Abstract
Background
In the context of a randomized controlled trial evaluating the efficacy of augmenting fluoxetine treatment in young adults with major depressive disorder (MDD) using a modified repeated partial sleep deprivation protocol contrasting two weeks of restricted time in bed (i.e., 6h TIB) to no time in bed restriction (i.e., 8h TIB) the study examines whether sleep duration and the timing of repeated partial sleep deprivation predicts patient reported affect ratings.
Participants
Participants included 58 young adults with DSM-IV-diagnosed MDD.
Methods
Daily ratings of affect and sleep were collected during the first two weeks of initiating fluoxetine treatment, yielding 630 person-days. Actigraphy monitoring was employed to assess compliance with time in bed condition.
Results
Negative affect ratings and positivity ratios in the morning were more improved among participants assigned to the 6h TIB condition compared to the 8h TIB group. Participants whose bedtime was delayed by 2 hours nightly demonstrated the most significant improvement in negative affect and positivity ratio during the first two weeks of fluoxetine therapy. Moreover, the trajectory of morning negative affect ratings in the first two weeks was predictive of remission after 4 weeks of fluoxetine therapy.
Conclusions
These findings suggest that monitoring changes in daily affect may be a valuable marker of early treatment response in young adults with MDD.
TRIAL REGISTRATION
ClinicalTrials.gov identifier: NCT01545843
Keywords: sleep, affect, adult, depression, ecological momentary assessment, actigraphy
Evidence-based pharmacotherapy for major depressive disorder (MDD) is readily available, commonly employed and associated with a 60% improvement in symptoms during the first two weeks of therapy (Posternak & Zimmerman, 2005). This early improvement is positively correlated with long-term treatment outcomes (Henkel et al., 2009; Stassen, Angst, Hell, Scharfetter, & Szegedi, 2007; Szegedi et al., 2009; Tadic et al., 2010). However, some patients experience delays in response time or do not respond, with failure rates as high as 30–40% (Bech et al., 2000; Nierenberg et al., 2000; Trivedi et al., 2006). Thus, identifying characteristics that are connected to treatment response early in antidepressant treatment is of clinical importance.
Important aspects of emotional functioning associated with treatment response may be overlooked during the initial stages of antidepressant treatment when relying on periodic symptom severity assessments (Bagby, Ryder, Schuller, & Marshall, 2004). Experience sampling methods (ESM), by contrast, may provide a window into the dynamic mechanisms underlying treatment response since these methods prospectively sample an individual’s experiences (e.g., affect, behavior) overtime (Portell, Anguera, Hernandez-Mendo, & Jonsson, 2015). They also have the advantage of reducing recall bias in depressed patients (Hamilton & Gotlib, 2008) relative to weekly or bi-monthly assessments. Using ESM to elucidate underlying affective states may aid treatment strategies and clinical decision making by identifying factors that impact antidepressant treatment response over time.
Previous studies using ESM characterize mood by assessing two underlying affective states- positive affect (PA) and negative affect (NA) - that represent separate orthogonal dimensions and are negatively correlated within a person (Clark, Watson, & Leeka, 1989). Within this framework, depression is characterized by a state of high NA and low PA or a low PA/NA ratio, referred to as the positivity ratio (Diener, 2000) and an indicator of emotional well-being (Fredrickson, 2001). Individuals with depression have a later peak in PA relative to healthy controls (Murray, Allen, & Trinder, 2002; Watson, Wiese, Vaidya, & Tellegen, 1999) and a distinct ∩-shaped diurnal pattern for NA, which is absent in healthy controls (Gordijn, Beersma, Bouhuys, Reinink, & Van den Hoofdakker, 1994; Murray, 2007; Peeters, Berkhof, Delespaul, Rottenberg, & Nicolson, 2006). These affective states are sensitive to antidepressant treatment, with PA increasing and NA decreasing over the course of a 6-week medication trial. Moreover, significant PA increases during the initial week of treatment have been associated with a reduction in depression symptom severity following antidepressant treatment (Geschwind et al., 2011). While previous studies suggest that NA and PA have different trajectories during depression treatment, no study has evaluated whether experimental manipulations in the early stages of antidepressant therapy impact these affective states.
In healthy adults, sleep deprivation can have adverse effects (c.f., Pilcher & Huffcutt, 1996) specially impairments in affect (Krause et al., 2017), cognitive processing (e.g., attention and working memory, cognitive control; Acheson, Richards, & de Wit, 2007; Demos et al., 2016; Krause et al., 2017), and stress reactivity (Minkel et al., 2014), but it has also been used therapeutically in patients with depression, including both total (Dallaspezia & Benedetti, 2015) and partial-night sleep deprivation (PSD), (Dallaspezia & Benedetti, 2015; Hemmeter, Seifritz, Hatzinger, Muller, & Holsboer-Trachsler, 1995; Holsboer-Trachsler & Seifritz, 2000; Holsboer-Trachsler, Wiedemann, & Holsboer, 1988).). Limited evidence from largely uncontrolled PSD studies indicates that late-PSD (i.e., restricting wakefulness to the second half of the night by moving rise time earlier, i.e., advancing rise time) is more effective than early-PSD (i.e., delaying bedtime) for depression (Sack, Duncan, Rosenthal, Mendelson, & Wehr, 1988).
In a clinical trial where we experimentally manipulated time in bed (TIB) during the initial two weeks of antidepressant treatment, we found that remission rates after 8 weeks were lower among depressed participants randomized to 6 hours’ time in bed (TIB) relative to those assigned to 8 hours’ TIB. We further found that there were no differences in mood outcomes between participants assigned to a 2-hour delay of bedtime (Late Bedtime group) vs. those assigned to a 2-hour advance of rise time (Early Rise Time) (Arnedt et al., 2016). In the current study, we extended these findings and fill an important gap in the literature by evaluating the effects of a TIB manipulation on daily affect ratings (with consideration of time of day) in the context of initiating antidepressant therapy using ESM. In addition, we evaluate whether this information might have clinical utility by assessing whether changes in affect are associated with antidepressant treatment response. The aims were to examine: (1) the effect of time in bed (i.e., 6h TIB vs. 8h TIB) on affect i.e., NA, PA and PA/NA ratio; (2) whether the dynamics of NA, PA and PA/NA ratio relate to the timing of PSD (i.e., Late Bedtime vs Early Risetime); and (3) whether the dynamics of NA, PA, and PA/NA ratio during the first two weeks are associated with treatment response at 2, 4, and 8 weeks.
Method
Participant data were obtained from a randomized controlled clinical trial (ClinicalTrials.gov identifier: NCT01545843) comparing treatment response following two weeks of 6h TIB or 8 h TIB delivered adjunctive to antidepressant therapy in outpatient adults with MDD. Study characteristics are briefly summarized here with additional details regarding study design and methods described elsewhere (Arnedt et al., 2016).
Participants
Participants were recruited through advertisements and clinical referrals and study procedures were approved by the University of Michigan Medical School Institutional Review Board. All participants provided written informed consent. To be eligible, participants had to be 18–65 years old, meet DSM-IV criteria for MDD of at least moderate severity (≥ 18 on the 17-item Hamilton Rating Scale for Depression, HAMD-17), habitually spend 7–10 hours in bed at night, and be free of antidepressants ≥2 weeks (≥4 weeks for longer-acting antidepressants). Exclusion criteria included: (1) lifetime DSM-IV diagnosis other than MDD or generalized anxiety disorder; (2) past 6-month DSM-IV diagnosis of alcohol abuse; (3) medical conditions associated with depression or that interfered with sleep; (4) sleep disorder other than insomnia; (5) use of prescription or over-the-counter remedy for sleep or depression; (6) failed fluoxetine trial within the past six months; (7) overnight shift work; (8) pregnancy, breastfeeding, or inadequate contraception in women of childbearing potential; (9) known contraindication to fluoxetine; and (10) clinical laboratory values outside normal limits. Eight participants were excluded for suspicion of either sleep-related breathing disorder (6 participants) or periodic limb movement disorder (2 participants), based on International Classification of Sleep Disorders-2 criteria during in-laboratory screening with polysomnography that followed standard procedures (Iber, Ancoli-Israel, Chesson, & Quan, 2007).
Study Design and Procedures
In this randomized, controlled parallel trial design, participants received open-label fluoxetine 20–40 mg for eight weeks and were randomized (1:1:1) to the following TIB conditions: 8h TIB (e.g., 23:00 to 07:00) or one of two 6h TIB schedules: Late Bedtime (a two-hour delay of bedtime, e.g., 01:00 to 07:00); or Early Risetime (a two-hour advance of rise time, e.g., 23:00 to 05:00) for the first two weeks of the study. Following a 3-night sleep laboratory assessment, participants maintained their assigned TIB schedule for 14 (SD = 1.6) nights and took fluoxetine each morning. At-home compliance with the assigned TIB condition was evaluated with wrist-worn actigraphy (Actiwatch-2™, Philips Respironics, Murrysville, PA).
Outcome Measures
The Positive and Negative Affect Scale (PANAS; Watson, Clark, & Tellegen, 1988) was the primary outcome measure with ratings completed twice daily, in the morning and evening, for the first 2 weeks of the study. The PANAS is comprised of two 10-item scales measuring positive affect (PA) and negative affect (NA). Scales consist of adjectives rated on a 5-point scale ranging from 1 (very slightly or not at all) to 5 (extremely). Scores range from 10 – 50 with higher scores indicating a greater level of positive (sample M = 15.2, SD = 5.2; range = 10 – 40) or negative affect (sample M = 14.8, SD = 5.6; range = 10 – 41). In the present study, the PANAS had Cronbach’s alphas of .91and .85 for the PA and NA subscales, respectively. To explore the relative balance between positive and negative affect, PA/NA ratios were computed by dividing PA by NA scores with a higher PA/NA ratio indicting greater well-being (sample M = 1.1, SD = 0.4; range 0.3 – 3.0).
Self-reported depression severity was assessed using the 16-item Quick Inventory of Depressive Symptomatology-Self-Report (QIDS-SR; Rush et al., 2003). Total scores range from 0 to 27 with higher scores indicating greater depression severity and scores ≤ 5 indicate remission (sample M = 13.1, SD = 3.9; range = 4 – 21). Ratings were completed at baseline and weeks 2, 4, and 8 of treatment.
Participants recorded their daily sleep and wake patterns each morning for the first 2 weeks of the study by completing a brief online sleep/wake diary via SurveyMonkey. The primary dependent variables derived from the diaries included: total sleep time (TST) and sleep efficiency (SE; TST/TIB*100). Sleep diaries have been shown to be a reliable estimate of sleep parameters and are considered the gold standard measure of subjective sleep (Buysse, Ancoli-Israel, Edinger, Lichstein, & Morin, 2006).
Statistical Analyses
All analyses were performed using Stata 13 (StataCorp LP, College Station, TX). Data are reported as mean (standard deviation) or n (%) and an alpha level of .05 was set a priori. Outcome measures for 58 participants were recorded daily for 14 days and this nesting of repeated measures within a participant makes our data multilevel. The statistical dependence that arises from multilevel data structures needs to be appropriately accounted for in statistical modeling to arrive at the appropriate statistical inference. Since linear mixed models (also known as hierarchical linear models or mixed models) are a used to analyze relationships while accommodating statistical dependence we employed these methods to assess the relationship between daily affect outcomes (NA, PA, and PA/NA ratio) and the predictors of day and TIB condition.
The models included fixed effects for day, TIB condition, and an interaction between day and TIB condition. This interaction term tests whether or not the TIB conditions had different trends over the two-week intervention period, which is of primary interest. Failing to find meaningful interactions between TIB condition and day would indicate that the effect of TIB condition on daily affect was stable over the intervention period. The 8h TIB group was used as the reference in all models; therefore a significant coefficient for an interaction term would indicate a change or difference relative to the trend observed for the 8h TIB group. NA and PA ratings from the previous evening were included in all models to account for the possible influence of affective spillover (i.e., affect from previous day). All models contained a random intercept per participant and a random slope associated with day was included to allow trajectories over the study period to vary by participant. For each outcome (NA, PA, and PA/NA ratio) two models were estimated to address Aims 1 (i.e., 6h TIB vs 8h TIB) and 2 (i.e., the timing of PSD). Standardized empirical best linear unbiased predictors (EBLUPs) were generated for the model’s random slope for day (Raudenbush & Bryk, 2002) and represent participant specific linear trajectory measures. Multivariable logistic regressions were used to model self-rated remission (QIDS-SR ≤ 5) at weeks 2, 4 and 8. These models controlled for baseline depression severity and included the EBLUPs from the prior linear mixed models to determine if the trajectory of NA, PA, or PA/NA ratio predicted treatment response (Rowe, Raudenbush, & Goldin-Meadow, 2012) (i.e., Aim 3).
Results
Demographic and Sleep Characteristics
Sixty-eight participants were initially randomized to one of three TIB conditions and 58 participants (85.2%) completed two weeks of daily assessment (51.7% [n = 30] female; 81.0% [n = 47] Caucasian; mean age 25.8, SD = 7.0 years). Dropouts did not differ from completers on any demographic or clinical variables assessed. Study participants were well-matched across groups among demographic and clinical characteristics (Table 1). Preliminary analyses indicated that the 8h TIB group had a higher level of education relative to the 6h TIB groups (p’s < .05).
Table 1.
Participant Demographic and Psychiatric Characteristics (N = 58)
| 8h TIB |
6h TIB |
|||||
|---|---|---|---|---|---|---|
| Late Bedtime |
Early Risetime |
|||||
| (n = 16) | (n = 20) | (n = 22) | ||||
| n | % | n | % | n | % | |
| Sex, % female | 16 | 1.6 | 14.5 | 1.7 | 14.5 | 1.5 |
| M | SD | M | SD | M | SD | |
| Age (years) | 26.9 | 8 | 24.7 | 6.1 | 26.1 | 7.2 |
| Education (years)* | 16 | 1.6 | 14.0* | 1.7 | 14.6* | 1.5 |
| Baseline depression severity (QIDS-SR) | 12.4 | 3.0 | 12.4 | 4.1 | 14.2 | 4.2 |
| Length of current depressive episode (months) | 10.7 | 8.1 | 16.1 | 23.9 | 10.1 | 8.8 |
Note. QIDS-SR indicates the 16-item Quick Inventory of Depressive Symptomatology-Self-Report. Post-hoc comparisons indicated 8h TIB > both Late Bedtime and Early Risetime.
p < .05.
Sleep diary and actigraphy variable means adjusted for group, day, and education are displayed in Table 2. Consistent with study requirements the 6h TIB group slept less than the 8h TIB groups (p’s < .001; Table 2) according to both sleep diary and actigraphy estimates. There were no group differences in sleep efficiency. The Early Risetime group demonstrated less compliance with TIB requirements compared to the 8hr TIB group.
Table 2.
Adjusted Means (Standard Errors) for Sleep Diary and Actigraphy Variables During the First Two Weeks of Antidepressant Therapy
| 8h TIB |
6 h TIB |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Late Bedtime |
Early Risetime |
||||||||
| (n = 16) | (n = 20) | (n = 22) | Pairwise comparisons (z) | ||||||
| M | SE | M | SE | M | SE | a | b | c | |
| Sleep Diary | |||||||||
| Total sleep time (min.) | 381 | 14.5 | 270 | 13 | 253.7 | 12.5 | −5.67*** | −6.50*** | −0.92 |
| Sleep efficiency (%) | 78.7 | 3.7 | 73.7 | 3.3 | 68.2 | 3.2 | −1.01 | −2.11 | −1.21 |
| Actigraphy | |||||||||
| Total sleep time (min.) | 438 | 7.9 | 338.2 | 7.1 | 350.7 | 6.7 | −9.36*** | −8.13*** | 1.28 |
| Sleep efficiency (%) | 84.1 | 1.9 | 85.4 | 1.7 | 88.7 | 1.6 | 0.5 | 1.76 | 1.38 |
| Compliance | |||||||||
| Mean TIB schedule deviation(min.) | 5.6 | 15.3 | 39.5 | 14.1 | 58.8 | 13.1 | 1.62 | 2.63* | 1.00 |
Note. TIB indicates time in bed. NA indicates negative affect. PA indicates positive affect.
p < .05.
p< .001.
Daily Affect Ratings by Time in Bed (TIB) Condition
Results regarding the prediction of affect ratings by TIB condition and day are shown in Table 3. Morning NA and PA/NA ratio were associated with a TIB condition by day interaction, with the 6h TIB group reporting lower morning NA and higher morning PA/NA ratio than the 8h TIB group. Meaningful TIB condition by day interactions were not observed for evening affect ratings and there were no associations between affect ratings and sleep diary or actigraphy derived variables (data not shown).
Table 3.
Multilevel Model Coefficients for NA, PA and PA/NA Ratio for 8h TIB compared to 6h TIB group
| NA |
PA |
Positivity ratio (PA/NA) |
|
|---|---|---|---|
| B (SE) | B (SE) | B (SE) | |
| Level 2: Person-level | |||
| 8h TIB (Reference) | – | – | – |
| 6h TIB | − 0.47 (1.18) | 0.98 (1.11) | 0.10 (0.10) |
| Level 1: Daily-level | |||
| Day | 0.19 (0.10)* | − 0.05 (0.08) | − 0.02 (0.01)* |
| NA spillover | 0.17 (0.03)*** | − 0.04 (0.03) | − 0.01 (0.003)*** |
| PA spillover | − 0.03 (0.03) | 0.17 (0.03) | 0.01 (0.003)*** |
| Group x day | |||
| 6h TIB x day | − 0.23 (0.11)* | 0.02 (0.10) | 0.02 (0.01)* |
Note. TIB indicates time in bed. NA indicates negative affect. PA indicates positive affect.
p < .05.
p< .001.
Association between Daily Affect Ratings and Timing of Partial Sleep Deprivation
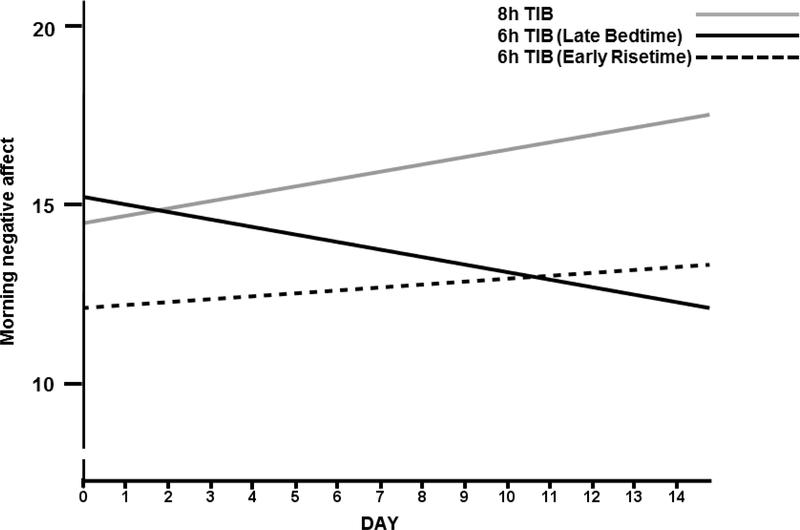
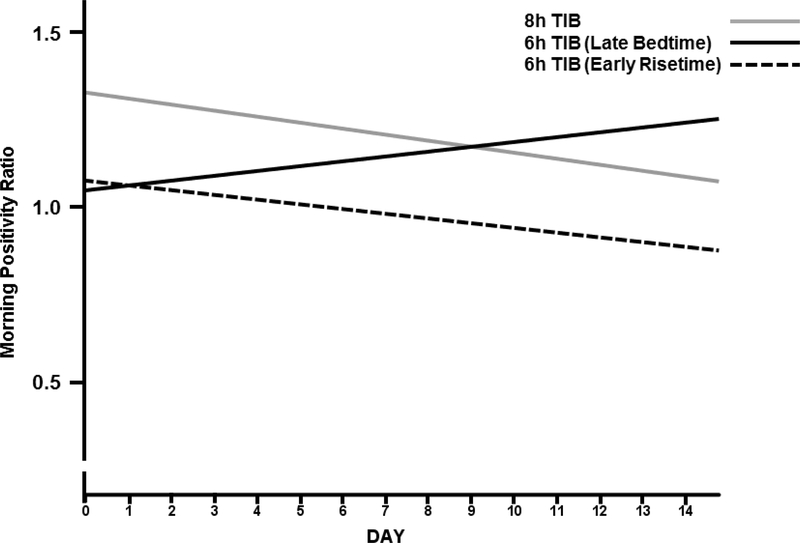
After separating the 6h TIB group into the Late Bedtime and Early Risetime groups, a day by TIB condition interaction was observed for the Late Bedtime group (Table 4). For morning NA (Figure 1) a downward trend (i.e., a decrease in NA) was observed for the 6h Late Bedtime group compared to the positive trend (i.e., an increase in NA) for morning NA observed for the 8h TIB group. Similarly, for the morning PA/NA ratio (Figure 2) a positive trend (i.e., an improvement in affect) was observed for the Late Bedtime group compared to a negative trend (i.e., a deterioration in affect) in the 8h TIB group. In both models, the Early Risetime group does not meaningfully change during the two week intervention period. These results, suggest that improvement in morning affect ratings was associated with staying up later than habitual bedtime when restricted to a 6h TIB schedule. There were no associations between affect ratings and sleep diary or actigraphy derived variables (data not shown).
Table 4.
Multilevel Model Coefficients for NA, PA and PA/NA Ratio for 8h TIB compared to 6h TIB subgroups
| NA |
PA |
Positivity ratio (PA/NA) |
|
|---|---|---|---|
| B (SE) | B (SE) | B (SE) | |
| Level 2: Person-level | |||
| 8h TIB (Reference) | – | – | – |
| 6h TIB (Late Bedtime) | 1.01 (1.26) | 0.68 (1.24) | −0.04 (0.11) |
| 6h TIB (Early Risetime) | −1.89 (1.25) | 1.27 (1.23) | 0.23 (0.11)* |
| Level 1: Daily-level | |||
| Day | 0.20 (0.10)* | − 0.05 (0.08) | − 0.02 (0.008)* |
| NA spillover | 0.17 (0.03)*** | − 0.04 (0.03) | −0.01 (0.003)*** |
| PA spillover | − 0.03 (0.03) | 0.17 (0.03)*** | 0.01 (0.003)*** |
| Group x day | |||
| 6h TIB (Late Bedtime) x day | − 0.32 (0.13)* | 0.03 (0.11) | 0.03 (0.01)** |
| 6h TIB (Early Risetime) x day | − 0.14 (0.12) | 0.02 (0.11) | 0.01 (0.01) |
Note. TIB indicates time in bed. NA indicates negative affect. PA indicates positive affect.
p < .05.
p < .01.
p < .001.
Figure 1.
Morning negative affect ratings (PANAS) across the first two weeks of antidepressant therapy for each sleep schedule condition. 8h TIB indicates randomization to no partial sleep deprivation. Late bedtime and Early Risetime indicate randomization to repeated partial sleep deprivation on a 6h TIB schedule. An increase in NA indicates a deterioration in affect.
Figure 2.
Morning positivity ratios (PANAS PA/NA ratio) across the first two weeks of antidepressant therapy for each sleep schedule condition. 8h TIB indicates randomization to no partial sleep deprivation. Late bedtime and Early Risetime indicate randomization to repeated partial sleep deprivation on a 6h TIB schedule. An increase in the positivity ratio indicates greater well-being.
Prediction of Depression Remission
The slope of morning NA ratings over the first two weeks of antidepressant treatment predicted QIDS-SR remission at week 4 (OR = 0.39, 95% CI, 0.15 – 0.96, p = .042) when controlling for sleep condition and accounting for baseline depression severity. More specifically, a 1 standard deviation increase in the slope of morning NA (i.e., worsening negative affect) reduced the odds of remission at week 4 on the QIDS-SR by 62%. A similar effect for morning NA predicting remission was observed at week 2 (OR = 0.45, 95% CI, 0.19 – 1.08, p = .075) but the effect did not persist at week 8 (OR = 0.59, 95% CI, 0.28 – 1.25, p = .60). The trajectories of morning PA and PA/NA ratio over the first two weeks were not predictive of remission at any time point (results not shown).
Discussion
This study utilized ESM to assess twice-daily affect and sleep/wake patterns across 600+ days (approximately 14 days per person), providing a novel window into the dynamics of MDD phenomenology and response to treatment in the context of an experimental TIB manipulation. Our study is the first to systematically characterize the associations between sleep duration on outpatient ratings of daily affect with a restricted TIB intervention delivered adjunctive to antidepressant therapy. During the initial two weeks of fluoxetine therapy, ratings of daily morning affect were related to nightly TIB duration and the timing of repeated PSD. Specifically, a delayed bedtime on a 6h TIB schedule reduced morning NA and increased morning PA/NA ratios more rapidly than 6h TIB with an advance of rise time or 8h TIB. Irrespective of TIB randomization, improvement in morning NA was predictive of self-rated depression remission after 4 weeks of fluoxetine therapy. These results suggest that the duration and timing of sleep when initiating fluoxetine may be important clinical considerations. Monitoring daily affect in the morning, with a focus on changes in NA or the PA/NA ratio, may help identify subtle changes to gauge early treatment response in individuals with MDD and represent an improvement over conventional cross-sectional depression severity assessment measures.
Our results indicate that the timing of repeated PSD was important to changes in patient reported daily affect ratings. Evidence regarding the timing of repeated PSD is mixed (Benedetti & Colombo, 2011) with some evidence indicating that sleep deprivation per se rather than the timing at which is take places is critical for mood improvement (Giedke, Geilenkirchen, & Hauser, 1992). Early studies indicating that restricting sleep to the first half of the night was superior to the second half (Sack et al., 1988) differed from the current study in several important ways including the frequency and duration of repeated PSD (2 nights of approximately 5 hours of total sleep vs. two consecutive weeks of 6 hours TIB); and demographics of the study sample (larger age range and diversity of psychopathology). In addition, assessment of mood outcomes has relied primarily on clinician-rated depression symptom inventories that measure several distinct symptom domains (e.g., affect, behavior, cognitive, and somatic) relative to the emphasis on an individual’s affective state as measured by the PANAS. These assessments were also not completed daily as in the present study and not validated for more frequent use, highlighting the utility of ESM in identifying subtle changes in patient’s subject experiences that may not be identified with longer assessment intervals (e.g., weekly, bi-monthly). The underlying sources of these differences requires additional clarification and future studies comparing the timing and duration of repeated PSD are required to replicate and extend these findings to more diverse clinical samples.
Of note, findings from the parent trial, which was focused on depression outcomes, indicate that the 6-hour TIB restriction did not augment treatment response or accelerate antidepressant response when using the clinician-rated Hamilton Rating Scale for Depression as the main outcome measure (Arnedt et al., 2016). In contrast, findings from the present study, which was focused on more subtle changes in affect and the role of these changes in predicting depression outcomes, found that that a 6-hour TIB schedule with a delayed bedtime may help improve affect across the first 2 weeks of antidepressant initiation. In turn, we also found that improvement in morning NA, regardless of TIB schedule, predicted depression remission using a self-report depression measure after 4 weeks of antidepressant therapy. Differences in the findings between the parent trial and the present study could be due to the use of different outcome measures for depression (e.g., the parent trial utilized the clinician-rated Hamilton Rating Scale for Depression, vs. the self-report measure used in the present study, the Quick Inventory of Depression Symptoms), and the discrepancies between affect and depression—although affect is one dimension of depression, negative affect alone does not fully characterize the construct of depression. The predictive utility of the improvement in morning NA being limited to predicting treatment response at only 4 weeks (2 weeks following the TIB intervention) may reflect to the short-term nature of the gain associated with the TIB intervention on affect. In addition, the daily affect and sleep patterns were not monitored after the initial two weeks of intervention so we cannot rule out additional moderators associated with clinical trajectories after week 2.
This study has several important strengths including prospective data collection using ESM to assess daily affect and careful monitoring of adherence to assigned sleep schedules. In the current study, the participants were predominantly young adults, which may have contributed to an evening preference that made the Early Risetime schedule more difficult to maintain, as observed by lower adherence in this group. Previous studies evaluating mood variation in depression have relied on one item measures of mood variability derived from clinician-rated scales and used uni-dimensional mood scales (Peeters et al., 2006). Inclusion of a multidimensional inventory like the PANAS affords the opportunity to measure correlated yet distinct affective states that are sensitive to change. In the current sample, the TIB groups were not differentiated by positive affect ratings, rather the TIB groups were differentiated by variability associated with the morning of negative affect. In addition, data collection in participants’ natural environments provides the study with high ecological validity and the ESM sampling framework reduces recall bias which is common in MDD (Beck, 1963).
While our study demonstrated several strengths, specifically the use of ESM, there were also limitations. Study participants were relatively young and the effects of repeated PSD on affect and symptom severity changes early in therapy may differ across the developmental spectrum. In addition, the sample was preliminary white which may limit the generalizability of our findings to more diverse clinical samples. Compliance with the sleep schedule was variable in the 6h TIB group, especially for participants assigned to the Early Risetime group, potentially limiting conclusions drawn about the timing of repeated PSD for improving mood ratings. It is possible that the timing of repeated PSD may be best informed by circadian preferences and this warrants further investigation. This issue may be particularly relevant to young adults with MDD who may have a stronger preference for an evening schedule, which in turn may make it difficult to consistently implement an earlier rise time since circadian preferences have been correlated with circadian period in young adults (Duffy, Rimmer, & Czeisler, 2001).
In summary, this study indicates that the timing and duration of sleep when starting antidepressant therapy may have implications for treatment response. Two weeks of repeated PSD (when timed so that bedtime is delayed by two hours) in young adults with MDD who are starting an antidepressant is associated with reduced NA and an improved PA/NA ratio in the morning. Importantly, reduction of morning NA in the first two weeks of therapy was predictive of depression remission after 4 weeks of antidepressant therapy. These novel findings suggest that close monitoring of morning affect, which can be easily implemented in clinical settings, may be a tool to predict early treatment response.
Acknowledgments
This research was, in part, supported by National Institute of Mental Health grant R01 MH077690 (J.T. Arnedt, Principal Investigator). The authors thank the Sleep & Circadian Research administrative staff and sleep technicians for their generous support. Portions of these data were presented at the 27th annual convention of the Meeting of the Associated Professional Sleep Societies. This work was completed while Dr. Huntley was a postdoctoral fellow in the University of Michigan Sleep & Chronophysiology Laboratory.
Contributor Information
Edward D. Huntley, Department of Psychiatry, University of Michigan
Leslie M. Swanson, Department of Psychiatry, University of Michigan
Giselle E. Kolenic, Program on Women’s Health Care Effectiveness Research, Department of Obstetrics and Gynecology, University of Michigan
Holli Bertram, Department of Psychiatry, University of Michigan.
Ann Mooney, Department of Psychiatry, University of Michigan.
Richard Dopp, Department of Psychiatry, University of Michigan.
J. Todd Arnedt, Department of Psychiatry, University of Michigan..
References
- Acheson A, Richards JB, & de Wit H (2007). Effects of sleep deprivation on impulsive behaviors in men and women. Physiolology & Behavior, 91(5), 579–587. doi: 10.1016/j.physbeh.2007.03.020 [DOI] [PubMed] [Google Scholar]
- Arnedt JT, Swanson LM, Dopp RR, Bertram HS, Mooney AJ, Huntley ED, … Armitage R. (2016). Effects of Restricted Time in Bed on Antidepressant Treatment Response: A Randomized Controlled Trial. Journal of Clinical Psychiatry, 77, e1218–e1225. doi: 10.4088/JCP.15m09879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagby RM, Ryder AG, Schuller DR, & Marshall MB (2004). The Hamilton Depression Rating Scale: has the gold standard become a lead weight? American Journal of Psychiatry, 161, 2163–2177. doi: 10.1176/appi.ajp.161.12.2163 [DOI] [PubMed] [Google Scholar]
- Bech P, Cialdella P, Haugh MC, Birkett MA, Hours A, Boissel JP, & Tollefson GD (2000). Meta-analysis of randomised controlled trials of fluoxetine v. placebo and tricyclic antidepressants in the short-term treatment of major depression. British Journal of Psychiatry, 176, 421–428. doi: 10.1192/bjp.176.5.421 [DOI] [PubMed] [Google Scholar]
- Beck A (1963). Thinking and depression: I. Idiosyncratic content and cognitive distortions. Archives of General Psychiatry, 9, 324–333. doi: 10.1001/archpsyc.1963.01720160014002 [DOI] [PubMed] [Google Scholar]
- Benedetti F, & Colombo C (2011). Sleep deprivation in mood disorders. Neuropsychobiology, 64, 141–151. doi: 10.1159/000328947 [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Ancoli-Israel S, Edinger JD, Lichstein KL, & Morin CM (2006). Recommendations for a standard research assessment of insomnia. Sleep, 29, 1155–1173. doi: 10.1093/sleep/29.9.1155 [DOI] [PubMed] [Google Scholar]
- Clark LA, Watson D, & Leeka J (1989). Diurnal variation in the positive affects. Motivation and Emotion, 13, 205–234. doi: 10.1007/BF00995536 [DOI] [Google Scholar]
- Dallaspezia S, & Benedetti F (2015). Sleep Deprivation Therapy for Depression. Current Topics in Behavioral Neurosciences, 25, 483–502. doi: 10.1007/7854_2014_363 [DOI] [PubMed] [Google Scholar]
- Demos KE, Hart CN, Sweet LH, Mailloux KA, Trautvetter J, Williams SE, … McCaffery JM. (2016). Partial sleep deprivation impacts impulsive action but not impulsive decision-making. Physiolology & Behavior, 164(Pt A), 214–219. doi: 10.1016/j.physbeh.2016.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diener E (2000). Subjective well-being. The science of happiness and a proposal for a national index. American Psychologist, 55, 34–43. doi: 10.1037/0003-066X.55.1.34 [DOI] [PubMed] [Google Scholar]
- Duffy JF, Rimmer DW, & Czeisler CA (2001). Association of intrinsic circadian period with morningness-eveningness, usual wake time, and circadian phase. Behavioral Neuroscience, 115, 895–899. doi: 10.1037/0735-7044.115.4.895 [DOI] [PubMed] [Google Scholar]
- Fredrickson BL (2001). The role of positive emotions in positive psychology. The broaden-and-build theory of positive emotions. American Psychologist, 56, 218–226. doi: 10.1037/0003-066X.56.3.218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geschwind N, Nicolson NA, Peeters F, van Os J, Barge-Schaapveld D, & Wichers M (2011). Early improvement in positive rather than negative emotion predicts remission from depression after pharmacotherapy. European Neuropsychopharmacology, 21, 241–247. doi: 10.1016/j.euroneuro.2010.11.004 [DOI] [PubMed] [Google Scholar]
- Giedke H, Geilenkirchen R, & Hauser M (1992). The timing of partial sleep deprivation in depression. Journal of Affective Disorders, 25, 117–128. doi: 10.1016/0165-0327(92)90074-G [DOI] [PubMed] [Google Scholar]
- Gordijn MC, Beersma DG, Bouhuys AL, Reinink E, & Van den Hoofdakker RH (1994). A longitudinal study of diurnal mood variation in depression; characteristics and significance. Journal of Affective Disorders, 31, 261–273. doi: 10.1016/0165-0327(94)90102-3 [DOI] [PubMed] [Google Scholar]
- Hamilton JP, & Gotlib IH (2008). Neural substrates of increased memory sensitivity for negative stimuli in major depression. Biological Psychiatry, 63, 1155–1162. doi: 10.1016/j.biopsych.2007.12.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatzinger M, Hemmeter UM, Brand S, Ising M, & Holsboer-Trachsler E (2004). Electroencephalographic sleep profiles in treatment course and long-term outcome of major depression: association with DEX/CRH-test response. Journal of Psychiatric Research, 38(5), 453–465. doi: 10.1016/j.jpsychires.2004.01.010 [DOI] [PubMed] [Google Scholar]
- Hemmeter U, Seifritz E, Hatzinger M, Muller MJ, & Holsboer-Trachsler E (1995). Serial partial sleep deprivation as adjuvant treatment of depressive insomnia. Progress in Neuro-Psychopharmacology & Biological Psychiatry, 19(4), 593–602. doi: 10.1016/0278-5846(95)00104-4 [DOI] [PubMed] [Google Scholar]
- Henkel V, Seemuller F, Obermeier M, Adli M, Bauer M, Mundt C, … Riedel M. (2009). Does early improvement triggered by antidepressants predict response/remission? Analysis of data from a naturalistic study on a large sample of inpatients with major depression. Journal of Affective Disorders, 115, 439–449. doi: 10.1016/j.jad.2008.10.011 [DOI] [PubMed] [Google Scholar]
- Holsboer-Trachsler E, & Seifritz E (2000). Sleep in depression and sleep deprivation: a brief conceptual review. The World Journal of Biological Psychiatry, 1(4), 180–186. doi: 10.3109/15622970009150589 [DOI] [PubMed] [Google Scholar]
- Holsboer-Trachsler E, Wiedemann K, & Holsboer F (1988). Serial partial sleep deprivation in depression--clinical effects and dexamethasone suppression test results. Neuropsychobiology, 19(2), 73–78. doi: 10.1159/000118438 [DOI] [PubMed] [Google Scholar]
- Iber C, Ancoli-Israel S, Chesson A, & Quan SF (2007). The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications (1st ed.). Westchester, Illinois: American Academy of Sleep Medicine. [Google Scholar]
- Krause AJ, Simon EB, Mander BA, Greer SM, Saletin JM, Goldstein-Piekarski AN, & Walker MP (2017). The sleep-deprived human brain. Nature Reviews Neuroscience, 18(7), 404–418. doi: 10.1038/nrn.2017.55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minkel J, Moreta M, Muto J, Htaik O, Jones C, Basner M, & Dinges D (2014). Sleep deprivation potentiates HPA axis stress reactivity in healthy adults. Health Psychology, 33(11), 1430–1434. doi: 10.1037/a0034219 [DOI] [PubMed] [Google Scholar]
- Murray G (2007). Diurnal mood variation in depression: a signal of disturbed circadian function? Journal of Affective Disorders, 102, 47–53. doi: 10.1016/j.jad.2006.12.001 [DOI] [PubMed] [Google Scholar]
- Murray G, Allen NB, & Trinder J (2002). Mood and the circadian system: investigation of a circadian component in positive affect. Chronobiology international 19, 1151–1169. doi: 10.1081/CBI-120015956 [DOI] [PubMed] [Google Scholar]
- Nierenberg AA, Farabaugh AH, Alpert JE, Gordon J, Worthington JJ, Rosenbaum JF, & Fava M (2000). Timing of onset of antidepressant response with fluoxetine treatment. American Journal of Psychiatry, 157, 1423–1428. doi: 10.1176/appi.ajp.157.9.1423 [DOI] [PubMed] [Google Scholar]
- Peeters F, Berkhof J, Delespaul P, Rottenberg J, & Nicolson NA (2006). Diurnal mood variation in major depressive disorder. Emotion, 6, 383–391. doi: 10.1037/1528-3542.6.3.383 [DOI] [PubMed] [Google Scholar]
- Pilcher JJ, & Huffcutt AI (1996). Effects of sleep deprivation on performance: a meta-analysis. Sleep, 19, 318–326. doi: 10.1093/sleep/19.4.318 [DOI] [PubMed] [Google Scholar]
- Portell M, Anguera MT, Hernandez-Mendo A, & Jonsson GK (2015). Quantifying biopsychosocial aspects in everyday contexts: an integrative methodological approach from the behavioral sciences. Psychology Research and Behavior Management, 8, 153–160. doi: 10.2147/PRBM.S82417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posternak MA, & Zimmerman M (2005). Is there a delay in the antidepressant effect? A meta-analysis. The Journal of Clinical Psychiatry, 66, 148–158. doi: 10.4088/JCP.v66n0201 [DOI] [PubMed] [Google Scholar]
- Raudenbush SW, & Bryk AS (2002). Hierarchical linear models : applications and data analysis methods (2nd ed.). Thousand Oaks: Sage Publications. [Google Scholar]
- Rowe ML, Raudenbush SW, & Goldin-Meadow S (2012). The pace of vocabulary growth helps predict later vocabulary skill. Child Dev, 83(2), 508–525. doi: 10.1111/j.1467-8624.2011.01710.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rush AJ, Trivedi MH, Ibrahim HM, Carmody TJ, Arnow B, Klein DN, … Keller MB. (2003). The 16-Item Quick Inventory of Depressive Symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biological Psychiatry, 54, 573–583. doi: 10.1016/S0006-3223(02)01866-8 [DOI] [PubMed] [Google Scholar]
- Sack DA, Duncan W, Rosenthal NE, Mendelson WE, & Wehr TA (1988). The timing and duration of sleep in partial sleep deprivation therapy of depression. Acta Psychiatrica Scandinavica, 77, 219–224. doi: 10.1111/j.1600-0447.1988.tb05104.x [DOI] [PubMed] [Google Scholar]
- Stassen HH, Angst J, Hell D, Scharfetter C, & Szegedi A (2007). Is there a common resilience mechanism underlying antidepressant drug response? Evidence from 2848 patients. Journal of Clinical Psychiatry, 68, 1195–1205. doi: 10.4088/JCP.v68n0805 [DOI] [PubMed] [Google Scholar]
- Szegedi A, Jansen WT, van Willigenburg AP, van der Meulen E, Stassen HH, & Thase ME (2009). Early improvement in the first 2 weeks as a predictor of treatment outcome in patients with major depressive disorder: a meta-analysis including 6562 patients. Journal of Clinical Psychiatry, 70, 344–353. doi: 10.4088/JCP.07m03780 [DOI] [PubMed] [Google Scholar]
- Tadic A, Helmreich I, Mergl R, Hautzinger M, Kohnen R, Henkel V, & Hegerl U (2010). Early improvement is a predictor of treatment outcome in patients with mild major, minor or subsyndromal depression. Journal of Affective Disorders, 120, 86–93. doi: 10.1016/j.jad.2009.04.014 [DOI] [PubMed] [Google Scholar]
- Trivedi MH, Rush AJ, Wisniewski SR, Nierenberg AA, Warden D, Ritz L, … Team SDS. (2006). Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. American Journal of Psychiatry, 163, 28–40. doi: 10.1176/appi.ajp.163.1.28 [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, & Tellegen A (1988). Development and validation of brief measures of positive and negative affect: the PANAS scales. Journal of Personality and Social Psychology, 54, 1063–1070. doi: 10.1037/0022-3514.54.6.1063 [DOI] [PubMed] [Google Scholar]
- Watson D, Wiese D, Vaidya J, & Tellegen A (1999). The two general activation systems of affect: Structural findings, evolutionary considerations, and psychobiological evidence. Journal of Personality and Social Psychology, 76, 820–838. doi: 10.1037/0022-3514.76.5.820 [DOI] [Google Scholar]