Abstract
Rationale.
Excessive fear and anxiety, coupled with corticolimbic dysfunction, are core features of stress- and trauma-related psychopathology, such as posttraumatic stress disorder (PTSD). Interestingly, low doses of Δ9-tetrahydrocannabinol (THC) can produce anxiolytic effects, reduce threat-related amygdala activation, and enhance functional coupling between the amygdala and medial prefrontal cortex and adjacent rostral cingulate cortex (mPFC/rACC) during threat processing in healthy adults. Together, these findings suggest the cannabinoid system as a potential pharmacological target in the treatment of excess fear and anxiety. However, the effects of THC on corticolimbic functioning in response to threat have not be investigated in adults with trauma-related psychopathology.
Objective.
To address this gap, the present study tests the effects of an acute low-dose of THC on corticolimbic responses to threat in three groups of adults: 1) non-trauma-exposed healthy controls (HC; n = 25), 2) trauma-exposed adults without PTSD (TEC; n = 27), and 3) trauma-exposed adults with PTSD (n = 19).
Methods.
Using a randomized, double-blind, placebo-controlled, between-subjects design, 71 participants were randomly assigned to receive either THC or placebo (PBO) and subsequently completed a well-established threat processing paradigm during functional magnetic resonance imaging.
Results.
In adults with PTSD, THC lowered threat-related amygdala reactivity, increased mPFC activation during threat, and increased mPFC-amygdala functional coupling.
Conclusions.
These preliminary data suggest that THC modulates threat-related processing in trauma-exposed individuals with PTSD, which may prove advantageous as a pharmacological approach to treating stress- and trauma-related psychopathology.
Keywords: cannabinoid, prefrontal cortex, amygdala, PTSD, threat processing
Introduction
Self-medication with Cannabis sativa to relieve negative affective symptoms, like anxiety, is common in individuals with posttraumatic stress disorder (PTSD) and related disorders (Bolton et al. 2009; Bowles 2012; Bujarski et al. 2012; Boden et al. 2013; Walsh et al. 2017; O’Neil et al. 2017). Cannabis exerts its anxiolytic effects via the psychoactive component, Δ9-tetrahydrocannabinol (THC), which acts as an agonist on cannabinoid type-1 receptors (CB1Rs) in the brain (Viveros et al. 2005a). Importantly, the engagement of the endocannabinoid (ECB) system in the brain plays a critical role in fear-related learning and memory (Ruehle et al. 2012). For instance, animal models show that acute systemic THC administration reduces the behavioral expression of fear following exposure to a stressor (e.g., physical restraint; Patel et al. 2005). Conversely, CB1R blockade via systemic pharmacological administration of a CB1R antagonist or CB1R genetic deletion increases anxiety-related behaviors in the elevated-plus maze (Haller et al. 2002) and interferes with successful extinction of conditioned fear responses (Marsicano et al. 2002; Chhatwal et al. 2005; Pamplona and Takahashi 2006; Bitencourt et al. 2008; Lin et al. 2009). Given that fear and anxiety are central features of PTSD and other stress-related pathologies, these observations have prompted interest in using THC and other ECB modulators to alleviate PTSD symptoms (Trezza and Campolongo 2013; Patel et al. 2017).
Corticolimbic brain regions, including the amygdala, medial prefrontal cortex and adjacent rostral anterior cingulate cortex (mPFC/rACC), are involved in threat processing; however, individuals with PTSD often display dysfunction within and between these regions. This dysfunction is thought to be a mechanism by which acute stress responses following trauma exposure persist and develop into psychopathology (Etkin and Wager 2007; Fenster et al. 2018). The amygdala, for instance, which encodes threat-related information and generates fear responses (LeDoux 2000), is hyperactive in PTSD in response to trauma-related imagery (Shin et al. 1997, 2004), combat-related sounds or smells (Liberzon et al. 1999; Pissiota et al. 2002; Vermetten et al. 2007), trauma-related photographs or words (Driessen et al. 2004; Neumeister et al. 2017), and threatening facial expressions (Rauch et al. 2000; Shin et al. 2005; Williams et al. 2006; Bryant et al. 2008; Simmons et al. 2011; Stevens et al. 2013; Killgore et al. 2014; Badura-Brack et al. 2018). Exaggerated amygdala reactivity is likely due, in part, to insufficient top-down regulation from the mPFC/rACC, consequently leading to hyperarousal and an inability to suppress attention and responses to trauma-related stimuli (Pitman et al. 2001; Liberzon 2006; Garfinkel and Liberzon 2009; Fenster et al. 2018; Andrewes and Jenkins 2019). Indeed, lower functional connectivity between the amygdala and mPFC/rACC during threat processing has been reported in patients with PTSD relative to healthy individuals (Stevens et al. 2013; Wolf and Herringa 2016).
Interestingly, CB1Rs are highly abundant in these regions of the corticolimbic system (Tsou et al. 1998; Marsicano and Lutz 1999; Patel et al. 2017) and recent neuroimaging studies in healthy adults show that THC modulates activity in the amygdala and mPFC/rACC. Specifically, an acute oral low dose of THC (7.5 mg) reduced amygdala reactivity, but enhanced coupling between the amygdala and mPFC/rACC, to social threat (i.e., fearful and angry facial expressions), as compared to placebo (PBO; Phan et al. 2008; Gorka et al. 2015a). These findings suggest that pharmacological enhancement of ECB signaling may help to address corticolimbic dysfunction in PTSD and other stress-related disorders. However, it should be noted that others have reported that administration of THC, particularly at a higher dose (10 mg) increases amygdala activation (Bhattacharyya et al. 2010, 2017), and modulates activation in frontal and parietal regions (Crippa et al. 2009), while increasing levels of anxiety and autonomic arousal to fearful faces (Crippa et al. 2009; Bhattacharyya et al. 2010, 2017). These divergent findings highlight the complexity of THC’s effect on threat responding that may be bimodal, such that low doses of THC may be anxiolytic (Wachtel et al. 2002), whereas higher doses of THC are typically anxiogenic (D’Souza et al. 2004; Genn et al. 2004; Viveros et al. 2005b; Bhattacharyya et al. 2015, 2017). To-date, the effects of THC on corticolimbic responses to threat have only been conducted in healthy individuals. Thus, it is unclear whether the observed effects of THC on corticolimbic function and functional connectivity would replicate in individuals with trauma exposure who are at risk for developing PTSD and/or meet criteria for PTSD.
To address this gap, the present study tests the effects of an acute oral low dose of THC on corticolimbic activation and functional coupling to social threat in three groups of adults: 1) non-trauma-exposed healthy controls (HC), 2) trauma-exposed adults without PTSD (TEC), and 3) trauma-exposed adults with PTSD (PTSD).
Materials and Methods
Participants
Eighty-six right-handed individuals met pre-screening eligibility and were enrolled in the present study. Of the 86, a total of 15 were subsequently excluded (see Fig. 1 for summary), thus, N = 71 participants were included in our final analyses (20–45 years of age; 36 females; see Table 1). Participants were recruited from the Detroit community via print and online advertisements and flyers. Full exclusionary criteria, comorbid psychiatric conditions, and daily medication usage is presented in Online Resource 1. All participants gave written informed consent after explanation of the experimental protocol, as approved by the Wayne State University Institutional Review Board. Of note, this study is part of a larger study investigating the effects of THC on fear extinction in participants with PTSD (NCT02069366).
Fig. 1.
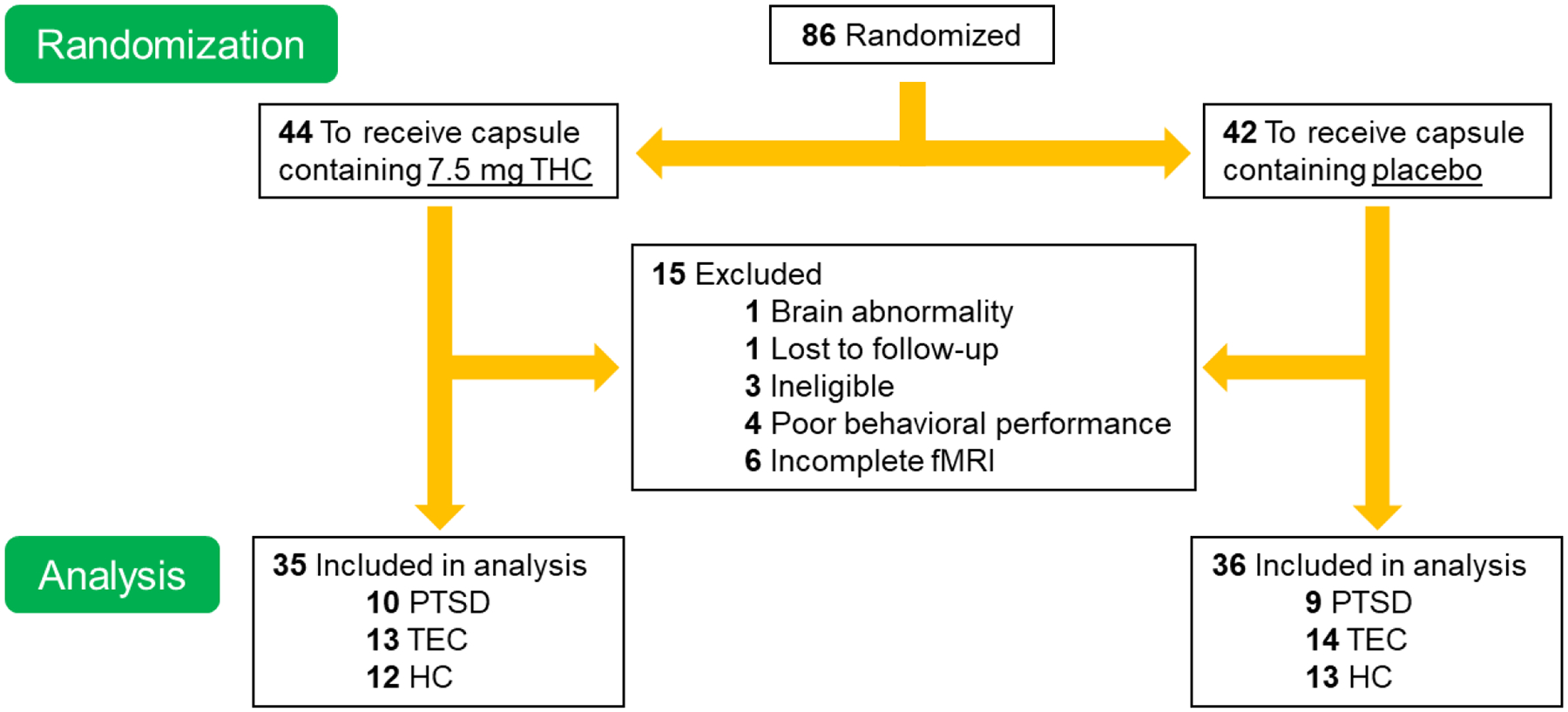
Screening and randomization breakdown of the present study. Eighty-six individuals that met inclusion criteria were randomized to THC or PBO. Of the 86, a total of 15 were subsequently excluded for the following reasons: 3 were ineligible based on participation criteria (i.e., positive drug screening, recently diagnosed mood disorder after enrollment), 4 had poor behavioral task performance (< 50% accuracy for shapes trials), 6 had incomplete functional magnetic resonance imaging (fMRI) data (i.e., did not complete scan or task, scanner malfunction), 1 participant’s structural MRI showed a brain abnormality, and 1 participant was lost to follow-up. Seventy-one participants had complete and reliable data to use for analyses. PTSD = posttraumatic stress disorder; TEC = trauma-exposed controls; HC = healthy controls; PBO = placebo; THC = Δ9-tetrahydrocannabidiol
Table 1.
Participant demographics
| THC (n = 35) | PBO (n = 36) | p-value | |||||||
|---|---|---|---|---|---|---|---|---|---|
| PTSD (n = 10) | TEC (n = 13) | HC (n = 12) | Total | PTSD (n = 9) | TEC (n = 14) | HC (n = 13) | Total | ||
| Age (mean years, SD) | 26.00 (5.46) | 28.31 (7.42) | 24.33 (2.53) | 26.29 (5.69) | 23.33 (3.08) | 26.43 (7.08) | 26.85 (6.66) | 25.81 (6.17) | 0.852 |
| Gender (n, female) | 9 | 5 | 5 | 19 | 5 | 8 | 4 | 17 | 0.552 |
| Race (n) | 0.803 | ||||||||
| African American | 2 | 4 | 0 | 6 | 1 | 3 | 2 | 6 | |
| Asian | 1 | 3 | 5 | 9 | 1 | 4 | 4 | 9 | |
| American Indian/ Alaskan Native | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | |
| Caucasian | 6 | 5 | 7 | 18 | 6 | 5 | 7 | 18 | |
| More than one race | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 2 | |
| Other | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | |
| Hispanic or Latino (n) | 1 | 1 | 0 | 2 | 0 | 1 | 2 | 3 | 0.468 |
| PTSD severity (CAPS-5) | |||||||||
| Total severity score (mean, SD) | 33.60 (10.95) | 2.69 (5.15) | -- | 11.60 (15.76) | 34.11 (6.83) | 4.00 (6.08) | -- | 11.11 (15.15) | 0.993 |
| Number of clinically significant symptoms (mean, SD) | 11.20 (2.57) | 0.92 (2.29) | -- | 3.83 (5.19) | 12.33 (2.87) | 1.14 (2.03) | -- | 3.83 (5.60) | 0.618 |
| Cannabis Use | |||||||||
| Used in the past 30 days (n) | 3 | 3 | 1 | 7 | 2 | 1 | 0 | 3 | 0.189 |
| Occasions (mean, SD) | 2.00 (1.00) | 1.00 (1.00) | 10.00 (0.00) | 3.00 (3.21) | 17.00 (18.00) | 1.00 (0.00) | -- | 11.67 (15.95) | 0.446 |
| Occasions lifetime (n) | 0.288 | ||||||||
| Never | 1 | 5 | 7 | 13 | 4 | 5 | 7 | 16 | |
| 1–10 | 1 | 4 | 2 | 7 | 1 | 4 | 4 | 9 | |
| 11–50 | 6 | 1 | 1 | 8 | 2 | 5 | 1 | 8 | |
| 51–100 | 0 | 2 | 0 | 2 | 0 | 0 | 0 | 0 | |
| < 100 | 2 | 1 | 2 | 5 | 2 | 0 | 1 | 3 | |
Abbreviations: THC, Δ9-tetrahydrocannabinol; PBO, placebo; PTSD, posttraumatic stress disorder; TEC, trauma-exposed control; HC, healthy control; CAPS-5, Clinician-Administered PTSD Scale for DSM-5. Items in BOLD, either refer to the highest category of demographics (age, gender, race, ethnicity, PTSD severity, or cannabis use) or the total number of participants/mean age across the three subgroups (PTSD/TEC/HC). Reported p-values are between-drug group comparisons.
Trauma-related psychopathology
The Life Events Checklist for DSM-5 (LEC-5) was used to identify potentially traumatic events in the participants’ lifetime (Weathers et al. 2013a) and total number of different types of traumatic events experienced and witnessed were used as an index of trauma load. A total of 65 of the N = 71 participants endorsed prior trauma exposure, and of these N = 46 had endorsed a traumatic event that met Criterion A per the Diagnostic and Statistical Manual of Mental Disorders (DSM-5; American Psychiatric Association 2014). Of the 46 Criterion A-exposed individuals, 22 were classified as meeting criteria for PTSD using The Clinician-Administered PTSD Scale for Diagnostic and Statistical Manual of Mental Disorders (CAPS-5; Page et al. 2013), by either meeting CAPS-5 diagnostic criteria or having CAPS-5 PTSD symptom severity scores ≥ 25. Twenty-four participants did not reach criteria for PTSD diagnosis or had severity scores < 25 and, thus, were labeled as TEC. Nineteen participants endorsed at least one traumatic event on the LEC-5 without any meeting Criterion A per the DSM-5. These participants, plus six that denied any exposure to a traumatic event over their lifetime, were therefore labeled as HC (N = 25). Importantly, HC, TEC, and PTSD groups did not differ in age, gender distribution, or race/ethnicity (p’s > 0.4). See Online Resource 1 for analyses comparing trauma load between groups. In addition, analyses comparing PTSD symptom severity and number of PTSD symptoms between PTSD and TEC were conducted and results can be found in Online Resource 1.
Drug groups
Participants were randomized to receive either THC (dronabinol; 7.5 mg capsule; Ascend Laboratories, LLC, Parsippany, NJ) or a matching capsule containing only dextrose (PBO). This dose was previously found to reduce amygdala reactivity to threat in healthy volunteers, without affecting activity in primary visual or motor cortices (Phan et al. 2008). Importantly, the drug was administered evenly across groups (χ2(2) = 0.116, p = 0.944) and individuals receiving THC vs PBO did not differ in sociodemographic factors or PTSD symptom severity (see Table 1 and Fig. 1). Self-reported cannabis use did not significantly differ between drug groups and did not affect the behavioral or brain results (see Online Resource 1).
Procedure
Participants ingested a capsule containing either PBO (N = 36) or THC (N = 35) by mouth approximately 120 minutes prior to the fMRI scan, which corresponds to the anticipated peak drug effects (Wachtel et al. 2002).The study’s principal investigator (CAR) performed drug randomization, and participants and research staff were blinded to the contents of the capsule and subsequent drug grouping.
Threat processing task
During fMRI scanning, participants completed an emotional face processing task developed by Hariri and colleagues (Hariri et al. 2002) that has been shown to reliably elicit threat-related amygdala responses. This social threat task has been extensively used in previous pharmacological fMRI studies (Hariri et al. 2002; Paulus et al. 2005; Tabbert et al. 2005), and consists of photographs of angry, fearful, and happy facial expressions of an equal number of adult male and female actors. During a given trial, participants were presented with a trio of faces selected from the validated Gur stimulus set (Gur et al. 2002). Participants were instructed to select which one of two bottom faces matched the expression of the top target face. The third, distracter face, was a neutral face. All three faces in a given trial were of different actors.
The task was administered in a block design, such that six blocks of each target expression (angry, fearful, happy) were presented. To allow for amygdala reactivity to return to baseline, blocks of shapes matching trials were interleaved with emotion blocks. During shapes trials, participants were instructed to match simple geometric shapes (i.e., circles, rectangles, or triangles). Of note, no target stimuli were repeated within or across blocks. This task design allows us to explore valid contrasts of social threat (angry and fearful faces) vs. shapes and non-threat (happy faces vs. shapes). The task lasted a total of 12 minutes, broken into two six-minute runs, with a total of 36 blocks (18 emotional faces, 18 shapes). Block order was counterbalanced across runs, and each 20 second block consisted of four 5-second trials.
Behavioral data
Accuracy and reaction time were measured for each condition. We performed a drug (PBO vs. THC) × group (PTSD vs. TEC vs. HC) × condition (threatening faces vs. non-threatening faces vs. shapes) ANOVA to test for main effects or interactions on accuracy and reaction time, using SPSS software (IBM SPSS Statistics 26). When needed, for each analysis we applied the Greenhouse-Geisser correction (if the probability of Mauchly’s Test of Sphericity was less than 0.05). Significant main effects and interactions were followed up by t-tests, and all results were considered significant at p < 0.05 (two-tailed), Bonferroni corrected for multiple comparisons.
Subjective anxiety ratings and drug effects
We collected subjective anxiety ratings using the State-Trait Anxiety Inventory (STAI state anxiety; Spielberger 1983) and Visual Analog Scales (VAS; Folstein and Luria 1973). See Online Resource 1 for details regarding these measures and results (Figs. S1 and S2).
fMRI
Data collection.
MRI data were collected on a 3T Siemens MAGNETOM Verio scanner with a 32-channel head coil at the Wayne State University MR Research Facility. See Online Resource 1 for scanning parameters.
Preprocessing.
FMRI data were analyzed using SPM8 software (Wellcome Department of Cognitive Neurology, London, UK; http://www.fil.ion.ucl.ac.uk/spm). The following preprocessing steps were applied, in order: (1) distortion correction, (2) realignment to the first image, (3) slice timing correction, (4) coregistration, (5) normalization to MNI space, (6) reslicing, and (7) spatial smoothing (6 mm FWHM Gaussian kernel). Data from 71 participants met criteria for high quality and scan stability with minimum motion correction and were subsequently included in the analyses (Online Resource 1).
Anatomically defined left and right amygdala were tested separately and defined using the AAL atlas (Tzourio-Mazoyer et al. 2002; Fig. 2a). For better anatomic localization in the amygdala, we also examined activation within cytoarchitectonically-defined amygdala subregions (basolateral [BL], centromedial [CM], superficial [SF], amygdalostriatal [AStr]) as defined in the SPM Anatomy Toolbox (Eickhoff et al. 2005; Amunts et al. 2005; Fig. 2b). The mPFC/rACC was defined using a 10 mm radius sphere centered on coordinates reported in a previous study of acute THC administration in healthy adults during social threat (x = 14, y = 42, z = 12; Gorka et al., 2015; Fig. 2c). All coordinates in this report are given in MNI convention.
Fig. 2.
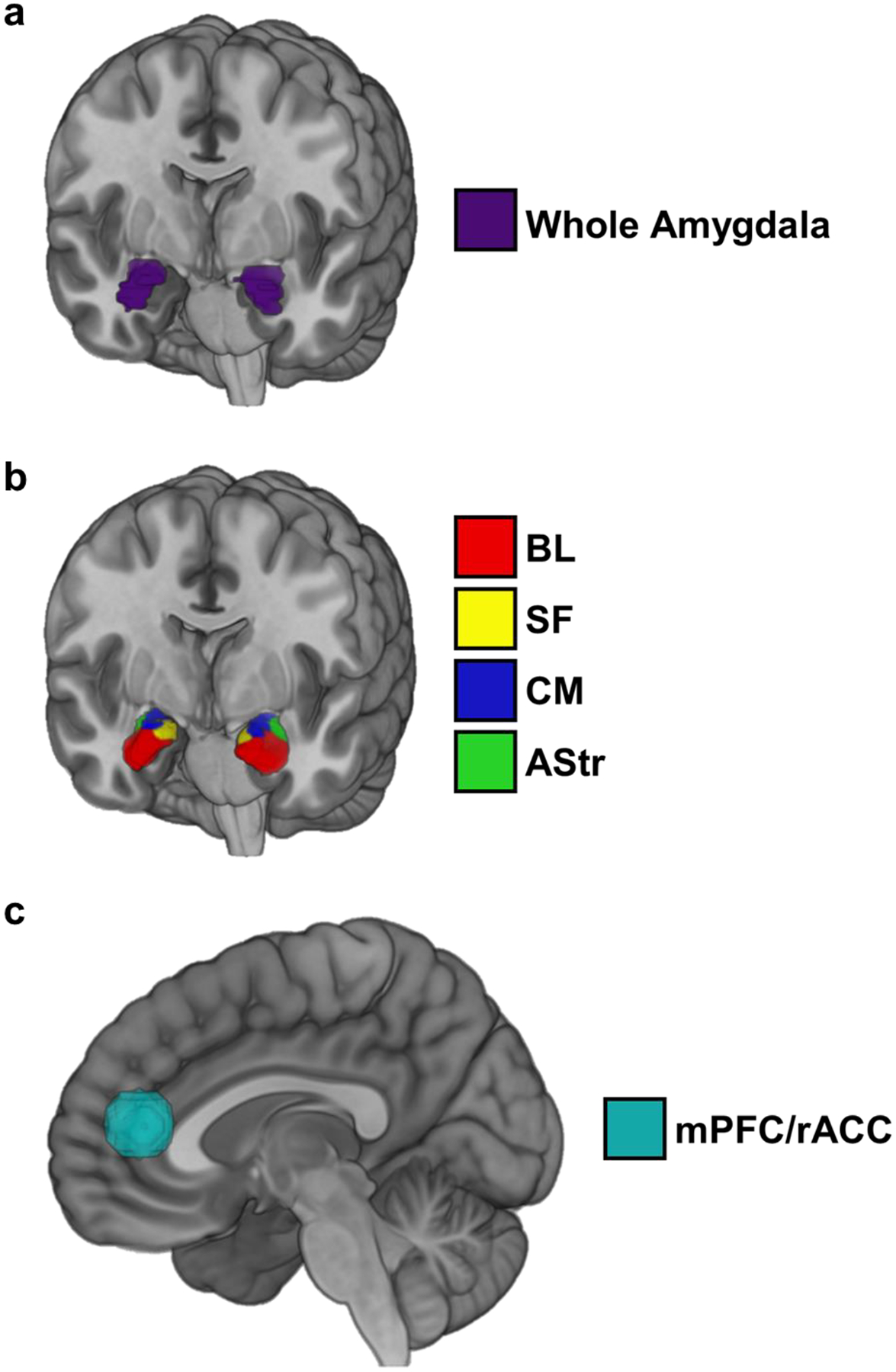
Anatomically defined left and right amygdala using the AAL atlas (Tzourio-Mazoyer et al. 2002) (a), cytoarchitectonically-defined amygdala subregions as defined in the SPM Anatomy Toolbox (Eickhoff et al. 2005; Amunts et al. 2005) (b), and mPFC/rACC defined using a 10 mm radius sphere centered on coordinates reported in a previous study of acute THC administration in healthy adults during social threat (x = 14, y = 42, z = 12; Gorka et al., 2015) (c). BL = basolateral subdivision; SF = superficial subdivision; CM = centromedial subdivision; AStr = amygdalostriatal subdivision; mPFC/rACC = medial prefrontal cortex/rostral anterior cingulate cortex
Analysis.
See Online Resource 1 for first-level model details. Following Phan et al. (2008), individual contrast maps for threatening (angry, fear) faces > shapes and non-threatening (happy) faces > shapes were estimated in SPM8 and subsequently submitted to second level analyses in a random-effects statistical model (Friston et al. 1998). The first principal component of the activation within each region of interest (ROI) for the contrasts threatening faces vs. shapes and non-threatening faces vs. shapes, was extracted for each participant.
For each ROI, a drug (PBO vs. THC) × group (PTSD vs. TEC vs. HC) × condition (threatening faces (vs. shapes) vs. non-threatening faces (vs. shapes)) repeated-measures ANOVA was performed on the extracted first-principal component β-estimates across the ROI, using SPSS software. When needed, for each analysis we applied the Greenhouse-Geisser correction (if the probability of Mauchly’s Test of Sphericity was less than 0.05). Significant main effects and interactions were followed up by t-tests, and all results were considered significant at p < 0.05 (two-tailed), Bonferroni corrected for multiple comparisons. Within SPM8, a complementary whole-brain corrected threshold pFWE < 0.05 was used for exploratory purposes (See Online Resource 1 for whole-brain results).
Functional connectivity.
Functional connectivity of the mPFC/rACC was assessed using a generalized psychophysiological interaction analysis (gPPI; McLaren et al. 2012) in SPM8. First, we created an mPFC/rACC seed region using the same mPFC/rACC ROI described above for the activation analysis, and deconvolved the time series of that seed region with the HRF to put the seed time series in “neuronal space”. Then, we created interaction terms (PPI) by multiplying the deconvolved time series from the mPFC/rACC with the onset times for threatening faces (vs. shapes) and non-threatening faces (vs. shapes). These PPIs were then used in a whole-brain regression, to obtain estimates of whole-brain voxelwise connectivity with the seed region during the task conditions. gPPI estimates were extracted from individual participant contrast images for mPFC/rACC connectivity during threat and non-threat using the anatomically defined left and right amygdala and cytoarchitectonically-defined amygdala subregions described above. These gPPI estimates were entered into repeated-measures ANOVAs using SPSS software. When needed, for each analysis we applied the Greenhouse-Geisser correction (if the probability of Mauchly’s Test of Sphericity was less than 0.05). Significant main effects and interactions were followed up by t-tests, and all results were considered significant at p < 0.05 (two-tailed), Bonferroni corrected for multiple comparisons. Within SPM8, a complementary whole-brain corrected threshold pFWE < 0.05 was used for exploratory purposes (See Online Resource 1 for whole-brain results).
Results
fMRI
Activation.
A drug (THC, PBO) × group (HC, TEC, PTSD) × condition (threatening faces vs. shapes, non-threatening faces vs. shapes) ANOVA showed a significant main effect of condition in bilateral whole amygdala (left: F(1,65) = 5.131, p = 0.027; right: F(1,65) = 4.396, p = 0.040; Fig. 3a), which was further localized to the BL subdivision (left: F(1,65) = 6.090, p = 0.016; right: F(1,65) = 4.538, p = 0.037) and left AStr (F(1,65) = 4.456, p = 0.039). Compared to non-threatening faces (vs. shapes), amygdala responding (bilateral BL, left AStr, bilateral amygdala as a whole) was greater to threat (vs. shapes). There was also a significant main effect of group in the BL subdivision of the left amygdala (F(2,65) = 5.032, p = 0.009; Fig. 3b). Compared to the HC group, the TEC group showed greater left BL activation across conditions (p = 0.007), however, there were no differences between TEC and PTSD or PTSD and HC (ps > 0.05). Importantly, there was a significant main effect of drug in the BL and SF subdivisions of the left (BL: F(1,65) = 7.182, p = 0.009; SF: F(1,65) = 4.740, p = 0.033) and right (BL: F(1,65) = 4.511, p = 0.037; SF: F(1,65) = 8.614, p = 0.005) amygdala (Fig. 3c). Overall, THC decreased bilateral BL and SF activation compared to PBO.
Fig. 3.
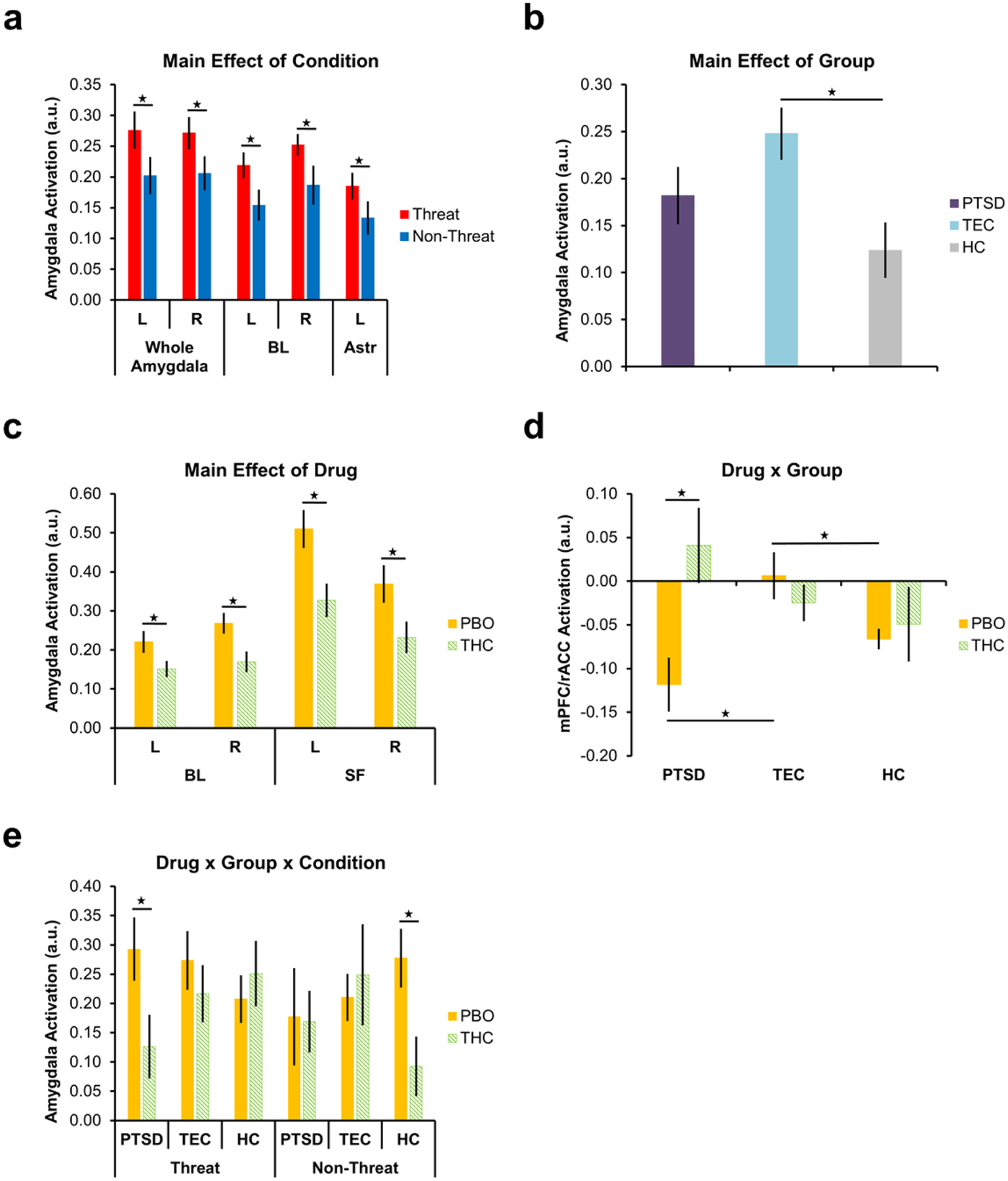
Significant main effect of condition on right and left amygdala activation as a whole, the BL subdivision of the left and right amygdala, and the AStr subdivision of the left amygdala (a) main effect of group on activation in the BL subdivision of the left amygdala (b), main effect of drug on activation in the BL and SF subdivision of the left and right amygdala (c), drug × group interaction on activation in the mPFC/rACC (d), and drug × group × condition interaction on activation in the AStr subdivision of the right amygdala (e). Stars indicate significant within- and between-group differences in corticolimbic activation. Error bars represent standard error of the mean. L = left; R = right; BL = basolateral subdivision; AStr = amygdalostriatal subdivision; SF = superficial subdivision; mPFC/rACC = medial prefrontal cortex/rostral anterior cingulate cortex; PTSD = posttraumatic stress disorder; TEC = trauma-exposed control; HC = healthy control; PBO = placebo; THC = Δ9-tetrahydrocannabidiol; a.u. = arbitrary units
There was a significant drug × group interaction in the mPFC/rACC (F(2,65) = 4.887, p = 0.011; Fig. 3d). Follow-up t-tests showed that within the PBO group, the TEC group showed higher mPFC/rACC activation compared to both PTSD (t(21) = 2.987, p = 0.007) and HCs (t(17.737) = 2.468, p = 0.024). There was no difference in mPFC/rACC activation between the PTSD and HC groups that had received PBO. Notably, within the PTSD group, THC increased mPFC/rACC activation compared to PBO (t(17) = 2.945, p = 0.009). There were no significant differences in mPFC/rACC activation between PTSD, TEC, and HC groups that had received THC or between THC and PBO within the TEC and HC groups (ps > 0.05).
Finally, there was a significant three-way interaction (drug × group × condition) in the AStr subdivision of the right amygdala (F(2,65) = 5.995, p = 0.004; Fig. 3e). Follow-up t-tests showed that within the PTSD group, THC decreased right AStr response to threat relative to PBO (t(17) = 2.161, p = 0.045). In the HC group, THC decreased right AStr response to non-threat relative to PBO (t(23) = 2.662, p = 0.011). There were no significant differences in AStr activation between drug groups within the TEC group for either condition (threat, non-threat) and no significant between group (HC, TEC, PTSD) differences for either condition (ps > 0.05). In our a priori regions of interest (i.e., mPFC/rACC, amygdala and subregions), there were no significant drug × condition or group × condition interactions (ps > 0.05).
Functional connectivity.
A drug (THC, PBO) × group (HC, TEC, PTSD) × condition (threatening faces vs. shapes, non-threatening faces vs. shapes) ANOVA showed a significant main effect of group on functional connectivity between the mPFC/rACC and the right amygdala as a whole (F(2,65) = 3.823, p = 0.023; Fig. 4a). Compared to TEC, the PTSD and HC groups showed greater functional connectivity between the mPFC/rACC and right amygdala, as a whole, across conditions (ps < 0.020), however, there were no differences between PTSD and HC (p > 0.05). There was also a significant group × condition interaction for mPFC/rACC functional connectivity with BL and SF subdivisions of the right amygdala (BL: F(2,65) = 4.946,p = 0.010; SF: F(2,65) = 4.115, p = 0.021; Fig. 4b). The group × condition interaction was driven by increased functional connectivity between the mPFC/rACC and right BL and SF to non-threat in the HC group compared to the TEC group (BL: t(50) = 3.030, p = 0.004; SF: t(50) = 2.237, p = 0.030). There were no significant differences to non-threat between PTSD and TEC or PTSD and HCs, nor to threat between any groups (ps > 0.05).
Fig. 4.
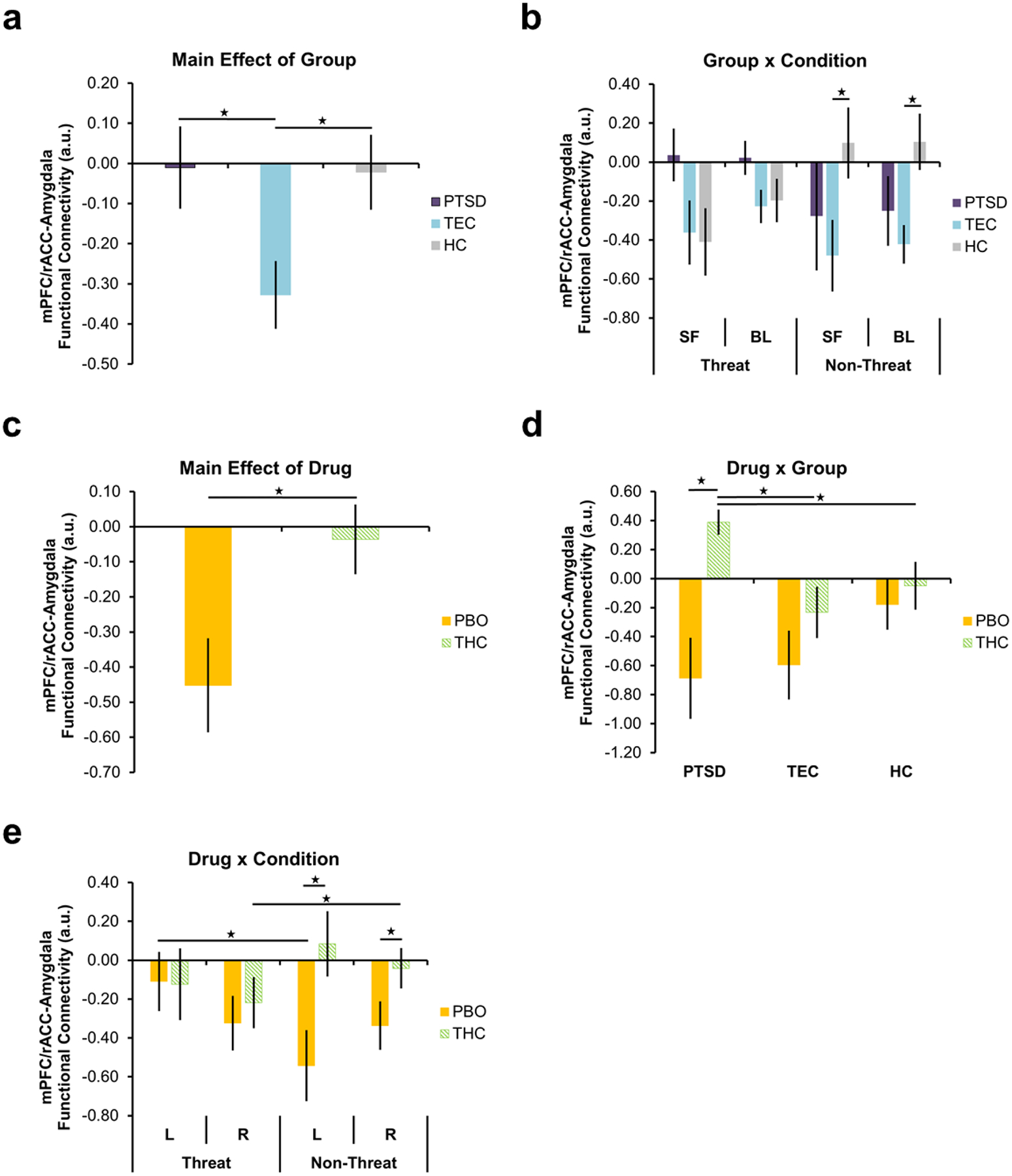
Significant main effect of group on functional connectivity between the mPFC/rACC and right amygdala as a whole (a), group × condition interaction on functional connectivity between the mPFC/rACC and SF and BL subdivisions of the right amygdala (b), main effect of drug on functional connectivity between the mPFC/rACC and SF subdivision of the right amygdala (c), drug × condition interaction on functional connectivity between the mPFC/rACC and SF subdivision of the left and right amygdala (d), and drug × group interaction on functional connectivity between the SF subdivision of the right amygdala (e). Stars indicate significant within- and between-group differences in corticolimbic functional connectivity. Error bars represent standard error of the mean. L = left; R = right; mPFC/rACC = medial prefrontal cortex/rostral anterior cingulate cortex; SF = superficial subdivision; BL = basolateral subdivision; PTSD = posttraumatic stress disorder; TEC = trauma-exposed control; HC = healthy control; PBO = placebo; THC = Δ9-tetrahydrocannabidiol; a.u. = arbitrary units
There was a significant main effect of drug on functional connectivity between the mPFC/rACC and the SF subdivision of the right amygdala (F(1,65) = 8.181, p = 0.006; Fig. 4c). Overall, THC increased functional connectivity between the mPFC/rACC and right SF compared to PBO. Moreover, there was a significant drug × condition interaction on mPFC/rACC functional connectivity with the SF subdivision of the left and right amygdala (left: F(1,65) = 7.070, p = 0.010; right: F(1,65) = 7.675, p = 0.007; Fig. 4d). Compared to PBO, THC increased functional connectivity between the mPFC/rACC and bilateral SF during the non-threat condition (left: t(69) = 2.525, p = 0.014; right: t(59.556) = 3.165, p = 0.002). There was no significant difference in mPFC/rACC-SF connectivity between THC and PBO during the threat condition (ps > 0.05). In the PBO group, mPFC/rACC-left SF functional connectivity to threat was increased compared to non-threat (t(35) = 2.385, p = 0.023), whereas, within the THC group, mPFC/rACC-right SF functional connectivity to threat was decreased compared to non-threat (t(34) = 2.258, p = 0.030).
Finally, there was a significant drug × group interaction for mPFC/rACC functional connectivity with the SF subdivision of the right amygdala (F(2,65) = 3.719, p = 0.030; Fig. 4e). Follow-up t-tests showed no difference in mPFC/rACC-right SF functional between the groups (i.e., PTSD, TEC, HC) that received PBO (ps > 0.05). However, within the THC group, the PTSD group showed increased mPFC/rACC-right SF functional connectivity compared to both TEC (t(21) = 2.869, p = 0.009) and HCs (t(16.381) = 3.039, p = 0.008). Notably, within the PTSD group, THC increased mPFC/rACC-right SF functional connectivity compared to PBO (t(9.539) = 3.673, p = 0.005). There were no significant differences in mPFC/rACC-SF connectivity between THC and PBO within the TEC and HC groups (ps > 0.05). In our a priori regions of interest, there was no significant main effect of condition and no significant three-way interactions for mPFC/rACC-SF connectivity (ps > 0.05).
Behavioral
Accuracy.
A drug (THC, PBO) × group (HC, TEC, PTSD) × condition (threatening faces vs. non-threatening faces vs. shapes) ANOVA showed a significant main effect of condition on accuracy (F(1.717,111.606) = 21.910, p < 0.001). There were no significant main effects of drug or group, and no group or drug interactions on accuracy (ps > 0.05). Follow-up t-tests revealed that accuracy was higher for non-threatening faces as compared to both threatening faces and shapes trials (ps < 0.001).
To test whether the observed main effect of condition remained significant within trauma-exposed participants alone, we ran two additional ANOVAs. First, we ran a drug (THC, PBO) × group (TEC, PTSD) × condition (threatening faces vs. non-threatening faces vs. shapes) ANOVA. The main effect of condition remained significant (F(1.618,67.960) = 10.760, p < 0.001). We found similar results when we took out the “group” factor and ran a drug (THC, PBO) × condition (threatening faces vs. non-threatening faces vs. shapes) ANOVA across trauma-exposed participants (i.e., TEC + PTSD; main effect of condition, F(1.588,69.860) = 10.312, p < 0.001).
Reaction time.
A drug (THC, PBO) × group (HC, TEC, PTSD) × condition (threatening faces vs. non-threatening faces vs. shapes) ANOVA showed a significant main effect of condition (F(1.419, 92.234) = 149.226, p < 0.001), such that reaction time was slower for threatening faces as compared to non-threatening faces and shapes trials (p’s < 0.001). In addition, reaction time to non-threatening faces was significantly slower as compared to shapes trials (p < 0.001). There were no significant main effects of drug or group and no significant drug or group interactions for reaction time (ps > 0.05).
As we did for accuracy, we ran two additional ANOVAs to test whether the observed main effect of condition remained significant within trauma-exposed groups. First, we ran a drug (THC, PBO) × group (TEC, PTSD) × condition (threatening faces vs. non-threatening faces vs. shapes) ANOVA. The main effect of condition remained significant (F(1.473, 61.3863) = 93.007, p < 0.001). We found similar results when we took out the “group” factor and ran a drug (THC, PBO) × condition (threatening faces vs. non-threatening faces vs. shapes) ANOVA across trauma-exposed participants (i.e., TEC + PTSD). The main effect of condition (F(1.471,64.709) = 101.832, p < 0.001) was significant. Interestingly, the drug × condition interaction became significant in the trauma-only subsample (F(1.471, 64.709) = 3.713, p = 0.042).
To further explore these potential drug effects in trauma-exposed individuals, we calculated a difference score, comparing reaction time to threatening – non-threatening (i.e., happy faces), with higher values indicating a slower response for threatening (vs. non-threatening) faces. We found that, relative to PBO, THC administration resulted in a faster response to threatening (vs. non-threatening; t(44) = 2.121; p = 0.040) faces (see Fig. 5). Similar results were observed for threatening faces vs. shapes (F(1,44) = 5.337; p = 0.026), suggesting that THC may reduce the observed slowing of response to threatening faces vs. non-threatening faces or shapes. There was no significant difference between drug groups for non-threatening faces vs shapes (F(1,44) = 1.559; p =0.218).
Fig. 5.
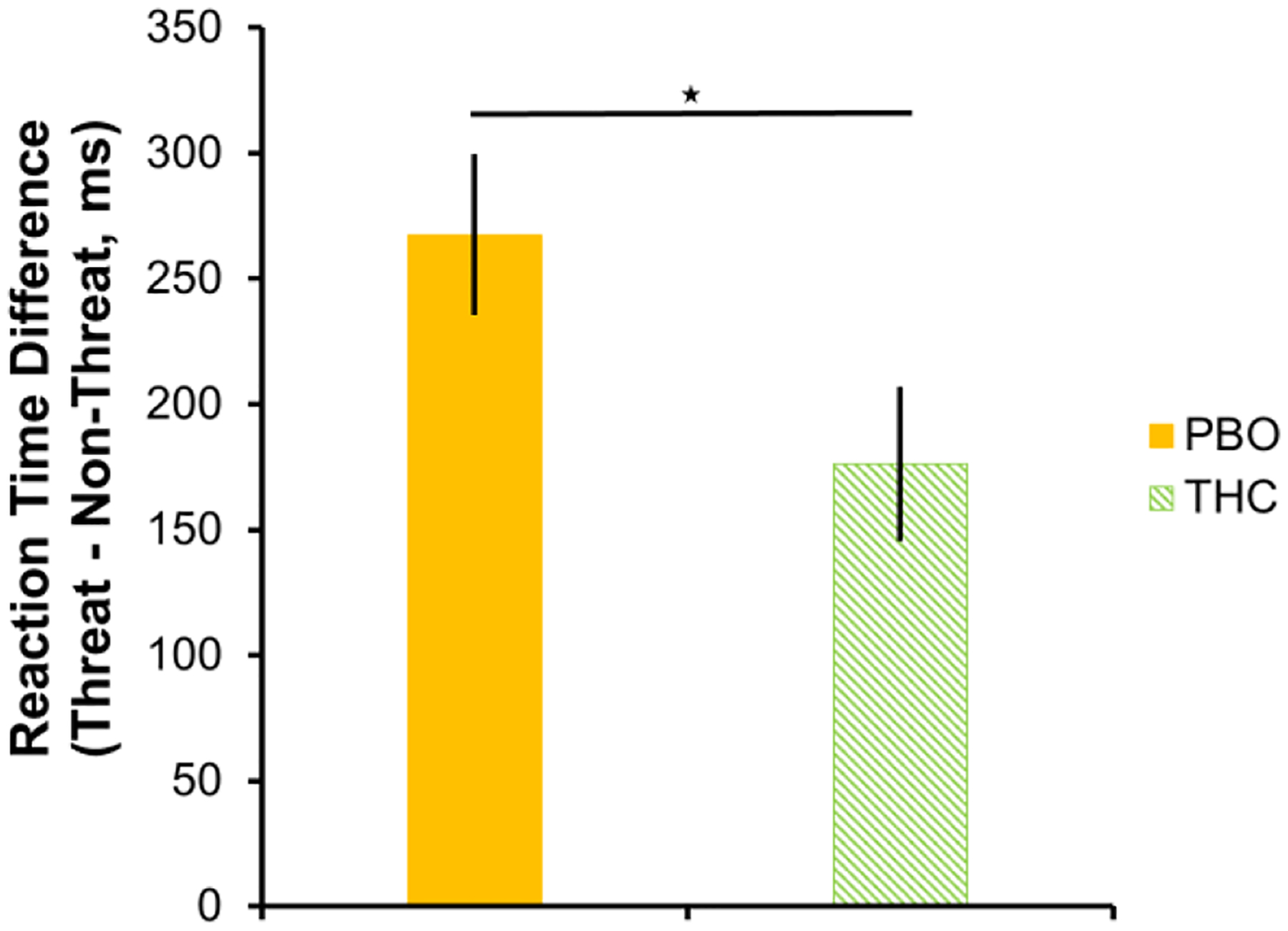
Within trauma-exposed individuals (PTSD+TEC), relative to PBO, THC administration is associated with less slowing of response to threatening vs non-threatening faces. Slowing in reaction time to threatening faces was calculated by subtracting reaction time to non-threatening faces from threatening faces, such that higher values indicate a slower response. Star indicates a significant between-group difference. Error bars represent standard error of the mean. PBO = placebo; THC = Δ9-tetrahydrocannabidiol.
Discussion
We assessed the effects of an acute low dose of oral THC on threat-related corticolimbic activity and coupling in a preliminary study of Criterion A trauma-naïve and trauma-exposed adults, both with and without PTSD. Consistent with previous findings in healthy adults, we found that, within the PTSD group, THC attenuated amygdala activation, increased mPFC/rACC activation, and increased corticolimbic functional connectivity to threat compared to PBO (Phan et al. 2008; Gorka et al. 2015).
The amygdala is a region with a high density of CB1Rs and responds to threatening and other biologically-relevant stimuli in the environment (Gläscher and Adolphs 2003; Goossens et al. 2009). Notably, we found that the modulatory effects of THC were localized to the BL, SF, and, in PTSD specifically, the AStr subdivisions of the amygdala. Amygdala subregions have distinct circuitry and specialized roles in emotion and threat-related processing (Hurlemann et al. 2008; Goossens et al. 2009; Kamprath et al. 2011; Boll et al. 2011). For instance, the BL integrates sensory information regarding threats and relays this information to the CM, which modulates physiological responses associated with fear and anxiety via dense interconnections with hypothalamic and midbrain regions (LeDoux 2000; Robinson et al. 2010; Pessoa 2011; Bzdok et al. 2013). Like the BL, the AStr subdivision also receives sensory information regarding threats and is interconnected with the BL and CM (Shammah-Lagnado et al. 1999; Wang et al. 2002). Indeed, rodent studies suggest that connections between the lateral amygdala and AStr subdivision are responsible for fast and accurate transmission of information that is critical for initial emotional reactivity (i.e., reflex; Wang et al. 2002). In contrast, information flow from the lateral amygdala to the BL, which is comparatively slower and less efficient, has been suggested to be important in facilitating signal integration and learning (Wang et al. 2002). The CM subdivision is involved in linking contextual information to emotional facial expressions in order to decipher the significance of the faces for the observer (Boll et al. 2011). Specifically, CM activation is greater in response to angry faces when the observer is told the anger is directed at him or her, than when the observer is told that the anger is directed at another person (Boll et al. 2011). Similarly, the SF is also involved in processing socially relevant stimuli, such as facial expressions (Hurlemann et al. 2008; Goossens et al. 2009; Eickhoff et al. 2011; Bzdok et al. 2013). Given that the administered task involved social threat, it is not surprising that we observed modulatory effects on BL, SF, and AStr amygdala subdivisions since they are largely involved in processing the socioemotional aspects of the stimuli.
Whereas THC blunted threat-related amygdala reactivity in the PTSD group, THC increased activation in the mPFC/rACC - a key region in emotion regulation, attentional control, and conflict monitoring (Etkin et al. 2011). A modulatory effect of THC on mPFC reactivity is consistent with the reported dense expression of CB1Rs in frontal regions (Tsou et al. 1998). In addition, increased amygdala-mPFC/rACC coupling has been reported in healthy individuals during threat processing following administration of THC (Gorka et al. 2015). Similarly, we found that THC (vs. PBO) increased mPFC/rACC-amygdala functional connectivity in the PTSD group, which is consistent with the known top-down control of the mPFC/rACC over amygdala reactivity (Quirk et al. 2003; Quirk and Beer 2006; Yizhar and Klavir 2018). For instance, mPFC/rACC activity increases while amygdala activity decreases during down-regulation of negative affect, highlighting the oppositional role of these structures (Urry 2006; Ochsner et al. 2012; Kohn et al. 2014; Buhle et al. 2014). Activation of CB1Rs in prefrontal brain regions terminates stress and threat-related behavioral responses, suggesting a mechanism by which prefrontal brain regions exert top-down inhibitory control of the amygdala (Hill et al. 2011).
Our behavioral analyses showed, overall, that accuracy was reduced and reaction times slowed when processing threatening faces relative to non-threatening faces or shapes. Interestingly, in trauma-exposed individuals alone, THC administration reduced the observed slowing of response to threatening faces vs. non-threatening faces or shapes. This finding may reflect a normalization of behavioral responses to threat that accompanies the observed THC-related modulation of underlying corticolimbic circuitry.
Limitations of this study warrant mention. First, although the fMRI session and task were approximately timed to occur during peak blood levels of THC and its metabolites (120 minutes following ingestion) we did not collect blood samples to confirm that THC and its metabolites were at peak levels at the time of scanning. However, we did collect subjective drug effect ratings from participants across several time points following capsule administration to estimate peak subjective THC effects (Online Resource 1). Relative to PBO, those who received THC reported significantly higher ratings on “feeling a drug effect” and “feeling high” at 120 to 240-minutes post-drug administration, the latter time point being after the task was completed. Second, we examined acute effects of a single, low-dose of THC on corticolimbic reactivity and behavior. There is differential sensitivity to the effects of THC and other cannabinoid modulators between individuals (Henquet et al. 2006; Bhattacharyya et al. 2012; Atakan et al. 2013) that cannot be controlled for by using the same dose across individuals. However, we were interested in replicating and extending previous studies, which used the same acute dose of THC in healthy participants (Phan et al. 2008; Gorka et al. 2015). Third, we included individuals with a wide history of self-reported cannabis use—from denying any use to reporting over 100 uses during their lifetime—rather than limiting our sample to non-users, as previous cannabis use could affect the ECB system (Colizzi et al. 2018; Colizzi and Bhattacharyya 2018). However, we re-ran all analyses in only participants that had reported no prior use of cannabis and our findings did not change. Self-reported prior cannabis use also did not affect self-reported anxiety and drug-effect ratings in the present study. Additionally, ECB changes associated with cannabis use can reverse rapidly starting within two days following abstinence (D’Souza et al. 2016). In our study, all participants had to screen negative on a urine drug test in order to participate, which means that if they did use cannabis, they would have had to abstain using anywhere from 3–30+ days prior to participating in the study (Verstraete 2004).
Together, these preliminary findings add to the growing body of literature suggesting that pharmacologic modulation of the ECB may be a promising approach for addressing corticolimbic dysfunction, which is a core feature of PTSD and stress-related psychopathologies.
Supplementary Material
Acknowledgments:
The research reported was supported by National Institute of Mental Health (MH101123) awarded to CAR. Special thanks to the research participants who generously donated their time to participate in this study and to the Wayne State University MR Research Facility and Dr. Richard Genik for support in imaging data collection.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflict of Interest: All authors declare no conflicts of interest.
References:
- American Psychiatric Association (2013) Diagnostic and statistical manual of mental disorders, Fifth Ed American Psychiatric Association, Arlington, VA [Google Scholar]
- Amunts K, Kedo O, Kindler M, et al. (2005) Cytoarchitectonic mapping of the human amygdala, hippocampal region and entorhinal cortex: intersubject variability and probability maps. Anat Embryol (Berl) 210:343–352. doi: 10.1007/s00429-005-0025-5 [DOI] [PubMed] [Google Scholar]
- Andrewes DG, Jenkins LM (2019) The role of the amygdala and the ventromedial prefrontal cortex in emotional regulation: implications for post-traumatic stress disorder. Neuropsychol Rev 29:220–243. doi: 10.1007/s11065-019-09398-4 [DOI] [PubMed] [Google Scholar]
- Atakan Z, Bhattacharyya S, Allen P, et al. (2013) Cannabis affects people differently: intersubject variation in the psychotogenic effects of Δ 9 -tetrahydrocannabinol: a functional magnetic resonance imaging study with healthy volunteers. Psychol Med 43:1255–1267. doi: 10.1017/S0033291712001924 [DOI] [PubMed] [Google Scholar]
- Badura-Brack A, McDermott TJ, Heinrichs-Graham E, et al. (2018) Veterans with PTSD demonstrate amygdala hyperactivity while viewing threatening faces: a MEG study. Biol Psychol 132:228–232. doi: 10.1016/j.biopsycho.2018.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya S, Atakan Z, Martin-Santos R, et al. (2012) Preliminary report of biological basis of sensitivity to the effects of cannabis on psychosis: AKT1 and DAT1 genotype modulates the effects of δ−9-tetrahydrocannabinol on midbrain and striatal function. Mol Psychiatry 17:1152–1155. doi: 10.1038/mp.2011.187 [DOI] [PubMed] [Google Scholar]
- Bhattacharyya S, Atakan Z, Martin-Santos R, et al. (2015) Impairment of inhibitory control processing related to acute psychotomimetic effects of cannabis. Eur Neuropsychopharmacol 25:26–37. doi: 10.1016/j.euroneuro.2014.11.018 [DOI] [PubMed] [Google Scholar]
- Bhattacharyya S, Egerton A, Kim E, et al. (2017) Acute induction of anxiety in humans by delta-9-tetrahydrocannabinol related to amygdalar cannabinoid-1 (CB1) receptors. Sci Rep. doi: 10.1038/s41598-017-14203-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya S, Morrison PD, Fusar-Poli P, et al. (2010) Opposite effects of δ−9-tetrahydrocannabinol and cannabidiol on human brain function and psychopathology. Neuropsychopharmacology 35:764–774. doi: 10.1038/npp.2009.184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitencourt RM, Pamplona FA, Takahashi RN (2008) Facilitation of contextual fear memory extinction and anti-anxiogenic effects of AM404 and cannabidiol in conditioned rats. Eur Neuropsychopharmacol 18:849–859. doi: 10.1016/j.euroneuro.2008.07.001 [DOI] [PubMed] [Google Scholar]
- Boden MT, Babson KA, Vujanovic AA, et al. (2013) Posttraumatic stress disorder and cannabis use characteristics among military veterans with cannabis dependence. Am J Addict 22:277–284. doi: 10.1111/j.1521-0391.2012.12018.x [DOI] [PubMed] [Google Scholar]
- Boll S, Gamer M, Kalisch R, Büchel C (2011) Processing of facial expressions and their significance for the observer in subregions of the human amygdala. Neuroimage 56:299–306. doi: 10.1016/j.neuroimage.2011.02.021 [DOI] [PubMed] [Google Scholar]
- Bolton JM, Robinson J, Sareen J (2009) Self-medication of mood disorders with alcohol and drugs in the National Epidemiologic Survey on Alcohol and Related Conditions. J Affect Disord 115:367–375. doi: 10.1016/j.jad.2008.10.003 [DOI] [PubMed] [Google Scholar]
- Bowles DW (2012) Persons registered for medical marijuana in the United States. J Palliat Med 15:9–11. doi: 10.1089/jpm.2011.0356 [DOI] [PubMed] [Google Scholar]
- Bryant RA, Kemp AH, Felmingham KL, et al. (2008) Enhanced amygdala and medial prefrontal activation during nonconscious processing of fear in posttraumatic stress disorder: An fMRI study. Hum Brain Mapp 29:517–523. doi: 10.1002/hbm.20415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhle JT, Silvers JA, Wager TD, et al. (2014) Cognitive reappraisal of emotion: a meta-analysis of human neuroimaging studies. Cereb Cortex 24:2981–2990. doi: 10.1093/cercor/bht154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bujarski SJ, Feldner MT, Lewis SF, et al. (2012) Marijuana use among traumatic event-exposed adolescents: posttraumatic stress symptom frequency predicts coping motivations for use. Addict Behav 37:53–59. doi: 10.1016/j.addbeh.2011.08.009 [DOI] [PubMed] [Google Scholar]
- Bzdok D, Laird AR, Zilles K, et al. (2013) An investigation of the structural, connectional, and functional subspecialization in the human amygdala. Hum Brain Mapp 34:3247–3266. doi: 10.1002/hbm.22138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chhatwal JP, Davis M, Maguschak KA, Ressler KJ (2005) Enhancing cannabinoid neurotransmission augments the extinction of conditioned fear. Neuropsychopharmacology 30:516–524. doi: 10.1038/sj.npp.1300655 [DOI] [PubMed] [Google Scholar]
- Colizzi M, Bhattacharyya S (2018) Cannabis use and the development of tolerance: a systematic review of human evidence. Neurosci Biobehav Rev 93:1–25. doi: 10.1016/j.neubiorev.2018.07.014 [DOI] [PubMed] [Google Scholar]
- Colizzi M, McGuire P, Giampietro V, et al. (2018) Previous cannabis exposure modulates the acute effects of delta-9-tetrahydrocannabinol on attentional salience and fear processing. Exp Clin Psychopharmacol. doi: 10.1037/pha0000221 [DOI] [PubMed] [Google Scholar]
- Crippa JA, Seal M, Martin-Santos R, et al. (2009) Distinct Effects of $Δ$9-Tetrahydrocannabinol and Cannabidiol on Neural Activation During Emotional Processing. Arch Gen Psychiatry 66:95. doi: 10.1001/archgenpsychiatry.2008.519 [DOI] [PubMed] [Google Scholar]
- D’Souza DC, Cortes-Briones JA, Ranganathan M, et al. (2016) Rapid changes in cannabinoid 1 receptor availability in cannabis-dependent male subjects after abstinence from cannabis. Biol Psychiatry Cogn Neurosci Neuroimaging 1:60–67. doi: 10.1016/j.bpsc.2015.09.008 [DOI] [PubMed] [Google Scholar]
- D’Souza DC, Perry E, MacDougall L, et al. (2004) The psychotomimetic effects of intravenous delta-9-tetrahydrocannabinol in healthy individuals: Implications for psychosis. Neuropsychopharmacology 29:1558–1572. doi: 10.1038/sj.npp.1300496 [DOI] [PubMed] [Google Scholar]
- Driessen M, Beblo T, Mertens M, et al. (2004) Posttraumatic stress disorder and fMRI activation patterns of traumatic memory in patients with borderline personality disorder. Biol Psychiatry 55:603–611. doi: 10.1016/j.biopsych.2003.08.018 [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Bzdok D, Laird AR, et al. (2011) Co-activation patterns distinguish cortical modules, their connectivity and functional differentiation. Neuroimage 57:938–949. doi: 10.1016/j.neuroimage.2011.05.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Stephan KE, Mohlberg H, et al. (2005) A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage 25:1325–1335. doi: 10.1016/j.neuroimage.2004.12.034 [DOI] [PubMed] [Google Scholar]
- Etkin A, Egner T, Kalisch R (2011) Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn Sci 15:85–93. doi: 10.1016/j.tics.2010.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Wager TD (2007) Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry 164:1476–1488. doi: 10.1176/appi.ajp.2007.07030504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenster RJ, Lebois LAM, Ressler KJ, Suh J (2018) Brain circuit dysfunction in post-traumatic stress disorder: from mouse to man. Nat Rev Neurosci 19:535–551. doi: 10.1038/s41583-018-0039-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Luria R (1973) Reliability, validity, and clinical application of the visual analogue mood scale. Psychol Med 3:479–486. doi: 10.1017/S0033291700054283 [DOI] [PubMed] [Google Scholar]
- Friston KJ, Fletcher P, Josephs O, et al. (1998) Event-related fMRI: characterizing differential responses. Neuroimage 7:30–40. doi: 10.1006/nimg.1997.0306 [DOI] [PubMed] [Google Scholar]
- Garfinkel SN, Liberzon I (2009) Neurobiology of PTSD: a review of neuroimaging findings. Psychiatr Ann 39:370–381. doi: 10.3928/00485713-20090527-01 [DOI] [Google Scholar]
- Genn RF, Tucci S, Marco EM, et al. (2004) Unconditioned and conditioned anxiogenic effects of the cannabinoid receptor agonist CP 55,940 in the social interaction test. Pharmacol Biochem Behav. doi: 10.1016/j.pbb.2003.12.019 [DOI] [PubMed] [Google Scholar]
- Gläscher J, Adolphs R (2003) Processing of the arousal of subliminal and supraliminal emotional stimuli by the human amygdala. J Neurosci 23:10274–10282. doi: 10.1523/JNEUROSCI.23-32-10274.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goossens L, Kukolja J, Onur OAA, et al. (2009) Selective processing of social stimuli in the superficial amygdala. Hum Brain Mapp 30:3332–3338. doi: 10.1002/hbm.20755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorka SM, Fitzgerald DA, de Wit H, Phan KL (2015) Cannabinoid modulation of amygdala subregion functional connectivity to social signals of threat. Int J Neuropsychopharmacol 18:pyu104–pyu104. doi: 10.1093/ijnp/pyu104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorka SM, Phan KLL, Lyons M, et al. (2016) Cannabinoid modulation of frontolimbic activation and connectivity during volitional regulation of negative affect. Neuropsychopharmacology 41:1888–1896. doi: 10.1038/npp.2015.359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur RC, Sara R, Hagendoorn M, et al. (2002) A method for obtaining 3-dimensional facial expressions and its standardization for use in neurocognitive studies. J Neurosci Methods 115:137–43 [DOI] [PubMed] [Google Scholar]
- Haller J, Bakos N, Szirmay M, et al. (2002) The effects of genetic and pharmacological blockade of the CB1 cannabinoid receptor on anxiety. Eur J Neurosci 16:1395–1398. doi: 10.1046/j.1460-9568.2002.02192.x [DOI] [PubMed] [Google Scholar]
- Hariri AR, Tessitore A, Mattay VS, et al. (2002) The amygdala response to emotional stimuli: a comparison of faces and scenes. Neuroimage 17:317–323. doi: 10.1006/nimg.2002.1179 [DOI] [PubMed] [Google Scholar]
- Henquet C, Rosa A, Krabbendam L, et al. (2006) An experimental study of catechol-O-methyltransferase Val158Met moderation of Δ−9-tetrahydrocannabinol-induced effects on psychosis and cognition. Neuropsychopharmacology 31:2748–2757. doi: 10.1038/sj.npp.1301197 [DOI] [PubMed] [Google Scholar]
- Hill MN, McLaughlin RJ, Pan B, et al. (2011) Recruitment of prefrontal cortical endocannabinoid signaling by glucocorticoids contributes to termination of the stress response. J Neurosci 31:10506–10515. doi: 10.1523/JNEUROSCI.0496-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurlemann R, Rehme AK, Diessel M, et al. (2008) Segregating intra-amygdalar responses to dynamic facial emotion with cytoarchitectonic maximum probability maps. J Neurosci Methods 172:13–20. doi: 10.1016/j.jneumeth.2008.04.004 [DOI] [PubMed] [Google Scholar]
- Kamprath K, Romo-Parra H, Häring M, et al. (2011) Short-term adaptation of conditioned fear responses through endocannabinoid signaling in the central amygdala. Neuropsychopharmacology 36:652–663. doi: 10.1038/npp.2010.196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killgore WDS, Britton JC, Schwab ZJ, et al. (2014) Cortico-limbic responses to masked affective faces across PTSD, panic disorder, and specific phobia. Depress Anxiety 31:150–159. doi: 10.1002/da.22156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohn N, Eickhoff SB, Scheller M, et al. (2014) Neural network of cognitive emotion regulation — an ALE meta-analysis and MACM analysis. Neuroimage 87:345–355. doi: 10.1016/j.neuroimage.2013.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE (2000) Emotion circuits in the brain. Annu Rev Neurosci 23:155–184. doi: 10.1146/annurev.neuro.23.1.155 [DOI] [PubMed] [Google Scholar]
- Liberzon I (2006) Neuroimaging Studies of Emotional Responses in PTSD. Ann N Y Acad Sci 1071:87–109. doi: 10.1196/annals.1364.009 [DOI] [PubMed] [Google Scholar]
- Liberzon I, Taylor SF, Amdur R, et al. (1999) Brain activation in PTSD in response to trauma-related stimuli. Biol Psychiatry 45:817–826. doi: 10.1016/S0006-3223(98)00246-7 [DOI] [PubMed] [Google Scholar]
- Lin H-C, Mao S-C, Su C-L, Gean P-W (2009) The role of prefrontal cortex CB1 receptors in the modulation of fear memory. Cereb Cortex 19:165–175. doi: 10.1093/cercor/bhn075 [DOI] [PubMed] [Google Scholar]
- Marsicano G, Lutz B (1999) Expression of the cannabinoid receptor CB1 in distinct neuronal subpopulations in the adult mouse forebrain. Eur J Neurosci 11:4213–4225. doi: 10.1046/j.1460-9568.1999.00847.x [DOI] [PubMed] [Google Scholar]
- Marsicano G, Wotjak CT, Azad SC, et al. (2002) The endogenous cannabinoid system controls extinction of aversive memories. Nature 418:530–534. doi: 10.1038/nature00839 [DOI] [PubMed] [Google Scholar]
- McLaren DG, Ries ML, Xu G, Johnson SC (2012) A generalized form of context-dependent psychophysiological interactions (gPPI): a comparison to standard approaches. Neuroimage 61:1277–1286. doi: 10.1016/j.neuroimage.2012.03.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumeister P, Feldker K, Heitmann CY, et al. (2017) Interpersonal violence in posttraumatic women: brain networks triggered by trauma-related pictures. Soc Cogn Affect Neurosci 12:555–568. doi: 10.1093/scan/nsw165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neil ME, Nugent SM, Morasco BJ, et al. (2017) Benefits and harms of plant-based cannabis for posttraumatic stress disorder. Ann Intern Med 167:332. doi: 10.7326/M17-0477 [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Silvers JA, Buhle JT (2012) Functional imaging studies of emotion regulation: a synthetic review and evolving model of the cognitive control of emotion. Ann N Y Acad Sci 1251:E1–E24. doi: 10.1111/j.1749-6632.2012.06751.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pamplona FA, Takahashi RN (2006) WIN 55212–2 impairs contextual fear conditioning through the activation of CB1 cannabinoid receptors. Neurosci Lett 397:88–92. doi: 10.1016/j.neulet.2005.12.026 [DOI] [PubMed] [Google Scholar]
- Patel S, Hill MN, Cheer JF, et al. (2017) The endocannabinoid system as a target for novel anxiolytic drugs. Neurosci Biobehav Rev 76:56–66. doi: 10.1016/j.neubiorev.2016.12.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S, Roelke CT, Rademacher DJ, Hillard CJ (2005) Inhibition of restraint stress-induced neural and behavioural activation by endogenous cannabinoid signalling. Eur J Neurosci 21:1057–1069. doi: 10.1111/j.1460-9568.2005.03916.x [DOI] [PubMed] [Google Scholar]
- Paulus MP, Feinstein JS, Castillo G, et al. (2005) Dose-dependent decrease of activation in bilateral amygdala and insula by lorazepam during emotion processing. Arch Gen Psychiatry 62:282. doi: 10.1001/archpsyc.62.3.282 [DOI] [PubMed] [Google Scholar]
- Pessoa L (2011) Reprint of: Emotion and cognition and the amygdala: From “what is it?” to “what’s to be done?” Neuropsychologia 49:681–694. doi: 10.1016/j.neuropsychologia.2011.02.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan KL, Angstadt M, Golden J, et al. (2008) Cannabinoid modulation of amygdala reactivity to social signals of threat in humans. J Neurosci 28:2313–2319. doi: 10.1523/JNEUROSCI.5603-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pissiota A, Frans Ö, Fernandez M, et al. (2002) Neurofunctional correlates of posttraumatic stress disorder: a PET symptom provocation study. Eur Arch Psychiatry Clin Neurosci 252:68–75. doi: 10.1007/s004060200014 [DOI] [PubMed] [Google Scholar]
- Pitman RK, Shin LM, Rauch SL (2001) Investigating the pathogenesis of posttraumatic stress disorder with neuroimaging. J Clin Psychiatry 62:47–54 [PubMed] [Google Scholar]
- Quirk GJ, Beer JS (2006) Prefrontal involvement in the regulation of emotion: convergence of rat and human studies. Curr Opin Neurobiol 16:723–727. doi: 10.1016/j.conb.2006.07.004 [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Likhtik E, Pelletier JG, Paré D (2003) Stimulation of medial prefrontal cortex decreases the responsiveness of central amygdala output neurons. J Neurosci 23:8800–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinak CA, Angstadt M, Lyons M, et al. (2014) Cannabinoid modulation of prefrontal–limbic activation during fear extinction learning and recall in humans. Neurobiol Learn Mem 113:125–134. doi: 10.1016/j.nlm.2013.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch SL, Whalen PJ, Shin LM, et al. (2000) Exaggerated amygdala response to masked facial stimuli in posttraumatic stress disorder: a functional MRI study. Biol Psychiatry 47:769–776. doi: 10.1016/S0006-3223(00)00828-3 [DOI] [PubMed] [Google Scholar]
- Robinson JL, Laird AR, Glahn DC, et al. (2010) Metaanalytic connectivity modeling: delineating the functional connectivity of the human amygdala. Hum Brain Mapp 31:173–184. doi: 10.1002/hbm.20854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruehle S, Rey AA, Remmers F, Lutz B (2012) The endocannabinoid system in anxiety, fear memory and habituation. J Psychopharmacol 26:23–39. doi: 10.1177/0269881111408958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shammah-Lagnado SJ, Alheid GF, Heimer L (1999) Afferent connections of the interstitial nucleus of the posterior limb of the anterior commissure and adjacent amygdalostriatal transition area in the rat. Neuroscience. doi: 10.1016/S0306-4522(99)90280-4 [DOI] [PubMed] [Google Scholar]
- Shin LM, Kosslyn SM, McNally RJ, et al. (1997) Visual imagery and perception in posttraumatic stress disorder. Arch Gen Psychiatry 54:233. doi: 10.1001/archpsyc.1997.01830150057010 [DOI] [PubMed] [Google Scholar]
- Shin LM, Orr SP, Carson MA, et al. (2004) Regional cerebral blood flow in the amygdala and medial prefrontal cortex during traumatic imagery in male and female Vietnam veterans with PTSD. Arch Gen Psychiatry 61:168. doi: 10.1001/archpsyc.61.2.168 [DOI] [PubMed] [Google Scholar]
- Shin LM, Wright CI, Cannistraro PA, et al. (2005) A functional magnetic resonance imaging study of amygdala and medial prefrontal cortex responses to overtly presented fearful faces in posttraumatic stress disorder. Arch Gen Psychiatry 62:273. doi: 10.1001/archpsyc.62.3.273 [DOI] [PubMed] [Google Scholar]
- Simmons AN, Matthews SC, Strigo IA, et al. (2011) Altered amygdala activation during face processing in Iraqi and Afghanistani war veterans. Biol Mood Anxiety Disord 1:6. doi: 10.1186/2045-5380-1-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger CD (1983) State-Trait Anxiety Inventory (STAI). Mind Gard 94061:261–3500. doi: 10.1002/9780470479216.corpsy0943 [DOI] [Google Scholar]
- Stevens JS, Jovanovic T, Fani N, et al. (2013) Disrupted amygdala-prefrontal functional connectivity in civilian women with posttraumatic stress disorder. J Psychiatr Res 47:1469–1478. doi: 10.1016/j.jpsychires.2013.05.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabbert K, Stark R, Kirsch P, Vaitl D (2005) Hemodynamic responses of the amygdala, the orbitofrontal cortex and the visual cortex during a fear conditioning paradigm. Int J Psychophysiol 57:15–23. doi: 10.1016/j.ijpsycho.2005.01.007 [DOI] [PubMed] [Google Scholar]
- Trezza V, Campolongo P (2013) The endocannabinoid system as a possible target to treat both the cognitive and emotional features of post-traumatic stress disorder (PTSD). Front Behav Neurosci 7:1–5. doi: 10.3389/fnbeh.2013.00100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsou K, Brown S, Sañudo-Peña M., et al. (1998) Immunohistochemical distribution of cannabinoid CB1 receptors in the rat central nervous system. Neuroscience 83:393–411. doi: 10.1016/S0306-4522(97)00436-3 [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, et al. (2002) Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. doi: 10.1006/nimg.2001.0978 [DOI] [PubMed] [Google Scholar]
- Urry HL (2006) Amygdala and ventromedial prefrontal cortex are inversely coupled during regulation of negative affect and predict the diurnal pattern of cortisol secretion among older adults. J Neurosci 26:4415–4425. doi: 10.1523/JNEUROSCI.3215-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermetten E, Schmahl C, Southwick SM, Bremner JD (2007) Positron tomographic emission study of olfactory induced emotional recall in veterans with and without combat-related posttraumatic stress disorder. Psychopharmacol Bull 40:8–30 [PMC free article] [PubMed] [Google Scholar]
- Verstraete AG (2004) Detection times of drugs of abuse in blood, urine, and oral fluid. Ther Drug Monit 26:200–5 [DOI] [PubMed] [Google Scholar]
- Viveros M, Marco E, File S (2005a) Endocannabinoid system and stress and anxiety responses. Pharmacol Biochem Behav 81:331–342. doi: 10.1016/j.pbb.2005.01.029 [DOI] [PubMed] [Google Scholar]
- Viveros MP, Llorente R, Moreno E, Marco EM (2005b) Behavioural and neuroendocrine effects of cannabinoids in critical developmental periods. Behav Pharmacol 16:353–362. doi: 10.1097/00008877-200509000-00007 [DOI] [PubMed] [Google Scholar]
- Wachtel S, ElSohly M, Ross S, et al. (2002) Comparison of the subjective effects of Δ 9 - tetrahydrocannabinol and marijuana in humans. Psychopharmacology (Berl) 161:331–339. doi: 10.1007/s00213-002-1033-2 [DOI] [PubMed] [Google Scholar]
- Walsh Z, Gonzalez R, Crosby K, et al. (2017) Medical cannabis and mental health: a guided systematic review. Clin Psychol Rev 51:15–29. doi: 10.1016/j.cpr.2016.10.002 [DOI] [PubMed] [Google Scholar]
- Wang C, Kang-Park M-H, Wilson WA, Moore SD (2002) Properties of the Pathways From the Lateral Amygdal Nucleus to Basolateral Nucleus and Amygdalostriatal Transition Area. J Neurophysiol 87:2593–2601. doi: 10.1152/jn.2002.87.5.2593 [DOI] [PubMed] [Google Scholar]
- Weathers F, Blake D, Schnurr P, et al. (2013a) The Life Events Checklist for DSM-5 (LEC-5). Natl Cent PTSD 5:Instrument available from the National Center for. doi: 10.1177/1073191104269954 [DOI] [Google Scholar]
- Weathers FW, Blake DD, Schnurr PP, et al. (2013b) The Clinician-Administered PTSD Scale for DSM-5 (CAPS-5). Available from www.ptsd.va.gov [DOI] [PMC free article] [PubMed]
- Williams LM, Kemp AH, Felmingham K, et al. (2006) Trauma modulates amygdala and medial prefrontal responses to consciously attended fear. Neuroimage 29:347–357. doi: 10.1016/j.neuroimage.2005.03.047 [DOI] [PubMed] [Google Scholar]
- Wolf RC, Herringa RJ (2016) Prefrontal–amygdala dysregulation to threat in pediatric posttraumatic stress disorder. Neuropsychopharmacology 41:822–831. doi: 10.1038/npp.2015.209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yizhar O, Klavir O (2018) Reciprocal amygdala–prefrontal interactions in learning. Curr Opin Neurobiol 52:149–155. doi: 10.1016/j.conb.2018.06.006 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


