). Even in the presence of KB220Z, females’ increased ethanol consumption compared to males was not impacted. (D) S.Q. administration of KB220Z had no effect on 3% sucrose consumption. Bars represent Means ± SEM. * = compared to control treatment, # = Sex Comparison
3.3. S.Q. KB220Z administration led to decreased horizontal beam breaks in male and female P rats.
Next, we assessed the effect of KB220Z on other affective measures beginning with a test of locomotor activity (LCA) in the open field. A different cohort of young adult P rats, not previously subjected to ethanol drinking or other behavioral tests were used for locomotor activity and on the elevated zero maze. Since S.Q. seemed the most efficient route of administration of KB220Z in the previous experiment, it was used for other behavioral studies. P rats are known to exhibit hyperlocomotion in the open field compared to non-P rats of the same background [62]. In the Open Field Test (OFT), there was a main effect of treatment F(1, 44)=6.72, p=0.01and sex F(1, 44)=7.35, p<0.01 on locomotor activity. Following 4 consecutive days of S.Q. KB220Z administration, male P rats but not females exhibited a significant 21% reduction in horizontal beam breaks, p<0.05, n=12 per group per sex (Figure 4A).
Figure 4:

Examination of Locomotor Activity Following S.Q. KB220Z Treatment.
(A) S.Q. Administration of KB220Z had a negligible effect on Female locomotion activity in the open field however significantly reduced Horizontal Beam Breaks for male P rats (***p<0.001). Females Subjects typically show more locomotor activity than male counter parts regardless of treatment (##p<0.01). (B) S.Q. Administration of the KB220Z had no significant effect on the open areas time in the EZM for female and male subjects compared to their saline control, however male P rats record less open areas time than females after the S.Q. drug administration (##p<0.01). Lower graphs represent no treatment (NT) controls versus S.Q. saline. Bars represent means ± SEM.* = Treatment Effects # = Sex Comparison
3.4. S.Q. KB220Z administration led to decreased time spent in the open areas of EZM in male but not female P rats.
Rats were placed in the Elevated Zero Maze (EZM) to measure anxiety. The EZM has been shown to yield stable results for anxiogenic/anxiolytic measurements over multiple testing days for rodents compared to the elevated plus maze which is subject to the one trial tolerance phenomenon [63]. The EZM therefore allowed animals to serve as their own controls. P male rats exhibited a decrease in the open arm time as compared to no treatment (NT) and S.Q. saline groups following 4 consecutive days of KB220Z treatment. No change was observed in time spent in the open areas by P female rats (Figure 4B). To test if there were effects of repeated injection on behavior, we compared NT with SQ saline in the same animal and found no significance (lower graphs of 4A-4B).
3.5. In Situ Hybridization for DRD2 mRNA in Nucleus Accumbens.
To test for a potential mechanism of KB220Z in the brain, we next determined its impact on DRD2 mRNA expression in the NAc core and shell after 4 days of SQ exposure. In situ hybridization using DIG-labeled DRD2 probes was followed by quantification of mRNA punctae based on unbiased stereological principles. Figure 5A shows a representative stained section at 10X, and 63X images of the anterior commissure where, as expected, there are no DRD2 mRNA punctae (Figure 5B) and an the NAC where medium spiny neurons expressed abundant mRNA. Overall, the NAc shell expressed more DRD2 mRNA than NAc core F(1, 8) = 100.657; <0.05) (not shown), as well as more DRD2 mRNA units per cell than the NAc Core regardless of treatment (Figure 5C; F(1, 8) = 108.423; p<0.001). However, 4 days of SQ KB220Z treatment did not change DRD2 mRNA per cell in either NAc shell or Core (Figure 5D). Figure 5E represents the rat NAc as traced from Bregma +3.00 to +0.72 mm. Stereological parameters are shown represented in Table 2.
Figure 5. In situ hybridization for DRD2 mRNA in Nucleus Accumbens Core and Shell.
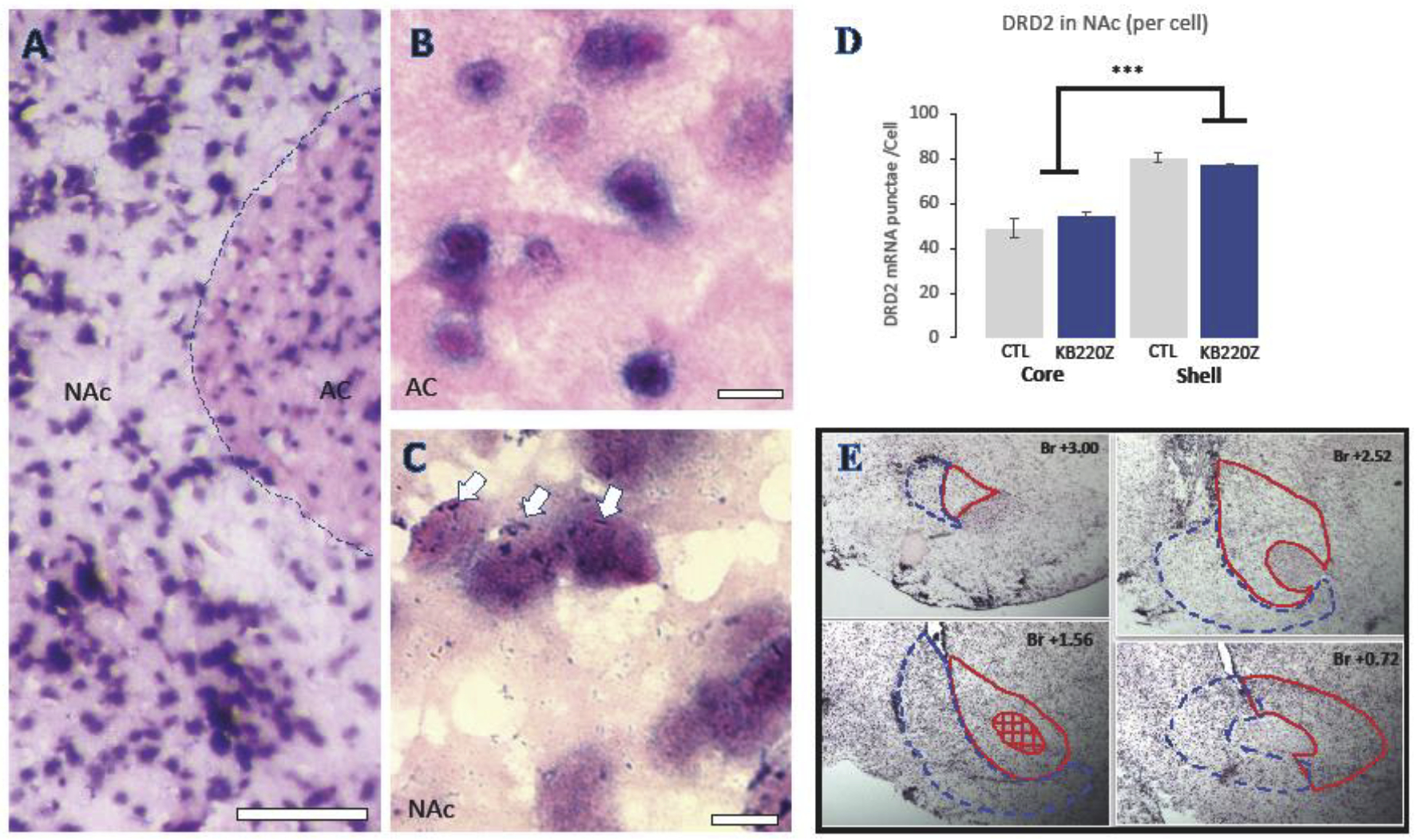
(A) Representative 10X image of a section with NAc and the anterior commissure (AC) and (B) a 63X image of the anterior commissure with no DRD2 mRNA puncta, and (C) an area of NAc with robust mRNA punctae clearly associated with neuronal cell bodies. (D) Unbiased stereological quantification of DRD2 mRNA punctae/cell in Nac core and shell of KB220 treated compared to non-treated animals. (E) Representative section tracings used for DRD2 stereology from anterior to posterior NAc. NAc Core is outlined in red and NAc Shell is in blue. AC = anterior commissure; NAc= Nucleus accumbens; ***= p<0.001. Bar = 100μm in A and 10μm in B and C.
Table 2:
Stereologer parameters for counting cells and mRNA in nucleus accumbens. Section Sampling Interval (SSI) is the interval on which the sections are collected. Reference Volume (Vref) is the volume measured for the region of interest. The coefficient of error (CE) should be <0.1.
| SSI | Vref (mm3) | CE (neuron counting) | CE (mRNA counting) | |
|---|---|---|---|---|
| CTL Nac Core | 65 | 2.068 | 0.0470 | 0.0525 |
| KB220Z-treated Nac Core | 65 | 1.623 | 0.0356 | 0.0536 |
| CTL Nac Shell | 69 | 2.301 | 0.0312 | 0.0573 |
| KB220Z-treated Nac Shell | 69 | 1.774 | 0.0261 | 0.0587 |
4.0. Discussion
Substance use disorders continue to be prevalent in the U.S. and internationally. Products that could repair the reward pathway of the brain, may prove useful in combating similar presentations associated with many types of substance use disorders and reward deficiency, in general. Here, we use an alcohol-preferring genetic model of excessive alcohol consumption to test a nutraceutical, KB220Z, believed to supply precursors that boost net dopamine in the brain. We report that KB220Z reduces drug seeking behavior by dampening the number of FR4 operant responses performed for an alcohol reinforcer, and this occured regardless of the route of administration of the KB220Z dose. Furthermore, overall EtOH consumed per kg is greater in females than males, even as the KB220Z treatment reduces consumption over several days of treatment in both sexes. A test of the nutraceutical on open field activity and anxiety, showed a reduction of P rat activity as well as times spent in the open areas of the EZM. These data suggest that this nutraceutical is effective in reducing aspects of drug-seeking behaviors and certain correlates of neuropsychiatric disease.
These studies were performed with a rat equivalent dose of 3.4 mL/kg, a dose which corresponds to the lowest daily KB220Z dose for human adults. The directions of using KB220Z as a dietary supplement on an empty stomach was achieved by fasting animals for 4 hours daily immediately prior to the treatment or with saline/vehicle. KB220Z is a variant formulation of a nutraceutical complex containing amino acid precursors, enkephalinase inhibitors, catabolic inhibitors that has been studied in both animals and humans [64]. The alcohol-preferring (P) rat was chosen because it is an established rodent model to study excessive alcohol consumption. Compared with nonalcohol-preferring (NP) rats, P rats have decreased dopaminergic neuronal projections and decreased expression of dopamine D2 receptors in the brain regions thought to contribute to AUD and associated behaviors [44, 45]. Furthermore, there are abnormalities in the serotonin (5-HT), GABA and opioid system of P rats also known to contribute to compulsive alcohol seeking behavior [42] and increased alcohol tolerance [48]. Based upon neurochemical and behavioral findings, it has been hypothesized that AUD and potentially all conditions associated with reward deficiency may share common molecular mechanisms that impige on suboptimal function of dopamine.
A close association between binge drinking and specific neurobehavioral deficits is well known in the literature, [65–67] and a causal role of disrupted dopamine homeostasis is well accepted [40, 68]. However, the link between binge drinking as well as associated behavioral deficits and a neuro-nutrient intervention has never been addressed. Our findings are therefore exciting for several reasons: First, the data herein suggests that the impaired dopamine homeostasis and reduced reward of the genetically alcohol preferring (P) rat is impacted by treatment with the putative prodopamine regulator (KB220Z) since it ameliorated occurrence of binge drinking behavior. Second, our data revealed that the dose of KB220Z administered elicited a greater effect by I.P. and S.C. than by P.O. in reducing the lever pressing for alcohol, possibly due to absorption efficacy differences of the nutraceutical in these routes. It is likely that KB220Z exhibited beneficial effects on behaviors of P rats via restoring decreased levels of dopamine and/or re-establishing the dopamine homeostasis within the reward circuitry of P rats.
Others have shown by BOLD resting state functional magnetic resonance imaging (rsfMRI) of rat brain that administration of KB220Z increased functional connectivity in reward and cognitive brain regions specifically in nucleus accumbens (NAc), hippocampus, anterior cingulate gyrus, anterior thalamic nuclei, and prelimbic and infralimbic loci [58]. Overall, KB220Z-induced dopamine homeostasis has been observed in both animal and human placebo-controlled fMRI experiments and electroencephalography recordings [69].
Pertinent to this, in the present study, we observed KB220Z-induced significant reduction in the number of lever presses by the P male and female rats suggesting decreased appetitive behavior for 10% ethanol. In addition, the P male and female rats had decreased consummatory behavior for 10% ethanol. Interestingly, KB220Z led to decreased horizontal beam breaks on the OFT suggesting reduced locomotor activity to a control state. Anxiety is a complex phenomenon known to be regulated by several brain regions including but not limited to the hippocampus, amygdala and the pre-frontal cortex (PFC) [70], influencers of brain reward. We did not observe significant effect of KB220Z treatment on the open areas times on the EZM compared to controls. A possible reason could be that 4 consecutive days of KB220Z treatment is not sufficient to induce changes in the neuroanatomical regions controlling this specific behavior. Nonetheless, males exhibited statistically significant anxiety-like behavior compared to females regardless of treatment. It is noteworthy, that the inability to cope with stress has been shown to load onto lower DRD2 receptors as shown in the work of the Comings group [71, 72] and others who implicate dopaminergic activity and stress related events including coping abilities [73, 74]. The anatomically distinct NAc core and shell regions modulate different aspects of reward seeking behavior and alcohol abuse [75, 76]. Previous studies suggest that P rats express reduced DRD2 receptors in both the VTA and NAc [45], and the excessive drinking phenotype can be attenuated with DRD2 gene transfer into the Acb core [46]. Thus we investigated if KB220Z might exert its effects on drinking by increasing DRD2 mRNA, and presumably DRD2 receptors in the Acb core. Our in situ findings suggest that the dose of KB220Z used (minimum recommended) does not increase DRD2 mRNA in the NAc as a mechanism for its behavioral rescue effects on excessive alcohol intake. Our approach did not account for expression of short vs. long forms of DRD2. Interestingly, NAc shell neurons generally express 30–50% more DRD2 than core neurons. Thus, the precise mechanism of KB220Z’s effect on drug abuse and behavioral health mechanisms are not fully known, as alcohol drinking and substance use disorder and co-morbid psychiatric disease, are complex disorders. Although KB220Z’s action on alcohol drinking may not be through the upregulation of DRD2 receptors, in specific, a boost in dopamine signaling may be achieved by other mechanisms that are enhanced by its components (Table 1).
5.0. Conclusion
In conclusion, data obtained in this study support our hypothesis that KB220Z reduces alcohol drinking and associated behavioral deficits, including psychological sequelae of open field activity and exploration of the EZM open area by P rats. Our conclusion is based on comparison with saline controls and with 3% sucrose as negative and positive controls. It is well known that hypodopaminergic reward circuitry leads to imbalance in dopamine homeostasis. This imbalance triggers the drug seeking behavior and other behavioral deficits. We suggest that KB220Z may act via a potent pro-dopamine regulator activity to help maintain optimum levels of dopamine within the reward circuit leading to decreased appetitive and consummatory behavior for ethanol. A nutrigenomic approach like this nutraceutical intervention can be beneficial alone or in combination with current treatment regimens, and might improve abstinence duration in substance use disorder patients.
Acknowledgement
Funding for this research was provided by National Institutes of Health’s Institute on Alcohol Abuse and Alcoholism (NIAAA) Grant #AA021262 to MGL. MGL and KB are recipients of NIMHD Grant #R41MD012318. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We thank Ms. Braeanna Hillman for technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Statement of Competing Interest: Kenneth Blum owns stock in some companies holding patents on genetic testing and KB220PAM. MGL serves on the Scientific Advisory Board of Geneus Health, which at the submission of this manuscript holds all rights to KB220Z. NS, TA, CG and PD have no other conflicts of interest to declare.
References
- [1].F.S. Centers for Disease Control and Prevention (CDC), Binge Drinking, Updated March 27, 2018.
- [2].Walsh K, Moreland AM, Hanson RF, Resnick HS, Saunders BE, Kilpatrick DG, Relationship violence victimization and binge drinking trajectories among a nationally representative sample of adolescents, J Adolesc 58 (2017) 49–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Hingson RW, Zha W, Binge Drinking Above and Below Twice the Adolescent Thresholds and Health-Risk Behaviors, Alcohol Clin Exp Res (2018). [DOI] [PubMed] [Google Scholar]
- [4].Popova S, Lange S, Probst C, Gmel G, Rehm J, Global prevalence of alcohol use and binge drinking during pregnancy, and fetal alcohol spectrum disorder, Biochem Cell Biol 96(2) (2018) 237–240. [DOI] [PubMed] [Google Scholar]
- [5].Zheng YL, Lian F, Shi Q, Zhang C, Chen YW, Zhou YH, He J, Alcohol intake and associated risk of major cardiovascular outcomes in women compared with men: a systematic review and meta-analysis of prospective observational studies, BMC Public Health 15 (2015) 773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Aberg F, Helenius-Hietala J, Puukka P, Jula A, Binge drinking and the risk of liver events: A population-based cohort study, Liver Int 37(9) (2017) 1373–1381. [DOI] [PubMed] [Google Scholar]
- [7].White AJ, DeRoo LA, Weinberg CR, Sandler DP, Lifetime Alcohol Intake, Binge Drinking Behaviors, and Breast Cancer Risk, Am J Epidemiol 186(5) (2017) 541–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Bassey RB, Gondré-Lewis MC, Combined early life stressors: Prenatal nicotine and maternal deprivation interact to influence affective and drug seeking behavioral phenotypes in rats, Behav Brain Res (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Gondre-Lewis MC, Warnock KT, Wang H, June HL Jr., Bell KA, Rabe H, Tiruveedhula VV, Cook J, Luddens H, Aurelian L, June HL Sr., Early life stress is a risk factor for excessive alcohol drinking and impulsivity in adults and is mediated via a CRF/GABA(A) mechanism, Stress 19(2) (2016) 235–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Gondre-Lewis MC, Addiction, Hyperactivity and Impulsivity: Consequences of Early Life Stress Exposure., Biological Psychiatry 77 (2015). [Google Scholar]
- [11].Radke AK, Held IT, Sneddon EA, Riddle CA, Quinn JJ, Additive influences of acute early life stress and sex on vulnerability for aversion-resistant alcohol drinking, Addict Biol (2019) e12829. [DOI] [PubMed] [Google Scholar]
- [12].Cruz FC, Quadros IM, Planeta Cda S, Miczek KA, Maternal separation stress in male mice: long-term increases in alcohol intake, Psychopharmacology (Berl) 201(3) (2008) 459–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Walters H, Kosten TA, Early life stress and the propensity to develop addictive behaviors, Int J Dev Neurosci 78 (2019) 156–169. [DOI] [PubMed] [Google Scholar]
- [14].Blum K, Thanos PK, Gold MS, Dopamine and glucose, obesity, and reward deficiency syndrome, Front Psychol 5 (2014) 919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Pavkovic B, Zaric M, Markovic M, Klacar M, Huljic A, Caricic A, Double screening for dual disorder, alcoholism and depression, Psychiatry Res 270 (2018) 483–489. [DOI] [PubMed] [Google Scholar]
- [16].Adinoff B, Neurobiologic processes in drug reward and addiction, Harv Rev Psychiatry 12(6) (2004) 305–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Blum K, Marcelo F, Dushaj K, Fried L, Badgaiyan RD, “Pro-dopamine regulation (KB220Z)” as a long-term therapeutic modality to overcome reduced resting state dopamine tone in opiate/opioid epidemic in America, J Syst Integr Neurosci 2(3) (2016) 162–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Volkow ND, Wang GJ, Telang F, Fowler JS, Alexoff D, Logan J, Jayne M, Wong C, Tomasi D, Decreased dopamine brain reactivity in marijuana abusers is associated with negative emotionality and addiction severity, Proc Natl Acad Sci U S A 111(30) (2014) E3149–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Ben Hamida S, Mendonca-Netto S, Arefin TM, Nasseef MT, Boulos LJ, McNicholas M, Ehrlich AT, Clarke E, Moquin L, Gratton A, Darcq E, Harsan LA, Maldonado R, Kieffer BL, Increased Alcohol Seeking in Mice Lacking Gpr88 Involves Dysfunctional Mesocorticolimbic Networks, Biol Psychiatry 84(3) (2018) 202–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Gondré-Lewis MC, Darius PJ, Wang H, Allard JS, Stereological analyses of reward system nuclei in maternally deprived/separated alcohol drinking rats, J Chem Neuroanat 76(Pt B) (2016) 122–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Willuhn I, Burgeno LM, Groblewski PA, Phillips PE, Excessive cocaine use results from decreased phasic dopamine signaling in the striatum, Nat Neurosci 17(5) (2014) 704–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Castrén S, Mäkelä N, Alho H, Selecting an appropriate alcohol pharmacotherapy: review of recent findings, Curr Opin Psychiatry 32(4) (2019) 266–274. [DOI] [PubMed] [Google Scholar]
- [23].Falk DE, O’Malley SS, Witkiewitz K, Anton RF, Litten RZ, Slater M, Kranzler HR, Mann KF, Hasin DS, Johnson B, Meulien D, Ryan M, Fertig J, Workgroup ACTIA, Evaluation of Drinking Risk Levels as Outcomes in Alcohol Pharmacotherapy Trials: A Secondary Analysis of 3 Randomized Clinical Trials, JAMA Psychiatry 76(4) (2019) 374–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Krupitsky E, Zvartau E, Blokhina E, Verbitskaya E, Wahlgren V, Tsoy-Podosenin M, Bushara N, Burakov A, Masalov D, Romanova T, Tyurina A, Palatkin V, Yaroslavtseva T, Pecoraro A, Woody G, Anhedonia, depression, anxiety, and craving in opiate dependent patients stabilized on oral naltrexone or an extended release naltrexone implant, Am J Drug Alcohol Abuse 42(5) (2016) 614–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Savant JD, Barry DT, Cutter CJ, Joy MT, Dinh A, Schottenfeld RS, Fiellin DA, Prevalence of mood and substance use disorders among patients seeking primary care office-based buprenorphine/naloxone treatment, Drug Alcohol Depend 127(1–3) (2013) 243–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Volkow ND, Frieden TR, Hyde PS, Cha SS, Medication-assisted therapies--tackling the opioid-overdose epidemic, N Engl J Med 370(22) (2014) 2063–6. [DOI] [PubMed] [Google Scholar]
- [27].Blum K, Gondré-Lewis MC, Baron D, Thanos P, Braverman ER, Neary J, Elman I, Badgaiyan RD, Introducing Precision Addiction Management of Reward Deficiency Syndrome, the Construct that Underpins All Addictive Behaviors, Frontiers in Psychiatry in Press; (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Blum K, Modestino EJ, Gondré-Lewis M, Downs BW, Baron D, Steinberg B, Siwicki D, Giordano J, McLaughlin T, Neary J, Hauser M, Fried L, Badgaiyan RD, “Dopamine homeostasis” requires balanced polypharmacy: Issue with destructive, powerful dopamine agents to combat America’s drug epidemic, J Syst Integr Neurosci 3 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Heilig M, Augier E, Pfarr S, Sommer WH, Developing neuroscience-based treatments for alcohol addiction: A matter of choice?, Transl Psychiatry 9(1) (2019) 255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Abijo T, Blum K, Gondré-Lewis MC, Neuropharmacological and Neurogenetic Correlates of Opioid Use Disorder (OUD) As A Function of Ethnicity: Relevance to Precision Addiction Medicine, Curr Neuropharmacol (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Eriksson K, The estimation of heritability for the self-selection of alcohol in the albino rat, Ann Med Exp Biol Fenn 47(2) (1969) 172–4. [PubMed] [Google Scholar]
- [32].Mardones J, Segovia-Riquelme N, Thirty-two years of selection of rats by ethanol preference: UChA and UChB strains, Neurobehav Toxicol Teratol 5(2) (1983) 171–8. [PubMed] [Google Scholar]
- [33].Li TK, Lumeng L, Doolittle DP, Selective breeding for alcohol preference and associated responses, Behav Genet 23(2) (1993) 163–70. [DOI] [PubMed] [Google Scholar]
- [34].Li TK, Lumeng L, Doolittle DP, Carr LG, Molecular associations of alcohol-seeking behavior in rat lines selectively bred for high and low voluntary ethanol drinking, Alcohol Alcohol Suppl 1 (1991) 121–4. [PubMed] [Google Scholar]
- [35].Cicero TJ, A critique of animal analogues of alcoholism., Biochemistry and Pharmacology of Ethanol 2 (1979) 533–560. [Google Scholar]
- [36].Lester D, Freed EX, Criteria for an animal model of alcoholism, Pharmacol Biochem Behav 1(1) (1973) 103–7. [DOI] [PubMed] [Google Scholar]
- [37].Balan I, Warnock KT, Puche A, Gondre-Lewis MC, Aurelian L, Innately activated TLR4 signal in the nucleus accumbens is sustained by CRF amplification loop and regulates impulsivity, Brain Behav Immun 69 (2018) 139–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].June HL, Liu J, Warnock KT, Bell KA, Balan I, Bollino D, Puche A, Aurelian L, CRF-amplified neuronal TLR4/MCP-1 signaling regulates alcohol self-administration, Neuropsychopharmacology 40(6) (2015) 1549–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Blum K, Reward Deficiency Syndrome, The SAGE Encyclopedia of Abnormal and Clinical Psychology (2017) 2888. [Google Scholar]
- [40].Bell RL, Rodd ZA, Lumeng L, Murphy JM, McBride WJ, The alcohol-preferring P rat and animal models of excessive alcohol drinking, Addict Biol 11(3–4) (2006) 270–88. [DOI] [PubMed] [Google Scholar]
- [41].Murphy JM, McBride WJ, Lumeng L, Li TK, Contents of monoamines in forebrain regions of alcohol-preferring (P) and -nonpreferring (NP) lines of rats, Pharmacol Biochem Behav 26(2) (1987) 389–92. [DOI] [PubMed] [Google Scholar]
- [42].Stewart RB, Li TK, The neurobiology of alcoholism in genetically selected rat models, Alcohol Health Res World 21(2) (1997) 169–76. [PMC free article] [PubMed] [Google Scholar]
- [43].Murphy JM, McBride WJ, Lumeng L, Li TK, Regional brain levels of monoamines in alcohol-preferring and -nonpreferring lines of rats, Pharmacol Biochem Behav 16(1) (1982) 145–9. [DOI] [PubMed] [Google Scholar]
- [44].Zhou FC, Zhang JK, Lumeng L, Li TK, Mesolimbic dopamine system in alcohol-preferring rats, Alcohol 12(5) (1995) 403–12. [DOI] [PubMed] [Google Scholar]
- [45].McBride WJ, Chernet E, Dyr W, Lumeng L, Li TK, Densities of dopamine D2 receptors are reduced in CNS regions of alcohol-preferring P rats, Alcohol 10(5) (1993) 387–90. [DOI] [PubMed] [Google Scholar]
- [46].Thanos PK, Taintor NB, Rivera SN, Umegaki H, Ikari H, Roth G, Ingram DK, Hitzemann R, Fowler JS, Gatley SJ, Wang GJ, Volkow ND, DRD2 gene transfer into the nucleus accumbens core of the alcohol preferring and nonpreferring rats attenuates alcohol drinking, Alcohol Clin Exp Res 28(5) (2004) 720–8. [DOI] [PubMed] [Google Scholar]
- [47].Liu J, Yang AR, Kelly T, Puche A, Esoga C, June HL Jr., Elnabawi A, Merchenthaler I, Sieghart W, June HL Sr., Aurelian L, Binge alcohol drinking is associated with GABAA alpha2-regulated Toll-like receptor 4 (TLR4) expression in the central amygdala, Proc Natl Acad Sci U S A 108(11) (2011) 4465–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Davis KM, Wu JY, Role of glutamatergic and GABAergic systems in alcoholism, J Biomed Sci 8(1) (2001) 7–19. [DOI] [PubMed] [Google Scholar]
- [49].Blum K, Elston SF, DeLallo L, Briggs AH, Wallace JE, Ethanol acceptance as a function of genotype amounts of brain [Met]enkephalin, Proc Natl Acad Sci U S A 80(21) (1983) 6510–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Chen W, Wang HJ, Shang NN, Liu J, Li J, Tang DH, Li Q, Moderate intensity treadmill exercise alters food preference via dopaminergic plasticity of ventral tegmental area-nucleus accumbens in obese mice, Neurosci Lett 641 (2017) 56–61. [DOI] [PubMed] [Google Scholar]
- [51].Robison LS, Swenson S, Hamilton J, Thanos PK, Exercise Reduces Dopamine D1R and Increases D2R in Rats: Implications for Addiction, Med Sci Sports Exerc (2018). [DOI] [PubMed] [Google Scholar]
- [52].Thanos PK, Hamilton J, O’Rourke JR, Napoli A, Febo M, Volkow ND, Blum K, Gold M, Dopamine D2 gene expression interacts with environmental enrichment to impact lifespan and behavior, Oncotarget 7(15) (2016) 19111–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Kjaer TW, Bertelsen C, Piccini P, Brooks D, Alving J, Lou HC, Increased dopamine tone during meditation-induced change of consciousness, Brain Res Cogn Brain Res 13(2) (2002) 255–9. [DOI] [PubMed] [Google Scholar]
- [54].Blum K, Liu Y, Wang W, Wang Y, Zhang Y, Oscar-Berman M, Smolen A, Febo M, Han D, Simpatico T, Cronje FJ, Demetrovics Z, Gold MS, rsfMRI effects of KB220Z on neural pathways in reward circuitry of abstinent genotyped heroin addicts, Postgrad Med 127(2) (2015) 232–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Blum K, Trachtenberg MC, Elliott CE, Dingler ML, Sexton RL, Samuels AI, Cataldie L, Enkephalinase inhibition and precursor amino acid loading improves inpatient treatment of alcohol and polydrug abusers: double-blind placebo-controlled study of the nutritional adjunct SAAVE, Alcohol 5(6) (1988) 481–93. [DOI] [PubMed] [Google Scholar]
- [56].McLaughlin T, Blum K, Steinberg B, Modestino EJ, Fried L, Baron D, Siwicki D, Braverman ER, Badgaiyan RD, Pro-dopamine regulator, KB220Z, attenuates hoarding and shopping behavior in a female, diagnosed with SUD and ADHD, J Behav Addict 7(1) (2018) 192–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].McLaughlin T, Blum K, Oscar-Berman M, Febo M, Demetrovics Z, Agan G, Fratantonio J, Gold MS, Using the Neuroadaptagen KB200z™ to Ameliorate Terrifying, Lucid Nightmares in RDS Patients: the Role of Enhanced, Brain-Reward, Functional Connectivity and Dopaminergic Homeostasis, J Reward Defic Syndr 1(1) (2015) 24–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Febo M, Blum K, Badgaiyan RD, Perez PD, Colon-Perez LM, Thanos PK, Ferris CF, Kulkarni P, Giordano J, Baron D, Gold MS, Enhanced functional connectivity and volume between cognitive and reward centers of naive rodent brain produced by pro-dopaminergic agent KB220Z, PLoS One 12(4) (2017) e0174774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Nair AB, Jacob S, A simple practice guide for dose conversion between animals and human, J Basic Clin Pharm 7(2) (2016) 27–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Shin J-W, Seol I-C, Son C-G, Interpretation of Animal Dose and Human Equivalent Dose for Drug Development, The Journal of Korean Oriental Medicine, 2010, pp. 1–7. [Google Scholar]
- [61].Liu J, Yang A, Kelly T, Puche A, Esoga C, June H, Elnabawi A, Merchenthaler I, Sieghart W, June HS, Aurelian L, Binge alcohol drinking is associated with GABAA α 2-regulated Toll-like receptor 4 (TLR4) expression in the central amygdala, Proc Natl Acad Sci USA, 2011, pp. 4465–4470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Badishtov BA, Overstreet DH, Kashevskaya OP, Viglinskaya IV, Kampov-Polevoy AB, Seredenin SB, Halikas JA, To drink or not to drink: open field behavior in alcohol-preferring and-nonpreferring rat strains, Physiol Behav 57(3) (1995) 585–9. [DOI] [PubMed] [Google Scholar]
- [63].Tucker LB, McCabe JT, Behavior of Male and Female C57BL/6J Mice Is More Consistent with Repeated Trials in the Elevated Zero Maze than in the Elevated Plus Maze, Front Behav Neurosci 11 (2017) 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Blum K, Oscar-Berman M, Stuller E, Miller D, Giordano J, Morse S, McCormick L, Downs WB, Waite RL, Barh D, Neal D, Braverman ER, Lohmann R, Borsten J, Hauser M, Han D, Liu Y, Helman M, Simpatico T, Neurogenetics and Nutrigenomics of Neuro-Nutrient Therapy for Reward Deficiency Syndrome (RDS): Clinical Ramifications as a Function of Molecular Neurobiological Mechanisms, J Addict Res Ther 3(5) (2012) 139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Harty SC, Gnagy EM, Pelham WE Jr., Molina BSG, Anger-irritability as a mediator of attention deficit hyperactivity disorder risk for adolescent alcohol use and the contribution of coping skills, J Child Psychol Psychiatry 58(5) (2017) 555–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Molnar SM, Beaton LE, Happer JP, Holcomb LA, Huang S, Arienzo D, Marinkovic K, Behavioral and Brain Activity Indices of Cognitive Control Deficits in Binge Drinkers, Brain Sci 8(1) (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Vinader-Caerols C, Talk A, Montanes A, Duque A, Monleon S, Differential Effects of Alcohol on Memory Performance in Adolescent Men and Women with a Binge Drinking History, Alcohol Alcohol 52(5) (2017) 610–616. [DOI] [PubMed] [Google Scholar]
- [68].Ding ZM, Ingraham CM, Rodd ZA, McBride WJ, Alcohol drinking increases the dopamine-stimulating effects of ethanol and reduces D2 auto-receptor and group II metabotropic glutamate receptor function within the posterior ventral tegmental area of alcohol preferring (P) rats, Neuropharmacology 109 (2016) 41–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Miller DK, Bowirrat A, Manka M, Miller M, Stokes S, Manka D, Allen C, Gant C, Downs BW, Smolen A, Stevens E, Yeldandi S, Blum K, Acute intravenous synaptamine complex variant KB220 “normalizes” neurological dysregulation in patients during protracted abstinence from alcohol and opiates as observed using quantitative electroencephalographic and genetic analysis for reward polymorphisms: part 1, pilot study with 2 case reports, Postgrad Med 122(6) (2010) 188–213. [DOI] [PubMed] [Google Scholar]
- [70].Patki G, Solanki N, Atrooz F, Allam F, Salim S, Depression, anxiety-like behavior and memory impairment are associated with increased oxidative stress and inflammation in a rat model of social stress, Brain Res 1539 (2013) 73–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Madrid GA, MacMurray J, Lee JW, Anderson BA, Comings DE, Stress as a mediating factor in the association between the DRD2 TaqI polymorphism and alcoholism, Alcohol 23(2) (2001) 117–22. [DOI] [PubMed] [Google Scholar]
- [72].Comings DE, Muhleman D, Gysin R, Dopamine D2 receptor (DRD2) gene and susceptibility to posttraumatic stress disorder: a study and replication, Biol Psychiatry 40(5) (1996) 368–72. [DOI] [PubMed] [Google Scholar]
- [73].Chandra R, Francis TC, Nam H, Riggs LM, Engeln M, Rudzinskas S, Konkalmatt P, Russo SJ, Turecki G, Iniguez SD, Lobo MK, Reduced Slc6a15 in Nucleus Accumbens D2-Neurons Underlies Stress Susceptibility, J Neurosci 37(27) (2017) 6527–6538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Campus P, Canterini S, Orsini C, Fiorenza MT, Puglisi-Allegra S, Cabib S, Stress-Induced Reduction of Dorsal Striatal D2 Dopamine Receptors Prevents Retention of a Newly Acquired Adaptive Coping Strategy, Front Pharmacol 8 (2017) 621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Ambroggi F, Ghazizadeh A, Nicola SM, Fields HL, Roles of nucleus accumbens core and shell in incentive-cue responding and behavioral inhibition, J Neurosci 31(18) (2011) 6820–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Purohit K, Parekh PK, Kern J, Logan RW, Liu Z, Huang Y, McClung CA, Crabbe JC, Ozburn AR, Pharmacogenetic Manipulation of the Nucleus Accumbens Alters Binge-Like Alcohol Drinking in Mice, Alcohol Clin Exp Res 42(5) (2018) 879–888. [DOI] [PMC free article] [PubMed] [Google Scholar]


