Abstract
Background:
While indwelling urinary and vascular catheters are valuable devices in patient care, prolonged or unnecessary use increases the risk of infectious and non-infectious catheter harms.
Methods:
To understand persistent barriers to detecting and removing unnecessary catheters, we conducted a multi-method qualitative study that included observations and in-person interviews with clinicians working on a progressive care unit of a large hospital. Observations consisted of shadowing nurses during shift change and while admitting patients, and observing physicians during morning rounds. Observational data were gathered using unstructured field notes. Interviews were conducted using a semi-structured guide, audio-recorded, and transcribed. Qualitative content analysis was conducted to identify main themes.
Results:
Barriers to timely removal identified during 19 interviews with clinicians and 133 hours of field observations included: physicians not routinely reviewing catheter necessity during rounds; catheters going unnoticed or hidden under clothing; common use of ‘do not remove’ orders; and little or no discussion of catheters among clinicians. Five overall themes emerged: 1) catheter data is hard to find, not accurate, or not available; 2) catheter removal is not a priority; 3) confusion exists about who has authority to remove catheters; 4) there is a lack of agreement on standard protocols and indications for removal; and 5) communication barriers among clinicians create challenges.
Conclusion:
To address barriers and facilitate detection and timely removal, clinicians need: ready access to accurate catheter data, more clearly delineated clinician roles for prompting removal, effective tools to facilitate discussions about catheter use, and standardized catheter removal protocols.
Keywords: Patient safety, healthcare quality improvement, infection control, nosocomial infections, qualitative research, catheter-associated urinary tract infection (CAUTI), central line-associated bloodstream infection (CLABSI), urinary catheter, vascular catheter, healthcare-associated infection, catheter-associated infections, infection prevention
INTRODUCTION
Catheters are essential in patient care and among the most commonly used medical devices in hospitals. However, recent research suggests that both indwelling urinary catheters (UCs) and central venous catheters (CVCs) are often placed in patients who may not need them;1–4 are, at times, used when risks outweigh benefits;5 and are sometimes kept in longer than medically indicated.3 Often, clinicians are not even aware that their patients have a catheter.6, 7 These devices place patients at a greater risk of acquiring healthcare-associated infections (HAI), with as many as 25% of all HAIs being linked with medical devices such as catheters.8 Two of the most common, serious, and often-preventable HAIs include catheter-associated urinary tract infection (CAUTI) and central line-associated bloodstream infection (CLABSI). Several efforts to standardize catheter insertion and maintenance practices have led to significant reductions in CLABSI,9–11 although few interventions have focused on removal of unnecessary catheters.12 Improvements in CAUTI have been more variable.13, 14
In addition to infections caused by catheters, non-infectious harms (such as pain, hematuria, and injury to the urethra from UCs, and deep vein thrombosis and catheter occlusion with CVCs), have recently been recognized as occurring even more frequently than catheter infections.15–17 These non-infectious harms are associated with increased length of hospital stay, can lead to increased risk of a catheter-associated infection, and a greater chance of needing additional procedures.18–23 The most common risk factor for catheter harms is extended catheter duration, because the longer catheters are left in, the greater the risk of infectious and non-infectious problems.16, 24–27
Findings presented in this paper are part of a larger study, conducted by our institution’s Patient Safety Learning Laboratory (Safety Lab), to develop and test ways to improve the safety of hospitalized patients. The focus of this stage of the study was to identify common barriers to timely and appropriate catheter removal with the goal of developing potential interventions at later stages of the study. While previous studies have also focused on catheter-related practices on inpatient units,6, 28–31 the unique contributions of this study include: selection of a hospital where several changes have already been implemented to improve timely catheter removal with the goal of revealing the most difficult and persistent barriers; inclusion of advanced practice professionals, along with physicians and nurses; and identification and synthesis of barriers that emerged at key points during the traditional Steps to Catheter Removal to inform future interventions.
METHODS
Study Design
To better understand clinician practices within the hospital setting, we designed a multi-method qualitative study32 that included field observations and interviews with clinicians. Using both observations and interviews allows for triangulation and thus more robust, comprehensive findings. This study was approved by our university’s Institutional Review Board.
Setting, Participants, Sampling
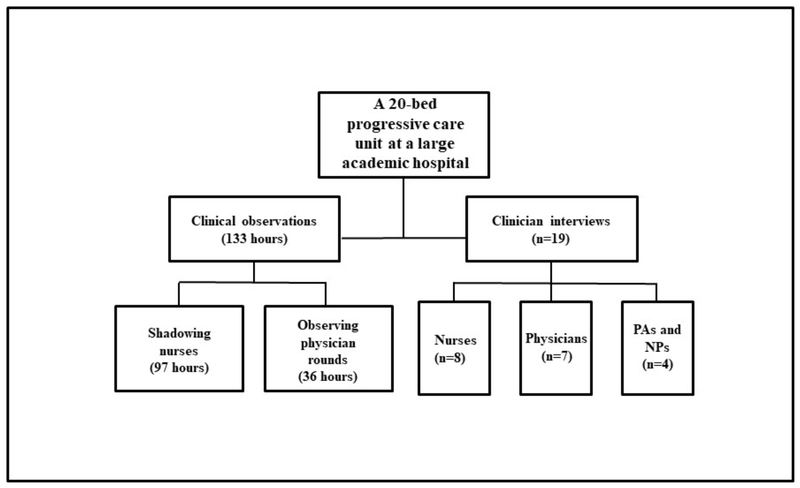
The setting was a 20-bed unit providing intermediate or progressive care in a large academically-affiliated tertiary care hospital. This unit is an “open” unit, meaning that several teams of physicians have patients in this unit including medical and surgical teams, though a single team of nurses is dedicated to the care of the patients. We selected this unit type because it is more common as opposed to the “closed” unit design, which has a single physician team caring for all patients and is often only present in intensive care units of large hospitals. In this unit, nurses work 12-hour shifts, caring for two to three patients with serious, complex conditions and who often require indwelling catheters. At the time of the study, the hospital had adopted several changes including: a fully-integrated electronic health record (EHR) system in June 2014 from the vendor Epic (Epic EpicCare Inpatient EMR, Verona Wisconsin); a unit-wide policy requiring nurses to document catheter presence and indication (i.e., reason for catheter use) in the EHR every shift; and, a policy empowering nurses to remove UCs (also known as Foleys or urethral catheters) without a physician order.
At the outset, the lead investigator (JM) for this project met with unit leadership to explain the purpose of the study and obtain permission to conduct observations and interviews. We purposefully sampled potential participants to include clinicians in various roles, with varied experience, and in both leadership and bedside positions. A general invitation email was sent to all unit nurses, nurse practitioners, and physician assistants (n=62) explaining the study and asking them if they would be interested in participating in interviews. Targeted, follow-up emails were then sent to specific clinician types (i.e., physicians, nurses, advanced practice providers) to ensure a mix of participants (n=39). In total, approximately 20 of the 101 invited, responded that they would be interested. A smaller number (n=7) were invited to participate in person by research staff while they were conducting observations. All interview participants received a $30 gift card. In addition, nurses who agreed to be shadowed, a specific observational technique in which the researcher closely follows an individual, received a bottle of hand sanitizer as a small token of appreciation.
Data Collection
Data collection occurred between May and August 2016. Field observations occurred at times during the day when we anticipated that catheter use was most likely to be assessed by clinicians. Nurses were observed during the morning nurse conference, nurse-to-nurse patient handoff, and at times when nurses were most likely to admit a new patient (e.g., after morning rounds when they received transfers from the ICU and new surgical admissions). For physicians, physician assistants (PAs), and nurse practitioners (NPs), we focused on surgery report (i.e., surgery night shift handoff to day shift) and morning rounds. Two to three members of the research team were present on the unit during observation periods, which lasted between two and four hours on average. All fieldwork was conducted in accordance with an Observation Guide created specifically for this study (Appendix 1). This guide included explicit instructions on what to listen and watch for during observation periods. Observers were aware of which patients had an indwelling UC (focusing on transurethral catheters) or a CVC (including internal jugular, subclavian, and femoral CVCs), or peripherally inserted central catheters (PICCs) as documented in the EHR and reported through a daily catheter census. Most observation periods started with the 7:00 am nurse conference where both day- and night-shift nurses provided short patient status updates. During these conferences, observers were listening for any discussion related to catheters. At the conclusion of the conference, members of the research team were paired with bedside nurses to shadow during the first part of their morning shift. Shadowing consisted of one observer following one nurse, allowing our observer to listen in on nurse handoff, sit near the nurse while s/he entered notes into the EHR, and follow the nurse into patient rooms as s/he did morning assessments. During slow periods, research staff had informal conversations with nurses related to catheter use and removal. Our team also observed physician rounds on the unit, including multidisciplinary, surgery, and pulmonary team rounds. One observer joined the rounding team of several physicians, listened in on their discussions, and took detailed field notes. Notes were later organized into a common format to help standardize data analysis.
During this same time period, we conducted semi-structured, in-person interviews with clinicians, either individually or, in a few cases when more convenient for interviewees, in pairs. The purpose was to ask about current practices regarding monitoring, documenting, and communicating catheter-related information and to solicit input from participants about common barriers and ways to improve current practices. Interviews were held in rooms close to the unit and when convenient to clinicians. Interviews were audio-recorded, transcribed, and de-identified. Our interview guide (Appendix 2) was reviewed and pilot tested by the study team, and tailored slightly to the role of the interviewee. Conducting both observations and interviews allowed us to triangulate our data from both methods and examine consistencies and/or contrasts between what was observed and what was reported by clinicians.
Data Analysis
All field notes were reviewed by two researchers. During this review, we conducted a directed qualitative content analysis,33 looking specifically for barriers and missed opportunities to timely removal of catheters that were observed directly by researchers and/or stated as barriers by clinicians during observation periods. These barriers were extracted from the field notes and aggregated across all observations. At the same time, qualitative content analysis was conducted on interview data using both deductive and inductive approaches. Four team members independently read a sample of interviews, met, and developed a preliminary coding scheme to help guide the analysis.34 A codebook was created that included both deductive codes, identified prior to coding based on broad categories most important to the study aims (e.g., communication, EHR), and inductive subcodes that emerged directly from interview responses. All interview transcripts were then read and independently coded (line-by-line) by two members of the research team. Coders met regularly to discuss and resolve any discrepancies in coding, until agreement was reached. Qualitative data were then entered into NVivo 11 software (QSR, International Pty. Ltd.), aggregated across all interview respondents, and common themes were identified.
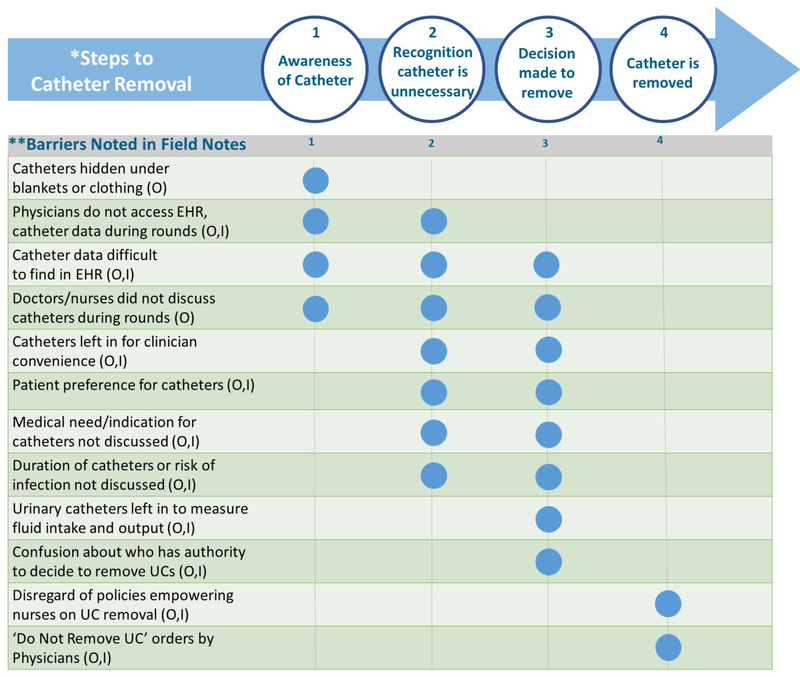
Observation and interview data were further integrated by using a common code scheme across both data sets and several common themes were found. Barriers noted during observations and/or stated during interviews were then itemized and entered into a matrix depicting the traditional ‘Steps to Catheter Removal’ (Table 1)35 and shared with the larger research team for additional review and feedback. By classifying the barriers in this way, we were able to quickly see which barriers were likely to interfere with specific steps in the removal process. The steps used for this analysis were defined to be consistent with the protocols specific to this unit and hospital. They also include nurses, NPs, and PAs, in addition to physicians, as all are empowered in this unit to remove UCs that do not meet specific clinical criteria. Using both observation and interview data sources allowed for triangulation, thus enhancing our findings.36, 37
Table 1.
Steps to Catheter Removal
| Steps | Description Used in This Study |
|---|---|
| 1. Awareness of catheter | Clinician (physician, nurse, physician assistant, nurse practitioner) who is responsible for catheter removal in this setting is aware of the catheter’s presence and continued use |
| 2. Recognition catheter is unnecessary | Clinician recognizes that catheter is no longer medically indicated |
| 3. Decision made to remove catheter | Clinician decides catheter should be removed |
| 4. Catheter is removed | Catheter is removed by the appropriate clinician based on device type and unit- and hospital-specific protocols |
RESULTS
Clinical observations on the unit were conducted over 133 hours with the majority of time spent shadowing nurses (97 hours) and less time observing physician rounding teams (36 hours). These observations generated 47 individual field note documents. A total of 19 clinicians were interviewed including 8 nurses, 7 physicians, 3 PAs, and 1 NP. Interviews lasted an average of 47 minutes (Figure 1).
Figure 1.
Setting and Sample
Findings from this study include: 1) a thematic assessment of the institutional and cultural factors related to catheter removal on this unit; and 2) a practical application of our findings related to common barriers to the Steps to Catheter Removal to help inform the development of future interventions.
THEMES IDENTIFIED FROM INTERVIEWS AND OBSERVATIONS
In general, five distinct and overarching themes emerged related to the organizational culture of catheter removal on this unit, including: 1) catheter data is hard to find, not accurate, or not available; 2) catheter removal is not a priority; 3) confusion exists about who has the authority to remove catheters; 4) there is a lack of agreement on, and awareness of, standard protocols and indications for removal; and 5) communication barriers create challenges. Table 2 includes themes identified, exemplary quotations from clinician interviews, and related examples and excerpts from observation field notes. Integrating data from both interviews and observations allowed us to synergize our findings.
Table 2.
Most Common Themes from Interviews and Observations
| Themes | Exemplary Quotes from Interviews | Excerpts from Field Notes |
|---|---|---|
| Theme 1: Catheter data is hard to find, not accurate, or not available | ||
| Data on catheters (e.g., medical need, duration) is hard to find in EHR |
“… with an electronic health record, you know, you have to learn how to find that information and that’s another barrier… ” (Attending Physician) “I think if we had like a banner that popped up … [showing] whenever the line was put in or discontinued … that would help everybody. ” (Nurse Practitioner) |
Observation 37 Day19: PA says there is no good way to see when a Foley was placed in the EHR. Observation 45 Day 22 nurse shadowing in team room: Physician Assistant (PA) 1 gets a call back from a page about access on the earlier patient, she is told to put the order in the computer for access in her dominant arm. PA 1 says: “the computer system is so complicated to find lines and drains specifics; the computer sometimes hurts more than it helps.” Observation 14 Day 6 morning rounds: Three different physician teams rounded on six patients. In general, teams were large (~10 doctors) and happened very quickly. None of the teams rounded with computers or accessed EHR during rounds. |
| Data often not accurate or completely missing in EHR, resulting in a lack of trust in data by clinicians |
“Sometimes, that information is incorrect though. If a patient is admitted, gets discharged, and gets readmitted … let’s say 20 days later… that old information is still in the system so it keeps inputting that, even if the *PICC is already out. ” (Physician Assistant) “I guess I wouldn’t say that I would trust that [data] 100%. ” (Physician Assistant) |
|
| Catheter information is missing at patient bedside |
“I think having something at the bedside, like on a monitor… near the bed … like the number of days [the catheter has been in]. or something that the family is looking at too, it might create a sense of urgency in their minds. ” (Nurse) “I feel like that might spark someone’s you know, feeling of ‘wow maybe I should get this out’ if it has been in for 58 days… ” (Nurse) |
|
| Theme 2: Catheter removal is not a priority | ||
| Clinicians don’t discuss or think about catheters unless there is a problem | You know, there are so many things to keep your eye on. I think if it’s not something life threatening, it’s low on the totem pole. I don’t think it’s on the forefront of physicians ‘ or nurses ‘ minds. ” (Nurse) | Observation 7 Day 3 nurse shadowing: Morning workflow, according to nurse 1, includes looking at orders after report-out, looking at recent labs, supposed to do “bedside double check” with the night shift nurse at the end of report-out but the practice has fallen off as of late because report-out takes a while and often nurses will have the same patient over and over. Times when medications are administered (9am and 9pm) are times with highest workload for nurses, nurse says s/he would like more techs, currently only one tech for 10 patients. |
| Not as important as more serious health issues |
“Critical patient care issues may overtake routine checking for devices. ” (Attending Physician) “It’s definitely not the first thing on your list in the morning, like maybe it should be, but it’s not. ” (Nurse) |
|
| Heavy clinician workload means sometimes catheters left in for clinician convenience |
“That’s the reality … like our patients really can’t get up by themselves, most of them, and so the act of not having the Foley and having to get ‘em up and urinate or use the bedpan or get to the commode is a really big workload issue.” (Nurse) “If I was having a crazy day, I am probably not going to push to take that Foley out as quickly as I would on a day where I had a little more time. ” (Nurse) “Nurses want more access for convenience so they can get their workload done. God love them. I understand. ” (Nurse) |
|
| Theme 3: Confusion exists about who has authority to remove | ||
| Authority for removal differs depending on type of catheter and is not well understood |
“The order to remove [a urinary catheter] has to come from a doctor. I mean I think it has to come from a doctor. ” (Attending Physician) “The majority of the time, the medical team initiates it because they know the treatment plan. They say, okay, their therapy is done. Take the line out. ” (Nurse) |
Observation 36 Day 18 morning rounds: Patient E had a nephrectomy yesterday, I & O discussion, urine output has slowed. Intern says: “I’d like to take out the Foley, is that some thing that urology has to okay?” Chief Resident: “Contact urology and talk with them about the Foley and drain plan and see what they say.” Observation 19 Day 9 nurse shadowing: We discuss the new policy that nurses do not need a physician order to remove a Foley but Nurse 2 said she would always talk with the physician before removing one because, “some doctors, especially surgeons will get angry if you remove it without talking with them first.” |
| Despite hospital policy empowering nurses to decide when to remove urinary catheters, nurses still consult doctors or wait for an order to remove |
“I know they started this initiative where … certain Foleys [nurses] can just take out without checking with the provider, but I found that even when that is in place, the provider is not happy when the Foley comes out. So I find it’s just prudent to give them a call and say, ‘hey, I don’t think we need this Foley anymore. I would like to take it out. Are you okay with that?’” (Nurse) “Yeah, the nurses don’t usually pull out a Foley unless they come talk to us about it. ” (Physician Assistant) |
|
| ‘Do Not Remove’ orders from doctors are common and supersede other policies | “A lot of times what we will see is it will say ‘do not remove ‘ [in EHR]. Now we are starting to see that pop up more. It says ‘do not remove unless there’s a physician order. ” (Nurse) | |
| Theme 4: Lack of agreement on and awareness of standard protocols and indications for removal | ||
| Current indications for removing urinary catheters are vague |
“The physicians … sometimes they think that having an epidural will constitute having a catheter, which is not part of the algorithm. So there is still that bias so, sometimes, catheters stay in for that reason. ” (Nurse) “I started doing audits on every single patient who had a Foley and if I couldn’t tell in the documentation why the patient needed the Foley, then I would talk to the nurse. Nine times out of 10 staff were selecting ‘accurate measurement of urine output in the critically ill ‘ [as the reason].” (Nurse) |
Observation 17 Day 8 nurse shadowing: Nurse 2: we are supposed to look at whether it meets CDC guidelines listed in the EHR but it’s a lot of clicks to get to the page. Once per shift we’re supposed to go in there and pick an indication but there are many things we are supposed to document and it gets overwhelming… |
| No known indications for assessing PICC lines | “No, there’s no indications like when you are doing the chart. At another job [there were] selections: ‘Are they having antibiotics for 14 or more days?’ ‘Are they like hemodynamically unstable?” (Nurse) | Observation 19 Day 9 nurse shadowing: I asked the nurse if she was familiar with the decision tree for catheters posted in this unit. She said only vaguely and that she really had not looked at it and doesn’t use it. |
| Nurses defer to physicians on indications for removing venous catheters |
“The physician [decides]… it would be an order, or they might send a page too but no one would remove it without an order. ” (Nurse) “We would have to change our mindset because I think we are so used to saying, oh, ‘double lumen PICC. ‘ It is there and its saline locked; they get an antibiotic every 12 hours. Does that patient really need a line for that? If they are a difficult stick, yes, but in the grand scheme of things, we would have to change our mindset too, to be honest with you. ” (Nurse) |
|
| Theme 5: Communication barriers create challenges | ||
| Poor communication between clinicians |
“The communication with nursing . is just really poor, particularly, physicians to nurses… And honestly we have done a number of things to try and improve this but honestly it’s a culture and it needs to come from the top down. ” (Nurse) “We also need to breakdown the communication silos here at the hospital. ” (Attending Physician) |
Observation 7 Day 3 nurse shadowing: Nurses are able to see if patient has Foley in the chart, but often not talked about in nurse handoff. Observation 9 Day 4 care management rounds: hallway rounds with updates on about 15 patients. Catheters were discussed with only 2 patients. While the two patients cared for by the nurse I was shadowing have 1) a PICC line and 2) a Foley, the catheters for these patients were not discussed at all during care management rounds. |
| Morning rounds is a ‘missed opportunity’ for communication and discussion of catheters |
“What we try to do is have the nurse present for rounding however, that hasn’t really been possible because they also … have to be in different [patient] rooms and things like that. ” (Resident) “They didn’t round with me, so that was a problem. They rounded when I was in another room for like 35 seconds so we didn’t talk about |
|
PICC = Peripherally Inserted Central Catheter
Theme 1: Catheter data is hard to find, not accurate, or not available
One of the most frequently reported interview themes was that information on catheters, such as catheter presence, when it was inserted, and the medical indication for it, was hard to find and often not accurate in the EHR. One nurse said, “I think [it’s] the weak link … the stuff is buried! You’ve got five clicks before you can actually get to that. There’s so much information to glean through.” Clinicians also stated that catheter data was often missing completely from the EHR, or not updated. These data deficiencies led to a general mistrust of information. During observations clinicians also noted that catheter data in the EHR was not readily available during morning rounds because these physician teams did not typically round with laptops or tablets.
Theme 2: Catheter removal is not a priority
Another common theme was that catheter removal was not a high priority for clinicians, particularly on a progressive care unit for patients with serious and often multiple health problems. During interviews, clinicians reported that catheters were only perceived as important when patients started showing signs of infection. While on the unit, we observed that clinicians rarely discussed catheters but when they did, it was to clarify if the patient had a catheter in place and the catheter type; however, they seldom talked about catheter duration or the medical need for the device. During nurse handoff, most nurses used a standard printed checklist of items to brief the nurse taking over the care of their patients. It was common for the nurses to say, for example, ‘double lumen PICC, right arm’ but neither the duration nor the medical indication for the catheter was part of this standard checklist of items. Conversation around duration and indication was usually only prompted when the patient started showing signs of infection.
Interviewees also said that physicians and nurses were extremely busy on these units and that large patient caseloads and heavy workloads may have meant that catheters were left in longer than necessary. During observations some clinicians told us that catheters were sometimes left in for convenience because removing them would increase their workload, particularly related to helping patients get to the restroom or assisting with bedpans. Nurses also said during observations that they liked to have an “extra line” in case they needed it or there was an emergency situation.
Theme 3: Confusion exists about who has the authority to remove
There was uncertainty expressed during both interviews and observations about who was ultimately responsible for the decision to remove UCs, and to a lesser extent, vascular catheters. For example, while clinicians agreed that it was up to physicians to remove CVCs, they disagreed on who had the authority to decide when to remove UCs and PICC lines. For UCs, many clinicians stated in interviews that they were unaware of a 2013 hospital policy empowering bedside nurses to remove UCs without a physician order. Other clinicians reported that the new policy was ignored and several nurses said that, though they were aware of the policy, they often waited for physician approval or sought permission from a doctor before removing UCs. Further adding to the confusion, doctors were found to frequently place ‘do not remove’ orders for UCs, which superseded nurse-empowered removal policies. There was similar confusion about PICC removal authority. Some clinicians thought that vascular nurses, along with physicians, had the authority to discontinue PICC lines. However, hospital policies, last updated in 2015, state that while ‘privileged providers’ (e.g., physicians, or advanced practice professionals under physician delegation such as PAs, NPs, CNMS, or CRNAs) have the authority to discontinue a PICC line, vascular nurses can only discontinue a PICC line when an order is placed by a privileged provider.
Theme 4: Lack of agreement on and awareness of standard protocols and indications for removal
During interviews, clinicians said there was disagreement among doctors and nurses about standard indications for UCs. For example, during observations of morning rounds we noted that a few physicians told their teams that the presence of an epidural and/or the need for an accurate measurement of fluid output were both valid indications for UCs. However, several nurses during interviews disagreed that these were appropriate indications and said more thought needed to be given to justify the additional risk to the patient. Similarly, while some standard PICC order sets requiring selection of an appropriate indication for PICC use were added to the hospital EHR in January 2016 (four months prior to our observations and interviews), many respondents were unaware of them. This was complicated by the fact that there were different teams that placed PICC lines at this hospital (similar to other hospitals), and the order sets differed by team (e.g. PICC nurses vs. interventional radiology). During our observations we discovered that the unit’s leadership had recently developed a decision tree algorithm to help guide appropriate urinary catheter removal decisions, however when asked about this tool during nurse shadowing, many nurses were unaware of it.
Theme 5: Communication barriers create challenges
Interviewees reported that there was poor communication between clinicians about catheters. “Unless there is a complication, we don’t talk about it [catheters],” said one resident. Nurses also reported that there was a culture of poor communication between doctors and nurses in particular and this culture affected timely removal. Others reported that most of the communication on catheters happened through pagers or through the EHR and that these one way communication devices created challenges. Lastly, several interviewees said morning rounds should have been a time to discuss catheters, but often resulted in a missed opportunity because they said, and we observed, that these discussions rarely occurred. We observed that nurses were usually unavailable to participate in rounds due to heavy workloads and the timing of rounds which usually occurred during nurse shift change (e.g., before most day shift nurses began work and as night shift nurses were getting ready to leave). Also, physicians may have had as many as 20 patients to see within a short time and they reported not having time to discuss each patient’s catheter. A more detailed examination of communication barriers is found in a related article by Manojlovich and colleagues.38
COMMON BARRIERS TO THE STEPS TO CATHETER REMOVAL
We identified several concrete barriers to, and missed opportunities for, timely removal of catheters from our observations and interviews. Figure 2 includes these barriers and illustrates at which step(s) in the Steps to Catheter Removal each barrier was deemed most likely to interfere with the timely removal of UCs or CVCs. Although each type of catheter is distinct and has unique issues related to removal, we found that several barriers to removal were similar across all catheter types. For example, the barrier of ‘catheters hidden under blankets or clothing’ applied to all catheter types and primarily affected awareness of the catheter by clinicians. Other barriers primarily affecting awareness included ‘catheter data difficult to find in EHR’ and ‘doctors/nurses did not discuss catheters during rounds.’ Barriers to the last step of removal included ‘disregard of policies empowering nurses on UC removal’ and ‘do not remove UC orders by physicians.’ Two barriers -- ‘catheter data difficult to find in the EHR’ and a general ‘lack of discussion about catheters during rounds’ -- appeared to have far-reaching effects on almost all of the Steps to Removal and figured prominently in the 5 overarching themes outlined above.
Figure 2.
Common Barriers to the Steps to Catheter Removal Identified during Observations and Clinician Interviews. Blue circles indicate where barriers are most likely to interfere with steps to removal (1-4)
*Steps to Removal excerpted from Meddings & Saint, 2011 in Disrupting the Life Cycle of the Urinary Catheter in CID 2011:52 1 June, 1291–1293
**While some barriers have the potential to affect several steps, we highlight the steps where the primary impact is most likely to be found. Abbreviations: O= noted in observations; I= stated during interviews
DISCUSSION
Our study found several persistent and ongoing barriers to the timely removal of catheters on a busy progressive care unit, despite implementation of multiple recent quality improvement initiatives. Perhaps our most important finding from both observations and interviews was that clinicians were not routinely discussing catheters in general, and specifically not during morning rounds, patient assessment, and nurse handoff, which were times that clinicians identified as the best opportunities for such discussions. When catheters were discussed, it was usually to note their presence but rarely to discuss the appropriateness, medical indication, or duration of the catheter. This lack of attention to catheters may stem from several factors we found in our study including: clinicians viewing catheters as less important than other more pressing health concerns; confusion about who has responsibility for timely removal; catheters going unnoticed because they are hidden; and difficulty accessing and trusting catheter data. These factors have far-reaching implications on patient safety and may place hospitalized patients at greater risk for catheter harms. It is important to note that the themes found in this study were interrelated, suggesting that timely catheter removal is complex and any intervention will likely require multiple components. For example, catheter data being difficult to find in the EHR suggests a low organizational priority to improve the EHR and confusion over authority to remove may stem from the hierarchical culture prevalent among hospital staff.
While our study supports other research identifying barriers to timely removal including heavy clinician workload and competing priorities,28, 30 patient preference,30 catheters going unnoticed,6, 7 and a need for clear guidelines and protocols on removal,39–41 our findings were unique in that we found that new organizational factors may create additional, unintended problems. For example, the rapid implementation of EHRs along with mandatory catheter reporting has great potential to improve device monitoring. However, clinicians in our study reported several challenges using data in the EHR and accessing it during rounds. Similarly, the nurse empowerment policy related to UCs may have increased confusion about who has the responsibility for removal and spurred use of new ‘do not remove’ orders by physicians. These unintended consequences underscore the need for ongoing quality improvement (QI) initiatives; regular and structured clinician feedback and assessment; and the development of hospital-wide QI committees to monitor and reward success.
While UCs and CVCs are distinct, we found several barriers common to both types of devices, suggesting that similar interventions could be used to encourage timely removal. Our study exposed gaps between the traditional Steps to Catheter Removal and actual practice. Specific interventions that could potentially close this gap may include initiatives designed to improve catheter awareness such as alerts in the EHR when catheters were in place for several days, electronic reminders, checklists, or tools to facilitate discussion. Similarly, some type of bedside display of catheter data could improve device awareness and prompt removal when no longer needed, by aiding as a virtual communication tool between nurses and physicians. Interventions designed to improve recognition that the catheter is no longer necessary might include implementing standard protocols for removal. Interventions targeted at prompting the decision to remove are also necessary and could involve better clarification of authority to remove. Interventions that seek to address barriers at multiple steps may be ideal. For example, making the details that are currently in the EHR about catheter presence, duration of use, and indication readily visible during bedside rounds could potentially 1) increase clinician awareness of the catheter’s presence, 2) prompt consideration and discussion of continued catheter necessity, 3) help facilitate removal if clinicians are confident it is no longer necessary, and 4) prompt recognition and correction of any incorrect catheter data in the EHR. While the implementation of improvement efforts such as EHR reminders and stop orders, nurse empowerment policies, and standardized protocols have become more common,11, 12, 30 we are unaware of any initiatives using bedside monitors to display this type of information.
Our study has both strengths and limitations. First, it was confined to one hospital unit and therefore may not be applicable in all settings. However, we believe this unit is similar in many ways to other hospital units that have implemented catheter-related improvements.42, 43 While we acknowledge the potential for a Hawthorne effect,44 we believe that our study design, which included embedding ourselves within the hospital unit, and collecting data from multiple sources, allowed us to reduce this possibility. Further, if there was a Hawthorne effect at play, it likely strengthens our findings because one could argue that if clinicians were not discussing catheters while being observed for a patient safety study, they would be even less likely to discuss catheters during normal workflow. A strength of our study was the inclusion of a broad sample of individuals from varied roles, disciplines, and levels of leadership. It was important to include not just doctors and nurses, but also advanced practice providers for whom less is known about their perceptions related to catheter removal, but who have an important role in catheter insertion, maintenance, and removal. Further, use of both interviews and observational methods, and the triangulation of data from these two sources, enriched our results, reduced the possibility of bias, and increased the validity of our findings.
CONCLUSION
This study provided a deeper understanding of the current challenges to timely catheter removal faced by frontline clinicians. Future interventions are needed to increase ready access to accurate catheter data; more clearly delineate clinician roles and responsibilities for removal; develop effective tools to improve catheter awareness; and standardize catheter removal protocols. Continuous monitoring and assessment of persistent barriers is required to understand the implications and unintended consequences of improvement efforts underway in hospital settings.
Supplementary Material
Acknowledgements:
We thank all members of the M-Safety Lab Research Team.
Funding/Support: This work was funded by the Agency for Healthcare Research and Quality (AHRQ) grant P30HS024385. Support was also provided by the Department of Veterans Affairs, Health Services Research and Development Service (RCS 11–222 to Dr. Krein). Dr. Meddings’ effort was initially partially funded by concurrent support from AHRQ (K08 HS19767).
Footnotes
Conflict of Interest Disclosures: Dr. Meddings has reported receiving honoraria for lectures and teaching related to prevention and value-based purchasing policies involving catheter associated urinary tract infection. The remaining authors report no conflicts of interest.
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent those of the sponsor, the Agency for Healthcare Research and Quality, or the U.S. Department of Veterans Affairs.
Patient Consent: Not required. Written informed consent was obtained from interview participants (clinicians).
Ethics Approval: This study was approved by The University of Michigan Medical School’s Institutional Review Board (HUM00106108).
Prior Presentations: Ameling, J, Quinn, M, Forman, J, Sankaran, R, Fowler, KE, Manojlovich, M, Petersen, L, & Meddings, J. Clinician-identified barriers to removing unnecessary urinary and vascular catheters. Poster presented at The Society for Healthcare Epidemiology of America Spring 2017 Conference, St. Louis, MO.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data Sharing Statement: To protect the confidentiality of interview participants, the datasets supporting this article are not available.
Open Access: Yes
REFERENCES
- 1.Fakih MG, et al. Urinary catheters in the emergency department: very elderly women are at high risk for unnecessary utilization. Am J Infect Control. 2010. November;38(9):683–688. [DOI] [PubMed] [Google Scholar]
- 2.Fakih MG, et al. Reducing inappropriate urinary catheter use: a statewide effort. Arch Intern Med. 2012. February 13;172(3):255–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jain P, et al. Overuse of the indwelling urinary tract catheter in hospitalized medical patients. Arch Intern Med. 1995. July 10;155(13):1425–1429. [PubMed] [Google Scholar]
- 4.Tejedor SC, et al. Temporary central venous catheter utilization patterns in a large tertiary care center: tracking the “idle central venous catheter”. Infect Control Hosp Epidemiol. 2012. January;33(1):50–57. [DOI] [PubMed] [Google Scholar]
- 5.Paje D, et al. Patterns and predictors of short-term peripherally inserted central catheter use: A multicenter prospective cohort study. J Hosp Med. 2018. February;13(2):76–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chopra V, et al. Do clinicians know which of their patients have central venous catheters?: A multicenter observational study. Ann Intern Med. 2014. October 21;161(8):562–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saint S, et al. Are physicians aware of which of their patients have indwelling urinary catheters? Am J Med. 2000. October 15;109(6):476–480. [DOI] [PubMed] [Google Scholar]
- 8.Magill SS, et al. Changes in Prevalence of Health Care-Associated Infections in U.S. Hospitals. N Engl J Med. 2018. November 1;379(18):1732–1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pronovost PJ, et al. Improving patient safety in intensive care units in Michigan. J Crit Care. 2008. June;23(2):207–221. [DOI] [PubMed] [Google Scholar]
- 10.Sawyer M, et al. Using evidence, rigorous measurement, and collaboration to eliminate central catheter-associated bloodstream infections. Crit Care Med. 2010. August;38(8 Suppl):S292–298. [DOI] [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention (CDC). Vital signs: central line-associated blood stream infections--United States, 2001, 2008, and 2009. MMWR Morb Mortal Wkly Rep. 2011. March 4;60(8):243–248. [PubMed] [Google Scholar]
- 12.Patel PK, et al. Review of strategies to reduce central line-associated bloodstream infection (CLABSI) and catheter-associated urinary tract infection (CAUTI) in adult ICUs. J Hosp Med. 2018. Feb 1;13(2):105–116. [DOI] [PubMed] [Google Scholar]
- 13.Dudeck MA, et al. National Healthcare Safety Network report, data summary for 2013, Device-associated Module. Am J Infect Control. 2015. March 1;43(3):206–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saint S, et al. A program to prevent catheter-associated urinary tract infection in acute care. N Engl J Med. 2016. June 2;374(22):2111–2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chopra V, et al. Variations in peripherally inserted central catheter use and outcomes in Michigan hospitals. JAMA Intern Med. 2016. April;176(4):548–551. [DOI] [PubMed] [Google Scholar]
- 16.Hollingsworth JM, et al. Determining the noninfectious complications of indwelling urethral catheters: a systematic review and meta-analysis. Ann Intern Med. 2013. September 17;159(6):401–410. [DOI] [PubMed] [Google Scholar]
- 17.Saint S, et al. A multicenter study of patient-reported infectious and noninfectious complications associated with indwelling urethral catheters. JAMA Intern Med. 2018. 178(8):1078–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aaronson DS, et al. National incidence and impact of noninfectious urethral catheter related complications on the Surgical Care Improvement Project. J Urol. 2011. May;185(5):1756–1760. [DOI] [PubMed] [Google Scholar]
- 19.Chopra V, et al. The Michigan Risk Score to predict peripherally inserted central catheter associated thrombosis. J Thromb Haemost. 2017. October;15(10):1951–1962. [DOI] [PubMed] [Google Scholar]
- 20.Leuck AM, et al. Complications of Foley catheters--is infection the greatest risk? J Urol. 2012. May;187(5):1662–1666. [DOI] [PubMed] [Google Scholar]
- 21.Parienti JJ, et al. Intravascular complications of central venous catheterization by insertion site. N Engl J Med. 2015. September 24;373(13):1220–1229. [DOI] [PubMed] [Google Scholar]
- 22.Spelman T, et al. Central line-associated bloodstream infections in Australian ICUs: evaluating modifiable and non-modifiable risks in Victorian healthcare facilities. Epidemiol Infect. 2017. October;145(14):3047–3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Timsit JF, et al. Central vein catheter-related thrombosis in intensive care patients: incidence, risks factors, and relationship with catheter-related sepsis. Chest. 1998. July;114(1):207–213. [DOI] [PubMed] [Google Scholar]
- 24.Huth TS, et al. Randomized trial of meatal care with silver sulfadiazine cream for the prevention of catheter-associated bacteriuria. J Infect Dis. 1992. January;165(1):14–18. [DOI] [PubMed] [Google Scholar]
- 25.Johnson JR, et al. Prevention of catheter-associated urinary tract infection with a silver oxide-coated urinary catheter: clinical and microbiologic correlates. J Infect Dis. 1990. November;162(5):1145–1150. [DOI] [PubMed] [Google Scholar]
- 26.Riley DK, et al. A large randomized clinical trial of a silver-impregnated urinary catheter: lack of efficacy and staphylococcal superinfection. Am J Med. 1995. April;98(4):349–356. [DOI] [PubMed] [Google Scholar]
- 27.Yoshida J, et al. Association between risk of bloodstream infection and duration of use of totally implantable access ports and central lines: a 24-month study. Am J Infect Control. 2011. September;39(7):e39–43. [DOI] [PubMed] [Google Scholar]
- 28.Harrod M, et al. Variations in risk perceptions: a qualitative study of why unnecessary urinary catheter use continues to be problematic. BMC Health Serv Res. 2013. April 26;13:151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Knoll BM, et al. Reduction of inappropriate urinary catheter use at a Veterans Affairs hospital through a multifaceted quality improvement project. Clin Infect Dis. 2011. June;52(11):1283–1290. [DOI] [PubMed] [Google Scholar]
- 30.Krein SL, et al. Barriers to reducing urinary catheter use: a qualitative assessment of a statewide initiative. JAMA Intern Med. 2013. May 27;173(10):881–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meddings J, et al. Reducing unnecessary urinary catheter use and other strategies to prevent catheter-associated urinary tract infection: an integrative review. BMJ Qual Saf. 2014. April;23(4):277–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hesse-Biber S, Johnson R, editors. The Oxford handbook of multimethod and mixed methods research inquiry: Oxford University Press, 2015. June 4. [Google Scholar]
- 33.Hsieh HF, Shannon SE. Three approaches to qualitative content analysis. Qual Health Res. 2005. November;15(9):1277–1288. [DOI] [PubMed] [Google Scholar]
- 34.Forman J, Damschroder L. Qualitative content analysis. Empirical methods for bioethics: A primer: Emerald Group Publishing Limited; 2007. p. 39–62. [Google Scholar]
- 35.Meddings J, Saint S. Disrupting the life cycle of the urinary catheter. Clin Infect Dis. 2011. June;52(11):1291–1293. [DOI] [PubMed] [Google Scholar]
- 36.Patton MQ. Enhancing the quality and credibility of qualitative analysis. Health Serv Res. 1999. December;34(5 Pt 2):1189–1208. [PMC free article] [PubMed] [Google Scholar]
- 37.Denzin N Sociological methods: A sourcebook: Routledge, 2017. July 12. [Google Scholar]
- 38.Manojlovich M, et al. Contextual Barriers to Communication Between Physicians and Nurses About Appropriate Catheter Use. Am J Crit Care. 2019. July;28(4):290–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chopra V, et al. The Michigan Appropriateness Guide for Intravenous Catheters (MAGIC): Results From a Multispecialty Panel Using the RAND/UCLA Appropriateness Method. Ann Intern Med. 2015. September 15;163(6 Suppl):S1–40. [DOI] [PubMed] [Google Scholar]
- 40.Meddings J, et al. The Ann Arbor Criteria for Appropriate Urinary Catheter Use in Hospitalized Medical Patients: Results Obtained by Using the RAND/UCLA Appropriateness Method. Ann Intern Med. 2015. May 5;162(9 Suppl):S1–34. [DOI] [PubMed] [Google Scholar]
- 41.Gould CV, et al. Guideline for prevention of catheter-associated urinary tract infections 2009. Infect Control Hosp Epidemiol. 2010. April;31(4):319–326. [DOI] [PubMed] [Google Scholar]
- 42.U.S. Department of Health and Human Services. National action plan to prevent health care associated infections: road map to elimination. (Updated: Nov 21, 2018). Accessed Nov 21, 2018 https://health.gov/hcq/prevent-hai-action-plan.asp.
- 43.Fakih MG, et al. Implementing a national program to reduce catheter-associated urinary tract infection: a quality improvement collaboration of state hospital associations, academic medical centers, professional societies, and governmental agencies. Infect Control Hosp Epidemiol. 2013. October;34(10):1048–1054. [DOI] [PubMed] [Google Scholar]
- 44.Parsons HM. What happened at Hawthorne?: New evidence suggests the Hawthorne effect resulted from operant reinforcement contingencies. Science. 1974. March 8;183(4128):922–932. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.