Abstract
Introduction
Bladder cancer is a lethal human malignancy. Currently, treatment for bladder cancer is limited. The anti-tumor effects of leflunomide have attracted much more concern in multiple human cancers.
Materials and Methods
This study evaluated the anti-tumor effects of leflunomide on cell viability, colony formation, apoptosis, and cell cycle in two human bladder carcinoma cell lines, 5637 and T24. Meanwhile, the underlying mechanism including PI3K/Akt signaling pathway and autophagy modulation was also identified.
Results
Leflunomide markedly inhibited the growth of both bladder cancer cell lines and induced apoptosis and cell cycle arrest in S phase. The phosphorylation levels of Akt and P70S6K in both cell lines were significantly down-regulated with leflunomide treatment. Furthermore, the deceased formation of autophagosomes and the accumulation of LC3II and P62 suggested the blockade of autophagy by leflunomide. Modulation of autophagy with rapamycin and chloroquine markedly attenuated and enhanced the cytostatic effects of leflunomide, respectively.
Conclusion
Leflunomide significantly reduced the cell viability of bladder cancer cells via inducing apoptosis and cell cycle arrest and suppressing the PI3K/Akt signaling pathway. In addition, the blockade of autophagy was observed, and autophagy inhibition enhanced leflunomide-mediating anti-tumor effects. Our data presented here offer novel ideas for comprehensive therapeutic regimes on bladder cancer.
Keywords: leflunomide, autophagy, PI3K/Akt pathway, anti-tumor, bladder cancer
Introduction
Bladder cancer is the ninth leading cause of malignancy worldwide, with nearly 430,000 new cases diagnosed each year.1,2 Approximately 25% of patients are initially diagnosed with muscle-invasive bladder cancer (MIBC) or metastatic disease.3 Nevertheless, there are limited favorable outcomes from current therapy in the clinic, and the long-term survival of these patients remains dismal.4,5 Therefore, novel therapeutic regimes for bladder cancer need to be considered.
Dihydroorotate dehydrogenase (DHODH) is an essential enzyme in the de novo pyrimidine biosynthesis pathway.6 Previous studies have shown that inhibition of DHODH induces tumor cell cycle arrest in S phase as a failure on the expansion of pyrimidine poll in dividing cells,7,8 which indicates DHODH a potential therapeutic target for cancer suppression. Leflunomide [N-(4-trifluoromethylphenyl)-5-methylisoxazol-4-carboxamide] is a widely used immunomodulatory drug, approved for the treatment of rheumatoid arthritis (RA) and allograft rejection in the clinic.9 After oral administration, leflunomide is metabolized to its activated form, teriflunomide, a potent DHODH blocker, and is tolerated in the plasma with a concentration up to 200μM with low toxicity.10,12 Recently, the anti-growth and apoptosis-inducing effects of leflunomide on multiple type of human cancers have been demonstrated.13–20 In addition, leflunomide was able to inhibit renal cell carcinoma cells, in which cell autophagy and WNT/β-catenin signaling pathway were involved.9
A recent study showed the anti-angiogenesis effect of leflunomide on bladder cancer.21 In the present study, we demonstrated that leflunomide reduced bladder cancer cell viability via inducing apoptosis and cell cycle arrest. Additionally, autophagy and Akt/mTOR signaling pathway were involved in the cytotoxicity of leflunomide on bladder cancer cells. Modulation of autophagy with rapamycin and chloroquine (CQ) significantly affected leflunomide-induced cytotoxicity, suggesting that autophagy plays a vital role in the cytotoxic effect of leflunomide on bladder cancer cells.
Materials and Methods
Cell Culture
Two human bladder cancer cell lines, T24 (Grade III) and 5637 (Grade II), were purchased from American Type Culture Collection. Both cell lines were cultured in 1640 medium (Gibco; USA) with 10% fetal bovine serum (Corning; USA) and incubated in a 5% CO2 humidified atmosphere at 37°C. Medium exchange was performed every 2–3 days or at the beginning of the treatment.
Reagents
Leflunomide, rapamycin and CQ were purchased from MCE (USA). According to the manufacturer’s recommendations, leflunomide and rapamycin were dissolved in dimethyl sulfoxide (DMSO) and stored at −80°C at 400mmol/L and 20mmol/L stock concentration, respectively. CQ was dissolved in PBS and stored at −80°C in stocks of 100μmol/L. Antibodies against Phospho-Akt (Ser473), Akt (pan), Phospho-p70S6Kinase (Thr389), p70S6Kinase, Phospho-mTOR (Ser2448), mTOR and cleaved-PARP were purchased from Cell Signaling Technology (USA). Mouse anti-Beta-actin, anti-Beta-tubulin antibodies was purchased from Zhongshan Jinqiao Biotechnology (China). Antibody against P62 was purchased from Abcam (USA). Antibody against LC3B (L7543) was purchased from Sigma-Aldrich (USA). Goat anti-rabbit IgG HRP-linked and anti-mouse IgG HRP-linked antibodies were purchased from Beyotime Biotechnology (China).
Cell Proliferation Assay
3-(4,5-Dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) assay was performed to evaluate cell proliferation. Briefly, T24 and 5637 cells were seeded in a 96-well plate at 1×104 cells/well density overnight, then cells were incubated with 1640 supplemented with 0.01% DMSO or increasing concentrations of leflunomide at 12.5, 25, 50, 100 and 200μM containing 0.01% DMSO. After incubation for 24, 48 and 96 hours, the MTS labeling reagent (Promega, USA) was added for 2 hours according to the manufacturer’s recommendations, and absorbance at 490nm and 690nm was determined using a VARIOSCAN FLASH microplate reader (Thermo Fisher, USA). All conditions were repeated in quadruplicate. Cell viability was represented by percentage values compared to the DMSO control.
Colony Formation Assay
As previously described,22 cells were seeded in 6-well culture plate at a density of 1×103 cells/well. After cell attachment, 1640 with 0.01% DMSO or varied dosages of leflunomide containing 0.01% DMSO were added into each well. All experimental samples were harvested after 14 days of incubation, and colonies were stained with crystal violet for 45 minutes. Three independent experiments were performed.
Analysis of Apoptosis and Cell Death
Annexin-V-FITC and propidium iodide (PI) staining kit (KeyGEN Biotech, China) was used to evaluate apoptosis by flow cytometry. The cells were collected after 24h or 48h treatment with 0.01% DMSO or varied dosages of leflunomide containing 0.01% DMSO and stained with Annexin-V and PI in the dark at room temperature for 15 min according to the manufacturer’s instructions. Annexin-V and PI fluorescent intensities were determined with FACScan (Becton–Dickinson, USA), with 20,000 cells analyzed for each sample.
Cell Cycle Analysis
Cell cycle phase was determined by flow cytometry according to protocol (KeyGEN Biotech, China). Briefly, the cells were harvested after treatment with 0.01% DMSO or varied dosages of leflunomide containing 0.01% DMSO for 48 hours and fixed with 75% ethanol at 4C overnight. The following day, samples were treated with 100μL RNase A and 400μL of PI for 30 minutes. Cells were detected by flow cytometry using Millipore (Becton–Dickinson, USA).
Western Blot Analysis
Cells were washed with PBS and lysed in lysis buffer containing protease inhibitors (MCE, USA). Protein concentrations were determined by using the Bio-Rad protein assay, followed by a standard Western blot assay to evaluate the expression of target proteins according to the protocol previously described.22 The blots were visualized using the ECL Western blot detection system (Syngene, Bangalore, India)
Acridine Orange Staining
Acridine Orange staining was performed to assess the volume of acidic vesicular organelles (AVOs), which increases with the induction of autophagy.23 Briefly, the bladder cancer cells were seeded in 6-well plates and treated with 0.01% DMSO or increasing doses of leflunomide containing 0.01% DMSO, or co-treated with rapamycin or CQ. After 48 hours of incubation, the cells were stained with 1µM acridine orange at 37°C in the dark for 15 minutes. The cells were washed with PBS and visualized with a laser scanning confocal microscope (Olympus FluoView FV1000, Tokyo, Japan) to detect the AVOs.
Statistical Analysis
All experiments were repeated at least three times and the data are presented as mean± standard deviation. The paired, 2-tailed Student’s t-test was run to analyze the statistical significance of the differences between the groups. Data were considered significant if p<0.05. * and ** represent p < 0.05 or p <0.01, respectively.
Results
Leflunomide Inhibited Proliferation of Bladder Cancer Cells
Bladder cancer cell lines 5637 and T24 were treated with increasing concentrations of leflunomide (12.5–200μM), with DMSO as the negative control. After 48 hours of incubation with leflunomide, cell viability and cell abundance were both diminished in the bladder cancer cell lines (Figure 1A and B). The cytostatic effect of leflunomide was further verified by MTS analysis. Cell proliferation of 5637 and T24 cells was significantly inhibited by leflunomide in a dose- and time-dependent manner (Figure 1C and D).
Figure 1.
Leflunomide reduced cell viability of bladder cancer cells. 5637 and T24 cells were treated with DMSO or increasing concentrations of leflunomide (12.5–200μM), the inferior cell condition and reduced cell viability were observed by microscopy (A and B, magnification details: 100×) and MTS assay (C and D). The data (C and D) represent the mean ± SD from three independent experiments.
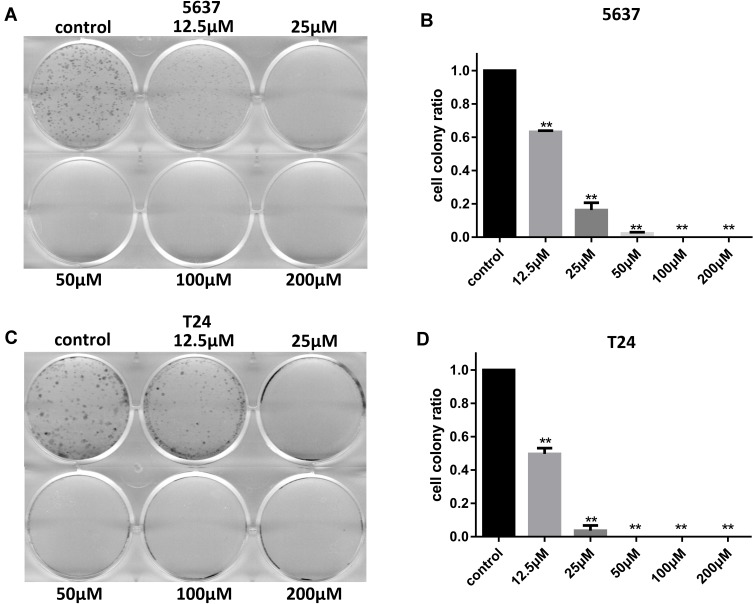
In addition, the inhibitive effect of leflunomide on both cell lines was evaluated with cell colony formation assay. Results revealed that the number of cell colonies were decreased under leflunomide treatment (Figure 2A-D). Furthermore, colonies were undetectable under high-doses of leflunomide (100μM and 200μM). These data provided strong evidence that leflunomide inhibits proliferation of human bladder cancer cells.
Figure 2.
Leflunomide inhibited cell colony formation of bladder cancer cells. 5637 (A and B) and T24 (C and D) cells were treated with DMSO or increasing concentrations of leflunomide (12.5–200μM), the cell colonies were stained with crystal violet. The data (B and D) represent the mean ± SD from three independent experiments. **P<0.01.
Leflunomide Induced Apoptosis in Bladder Cancer Cells
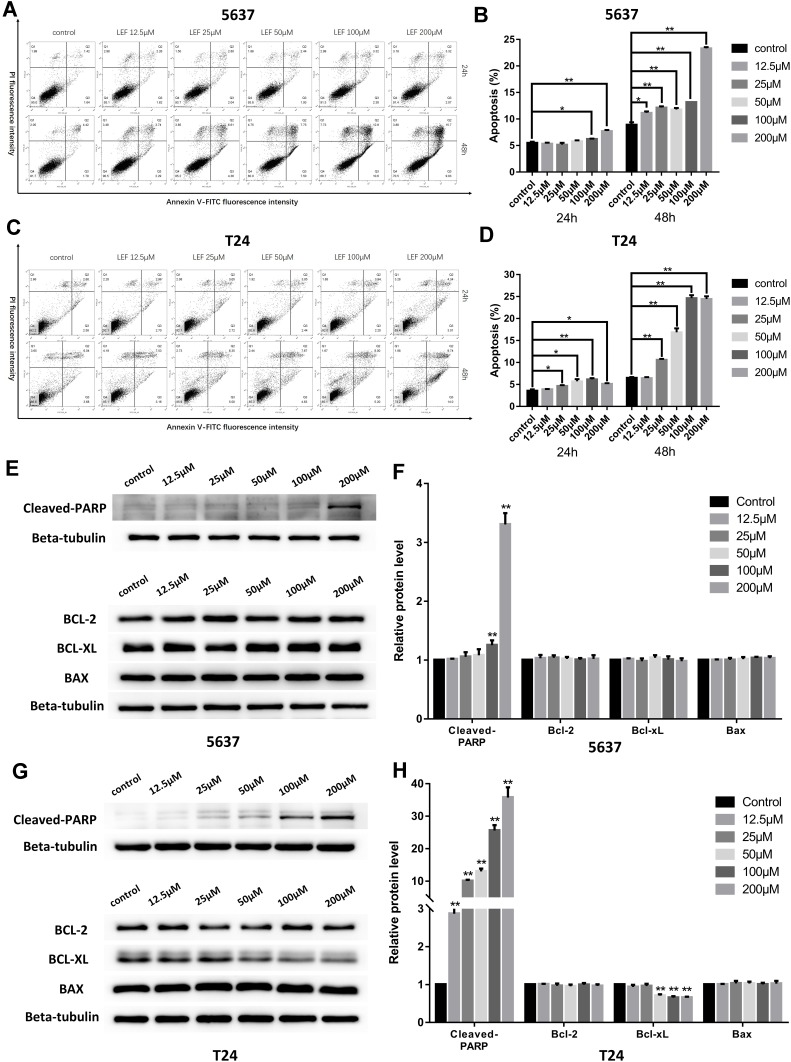
To explore whether apoptosis pathway was involved in the growth-inhibitive effect of leflunomide, flow cytometry analysis with Annexin V and PI double staining was performed. Results showed that treatment with leflunomide markedly induced apoptosis in both bladder cancer cell lines in a time- and dose-dependent manner (Figure 3A-D). After 24-hour treatment with leflunomide, significant increase of the apoptotic rate in 5637 cells was observed only at the higher doses (100μM and 200μM); however, 48 hours of treatment with 12.5μM to 200μM leflunomide significantly increased apoptosis in 5637 cells. In T24 cells, the apoptotic rate was significantly increased in only the higher doses of leflunomide treatment (25–200μM), with no difference observed at 12.5μM leflunomide treatment. In addition, Western blots were performed to determine the protein expression of apoptosis-related proteins in both cell lines treated with increasing concentrations of leflunomide (12.5–200μM) or DMSO for 48 hours. The results indicated a dose-dependent activation of PARP after leflunomide treatment (Figure 3E-H), which was consistent with the data from the flow cytometry analysis. Furthermore, the expression of BCL-2, BCL-XL and BAX was also evaluated and no significant differences were observed between the leflunomide and control groups in the 5637 cells. However, the anti-apoptotic factor BCL-XL showed a dose-dependent decrease in T24 cells.
Figure 3.
Leflunomide induced apoptosis in bladder cancer cells. 5637 and T24 cells were treated with increasing concentrations of leflunomide (12.5–200μM) or DMSO for 24 or 48 hours, the apoptotic rate was determined by flow cytometry analysis with Annexin-V/PI staining (A–D). Western blot was performed to evaluate the expression of apoptosis-related proteins in both cell lines treated with increasing concentrations of leflunomide (12.5–200μM) or DMSO for 48 hours (E–H). Beta-tubulin was used as internal loading reference. The results (B, D, F and H) represent the mean ± SD from three independent experiments. *P<0.05; **P<0.01.
Leflunomide Induced Cell Cycle Arrest in S Phase in Bladder Cancer Cells
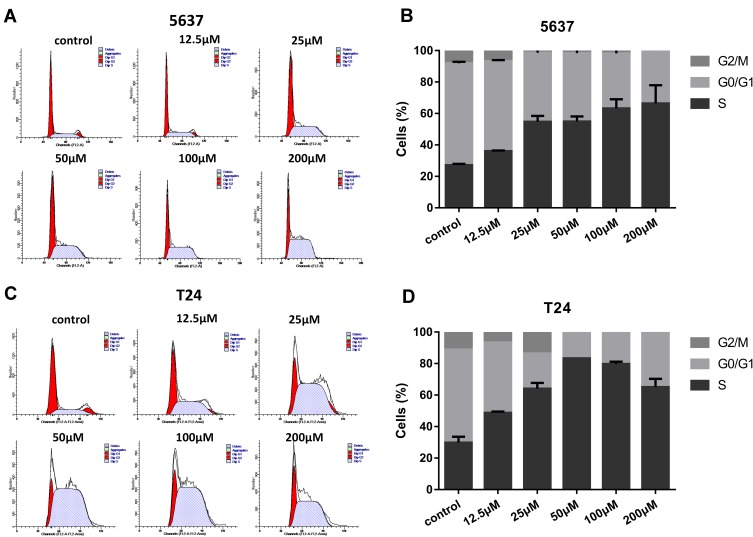
On the basis of evidence in previous studies,7,8 in tumor cells DHODH inhibitors could interfere with the cell cycle to arrest the cells in S phase as a failure in pyrimidine biosynthesis. In this study, we tested if leflunomide would also affect the cell cycle in bladder cancer cells. After incubation with increasing doses of leflunomide for 48 hours, the cells were collected and stained with PI then the cell cycle phase populations were determined with flow cytometry. The percentage of S phase in both cell lines were increased in a dose-dependent manner after leflunomide treatment (Figure 4A-D). These data indicated that leflunomide induces cell cycle arrest in S phase in bladder cancer cells, which might be attributed to the DHODH inhibition of leflunomide.
Figure 4.
Leflunomide induced cell cycle arrest in S phase in bladder cancer cells. 5637 (A, B) and T24 (C, D) cells were treated with DMSO or rising concentrations of leflunomide (12.5–200μM) for 48 hours, the cell phase populations were determined by flow cytometry with PI labeling. The results (B, D) represent the mean ± SD from three independent experiments.
Leflunomide Inhibited PI3K/Akt Signaling Pathway in Bladder Cancer Cells
The PI3K/Akt signaling pathway plays commanding roles in cell proliferation, differentiation, and cell cycle progress. Activation of genes in PI3K/Akt pathway drives tumorigenesis and tumor cell survival.24 To determine if PI3K/Akt signaling pathway was involved in the growth inhibitive effect of leflunomide, the expression of phosphorylated and total Akt, mTOR and P70S6K was examined by Western blot in both cell lines. As shown in Figure 5, treatment with 200μM leflunomide for 6 to 48 hours significantly inhibited the phosphorylation level of Akt, mTOR and P70S6K at varying times.
Figure 5.
Leflunomide inhibited PI3K/Akt signaling pathway in bladder cancer cells. 5637 and T24 cells were treated with leflunomide (200μM) or DMSO for 6 to 48 hours, the phosphorylation levels of PI3K/Akt pathway proteins in 5637 (A and B) and T24 (C and D) cells were determined by Western blot. The results (B and D) represent the mean ± SD from three independent experiments. *P<0.05; **P<0.01.
Leflunomide Blocked Autophagy in Bladder Cancer Cells
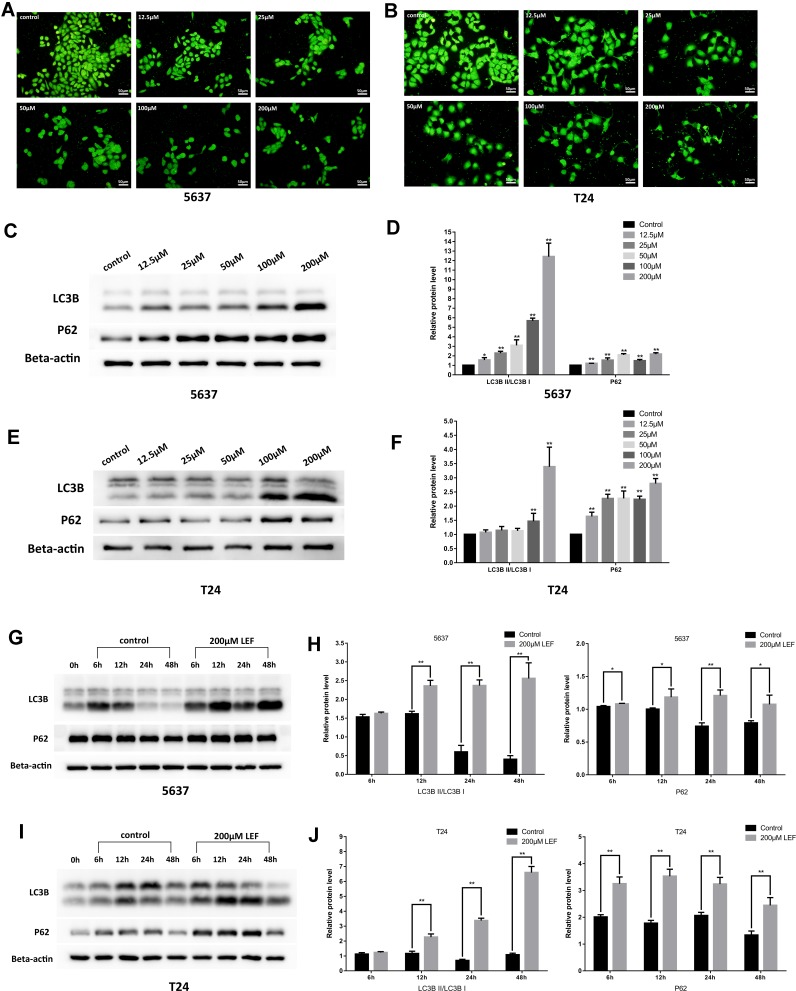
Next, we tested whether autophagy was involved in the cytostatic effect of leflunomide. We first detected the formation of autophagosome in both cell lines with the Acridine Orange staining, which revealed a descending trend of vacuoles with the rising concentration of leflunomide (Figure 6A and B). Next, LC3B and P62, two landmark proteins of autophagy were examined. Results showed an accumulation of both LC3B and P62 proteins in a time- and dose-dependent manner (Figure 6C-J), which indicated the blockade of autophagy flux. Our data show that autophagy was inhibited with the treatment of leflunomide in bladder cancer cells.
Figure 6.
Leflunomide blocked autophagy in bladder cancer cells. 5637 and T24 cells were treated with rising concentrations of leflunomide (12.5–200μM) or DMSO for 48 hours, the formation of autophagosome in 5637 (A) and T24 (B) cells was detected with the Acridine Orange staining, the expression of LC3II and P62 in 5637 (C and D) and T24 (E and F) cells was determined by Western blot. Both cell lines were treated with leflunomide (200μM) or DMSO for 6 to 48 hours, the expression of LC3II and P62 in 5637 (g and h) and T24 (I and J) cells was determined by Western blot. Beta-actin was used as internal loading reference. The results (D, F, H and J) represent the mean ± SD from three independent experiments. *P<0.05; **P<0.01.
Modulation of Autophagy with Rapamycin and Chloroquine Can Influence the Cytotoxicity of Leflunomide on Bladder Cancer Cells
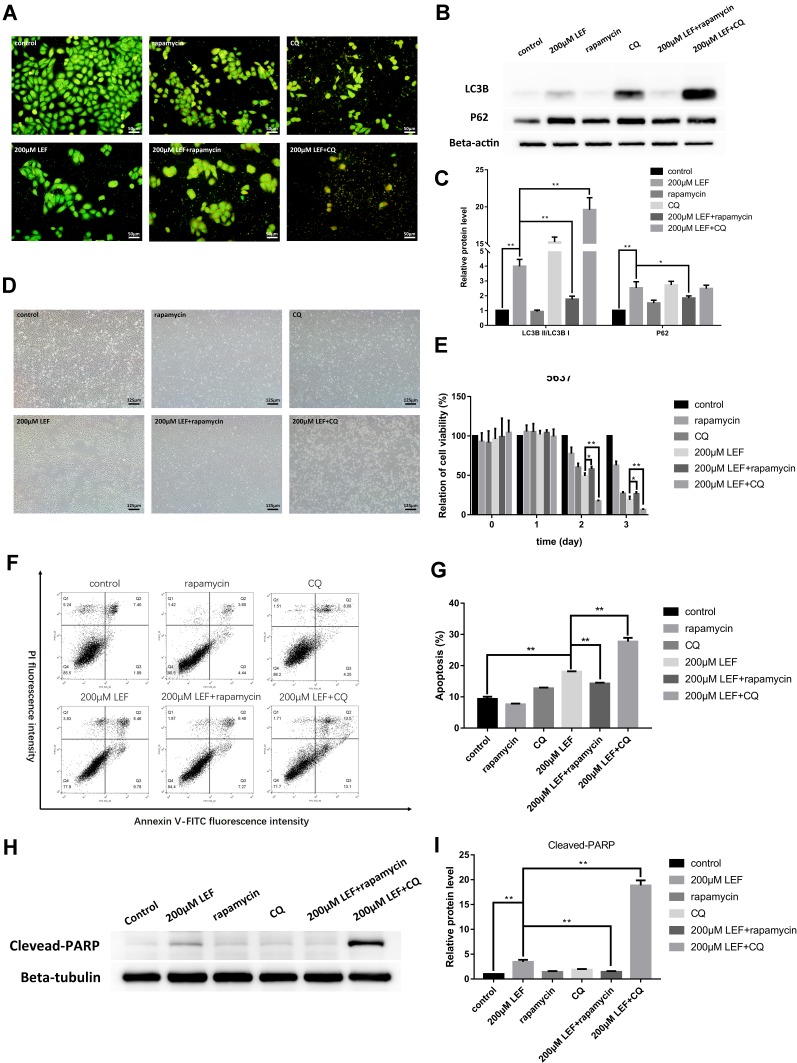
To confirm the involvement of autophagy in the inhibitive effect of leflunomide, an autophagy activator, rapamycin and an autophagy inhibitor, CQ was co-treated with leflunomide in 5637 cells. Combination treatment of leflunomide with 10nM rapamycin or 20nM chloroquine for 48 hours leads to a further increase or reduction, respectively, of the formation of autophagosome compared with the single use of leflunomide (Figure 7A). Western blot analysis was performed to detect the expression of LC3B and P62 (Figure 7B-C), and the results were consistent with the data of the Acridine Orange staining. Our results agree with the previous study.15
Figure 7.
Modulation of autophagy affected the cytotoxicity effect of leflunomide in 5637 cells. Combination use of leflunomide with 10nM rapamycin or 20nM CQ for 48 hours led to increased or reduced, respectively, autophagy levels compared with the single use of leflunomide, as determined by Acridine Orange staining (A) and Western blot (B and C). Microscopy observation (D) showed an inferior cell viability in the presence of leflunomide and chloroquine, compared with leflunomide alone. By contrast, the restored cell abundance and morphology was observed with the combined use of leflunomide and rapamycin. MTS assay (E), cell apoptosis analysis by flow cytometry (F and G) and Western blot (H and I) exhibited consistent results. Beta-actin or Beta-tubulin was used as internal loading reference. The results (C, E, G and I) represent the mean ± SD from three independent experiments. *P<0.05; **P<0.01.
Abbreviation: CQ, chloroquine.
The cell viability was also assessed under combined medication of leflunomide and autophagy modifiers. Microscopy results showed a decreased cell viability in the presence of leflunomide and chloroquine, compared with the single medication (Figure 7D). By contrast, a restored cell abundance and morphology was observed with the combination of leflunomide and rapamycin. MTS assay was performed to further assess the cell proliferation after combination drug therapy (Figure 7E), the results were consistent with the microscopic results. Flow cytometry analysis showed that the combined medication of leflunomide with chloroquine or rapamycin led to a significant increase or reduction, respectively, of the apoptotic rate, compared to the single use of leflunomide (Figure 7F-G). In addition, the expression of Cleaved-PARP was examined and the results indicated similar trend with flow cytometry analysis (Figure 7H-I). These results demonstrated that autophagy might exert protective effects which was blocked by leflunomide in bladder cancer cells.
Discussion
Bladder cancer is one of the most lethal human malignant tumors and is characterized by high incidence, high recurrence rate, high mortality and limited therapy schemes. Leflunomide is a widely used clinical drug in rheumatic diseases and has attracted much more attention due to its potent anti-tumor activity. Recently, leflunomide was reported to inhibit tumor angiogenesis by suppressing ephrin-A1/EphA2 in bladder cancer.21 However, other pathways affected by leflunomide such as apoptosis and autophagy mechanism in bladder cancer have not been well elucidated. In this study, we reported that leflunomide suppressed bladder cancer cells growth via inducing tumor cell apoptosis and cell cycle arrest in S phase. In addition, the PI3K/Akt signaling pathway and autophagy were also involved in leflunomide-induced cytotoxicity in human bladder cancer.
By MTS and cell colony assay, we demonstrated decreased tumor cell viability under leflunomide treatment in a dose- and time-dependent manner. Moreover, we evaluated the cytotoxic profile of leflunomide in both cell lines. The IC50 value (48h) of leflunomide in T24 and 5637 cells was 39.0μM and 84.4μM, respectively. Data showed that the high-grade bladder cancer cell line (T24) was more susceptible to leflunomide, which was consistent with the results of colony formation assay.
The PI3K/Akt/mTOR signaling pathway plays a vital role in the regulation of cell proliferation, differentiation and survival.24 PI3K directly activates Akt and initiates a signal transduction pathway that promotes tumorigenesis. mTOR is recognized as one of the key downstream effectors of Akt. Upon activation, mTOR activates its downstream effectors, 4E-BP1 and S6 kinase (S6K), leading to improved tumor cell growth and survival. In the present study, leflunomide inhibited the activation of phospho-Akt, and phospho-P70S6K in both bladder cancer cell lines, which may contribute to its inhibitive effects on bladder cancer cells.
To better elucidate the involvement of autophagy in the cytostatic effect of leflunomide, Western blot and AVO staining were performed. Leflunomide treatment led to an accumulation of LC3II and p62 with decreased formation of autophagosome, which showed the inhibition of autophagy. Cancer cells adapt to the stress by activation of the autophagy pathway primed for survival. The high basal level of autophagy has been reported in human bladder cancer cells.25 To better know the role of autophagy in bladder cancer, we modulated the autophagy process by treatment with rapamycin and CQ. Results showed that autophagy activator led to restored cell viability, whereas inhibition of autophagy resulted in decreased cell viability. Our data indicated that one of the mechanisms by which leflunomide exerted cytotoxicity was to block autophagy in bladder cancer cells.
mTOR inhibition is thought to induce activation of autophagy.26,27 However, in our present study, the phosphorylation of mTOR in T24 cell line was suppressed under leflunomide treatment, accompanied with inhibition of autophagy, which disagrees with other studies.28,29 Autophagy is a complicated process which is related to diverse signaling pathways, and we hypothesis that another signaling pathway, apart from mTOR pathway, might interfere with the autophagy process in bladder cancer with leflunomide treatment. Further studies are needed to understand the mechanism of autophagy in bladder cancer cells.
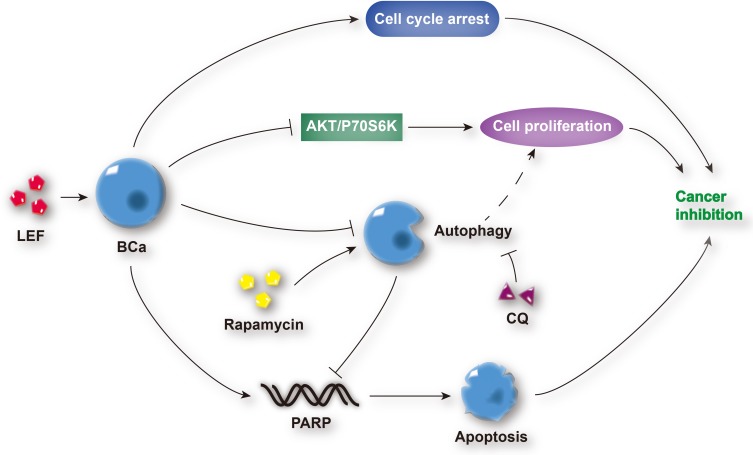
DHODH is one of the vital enzymes in cellular pyrimidine synthesis. DHODH depletion results in cell arrest and stunted cell proliferation, which makes it a potential therapeutic target for cancer therapy.30 In our study, the latent inhibitive effect of leflunomide on DHODH might also contribute to the bladder cancer suppression, which needs further investigation. In addition, leflunomide has been recently shown to work in combination with other chemotherapeutic agents to inhibit the growth of human cancer,31,32 which could be adapted to bladder cancer treatment in the future. Generally, the mechanism by which leflunomide exerts its anti-tumor effects on human bladder cells is shown in Figure 8.
Figure 8.
Schematic diagram depicting the anti-tumor effects of leflunomide on human bladder cancer cells. Leflunomide inhibited the cell proliferation of bladder cancer cells by the suppression of the PI3K/Akt signaling pathway and induction of cell apoptosis and cell cycle arrest. In addition, the blockade of autophagy was observed, and autophagy inhibition enhanced leflunomide-mediating anti-tumor effects.
Abbreviations: LEF, leflunomide; BCa, bladder cancer; CQ, chloroquine.
Conclusion
This study indicated that leflunomide exerted anti-tumor effects in human bladder cancer cells by the induction of apoptosis, cell cycle arrest and the inactivation of PI3K/Akt signaling pathway. In addition, the blockade of autophagy was involved in this process and the modulation of autophagy affected the leflunomide-induced cytotoxicity. Our study provides the basic mechanisms for novel comprehensive therapeutic regimes on bladder cancer.
Funding Statement
This study was funded by the National Natural Science Foundation of China (No. 81670617).
Disclosure
The abstract of this paper was presented at the ESMO 2019 Congress as a poster presentation. The poster’s abstract was published in ‘Poster Abstracts’ in Annals of Oncology: [https://www.annalsofoncology.org/article/S0923-7534(19)58295-3/pdf; https://www.researchgate.net/publication/336193248_61PAutophagy_inhibition_enhances_leflunomide-induced_cytotoxicity_in_human_bladder_cancer_cells]. The authors declare no conflicts of interest in this work.
References
- 1.Kamat AM, Hahn NM, Efstathiou JA, et al. Bladder cancer. Lancet (London, Engl). 2016;388(10061):2796–2810. doi: 10.1016/S0140-6736(16)30512-8 [DOI] [PubMed] [Google Scholar]
- 2.Dobruch J, Daneshmand S, Fisch M, et al. Gender and bladder cancer: a collaborative review of etiology, biology, and outcomes. Eur Urol. 2016;69(2):300–310. doi: 10.1016/j.eururo.2015.08.037 [DOI] [PubMed] [Google Scholar]
- 3.Robertson AG, Kim J, Al-Ahmadie H, et al. Comprehensive molecular characterization of muscle-invasive bladder cancer. Cell. 2017;171(3):540–556.e525. doi: 10.1016/j.cell.2017.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abufaraj M, Dalbagni G, Daneshmand S, et al. The role of surgery in metastatic bladder cancer: a systematic review. Eur Urol. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Funt SA, Rosenberg JE. Systemic, perioperative management of muscle-invasive bladder cancer and future horizons. Nat Rev Clin Oncol. 2017;14(4):221–234. doi: 10.1038/nrclinonc.2016.188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Madak JT, Bankhead A 3rd, Cuthbertson CR, Showalter HD, Neamati N. Revisiting the role of dihydroorotate dehydrogenase as a therapeutic target for cancer. Pharmacol Ther. 2018. [DOI] [PubMed] [Google Scholar]
- 7.Koundinya M, Sudhalter J, Courjaud A, et al. Dependence on the pyrimidine biosynthetic enzyme DHODH is a synthetic lethal vulnerability in mutant KRAS-driven cancers. Cell Chem Biol. 2018;25(6):705–717.e711. doi: 10.1016/j.chembiol.2018.03.005 [DOI] [PubMed] [Google Scholar]
- 8.Mohamad Fairus AK, Choudhary B, Hosahalli S, Kavitha N, Shatrah O. Dihydroorotate dehydrogenase (DHODH) inhibitors affect ATP depletion, endogenous ROS and mediate S-phase arrest in breast cancer cells. Biochimie. 2017;135:154–163. doi: 10.1016/j.biochi.2017.02.003 [DOI] [PubMed] [Google Scholar]
- 9.Chen Y, Huang Q, Zhou H, Wang Y, Hu X, Li T. Inhibition of canonical WNT/beta-catenin signaling is involved in leflunomide (LEF)-mediated cytotoxic effects on renal carcinoma cells. Oncotarget. 2016;7(31):50401–50416. doi: 10.18632/oncotarget.10409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chon WJ, Josephson MA. Leflunomide in renal transplantation. Expert Rev Clin Immunol. 2011;7(3):273–281. doi: 10.1586/eci.11.20 [DOI] [PubMed] [Google Scholar]
- 11.Teschner S, Burst V. Leflunomide: a drug with a potential beyond rheumatology. Immunotherapy. 2010;2(5):637–650. doi: 10.2217/imt.10.52 [DOI] [PubMed] [Google Scholar]
- 12.Breedveld FC, Dayer JM. Leflunomide: mode of action in the treatment of rheumatoid arthritis. Ann Rheum Dis. 2000;59(11):841–849. doi: 10.1136/ard.59.11.841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hwang C, Heath EI. Angiogenesis inhibitors in the treatment of prostate cancer. J Hematol Oncol. 2010;3(1):26. doi: 10.1186/1756-8722-3-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bahr HI, Toraih EA, Mohammed EA, Mohammad HM, Ali EA, Zaitone SA. Chemopreventive effect of leflunomide against Ehrlich’s solid tumor grown in mice: effect on EGF and EGFR expression and tumor proliferation. Life Sci. 2015;141:193–201. doi: 10.1016/j.lfs.2015.10.003 [DOI] [PubMed] [Google Scholar]
- 15.Doscas ME, Williamson AJ, Usha L, et al. Inhibition of p70 S6 kinase (S6K1) activity by A77 1726 and its effect on cell proliferation and cell cycle progress. Neoplasia (New York, NY). 2014;16(10):824–834. doi: 10.1016/j.neo.2014.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kis E, Nagy T, Jani M, et al. Leflunomide and its metabolite A771726 are high affinity substrates of BCRP: implications for drug resistance. Ann Rheum Dis. 2009;68(7):1201–1207. doi: 10.1136/ard.2007.086264 [DOI] [PubMed] [Google Scholar]
- 17.Dietrich S, Kramer OH, Hahn E, et al. Leflunomide induces apoptosis in fludarabine-resistant and clinically refractory CLL cells. Clin Cancer Res. 2012;18(2):417–431. doi: 10.1158/1078-0432.CCR-11-1049 [DOI] [PubMed] [Google Scholar]
- 18.Hail N Jr., Chen P, Bushman LR. Teriflunomide (leflunomide) promotes cytostatic, antioxidant, and apoptotic effects in transformed prostate epithelial cells: evidence supporting a role for teriflunomide in prostate cancer chemoprevention. Neoplasia (New York, NY). 2010;12(6):464–475. doi: 10.1593/neo.10168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alhefdhi A, Burke JF, Redlich A, Kunnimalaiyaan M, Chen H. Leflunomide suppresses growth in human medullary thyroid cancer cells. J Surg Res. 2013;185(1):212–216. doi: 10.1016/j.jss.2013.05.089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baumann P, Mandl-Weber S, Volkl A, et al. Dihydroorotate dehydrogenase inhibitor A771726 (leflunomide) induces apoptosis and diminishes proliferation of multiple myeloma cells. Mol Cancer Ther. 2009;8(2):366–375. doi: 10.1158/1535-7163.MCT-08-0664 [DOI] [PubMed] [Google Scholar]
- 21.Chu M, Zhang C. Inhibition of angiogenesis by leflunomide via targeting the soluble ephrin-A1/EphA2 system in bladder cancer. Sci Rep. 2018;8(1):1539. doi: 10.1038/s41598-018-19788-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheng L, Wang H, Guo K, et al. Reversine, a substituted purine, exerts an inhibitive effect on human renal carcinoma cells via induction of cell apoptosis and polyploidy. Onco Targets Ther. 2018;11:1025–1035. doi: 10.2147/OTT.S158198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thome MP, Filippi-Chiela EC, Villodre ES, et al. Ratiometric analysis of Acridine Orange staining in the study of acidic organelles and autophagy. J Cell Sci. 2016;129(24):4622–4632. doi: 10.1242/jcs.195057 [DOI] [PubMed] [Google Scholar]
- 24.Emerling BM, Akcakanat A. Targeting PI3K/mTOR signaling in cancer. Cancer Res. 2011;71(24):7351–7359. doi: 10.1158/0008-5472.CAN-11-1699 [DOI] [PubMed] [Google Scholar]
- 25.Lin YC, Lin JF, Wen SI, et al. Inhibition of high basal level of autophagy induces apoptosis in human bladder cancer cells. J Urol. 2016;195(4 Pt 1):1126–1135. doi: 10.1016/j.juro.2015.10.128 [DOI] [PubMed] [Google Scholar]
- 26.Lin JF, Lin YC, Yang SC, et al. Autophagy inhibition enhances RAD001-induced cytotoxicity in human bladder cancer cells. Drug Des Devel Ther. 2016;10:1501–1513. doi: 10.2147/DDDT.S95900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin JF, Tsai TF, Liao PC, et al. Benzyl isothiocyanate induces protective autophagy in human prostate cancer cells via inhibition of mTOR signaling. Carcinogenesis. 2013;34(2):406–414. doi: 10.1093/carcin/bgs359 [DOI] [PubMed] [Google Scholar]
- 28.Liu X, Zhao P, Wang X, et al. Celastrol mediates autophagy and apoptosis via the ROS/JNK and Akt/mTOR signaling pathways in glioma cells. J Exp Clin Cancer Res. 2019;38(1):184. doi: 10.1186/s13046-019-1173-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kang M, Jeong CW, Ku JH, Kwak C, Kim HH. Inhibition of autophagy potentiates atorvastatin-induced apoptotic cell death in human bladder cancer cells in vitro. Int J Mol Sci. 2014;15(5):8106–8121. doi: 10.3390/ijms15058106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Madak JT, Bankhead A 3rd, Cuthbertson CR, Showalter HD, Neamati N. Revisiting the role of dihydroorotate dehydrogenase as a therapeutic target for cancer. Pharmacol Ther. 2019;195:111–131. doi: 10.1016/j.pharmthera.2018.10.012 [DOI] [PubMed] [Google Scholar]
- 31.Hanson K, Robinson SD, Al-Yousuf K, et al. The anti-rheumatic drug, leflunomide, synergizes with MEK inhibition to suppress melanoma growth. Oncotarget. 2018;9(3):3815–3829. doi: 10.18632/oncotarget.23378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brown KK, Spinelli JB, Asara JM, Toker A. Adaptive reprogramming of de novo pyrimidine synthesis is a metabolic vulnerability in triple-negative breast cancer. Cancer Discov. 2017;7(4):391–399. doi: 10.1158/2159-8290.CD-16-0611 [DOI] [PMC free article] [PubMed] [Google Scholar]