Abstract
Background
Multiparity is associated with a greater risk of incident cardiovascular disease. However, the relationship of parity with cardiovascular health, as measured by the American Heart Association Life’s Simple 7 metrics, is uncertain.
Objective
We aimed to examine the association between parity and ideal cardiovascular health among 3,430 women, aged 45–84 years, free of clinical cardiovascular disease enrolled in the Multi-Ethnic Study of Atherosclerosis.
Study design
The Multi-Ethnic Study of Atherosclerosis is a prospective cohort study that recruited middle aged to older women and men from six centers in the United States between 2000 and 2002. The study population comprised 38% White, 28% Black, 23% Hispanic and 11% Chinese Americans. Parity (total number of live births) was self-reported and categorized as 0, 1–2, 3–4 and ≥5. The Life’s Simple 7 metrics, defined according to American Heart Association criteria include health behaviors (smoking, physical activity, body mass index, diet) and health factors, (blood pressure, total cholesterol and blood glucose). We categorized each metric into ideal (2-points), intermediate (1-point) and poor (0-points). A total cardiovascular health score of 0–8 was considered inadequate; 9–10, average, and 11–14, optimal. We used multinomial logistic regression to examine the cross-sectional association between parity and cardiovascular health score, adjusted for socio-demographics, field site, hormone therapy and menopause.
Results
The mean (SD) age was 62 (10) years. The mean (SD) cardiovascular health score was lower with higher parity [8.9 (2.3), 8.7 (2.3), 8.5 (2.2), and 7.8 (2.0) for 0, 1–2, 3–4 and ≥5 live births, respectively]. In comparison to inadequate cardiovascular health scores, the adjusted odds of average cardiovascular health scores were significantly lower for all parity categories relative to nulliparity [prevalence Odds Ratios (OR) for parity of 1–2, 0.64 (95% CI 0.49–0.83); 3–4, 0.65 (0.49–0.86); ≥5, 0.64 (0.45–0.91)]. Women with ≥5 live births had a lower prevalence of optimal cardiovascular health scores [OR 0.50 (0.30–0.83)]. In the fully adjusted models, the association between parity and each Life’s Simple 7 metric was only statistically significant for body mass index. Women with ≥5 live births had lower prevalence of ideal body mass index [OR 0.52 (0.35–0.80)]. In addition, the test for interaction showed that the association between parity and cardiovascular health was not modified by race/ethnicity (P=0.81 for average cardiovascular health scores and 0.20 for optimal cardiovascular health scores).
Conclusion
Multiparity was associated with poorer cardiovascular health, especially for women with ≥5 live births. More research is required to explore the mechanisms by which parity may worsen cardiovascular health.
Keywords: Parity, live births, pregnancy, ideal cardiovascular health metrics, Life’s Simple 7
Introduction
The burden of cardiovascular disease (CVD) among women is substantial, with 1 in 3 women dying from CVD globally.1, 2 In the United States, approximately 48 million women aged ≥20 years, had CVD between 2011–2014, accounting for 36% of the total female population.3 The normal physiologic changes occurring during pregnancy (for example, changes in lipids, glucose, and weight), and additional stressors (such as endothelial dysfunction, inflammation and hemostatic processes), 4, 5 may lead to an increased risk of incident CVD later in life. Prior studies have reported that both a history of nulliparity (0 live births)6 and high parity (≥4 live births)7 are associated with an increased risk of CVD6, 7. However, other studies have reported different results.8, 9
With the introduction of the concept of ideal cardiovascular health (CVH) measured by the Life’s Simple 7 (LS7) metrics, the American Heart Association (AHA) emphasizes primordial prevention of CVD risk factors as a means to decrease the CVD burden in the general population.10 A person is considered to be in ideal CVH if they meet the ideal requirements for each LS7 metric.10 Numerous studies have documented ideal CVH is inversely related to the incidence of CVD.11, 12 However, only one of these studies has explored the association between parity and ideal CVH.13 Furthermore, it is not known if parity is more strongly associated with any of the individual LS7 metrics (i.e. smoking, physical activity, body mass index (BMI), diet, total cholesterol, blood pressure, blood glucose). Lastly, it is unclear if there are racial/ethnic differences in CVH related to parity. Thus, the aim of this study is to determine if parity is associated with ideal CVH among women in the Multi-Ethnic Study of Atherosclerosis (MESA). A secondary aim is to assess for interactions by race/ethnicity. We hypothesized that multiparity will be associated with poorer CVH irrespective of race/ethnicity.
Methods
Study population
MESA is a longitudinal study that recruited 6,814 women and men between the ages of 45–84 years from 6 centers (Baltimore, MD; Chicago, IL; Forsyth County, NC; Los Angeles, CA; New York, NY and St Paul, MN) between years of 2000–2002.14 The MESA study population was 38% White, 28% Black, 23% Hispanic and 11% Chinese American. Participants did not have a history of clinical CVD at the time of enrollment. The institutional review boards of each recruitment center approved the study protocol, and all participants gave informed consent. Data were collected from standardized questionnaires, physical examination findings and fasting laboratory blood draw. A total of 3,430 women were included in this cross-sectional analysis from the baseline examination, after the exclusion of men (n= 3,213) and participants without complete information on parity (n=2) and the LS7 metrics (n=169).
Assessment of cardiovascular health
The CVH score is the dependent variable of this study derived from the components of the LS7 metrics which consists of 4 health behaviors (smoking, physical activity, BMI, diet) and 3 health factors (total cholesterol, blood pressure, blood glucose).10 According to the AHA, ideal CVH is defined as non-smoking, physical activity at goal levels, BMI <25kg/m2 and a healthy diet consistent with the Dietary Approaches to Stop Hypertension (DASH) guideline, in addition to the nonuse of medications to achieve total cholesterol <200mg/dL, blood pressure <120/<80mmHg, and fasting blood glucose <100mg/dL.10 Smoking status was self-reported with 3 categories: nonsmokers (participants who never smoked or quit >12months ago), former smokers (participants who quit within past 12 months) and current smokers.10 Physical activity was measured using a self-report survey instrument modified from the Cross-Cultural Activity Participation Study.15 The survey has 28 questions on time and frequency of activities during a week in the previous month. The questions were used to estimate the total minutes of moderate and vigorous exercise.16 BMI was expressed in kg/m2 and was calculated from the weight and height measurements.
A validated food frequency questionnaire with 120 items was employed in the assessment of diet. The questionnaire was modified from the Insulin Resistance Atherosclerosis Study instrument.17, 18 A healthy diet included 5 items: fruits and vegetables, fish, whole grains, intake of sodium <1500mg per day and sugar-sweetened beverages ≤450 kcal (36 oz.) per week.10 Three blood pressure readings were taken from each participant in a seated position after 5 minutes of rest. The average value of the 2nd and 3rd readings were recorded. Total cholesterol and blood glucose measurements were obtained from 12-hour fasting blood samples.
The definition of the LS7 metrics and their categories (poor, intermediate, and ideal)10 are presented in Supplemental Table 1. Points were assigned to each category, where 0 indicates poor; 1, intermediate; and 2, ideal. The points from the 7 metrics were then summed to give a total CVH score (dependent variable) that ranged from 0 to 14.19 As previously reported, CVH scores of 0 to 8 were considered inadequate, 9 to 10, average and 11 to 14, optimal.12, 16, 20, 21
Assessment of parity
Parity is the independent variable of this study. Parity was self-reported and defined as the total number of live births. We used a previously defined algorithm for the gravidity and parity assessment in MESA.22 According to prior studies,23 the validity of self-report for parity vs. chart review is very high (kappa 0.93–0.98). As per prior studies,24, 25 we categorized parity as 0, 1–2, 3–4 and ≥5, and 0 (nulliparity) was the reference category.
Assessment of covariates
Age, race/ethnicity, education, income, health insurance, marital status, MESA field site, menopause status, and current use of hormone therapy were the covariates included in this study. Age ranged from 45–84 years (continuous), but we considered 2 categories, <65 and ≥65 years, in stratified analyses. Race/ethnicity was categorized as White, Black, Hispanic, or Chinese-American. Education had 9 categories, but was dichotomized as ≥ bachelor’s degree and < bachelor’s degree in stratified analyses. Income had 13 categories, but was dichotomized as ≥$40,000 and < $40,000 for stratified analyses. Menopause status was defined using a previously described algorithm and categorized as “Yes” and “No”.26 Marital status was categorized as married, widowed, divorced, separated, and never married. Health insurance and current use of hormone therapy were categorized as “Yes” and “No.”
Statistical Analyses
The baseline characteristics of the study participants were reported by parity categories. Continuous variables were presented as means with standard deviation (SD) while categorical variables were presented as frequencies with percentages. We used the ANOVA and chi-square tests to compare the differences between continuous and categorical variables, respectively. We also reported the proportions for each LS7 metrics by parity categories.
Multinomial logistic regression models were used to examine the association between parity (independent variable) and the CVH score categories (dependent variable) in the total cohort. Three separate regression models were fitted. Model 1 was unadjusted. Model 2 was adjusted for sociodemographic factors [age (continuous), race/ethnicity (4 categories), education (9 categories), income (13 categories), health insurance status (yes/no), marital status (5 categories), and MESA field site (6 categories)]. We adjusted for education and income using the original 9 and 13 categories collected at baseline to minimize residual confounding. Model 3 was additionally adjusted for current use of hormone therapy (yes/no) and menopause (yes/no). Prevalence Odds Ratios (ORs) and 95% confidence intervals (CIs) were calculated for the CVH score categories across the categories of parity. The reference groups were 0 live births (nulliparity) for parity and the inadequate score for the CVH score. In addition, the associations between parity and each of the individual LS7 metrics were examined by comparing the intermediate and ideal categories of the respective metrics to the poor category. We also examined the association between parity and the number of LS7 metrics in the “ideal” category, reporting prevalence ORs and 95% CIs. Participants with 2, 3, 4, 5 and 6–7 ideal metrics were compared to those with 0–1 ideal metrics.
We examined the interaction of parity with race/ethnicity using the likelihood ratio chi-square test, by including interaction terms in model 3. We performed this analysis to assess if race/ethnicity is an effect modifier of the relationship between parity and CVH. Given a priori interest in racial/ethnic and sociodemographic differences, we presented results stratified by race/ethnicity, age, education, and health insurance status regardless if a significant interaction was found (presented in supplemental material). In supplemental analyses, we also explored results stratified by gravidity instead of parity. Lastly, we calculated predicted probabilities of achieving CVH scores by parity categories. All analyses were performed using STATA version 15.0 (StataCorp LP, College Station, TX).
Results
Among women in our study population (N=3,430), the mean (SD) age was 62 (10) years and 18% were nulliparous, 39% had 1–2 live births, 19% had 3–4 live births and 13% had ≥5 live births. The baseline characteristics of study participants varied by parity categories as illustrated in Table 1. Women with ≥5 live births were more likely to be Hispanic or have less than a bachelor’s degree or earn <$40,000 annually or have a higher systolic blood pressure or higher fasting blood glucose. These women were also less likely to be physically active.
Table 1-.
Characteristics of study participants by parity
| Total (3430) | 0 (627) | 1–2 (1349) | 3–4 (644) | ≥5 (453) | P-value | |
|---|---|---|---|---|---|---|
| Age, mean (SD), y | 62 (10) | 60 (11) | 60 (10) | 63 (10) | 68 (9) | <0.001 |
| Age, y | ||||||
| <65 n (%) | 1972 (57) | 395 (63) | 895 (66) | 541 (54) | 141 (31) | <0.001 |
| ≥65 n (%) | 1458 (43) | 232 (37) | 454 (34) | 460 (46) | 312 (69) | |
|
Race/Ethnicity | ||||||
| White n (%) | 1,320 (38) | 314 (50) | 538 (40) | 362 (36) | 106 (23) | |
| Chinese American n (%) | 409 (12) | 39 (6) | 176 (13) | 143 (14) | 51 (11) | <0.001 |
| Black n (%) | 957 (28) | 194 (31) | 410 (30) | 245 (24) | 108 (24) | |
| Hispanic n (%) | 744 (22) | 80 (13) | 225 (17) | 251 (25) | 188 (42) | |
|
Education | ||||||
| ≥ Bachelor’s degree n (%) | 1044 (30) | 326 (52) | 455 (34) | 235 (23) | 28 (6) | <0.001 |
| < Bachelor’s degree n (%) | 2386 (70) | 301 (48) | 894 (66) | 766 (77) | 425 (94) | |
|
Income | ||||||
| ≥$40,000 n (%) 1 | 1453 (42) | 322 (51) | 658 (49) | 397 (40) | 76 (17) | <0.001 |
| <$40,000 n (%) 2 | 1977 (58) | 305 (49) | 691(51) | 604 (60) | 377 (83) | |
|
Health insurance | ||||||
| Yes n (%) | 3123 (91) | 597 (95) | 1,230 (91) | 916 (92) | 380 (84) | <0.001 |
| No n (%) | 307 (9) | 30 (5) | 119 (9) | 85 (8) | 73 (16) | |
| Menopause status | ||||||
| Yes | 2933 (86) | 497 (79) | 1112 (82) | 890 (89) | 434 (96) | <0.001 |
| No | 497 (14) | 130 (21) | 237 (18) | 111 (11) | 19 (4) | |
| Hormone therapy (post-menopause) | ||||||
| Current user | 986 (32) | 182 (34) | 412 (35) | 297 (32) | 95 (22) | <0.001 |
| Non-current user | 2104 (68) | 357 (66) | 780 (65) | 629 (68) | 338 (78) | |
| LS7 metrics* | ||||||
| Current smoking n (%) | 398 (12) | 79 (13) | 176 (13) | 98 (10) | 45 (10) | 0.11 |
| Body mass index (kg/m2) | 29 (6) | 28 (6) | 28 (6) | 29 (6) | 30 (6) | <0.001 |
| Physical activity (MET-min/week) | 342 (515) | 359 (479) | 356 (528) | 357 (561) | 244 (396) | <0.001 |
| Healthy diet score (0–5) | 1.7 (0.9) | 1.7 (0.9) | 1.7 (0.9) | 1.7 (0.9) | 1.6 (0.8) | 0.23 |
| Total cholesterol (mg/dL) | 200 (36) | 199 (35) | 200 (36) | 199 (35) | 199 (37) | 0.97 |
| Systolic blood pressure (mmHg) | 127 (23) | 124 (23) | 125 (23) | 128 (23) | 134 (24) | <0.001 |
| Diastolic blood pressure (mmHg) | 69 (10) | 69 (10) | 69 (10) | 69 (10) | 69 (10) | 0.22 |
| Fasting blood glucose (mg/dL) | 95 (28) | 90 (22) | 94 (26) | 96 (31) | 101 (31) | <0.001 |
| CVH score | ||||||
| Inadequate | 1614 (47) | 240 (38) | 612 (45) | 483 (48) | 279 (62) | |
| Average | 1118 (33) | 228 (36) | 424 (31) | 327 (33) | 139 (31) | <0.001 |
| Optimal | 698 (20) | 159 (25) | 313 (23) | 191 (19) | 35 (8) | |
Abbreviations: SD, standard deviation; LS7, Life’s Simple 7; CVH, Cardiovascular health
Mean(SD) of LS7 metrics are shown with the exception of smoking
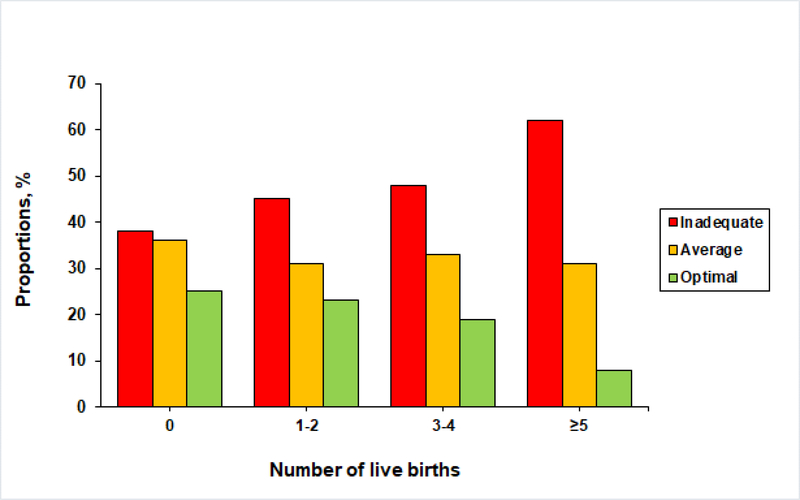
The mean (SD) for the CVH score was lower with higher parity as follows: 8.9 (2.3), 8.7 (2.3), 8.5 (2.2), and 7.8 (2.0) for 0, 1–2, 3–4 and ≥5 live births, respectively (P<0.001) (Supplemental Table 2). The distribution of the CVH scores within parity categories for the total cohort and stratified by race/ethnicity are presented in the Figure and supplemental material, respectively. The proportion of women with optimal CVH scores (11–14 points) was lower with higher parity.
Figure.
Distribution of cardiovascular health scores within parity categories
The associations between parity and CVH score categories in the overall study population are presented in Table 2. After adjusting for sociodemographic characteristics, MESA field site, current use of hormone therapy and menopause status (model 3), women with 1–2, 3–4 and ≥5 live births had lower prevalence of average CVH scores (9–10 points) compared to women with 0 live births [prevalence OR for 1–2 live births, 0.64 (95% CI 0.49–0.83); 3–4, 0.65 (0.49–0.86); ≥5, 0.64 (0.45–0.91)]. In addition, multiparity was associated with lower prevalence of optimal CVH scores although the association was only statistically significant for women with ≥5 live births [prevalence OR 0.50 (95% CI, 0.30–0.83)].
Table 2-.
Association between parity and cardiovascular health
| Parity | CVH score |
|
|---|---|---|
| Average vs. Inadequate | Optimal vs. Inadequate | |
|
OR (95% CI) | ||
| Model 1: Unadjusted | ||
| 0 | 1 (reference) | 1 (reference) |
| 1–2 | 0.73 (0.59–0.91) | 0.77 (0.61–0.98) |
| 3–4 | 0.71 (0.57–0.90) | 0.60 (0.46–0.78) |
| ≥5 | 0.52 (0.40–0.69) | 0.19 (0.13–0.28) |
| Model 2: Adjusted | ||
| 0 | 1 (reference) | 1 (reference) |
| 1–2 | 0.68 (0.52–0.87) | 0.82 (0.61–1.11) |
| 3–4 | 0.70 (0.53–0.92) | 0.79 (0.57–1.10) |
| ≥5 | 0.69 (0.49–0.95) | 0.48 (0.29–0.77) |
| Model 3: Adjusted | ||
| 0 | 1 (reference) | 1 (reference) |
| 1–2 | 0.64 (0.49–0.83) | 0.84 (0.61–1.17) |
| 3–4 | 0.65 (0.49–0.86) | 0.75 (0.53–1.08) |
| ≥5 | 0.64 (0.45–0.91) | 0.50 (0.30–0.83) |
Abbreviations: CVH, cardiovascular health; OR, odds ratio; CI, confidence interval. Model 2: adjusted for sociodemographic factors (age, race/ethnicity, education, income, health insurance status, marital status) and MESA site. Model 3: adjusted for Model 2 variables + current use of hormone therapy and menopause status. No statistically significant interaction for parity with race/ethnicity (p=0.81 for average scores, 0.20 for optimal scores). N for models 1 & 2= 3,430; N for model 3= 3,090
The distribution of each of the LS7 metrics by parity categories is shown in Supplemental Table 2, and the adjusted associations between parity and each LS7 metrics are presented in Tables 3 & 4. We did not find any associations of the metrics of diet, smoking, and total cholesterol with parity. For the metrics of physical activity, blood pressure, and glucose, there was a graded association between parity and the LS7 metrics, where multiparity was associated with lower prevalence of intermediate or ideal metrics; however these associations were not statistically significant in the fully adjusted model. However, for BMI, this graded association was seen even in adjusted analyses, where women with ≥5 live births had lower prevalence of ideal BMI [prevalence OR 0.52 (0.35–0.80), Table 3, model 3]. In adjusted models, we did not find a statistically significant association between parity and the number of LS7 metrics in the “ideal” category as shown in Supplemental Table 3.
Table 3 -.
Association between parity and Life’s Simple 7 metrics (Health behaviors)
| Parity | Smoking | Body Mass Index | Physical Activity | Diet | ||||
|---|---|---|---|---|---|---|---|---|
| Intermediate vs. poor | Ideal vs. Poor | Intermediate vs. poor | Ideal vs. Poor | Intermediate vs. poor | Ideal vs. Poor | Intermediate vs. poor | Ideal vs. Poor | |
| OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | |||||
| Model 1: Unadjusted | ||||||||
| 0 | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) |
| 1–2 | 1.62 (0.58,4.51) | 0.95 (0.72,1.27) | 1.06 (0.84,1.34) | 0.89 (0.71,1.12) | 0.96 (0.71,1.29) | 0.92 (0.72,1.17) | 1.07 (0.88,1.30) | 0.88 (0.41,1.86) |
| 3–4 | 1.45 (0.47,4.50) | 1.33 (0.97,1.82) | 1.04 (0.81,1.32) | 0.76 (0.60,0.97) | 0.67 (0.49,0.92) | 0.72 (0.56,0.92) | 1.15 (0.90,1.36) | 1.34 (0.64,2.81) |
| ≥5 | 1.05 (0.24,4.62) | 1.31 (0.89,1.93) | 0.77 (0.58,1.03) | 0.41 (0.30,0.56) | 0.65 (0.46,0.93) | 0.49 (0.37,0.66) | 1.01 (0.78,1.29) | 0.63 (0.21,1.84) |
| Model 2: Adjusted | ||||||||
| 0 | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) |
| 1–2 | 1.60 (0.52,4.89) | 0.90 (0.65,1.25) | 0.98 (0.75,1.27) | 0.90 (0.68,1.19) | 1.10 (0.79,1.53) | 1.04 (0.79,1.37) | 0.98 (0.78,1.23) | 0.69 (0.30,1.55) |
| 3–4 | 1.43 (0.40,5.04) | 1.16 (0.80,1.68) | 0.86 (0.65,1.15) | 0.74 (0.54,1.00) | 0.85 (0.60,1.22) | 0.95 (0.71,1.27) | 1.00 (0.79,1.28) | 1.08 (0.48,2.47) |
| ≥5 | 0.81 (0.15,4.42) | 0.97 (0.61,1.55) | 0.61 (0.43,0.86) | 0.49 (0.33,0.73) | 0.96 (0.62,1.47) | 0.91 (0.64,1.29) | 0.98 (0.72,1.33) | 0.66 (0.20,2.23) |
| Model 3: Adjusted | ||||||||
| 0 | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) |
| 1–2 | 1.44 (0.40,5.16) | 0.88 (0.62,1.26) | 1.01 (0.76,1.33) | 0.94 (0.70,1.28) | 1.11 (0.78,1.59) | 1.00 (0.74,1.34) | 1.02 (0.80,1.30) | 0.63 (0.27,1.44) |
| 3–4 | 1.52 (0.38,6.04) | 1.06 (0.72,1.58) | 0.89 (0.66,1.20) | 0.77 (0.55,1.07) | 0.79 (0.54,1.16) | 0.89 (0.65,1.22) | 1.03 (0.80,1.34) | 1.07 (0.46,2.46) |
| ≥5 | 0.80 (0.13,4.92) | 0.89 (0.55,1.45) | 0.65 (0.45,0.93) | 0.52 (0.35,0.80) | 1.00 (0.64,1.57) | 0.89 (0.61,1.28) | 1.00 (0.73,1.38) | 0.63 (0.18,2.14) |
Abbreviation: OR indicates odds ratio; CI, confidence interval; Model 2: adjusted for sociodemographic factors (age, race/ethnicity, education, income, health insurance status, marital status) and MESA site. Model 3: adjusted for Model 2 variables + current use of hormone therapy and menopause status. OR were rounded up to 2 decimal places; N for models 1 & 2= 3,430; N for model 3= 3,090
Table 4 -.
Association between parity and Life’s Simple 7 metrics (Health factors)
| Parity | Total Cholesterol | Blood Pressure | Blood Glucose | |||
|---|---|---|---|---|---|---|
| Intermediate vs. poor | Ideal vs. Poor | Intermediate vs. poor | Ideal vs. Poor | Intermediate vs. poor | Ideal vs. Poor | |
| OR (95% CI) | OR (95% CI) | OR (95% CI) | ||||
| Model 1: Unadjusted | ||||||
| 0 | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) |
| 1–2 | 1.11 (0.84,1.46) | 0.99 (0.75,1.30) | 0.80 (0.62,1.03) | 0.89 (0.71,1.11) | 0.85 (0.53,1.36) | 0.63 (0.43,0.91) |
| 3–4 | 1.27 (0.94,1.70) | 1.03 (0.77,1.38) | 0.76 (0.59,0.98) | 0.65 (0.52,0.83) | 0.85 (0.53,1.38) | 0.56 (0.38,0.83) |
| ≥5 | 0.88 (0.62,1.24) | 0.82 (0.58,1.15) | 0.68 (0.50,0.92) | 0.37 (0.28,0.50) | 0.85 (0.51,1.42) | 0.32 (0.21,0.48) |
| Model 2: Adjusted | ||||||
| 0 | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) |
| 1–2 | 1.02 (0.74,1.39) | 0.88 (0.65,1.20) | 0.91 (0.69,1.21) | 1.05 (0.80,1.40) | 0.81 (0.48,1.34) | 0.63 (0.42,0.96) |
| 3–4 | 1.19 (0.85,1.67) | 1.04 (0.74,1.46) | 0.96 (0.71,1.30) | 0.99 (0.73,1.34) | 0.89 (0.52,1.53) | 0.71 (0.46,1.10) |
| ≥5 | 0.99 (0.66,1.50) | 1.09 (0.72,1.65) | 1.13 (0.79,1.62) | 0.95 (0.65,1.41) | 1.06 (0.58,1.94) | 0.70 (0.42,1.14) |
| Model 3: Adjusted | ||||||
| 0 | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) |
| 1–2 | 0.94 (0.68,1.30) | 0.87 (0.63,1.21) | 0.96 (0.72,1.30) | 1.04 (0.77,1.40) | 0.78 (0.46,1.34) | 0.59 (0.38,0.92) |
| 3–4 | 1.11 (0.78,1.58) | 1.01 (0.70,1.44) | 0.99 (0.73,1.36) | 0.95 (0.69,1.31) | 0.87 (0.49,1.54) | 0.66 (0.42,1.05) |
| ≥5 | 0.92 (0.60,1.42) | 1.04 (0.68,1.60) | 1.13 (0.78,1.65) | 0.95 (0.64,1.42) | 1.04 (0.55,1.95) | 0.68 (0.40,1.14) |
Abbreviation: OR indicates odds ratio; CI, confidence interval; Model 2: adjusted for sociodemographic factors (age, race/ethnicity, education, income, health insurance status, marital status) and MESA site. Model 3: adjusted for Model 2 variables + current use of hormone therapy and menopause status. OR were rounded up to 2 decimal places; N for models 1 & 2= 3,430; N for model 3= 3,090
The test for interaction showed that the association between parity and CVH was not modified by race/ethnicity (P=0.81 for average scores and 0.20 for optimal scores). The associations between parity and CVH score categories stratified by race/ethnicity are shown in Supplemental Table 4 & 5. In the fully adjusted stratified analysis, White women with ≥5 live births had lower prevalence of optimal scores [prevalence OR 0.23 (0.09–0.63)], while Hispanic women with 3–4 live births had lower prevalence of optimal scores [prevalence OR 0.29 (0.10–0.82)]. The predicted probabilities of CVH scores by parity categories showed that women with ≥5 live births had highest probability for achieving inadequate scores [margin 0.53 (0.47–0.59)] and the lowest probability for achieving optimal scores [margin 0.11 (0.08–0.15) (Supplemental Table 6)]. The associations of parity and CVH scores stratified by age, education, and health insurance status are presented in Supplemental Table 7. The associations of gravidity and CVH are presented in Supplemental Table 8 and show similar patterns to parity.
Discussion
Principal findings
In this multi-ethnic community-based cohort of women free of CVD at baseline, we found that after taking into consideration sociodemographic characteristics (age, race/ethnicity, education, income, health insurance, marital status), MESA field site, current use of hormone therapy and menopause status, women with a history of 1–2, 3–4 and ≥5 live births had lower prevalence of average CVH scores compared to women with 0 live births. In addition, women with ≥5 live births had lower prevalence of optimal CVH scores compared to women with 0 live births. Women with a history of ≥5 live births also had lower prevalence of ideal BMI.
Comparison to prior research
A previous meta-analysis including 10 cohorts and over 3 million women found an increased risk of incident CVD events associated with parity (compared to nulliparity) in a non-linear dose-dependent fashion6. The reasons for this greater CVD risk associated with parity are uncertain, but several potential biologic mechanisms have been proposed including unfavorable changes in lipids, glucose, and weight with each pregnancy, as well as endothelial dysfunction, inflammatory and hemostatic processes.4, 5 Few studies have examined the association of parity with markers of cardiovascular wellness, which was the aim of the present study. The findings of our study are comparable to those of a prior cross-sectional study that examined the cumulative effect of reproductive factors including parity on ideal CVH among postmenopausal Chinese women.13 In that study, participants with more than 3 children (compared with women with 3 children) were less likely to have better CVH. After adjusting for age, these women had lower prevalence of 2, 3, 4, 5, and 6–7 ideal metrics (prevalence OR 0.72 (0.62–0.82), 0.66 (0.58–0.76), 0.62 (0.52–0.68), 0.56 (0.48–0.66) and 0.42 (0.38–0.56), respectively compared to 0–1 ideal metrics. Additionally, the odds of meeting the ideal criteria for BMI and physical activity were 24% and 34% lower, respectively among these women.
Possible mechanisms
The main finding of our study in a multi-ethnic cohort showed that women with a history of greater number of live births were more likely to have worse CVH when assessed in mid- to later adulthood. For example, women with a history of multiparity had lower prevalence of ideal BMI later in life. This finding can be explained by several physiological changes that occur during pregnancy and persist many years postpartum.27, 28 These changes include, insulin resistance,29 hormonal alterations (such as decreased estrogen levels) caused by fewer ovulatory cycles,30 and the excessive accumulation of adipose tissue.31 Behavioral and lifestyle changes during pregnancy, such as reduction in physical activity levels32 and increase in caloric intake may also contribute to higher BMI.33 Patterns of weight gain in early- and mid-adulthood have been shown to track into later adulthood and are associated with increased CVD risk.34
In our unadjusted models, we observed a worse physical activity profile among multiparous women, although upon adjustment for covariates the association was not significant. Pregnancy often leads to a reduction in physical activity, particularly vigorous activities such as sports and exercise.32 Post-pregnancy, due to the time demands allocated to their caregiver roles, mothers may have less opportunities to meet the AHA recommended exercise levels, and again these behavioral patterns can track long-term. Studies have found that younger women are less physically active, on average, compared to similarly aged men.35 As life events such as having children are associated with reduction in physical activity levels in women,36 better strategies are needed to promote the maintenance of physical activity during these vulnerable life transitions.
We also observed lower prevalence of ideal blood pressure levels with a higher number of live births in our unadjusted models. Prior studies have documented that parity is inversely correlated with blood pressure levels37–39. Additionally, in the unadjusted models, women with a higher number of live births had lower prevalence of ideal blood glucose levels. Adjusting for covariates showed women with ≥5 live births were still less likely to have ideal glucose levels although the association was not statistically significant. These results are consistent with a meta-analysis of 7 cohort studies40 (pooled total of over 9,000 cases and approximately 290,000 participants) that reported women with ≥5 live births had a 42% increased risk of incident type 2 diabetes mellitus compared to those with 0 or 1 live birth.40 During a normal pregnancy, insulin levels rise, and there is an increased resistance of peripheral tissues to insulin caused by the antagonistic effects of hormones secreted during pregnancy such estrogen, progesterone, chorionic somatomammotropin and corticosteroids.41 Significantly increased insulin resistance during pregnancy presenting as gestational diabetes is associated with greater risk of later-life type 2 diabetes and maternal CVD.42 Unfortunately, there was insufficient data collection from MESA participants regarding a history of gestational diabetes, pre-eclampsia, or pre-term delivery, so we could not examine these adverse pregnancy outcomes in our analyses.
Implications
In summary, our study showed that a higher number of live births was associated with poorer CVH, an established risk for developing CVD.10 This study adds to the growing literature that emphasizes the use of primordial prevention of CVD risk factors as a strategy to reduce the burden of CVD, a shift from disease prevention to promotion of cardiovascular wellness.10, 43, 44 Primordial prevention of CVD risk factors is especially important among women given the poorer cardiovascular outcomes observed in women compared to men.45 The metabolic processes occurring during pregnancy such as changes in lipids, glucose, and weight may partly explain the increased burden of CVD among multi-parous women later in life.4, 5
Many younger women who do not regularly seek healthcare may do so during pregnancy; thus the time surrounding a pregnancy can represent an opportunity to reinforce healthy lifestyle habits such as regular physical activity, healthy diet, and maintenance of a normal BMI. Our findings, in addition to those of previous studies, highlight the importance of increasing awareness of CVD prevention among women and also the implementation of policies that encourage efforts to decrease considerably the morbidity and mortality of CVD among women.45
Strengths and limitations
This study has many strengths which include the evaluation of an ethnically diverse group of women and the standardized methodology for data collection. However, the following limitations should be taken into consideration in the interpretation of our findings. First, causal inferences cannot be drawn because of the observational study design. Second, recall bias may have been introduced through the use of self-report questionnaires for data collection on smoking, physical activity and diet. Third, CVH status was measured at the baseline exam when the women were mid- to older age (mean age was 62 years) and thus may not be representative of the past or future CVH status of participants. Information regarding a woman’s CVH at the time of their pregnancies was not available. Fourth, data were not available in MESA for variables such as birth spacing and breastfeeding that may also influence the association between parity and CVH. Fifth, diet was only assessed at the baseline exam, so we could not examine change in CVH score over longitudinal MESA follow-up. Lastly, MESA did not collect data on parity among men, so we could not assess this relationship in men to rule out residual confounding by sociodemographic factors.
Conclusion
We found that women with a history of multiparity had a lower prevalence of favorable CVH in middle to later adulthood. Future studies should be conducted to examine the implications of our findings, including exploring the mechanisms by which multiparity contributes to poor CVH and studying whether targeted preventive interventions geared at women at the time of pregnancy can improve their CVH later in life.
Supplementary Material
AJOG at a Glance.
A: To examine the relationship between parity and ideal cardiovascular health, as measured by the American Heart Association’s Life’s Simple 7 metrics.
B: Multiparity was associated with poorer cardiovascular health in middle or late adulthood particularly among women with ≥5 live births.
C: This study emphasizes the importance of primordial prevention of cardiovascular disease risk factors as a strategy to improve cardiovascular health among women, a shift from disease prevention to promotion of cardiovascular wellness.
Acknowledgments
The authors thank the other investigators, the staff, and the participants of the Multi-Ethnic Study of Atherosclerosis for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
Sources of funding
The Multi-Ethnic Study of Atherosclerosis is supported by contracts N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168, N01-HC-95169 and HHSN268201500003I from the National Heart, Lung, and Blood Institute (NHLBI) and by grants UL1-RR-024156 and UL1-RR-025005 from the National Center for Research Resources (NCRR). Dr. Michos is additionally supported by the Blumenthal Scholars Fund in Preventive Cardiology.
Footnotes
Conflicts of interest
The authors report no conflict of interest
Condensation
Multiparity is associated with poorer cardiovascular health among women in middle or late adulthood.
References
- 1.Shah RU, Klein L and Lloyd-Jones DM. Heart failure in women: epidemiology, biology and treatment. Women’s health (London, England). 2009;5:517–27. [DOI] [PubMed] [Google Scholar]
- 2.Mathers CD, Ezzati M and Lopez AD. Measuring the burden of neglected tropical diseases: the global burden of disease framework. PLoS neglected tropical diseases. 2007;1:e114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cheng S, Chiuve SE, Cushman M, Delling FN, Deo R, de Ferranti SD, Ferguson JF, Fornage M, Gillespie C, Isasi CR, Jimenez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Lutsey PL, Mackey JS, Matchar DB, Matsushita K, Mussolino ME, Nasir K, O’Flaherty M, Palaniappan LP, Pandey A, Pandey DK, Reeves MJ, Ritchey MD, Rodriguez CJ, Roth GA, Rosamond WD, Sampson UKA, Satou GM, Shah SH, Spartano NL, Tirschwell DL, Tsao CW, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P, American Heart Association Council on E, Prevention Statistics C and Stroke Statistics S. Heart Disease and Stroke Statistics-2018 Update: A Report From the American Heart Association. Circulation. 2018;137:e67–e492. [DOI] [PubMed] [Google Scholar]
- 4.Parikh NI, Cnattingius S, Dickman PW, Mittleman MA, Ludvigsson JF and Ingelsson E. Parity and risk of later-life maternal cardiovascular disease. American heart journal. 2010;159:215–221.e6. [DOI] [PubMed] [Google Scholar]
- 5.Peters SA, van der Schouw YT, Wood AM, Sweeting MJ, Moons KG, Weiderpass E, Arriola L, Benetou V, Boeing H, Bonnet F, Butt ST, Clavel-Chapelon F, Drake I, Gavrila D, Key TJ, Klinaki E, Krogh V, Kuhn T, Lassale C, Masala G, Matullo G, Merritt M, Molina-Portillo E, Moreno-Iribas C, Nost TH, Olsen A, Onland-Moret NC, Overvad K, Panico S, Redondo ML, Tjonneland A, Trichopoulou A, Tumino R, Turzanski-Fortner R, Tzoulaki I, Wennberg P, Winkvist A, Thompson SG, Di Angelantonio E, Riboli E, Wareham NJ, Danesh J and Butterworth AS. Parity, breastfeeding and risk of coronary heart disease: A pan-European case-cohort study. European journal of preventive cardiology. 2016;23:1755–1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li W, Ruan W, Lu Z and Wang D. Parity and risk of maternal cardiovascular disease: A dose-response meta-analysis of cohort studies. European journal of preventive cardiology. 2019;26:592–602. [DOI] [PubMed] [Google Scholar]
- 7.Shen L, Wu J, Xu G, Song L, Yang S, Yuan J, Liang Y and Wang Y. Parity and Risk of Coronary Heart Disease in Middle-aged and Older Chinese Women. Scientific reports. 2015;5:16834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Colditz GA, Willett WC, Stampfer MJ, Rosner B, Speizer FE and Hennekens CH. A prospective study of age at menarche, parity, age at first birth, and coronary heart disease in women. American journal of epidemiology. 1987;126:861–70. [DOI] [PubMed] [Google Scholar]
- 9.Steenland K, Lally C and Thun M. Parity and coronary heart disease among women in the American Cancer Society CPS II population. Epidemiology (Cambridge, Mass). 1996;7:641–3. [DOI] [PubMed] [Google Scholar]
- 10.Lloyd-Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, Greenlund K, Daniels S, Nichol G, Tomaselli GF, Arnett DK, Fonarow GC, Ho PM, Lauer MS, Masoudi FA, Robertson RM, Roger V, Schwamm LH, Sorlie P, Yancy CW, Rosamond WD, American Heart Association Strategic Planning Task F and Statistics C. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association’s strategic Impact Goal through 2020 and beyond. Circulation. 2010;121:586–613. [DOI] [PubMed] [Google Scholar]
- 11.Folsom AR, Yatsuya H, Nettleton JA, Lutsey PL, Cushman M and Rosamond WD. Community prevalence of ideal cardiovascular health, by the American Heart Association definition, and relationship with cardiovascular disease incidence. Journal of the American College of Cardiology. 2011;57:1690–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ogunmoroti O, Michos ED, Aronis KN, Salami JA, Blankstein R, Virani SS, Spatz ES, Allen NB, Rana JS, Blumenthal RS, Veledar E, Szklo M, Blaha MJ and Nasir K. Life’s Simple 7 and the risk of atrial fibrillation: The Multi-Ethnic Study of Atherosclerosis. Atherosclerosis. 2018;275:174–181. [DOI] [PubMed] [Google Scholar]
- 13.Cao X, Zhou J, Yuan H and Chen Z. Cumulative effect of reproductive factors on ideal cardiovascular health in postmenopausal women: a cross-sectional study in central south China. BMC cardiovascular disorders. 2015;15:176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacob DR Jr., Kronmal R, Liu K, Nelson JC, O’Leary D, Saad MF, Shea S, Szklo M and Tracy RP. Multi-Ethnic Study of Atherosclerosis: objectives and design. American journal of epidemiology. 2002;156:871–81. [DOI] [PubMed] [Google Scholar]
- 15.Ainsworth BE, Irwin ML, Addy CL, Whitt MC and Stolarczyk LM. Moderate physical activity patterns of minority women: the Cross-Cultural Activity Participation Study. Journal of women’s health & gender-based medicine. 1999;8:805–13. [DOI] [PubMed] [Google Scholar]
- 16.Unger E, Diez-Roux AV, Lloyd-Jones DM, Mujahid MS, Nettleton JA, Bertoni A, Badon SE, Ning H and Allen NB. Association of neighborhood characteristics with cardiovascular health in the multi-ethnic study of atherosclerosis. Circulation Cardiovascular quality and outcomes. 2014;7:524–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Block G, Woods M, Potosky A and Clifford C. Validation of a self-administered diet history questionnaire using multiple diet records. Journal of clinical epidemiology. 1990;43:1327–35. [DOI] [PubMed] [Google Scholar]
- 18.Mayer-Davis EJ, Vitolins MZ, Carmichael SL, Hemphill S, Tsaroucha G, Rushing J and Levin S. Validity and reproducibility of a food frequency interview in a Multi-Cultural Epidemiology Study. Annals of epidemiology. 1999;9:314–24. [DOI] [PubMed] [Google Scholar]
- 19.Lloyd-Jones DM. Improving the cardiovascular health of the US population. Jama. 2012;307:1314–6. [DOI] [PubMed] [Google Scholar]
- 20.Mathews L, Ogunmoroti O, Nasir K, Blumenthal RS, Utuama OA, Rouseff M, Das S, Veledar E, Feldman T, Agatston A, Zhao D and Michos ED. Psychological Factors and Their Association with Ideal Cardiovascular Health Among Women and Men. Journal of women’s health (2002). 2018;27:709–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ogunmoroti O, Utuama OA, Michos ED, Valero-Elizondo J, Okunrintemi V, Taleb ZB, Bahelah R, Das S, Rouseff M, Parris D, Agatston A, Feldman T, Veledar E, Maziak W and Nasir K. Does education modify the effect of ethnicity in the expression of ideal cardiovascular health? The Baptist Health South Florida Employee Study. Clinical cardiology. 2017;40:1000–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vaidya D, Bennett WL, Sibley CT, Polak JF, Herrington DM and Ouyang P. Association of parity with carotid diameter and distensibility: multi-ethnic study of atherosclerosis. Hypertension (Dallas, Tex : 1979). 2014;64:253–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Buka SL, Goldstein JM, Spartos E and Tsuang MT. The retrospective measurement of prenatal and perinatal events: accuracy of maternal recall. Schizophrenia research. 2004;71:417–26. [DOI] [PubMed] [Google Scholar]
- 24.Catov JM, Newman AB, Sutton-Tyrrell K, Harris TB, Tylavsky F, Visser M, Ayonayon HN and Ness RB. Parity and cardiovascular disease risk among older women: how do pregnancy complications mediate the association? Annals of epidemiology. 2008;18:873–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fowler-Brown AG, de Boer IH, Catov JM, Carnethon MR, Kamineni A, Kuller LH, Siscovick DS and Mukamal KJ. Parity and the association with diabetes in older women. Diabetes care. 2010;33:1778–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ying W, Zhao D, Ouyang P, Subramanya V, Vaidya D, Ndumele CE, Sharma K, Shah SJ, Heckbert SR, Lima JA, deFilippi CR, Budoff MJ, Post WS and Michos ED. Sex Hormones and Change in N-Terminal Pro-B-Type Natriuretic Peptide Levels: The Multi-Ethnic Study of Atherosclerosis. The Journal of clinical endocrinology and metabolism. 2018;103:4304–4314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.den Tonkelaar I, Seidell JC, van Noord PA, Baanders-van Halewijn EA and Ouwehand IJ. Fat distribution in relation to age, degree of obesity, smoking habits, parity and estrogen use: a cross-sectional study in 11,825 Dutch women participating in the DOM-project. International journal of obesity. 1990;14:753–61. [PubMed] [Google Scholar]
- 28.Bastian LA, West NA, Corcoran C and Munger RG. Number of children and the risk of obesity in older women. Preventive medicine. 2005;40:99–104. [DOI] [PubMed] [Google Scholar]
- 29.Ness RB, Harris T, Cobb J, Flegal KM, Kelsey JL, Balanger A, Stunkard AJ and D’Agostino RB. Number of pregnancies and the subsequent risk of cardiovascular disease. The New England journal of medicine. 1993;328:1528–33. [DOI] [PubMed] [Google Scholar]
- 30.Gunderson EP and Abrams B. Epidemiology of gestational weight gain and body weight changes after pregnancy. Epidemiologic reviews. 2000;22:261–74. [DOI] [PubMed] [Google Scholar]
- 31.Kaye SA, Folsom AR, Prineas RJ, Potter JD and Gapstur SM. The association of body fat distribution with lifestyle and reproductive factors in a population study of postmenopausal women. International journal of obesity. 1990;14:583–91. [PubMed] [Google Scholar]
- 32.Fell DB, Joseph KS, Armson BA and Dodds L. The impact of pregnancy on physical activity level. Maternal and child health journal. 2009;13:597–603. [DOI] [PubMed] [Google Scholar]
- 33.Nicholson WK, Asao K, Brancati F, Coresh J, Pankow JS and Powe NR. Parity and risk of type 2 diabetes: the Atherosclerosis Risk in Communities Study. Diabetes care. 2006;29:2349–54. [DOI] [PubMed] [Google Scholar]
- 34.Fliotsos M, Zhao D, Rao VN, Ndumele CE, Guallar E, Burke GL, Vaidya D, Delaney JCA and Michos ED. Body Mass Index From Early-, Mid-, and Older-Adulthood and Risk of Heart Failure and Atherosclerotic Cardiovascular Disease: MESA. Journal of the American Heart Association. 2018;7:e009599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Michos ED and Blaha MJ. Encouraging Young Women to Move More: Linking Physical Activity in Young Adulthood to Coronary Risk in Women. Circulation. 2016;134:300–3. [DOI] [PubMed] [Google Scholar]
- 36.Brown WJ and Trost SG. Life transitions and changing physical activity patterns in young women. Am J Prev Med. 2003;25:140–3. [DOI] [PubMed] [Google Scholar]
- 37.Jang M, Lee Y, Choi J, Kim B, Kang J, Kim Y and Cho S. Association between Parity and Blood Pressure in Korean Women: Korean National Health and Nutrition Examination Survey, 2010–2012. Korean journal of family medicine. 2015;36:341–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Haug EB, Horn J, Markovitz AR, Fraser A, Macdonald-Wallis C, Tilling K, Romundstad PR, Rich-Edwards JW and Asvold BO. The impact of parity on life course blood pressure trajectories: the HUNT study in Norway. European journal of epidemiology. 2018;33:751–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Haug EB, Horn J, Markovitz AR, Fraser A, Macdonald-Wallis C, Tilling K, Romundstad PR, Rich-Edwards JW and Asvold BO. Correction to: The impact of parity on life course blood pressure trajectories: the HUNT study in Norway. European journal of epidemiology. 2018;33:895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guo P, Zhou Q, Ren L, Chen Y and Hui Y. Higher parity is associated with increased risk of Type 2 diabetes mellitus in women: A linear dose-response meta-analysis of cohort studies. Journal of diabetes and its complications. 2017;31:58–66. [DOI] [PubMed] [Google Scholar]
- 41.Barbour LA, McCurdy CE, Hernandez TL, Kirwan JP, Catalano PM and Friedman JE. Cellular mechanisms for insulin resistance in normal pregnancy and gestational diabetes. Diabetes care. 2007;30 Suppl 2:S112–9. [DOI] [PubMed] [Google Scholar]
- 42.Hauspurg A, Ying W, Hubel CA, Michos ED and Ouyang P. Adverse pregnancy outcomes and future maternal cardiovascular disease. Clinical cardiology. 2018;41:239–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Labarthe DR. From cardiovascular disease to cardiovascular health: a quiet revolution? Circulation Cardiovascular quality and outcomes. 2012;5:e86–92. [DOI] [PubMed] [Google Scholar]
- 44.Labarthe D and Lloyd-Jones DM. 50×50×50. Circulation. 2018;138:968–970. [DOI] [PubMed] [Google Scholar]
- 45.Gulati M Improving the Cardiovascular Health of Women in the Nation: Moving Beyond the Bikini Boundaries. Circulation. 2017;135:495–498. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.