Abstract
Although a great deal is known about neurobiological mechanisms of initial conditioning and extinction, relatively little is known about mechanisms involved in the return of behavior following extinction. Here, we examine the effects of temporarily inactivating the bed nucleus of the stria terminalis (BNST) on initial conditioning and post-extinction reconditioning. We investigate effects in unsignaled contextual fear conditioning, in which animals initially receive strong contextual conditioning, followed by three sessions of nonreinforced context exposure (extinction), and then receive a single context-shock reconditioning trial. In two experiments with male Long Evans rats, we evaluated the effects of delivery of a muscimol/baclofen cocktail to the BNST prior to initial conditioning or reconditioning. In Experiment 1, we find that a single context-shock pairing results in more freezing following extinction than when it is the initial conditioning trial. This rapid reconditioning effect was impaired by BNST inactivation. In Experiment 2, we find that BNST inactivation also causes a deficit in freezing after strong initial conditioning. These findings suggest that the BNST is involved in both initial conditioning and post-extinction reconditioning. We discuss implications of these findings for current thinking about BNST function in learning and memory processes.
Keywords: learning, memory, BNST, fear conditioning, extinction
Many studies have found that nonreinforced exposure to a previously conditioned stimulus (CS) attenuates conditioned responding. This extinction effect suppresses the original association between a CS and an unconditioned stimulus (US) but leaves that association intact. Among the evidence for this is that conditioned responding re-emerges after extinction following a conditioning trial that on its own does not result in strong conditioning. This rapid reacquisition effect is pervasive and has been shown in a number of Pavlovian and operant procedures (e.g., Leung, Bailey, Laurent, & Westbrook, 2007; Perry & McNally, 2012; reviewed in Lattal & Lattal, 2012). In contrast to other post-extinction unmasking procedures (including spontaneous recovery, contextual renewal, and reinstatement), rapid reconditioning involves a re-pairing of the original associative contingences, which allows for a more direct comparison between the mechanisms of initial conditioning and the post-extinction return of behavior.
At a behavioral level, rapid reacquisition of context-evoked fear in rats occurs after few or many extinction sessions and results from the re-emergence of a specific context-shock association (Williams, Kim, & Lattal, 2019). Although a great deal is known about the behavioral mechanisms that underlie post-extinction reacquisition, including situations that may lead to slowed reacquisition, comparatively little is known about the neurobiology that underlies these effects.
There are multiple brain regions that may be involved and several studies suggest that the anterior region of the bed nucleus of the stria terminalis (BNST) may play an important role in processes like post-extinction reconditioning (e.g., Goode & Maren, 2017). The BNST is a member of the extended amygdala and shares similar connections and neuroanatomical makeup with the central nucleus of the amygdala (CeA; Alheid & Heimer, 1988; Alheid, DeOlmos, & Beltramino, 1995). One idea about the role of the BNST in conditioning is that it is particularly important for conditioning of sustained anxiety to long-duration cues, such as contextual fear conditioning (Davis et al., 2010; Schulz & Canbeyli, 1999; Sullivan et al., 2004; Waddell et al., 2006), but not for conditioning of discrete cues, for which the CeA is necessary (Zimmerman & Maren, 2011). The BNST is also important for corticotropin-releasing factor- and light-potentiated startle (Lee & Davis, 1997; Walker & Davis, 1997), as well as stress-induced reinstatement of drug seeking (Erb & Stewart, 1999) and reinstatement of conditioned cued fear behavior (Goode et al., 2015; Waddell et al., 2006, 2008).
More recently, studies have suggested a more nuanced role for the BNST in conditioning, with evidence suggesting that the BNST may be particularly involved in learning about conditioned stimuli that are less predictive of unconditioned stimuli (Goode, Ressler, Acca, Miles, & Maren, 2019; Goode, Acca, & Maren, 2019; Hammack, Todd, Kocho-Schellenberg, & Bouton, 2015). For example, with typical parameters in the case of unsignaled contextual conditioning, although the context predicts the shock, the temporal precision with which a long duration context predicts shock is much less than that of a short duration discrete CS. In the case of post-extinction reconditioning, a CS has a history of being associated with a US and also with nonreinforcement, which establishes a CS as having an ambiguous relation with the US (see also Bouton, 2002). Thus, one might expect that the BNST would regulate post-extinction reconditioning.
Experiment 1: Effects of BNST inactivation on rapid reconditioning after extinction
Methods
Subjects.
Sixty-four experimentally naive male Long Evans rats (Charles River Laboratories, Wilmington, MA) were purchased at 275-300 g (about 8 weeks of age) and were allowed to acclimate to the vivarium for one week. During acclimation, rats were housed two per cage. Rats were kept on a reverse light-dark schedule (lights off at 06:00 and lights on at 18:00) in a colony space that had constant temperature (22°C ± 1°C) and humidity (70%). Food and water were available ad libitum and all behavioral experiments occurred during the dark phase, between 09:00 and 15:00. The colony space was located within a suite that also included an anteroom where microinjections occurred and three procedure rooms for behavioral experiments. All experimental procedures were approved by the Oregon Health & Science University Institutional Animal Use and Care Committee and were conducted in accordance with National Institutes of Health (NIH) “Principles of Laboratory Animal Care” (NIH Publication No. 86-23, revised 1985).
Apparatus.
Fear conditioning occurred in conditioning chambers (exterior dimensions: 31.8 cm L × 25.4 cm W× 26.7 cm H) housed within sound-attenuating chambers (Med Associates). The operant chambers were fixed with a grid floor set to deliver a 1 sec, 0.75 mA scrambled shock and a house light that illuminated to signal the start of the session. Before and after each round of behavior, the grid floors and chamber walls were cleaned with 95% ethanol. Animals were loaded into chambers in red light conditions to maintain the dark circadian cycle.
Intracranial Cannula Placement Surgeries.
Rats received intracranial surgery for placement of guide cannulas (C315GS-5/SPC, 26 gauge, 17 mm, Plastics One, Roanoke, VA) and dummies (C315DCS-5/SPC, 30 gauge, 17.5 mm, Plastics One) above the BNST. Rats were given an intramuscular injection of ketamine/xylazine (85 mg/kg; 10 mg/kg) for initial anesthesia and then were maintained under vaporized isoflurane (1%) for the remainder of the surgery. Following anesthesia, rats’ heads were shaved and then they were placed in a stereotaxis (David Kopf Instruments). Guide cannula were lowered at a 25° angle to prevent injector placement within the lateral ventricles and were implanted 1 mm dorsal to the BNST with the following coordinates: 0.0 mm AP, ±4.20 mm ML, and −7.10 mm DV relative to bregma. Guide cannulas were secured with stainless steel screws and acrylic dental cement (Orthojet). The stainless steel dummies were then placed in the guide cannulas to prevent cannula blockage and infection. Prior to lifting anesthesia, rats were given a subcutaneous injection of Carprofen (5mg/kg; 5mg/ml; Putney) and antibiotic ointment (Neosporin) was applied to the sutures. These treatments were repeated for 3 days following surgery and animals were given 7 days to recover prior to behavior. Two animals were lost during surgery and 12 animals were removed from the main analysis due to broken or clogged guide cannula that prevented bilateral microinjections.
Behavioral Schedule.
All rats were habituated to transport to the anteroom and handling for three days. Rats in the reconditioning groups received 12-min initial conditioning sessions with an unsignaled footshock (1 sec, .75 mA) delivered at 2.5 min, 5 min, 9 min, and 11.5 min into the session on Days 1 and 2 (Phase 1). Extinction consisted of a 24-min non-reinforced exposure to the fear-conditioning context on Days 3-5 (Phase 2). Rats in the conditioning groups were transported to the ante room and handled instead of receiving conditioning and extinction (they were not placed in an alternative context because our previous work found that context exposure weakened initial conditioning with these parameters; Williams, et al. 2019). All rats then received a 3-min conditioning session with a single unsignaled footshock delivered 2.5 min into the session on Day 6 (Phase 3). For rats in Group Reconditioning (REC), this was a reconditioning session; for rats in Group Conditioning (CON), this was an initial conditioning session. Immediately prior to the reconditioning/conditioning session, half of the animals in each group received a microinjection of a muscimol + baclofen (M+B) cocktail and the other half received saline (SAL; see below). All rats received a 24-min nonreinforced test session on Day 7 (Phase 4). All sessions occurred at the same time of day separated by 24 hr.
Drugs.
A mixture of GABAa, muscimol (Sigma-Aldrich), and GABAb, Baclofen (Sigma-Aldrich), receptor agonists was prepared to inhibit activity within the BNST, which has both types of receptors for GABA (Margeta-Mitrovic, Mitrovic, Riley, Jan, & Basbaum, 1999; Wisden, Laurie, Monyer, & Seeburg, 1992), the major inhibitory neurotransmitter system in the brain. A 0.1mM muscimol + 1.0 mM baclofen mixture (M+B) in sterile saline was used as it has had previous success locally inhibiting neural activity in rodents in similar procedures (Buffalari & See, 2011; Pina, Young, Ryabinin, & Cunningham, 2015; Rogers, Ghee, & See, 2008). Sterile saline was used as the control vehicle for animals not receiving microinjections of M+B.
Microinjections.
Microinjectors were made in-house with 32 gauge stainless steel tubing (18 mm, Small Parts Inc.) to reach 1 mm past the end of the guide cannula and into the BNST. Polyethylene tubing (PE-20, Instech) connected the microinjectors to 10 ul Hamilton glass syringe (Hamilton Company, Reno, NV) on a mircosyringe pump (Fusion 100, Chemyx Inc.). Microinjectors were placed into the BNST of each animal immediately prior to Phase 3 (reconditioning/conditioning) and .3 μl of M+B or saline was bilaterally infused at an infusion rate of .3 μl/min. Microinjectors remained in the guide cannula for 1 min following infusion to ensure the entire volume was infused and then rats were placed in the context for conditioning.
Cannula Placement Confirmation.
Several days following Phase 4, rats were euthanized with isoflurane until all breathing had stopped and then brains were removed and placed in 4% PFA in PBS for no more than 24 hr. For cryoprotection, brains were then placed in a solution of 20% sucrose and .1% sodium azide (NaN3) in PBS until the brain was fully saturated with sucrose (generally 24 hr). The brains were then transferred to 30% sucrose and .1% NaN3 in PBS for no more than 1 month before sectioning. The brains were sectioned at 35 microns and then placed in well plates of PBS and 0.1% NaN3 until mounted to microscope slides. Placement of the guide cannula and microinjector location was confirmed on the microscope. Correct BNST placement was considered to be within the BNST at 0.12 mm to −0.60 mm around bregma. Microinjector placements within the anterior commissure between the dorsal and ventral BNST were also counted (Goode et al., 2015). Only animals with correct bilateral placement were included in analyses.
Freezing Assessment.
The level of contextual fear conditioning was assessed by the amount of freezing, the natural conditioned response upon re-exposure to a cue or context associated with shock (Fanselow & Bolles, 1979). Rats were run in squads of four and freezing was hand scored in real-time by visual time sampling of each animal every 8 s, which was the time it took to scan each chamber and record the freezing response. The scorer was blind to treatment assignments.
Data Analysis.
Statistical analyses consisted of 2 x 2 ANOVAs with Group (conditioning or reconditioning) and treatment (SAL or M+B) as factors. Additionally, adjusted effect size was calculated with omega-squared in R and the power to detect effects was calculated with G*Power (Faul, Erdfelder, Lang, & Buchner, 2007) for the freezing results in the Phase 4 test. Experiments were conducted in multiple replications with each factor level represented (Experiment 1 had 3 replications; Experiment 2 had two replications). Groups were collapsed across replications because there were no main effects of or interactions with replication before or after reconditioning (pre-reconditioning: F(1,20) = 2.02, p = .170; post-reconditioning: F(1,8) = .48, p = .508).
Results
Figure 1A shows a representative coronal section of guide cannula and microinjector placement in the BNST. Figure 1B shows representation of injector cannula tip placements. 23 injectors terminated in the anterior dorsal BNST, 4 injectors terminated in the anterior ventral BNST, 3 terminated in the posterior dorsal BNST, and 6 terminated in the anterior commissure. These placements resulted in the following groups: REC M+B, n = 8; REC SAL, n = 12; COND M+B, n= 7; COND SAL, n = 9. Unilateral misses (n = 9) and bilateral misses (n = 5) were removed from primary analyses.
Figure 1. Cannula Placements within the BNST for Experiment 1.
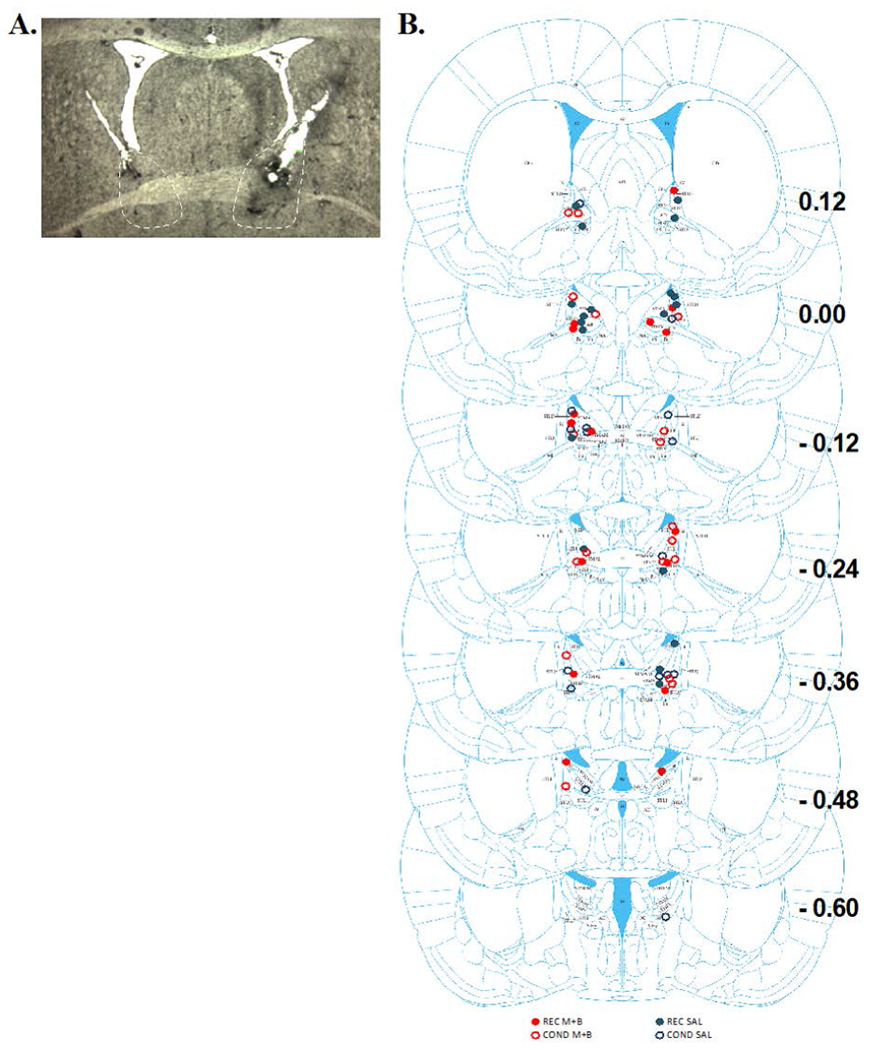
(A) displays a representative coronal slice of the BNST with cannula tracks ending above the BNST to allow microinjectors to infuse M+B or SAL. (B) Schematic of all the microinjector placements within the BNST for Experiment 1 across bregma coordinates 0.12 to −0.60. The filled circles represent REC and empty circles represent COND groups. Red circles represent M+B BNST treatment and blue circles represent saline treatment.
Conditioning
An overview of the experimental design of Experiment 1 is shown in Figure 2A. Two conditioning sessions led to a significant increase in conditioned freezing behavior in REC groups. A RMANOVA comparing the within-subject effect of Acquisition Session and between-subject effect of future BNST Treatment revealed a significant effect of Acquisition Session (F(1, 16) = 27.26, p < .001; session one: M = 46.95, SEM = 2.58, session two: M = 65.97, SEM = 4.04), but no effect of Treatment (F(1, 16) = .01, p = .910; M+B: M = 56.36, SEM = 3.83, SAL: M = 56.52, SEM = 4.00) or an interaction (F(1, 16) = .01, p = .931). These results showed that conditioning led to a successful acquisition of conditioned freezing in groups balanced for future BNST treatment.
Figure 2. Inactivation of the BNST Prevents Expression of Rapid Reacquisition of Contextual Fear, but Not Mild Acquisition in Experiment 1.
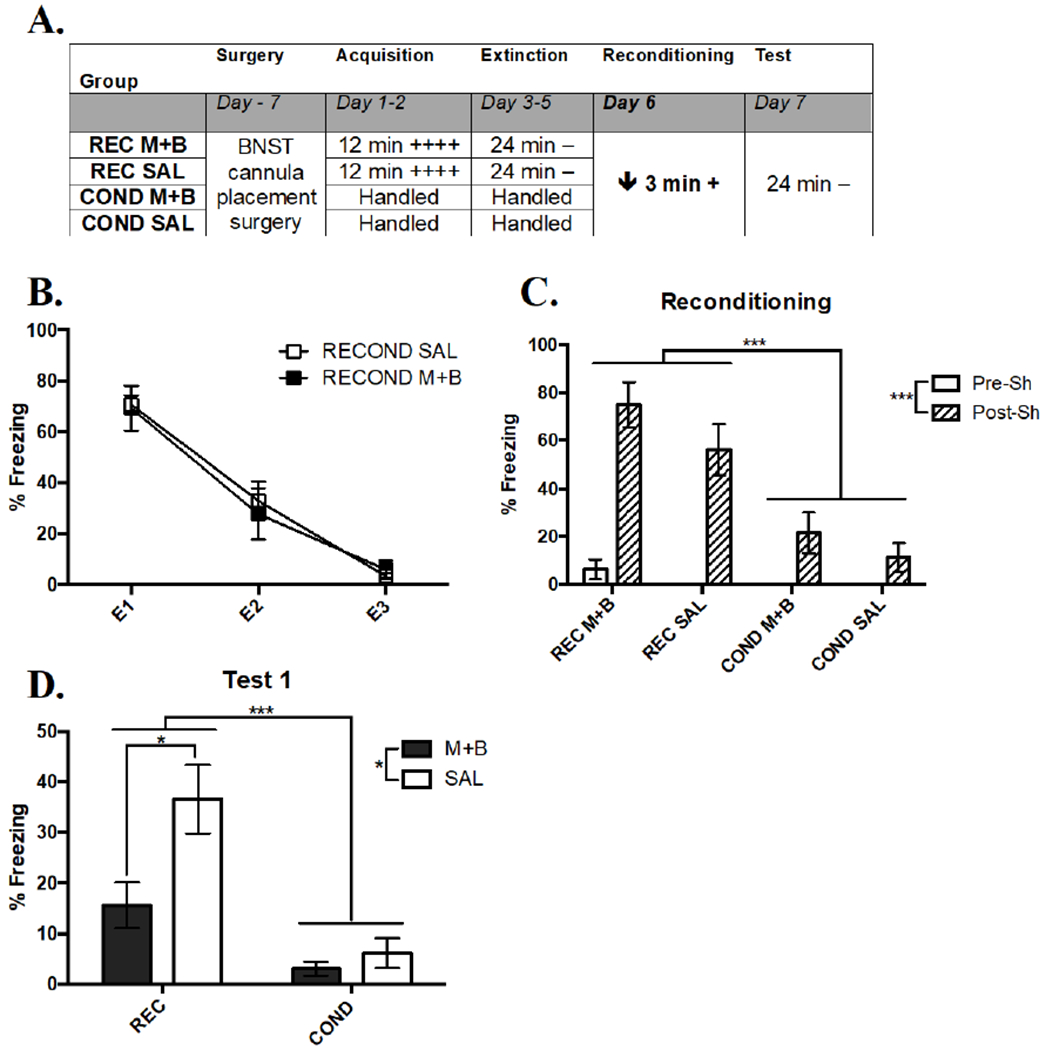
(A) Overall design of Experiment 1. The times listed represent the total time of exposure to the context for a given session. A plus sign indicates a single .75 mA shock and a minus sign indicates exposure to the context without shock. (B) Extinction curve of REC SAL and REC M+B as shown by the average percent freezing for each session; E = extinction session (C) Mean percent freezing in the 30 sec pre and post shock on Day 6 during reconditioning. Rapid reacquisition of freezing relative to initial acquisition occurs in both REC groups. (D) Mean percent freezing during the Test on Day 7, where expression rapid reacquisition, but not weak acquisition was blocked by BNST inactivation during Day 6. Significance between groups is represented by *** p < .001; ** p < .01; * p < .05. REC SAL, n = 12; REC M+B, n = 8; COND SAL, n = 9; COND M+B, n = 7. Error bars represent standard error of the mean.
Extinction
Extinction reduced conditioned freezing in both REC groups prior to reconditioning (Figure 2B). A RMANOVA investigating the effect of repeated Extinction Sessions and future BNST Treatment on freezing behavior revealed a significant within-subject effect of Extinction Session (F(2, 32) = 89.19, p < .001), but no effect of future Treatment (F(1, 16) = .43, p = .522) or an interaction (F(2, 32) = .52, p = .602). The significant effect of Extinction Session was caused by significantly more freezing in E1 relative to E2 (q (32) = 9.29, p < .001) and E3 (q (32) = 15.56, p < .001) and E2 relative to E3 (q (32) = 6.26, p < .001), showing that three Extinction Sessions were necessary to lower freezing behavior prior to reconditioning. This result also suggested that groups had equivalent behavior prior to BNST manipulations.
Reconditioning
A reconditioning shock led to immediate rapid reacquisition of contextual fear that was not altered by BNST inactivation prior to Phase 3 (Figure 2C). A three-way RMANOVA comparing percent time spent freezing in the 30 sec before and after shock by each Group and Treatment (Time X Group X Treatment) found a significant main effect of Group (F(1, 29) = 27.98, p < .001), driven by REC freezing more than COND. There was also a significant within-subjects effect of Time (F(1, 29) = 71.77, p < .001) that was due to more freezing after shock relative to before. There was no reliable main effect of Treatment (F(1, 29) = 2.98, p = .095), but there was a significant Group X Time interaction (F(1, 29) = 24.28, p < .001), caused by REC freezing more than COND after shock (q (29) = 16.78, p < .001), but not before (q (29) = .07, p = .983). These results suggest that rapid reacquisition occurred immediately following a reconditioning shock regardless of BNST treatment, which indicated that activity within the BNST was not involved in the reacquisition of fear during reconditioning.
Test
Temporary inactivation of the BNST with M+B during reconditioning impaired retention of the rapid reacquisition effect 24 hr later in the test (Figure 2D). A two-way ANOVA found a significant main effect of Group (F(1, 32) = 15.15, p < .001) that was due to greater freezing shown by REC compared to COND. The ANOVA also revealed a significant main effect of Treatment (F(1, 32) = 5.08, p < .05), which was caused by M+B group freezing less relative to SAL treatment. There was not a significant interaction of Group X Treatment (F(1, 32) = 2.82, p = .102), however, a t-test comparing the impact of the two treatments on REC groups revealed a significant impairment of M+B on retention of reacquisition freezing relative to SAL (t(21) = 2.82, p < .05). A t-test comparing COND groups did not find a significant effect of Treatment (t(16) = .60, p = .555). These results suggest that activity within the BNST during a reacquisition trial is needed for retention of the reacquisition memory 24 hr later. However, there was no effect of BNST inactivation during that conditioning procedure in rats that experienced it as their initial conditioning experience. Because this weak conditioning episode resulted in low freezing in control animals, it is unclear if the absence of an effect of BNST inactivation was due to an absence of a requirement for the BNST in acquisition or due to a floor effect. In Experiment 2, we increased the strength of initial conditioning to address this issue.
Experiment 2: Effects of BNST inactivation on stronger initial conditioning
In the previous experiment, BNST inactivation with M+B did not impair initial acquisition of contextual fear, as has been previously found (Sullivan et al., 2004; Waddell et al., 2006; Zimmerman & Maren, 2011). In this experiment, we assessed the role of the BNST in acquisition of contextual fear by temporarily inactivating the BNST prior to stronger conditioning parameters that should increase freezing levels.
Methods
40 Long-Evans male rats underwent surgery as described in Experiment 1 to place a guide cannula 1 mm above the BNST. Surgeries and injection in Experiment 2 were identical to procedures from Experiment 1. Fear conditioning consisted of a single 12-min context exposure with 4 unsignaled shocks on Day 1, as in Day 1 of Experiment 1. Prior to conditioning, rats received an intra-BNST injection of M+B or SAL. On Day 2, animals were exposed to the context for 24 min without shock to test their memory of the context and as an initial extinction session (Test1/Extinction Session 1 or T1/E1). Days 3 through 4 were repeated tests, which act as extinction sessions (T2/E2 and T3/E3). The following day, reconditioning (3 min context exposure with 1 shock) occurred (Day 5). Day 6 was a post-reconditioning test, which was identical to treatments on Days 2-4. All sessions were separated by 24 hrs.
As in Experiment 1, there was no effect of replication, so replications were combined in the analysis (F(1, 24) = .58, p = .454). Two animals died during surgery and 6 animals were removed from the main analysis due to broken or clogged guide cannula that prevented bilateral microinjections.
Results
Cannula Placements
Figure 3 shows microinjector placement in the BNST. For this experiment, 23 injectors terminated in the anterior dorsal BNST, 2 terminated in the posterior dorsal BNST, and 1 terminated in the anterior commissure. The cannulae placements resulted in the following group numbers: M+B, n= 14; SAL, n = 12. Unilateral misses (n = 3) and bilateral misses (n = 3) were removed from primary analyses.
Figure 3. Cannula Placements within the BNST for Experiment 2.
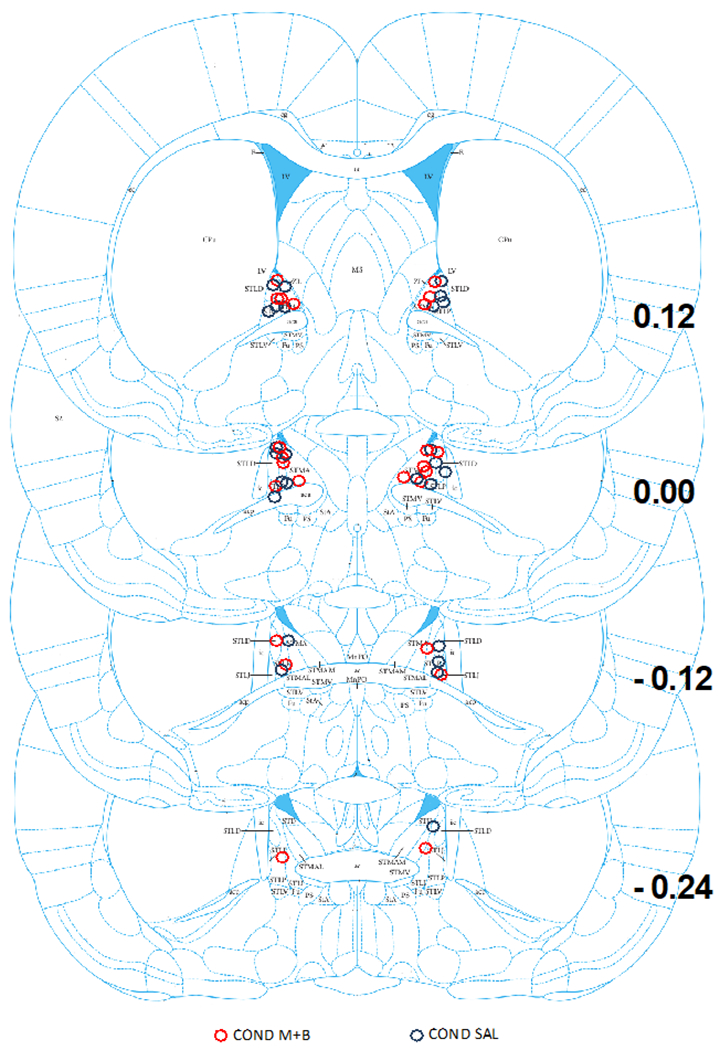
This figure is a schematic of all the microinjector placements within the BNST for Experiment 2 across bregma coordinates 0.12 to −0.24. Red circles represent M+B BNST treatment and blue circles represent saline treatment.
Conditioning
An overview of the design of Experiment 2 is shown in Figure 4A. Inactivation of the BNST caused an enhancement of freezing during initial strong acquisition (Figure 4B). A t-test comparing the percent freezing across Treatment found significantly greater freezing in M+B compared to SAL (t(24) = 2.52, p < .05). This effect emerged over the course of the acquisition session, with no group difference in the first 3 min (t(24) < 1.0, p = .40). This result suggests that pre-treating the BNST with M+B might enhance short-term conditioned freezing following shock presentations.
Figure 4. Inactivation of the BNST Prevents Strong Acquisition.
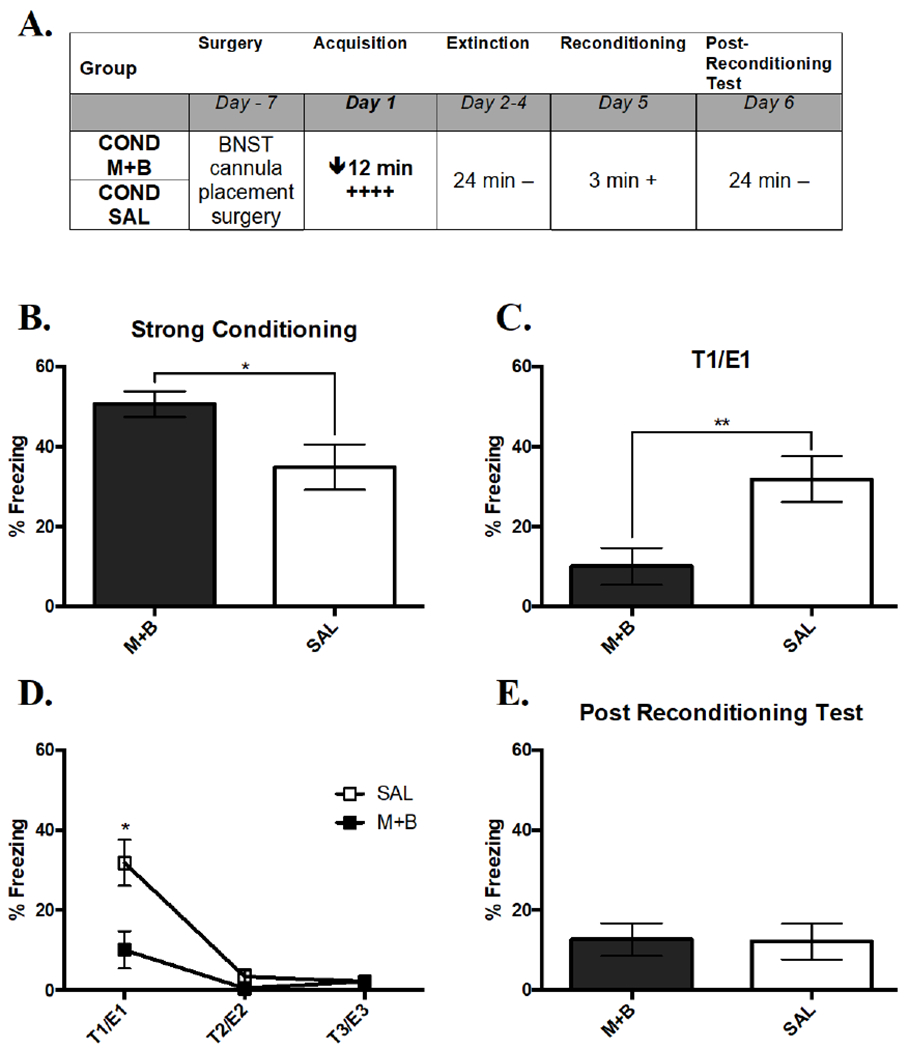
(A) Overall design of Experiment 2. The times listed represent the total time of exposure to the context for a given session. A plus sign indicates a single .75 mA shock and a minus sign indicates exposure to the context without shock. (B) Mean percent freezing during acquisition of strong conditioning with an active or inactive BNST. An inactive BNST enhanced freezing. (C) Mean percent freezing during the expression of acquisition in the test on Day 2, where retention of acquisition was impaired by an inactive BNST during conditioning (D) Extinction curve on Days 2 through 4 as shown by a line graph of both groups’ decreasing average percent time freezing. (E) Mean percent freezing during the test of reacquisition on Day 6 that was unaffected by initial acquisition differences. Significance between groups is represented by *** p < .001; ** p < .01; * p < .05. COND SAL, n = 12; COND M+B, n = 14. Error bars represent standard error of the mean.
Test 1
M+B treatment before conditioning caused a marked reduction in expression of freezing during Test 1 24 hr later (Figure 4C; t(24) = 2.97, p < .01), which indicated that activity in the BNST was involved in initial acquisition of a strong memory.
Extinction
Over the course of three test sessions, conditioned responding in both treatments extinguished (Figure 4D). A RMANOVA showed a significant main effect of Treatment (F(1, 18) = 4.73, p < .05), Extinction Session (F(2, 36) = 22.21, p < .001), and Treatment X Extinction Session interaction (F(2, 36) = 4.22, p < .05). The significant main effect of Treatment was caused by SAL freezing more than M+B (Figure 4D). More freezing in E1 relative to E2 (q (36) = 16.29, p < .001) and E3 (q (32) = 16.73, p < .001) caused the significant within-subjects effect of Extinction Session. The interaction was due to SAL freezing more than M+B in E1 (q (32) = 15.97, p < .001), but not in E2 (q (32) = .09, p = .985) or E3 (q (32) = .00, p = 1.0). This interaction showed that inactivation the BNST during acquisition caused a significant impairment in freezing that dissipated after one extinction session (i.e., after Test 1).
Reconditioning and Post-Reconditioning Test
Reconditioning did not lead to differences in reacquisition of freezing as a function of previous treatment (data not shown). A two-way RMANOVA comparing percent time spent freezing in the 30 sec before and after shock found a significant within-subjects effect of Time (F(1, 18) = 32.31, p < .001), driven by significantly more freezing after (M = 40.38, SEM = 6.06) shock compared to before (M = .00, SEM = .00). However, there was no effect of past Treatment (F(1, 18) = 1.19, p = .290; M+B: M = 15.18, SEM = 4.70, SAL: M = 26.04, SEM = 6.97) or an interaction effect (F(1, 18) = 1.19, p = .290) on freezing, suggesting that post-shock reacquisition was independent of past BNST inactivation and differences in conditioning.
Additionally, a t-test comparing the expression of reacquisition in a test 24 hr later did not show an effect of previous Treatment on reacquisition freezing (t(24) = .07, p = .941), which again suggested that reacquisition was unaffected by the prior impairment of initial acquisition due to an inactive BNST (Figure 4E).
General Discussion
Inactivating the BNST with GABA receptor agonists prior to reconditioning prevented retention of rapid reacquisition of contextual fear. Although there was no effect on initial conditioning with a brief context-shock pairing, BNST inactivation impaired stronger initial conditioning. These results confirm the identified role of the BNST in contextual fear conditioning and they extend it to post-extinction reconditioning.
Rapid reconditioning involves a large, rapidly acquired sustained fear response that is a disproportionate (relative to initial conditioning) to the conditioning events (a brief context-shock pairing). In this sense, our findings are consistent with ideas that the BNST is involved in sustained fear responses (Sullivan et al., 2004; Waddell et al.,2006) and fear conditioning of long-duration cues (Hammack, et al. 2015; Walker & Davis, 1997; Walker, Miles, & Davis, 2009). Our findings also are consistent with the idea that the BNST is involved in learning in ambiguous situations that is based on findings showing a BNST requirement for conditioning in situations in which the CS is not a reliable predictor of the US (Goode, et al. 2019a). In the case of post-extinction reconditioning, a CS has a history of reinforcement and nonreinforcement, leading to some level of ambiguity during each presentation.
This ambiguity idea is also consistent with our findings in Experiment 2 that inactivating the BNST impaired initial acquisition of contextual fear with a long session and temporally unpredictable shock. We have previously found that the adBNST is engaged by weak contextual fear using an immediate early gene readout (Williams, et al. 2019), but we saw no reliable effect on conditioning in Experiment 1 with a brief context exposure paired with a single US. In some ways, this finding is similar to that reported by Hammack et al. (2015), who found that conditioning induced by a single shock after a brief (1 min), but not long (10 min) context exposure, was unaffected by BNST lesions. More recently, Goode, et al. (2019b) isolated a critical role for the BNST in this effect to expression of fear after conditioning with a longer context-shock interval. Because freezing was so low in our control animals, it is difficult to know how much to make of the absence of an effect of BNST inactivation on initial conditioning in Experiment 1.
We targeted our injections to the anterior dorsal region of the BNST. The BNST is an heterogeneous brain region, comprised of at least 18 subregions (Ju & Swanson, 1989; Ju, Swanson, & Simerly, 1989), some of which have been proposed to have opposing roles in anxiety; the oval nucleus within the adBNST is thought to be anxiogenic, but the remainder of adBNST has shown anxiolytic properties (Sung-Yon Kim et al., 2013). Further, our pharmacological studies targeted all of the BNST, dorsal and ventral, and our previous significant c-Fos results were found only in the dorsal BNST. Thus, inactivating the whole BNST could cause opposing processes that resulted in no effect on milder contextual fear manipulations.
We also found that during conditioning or reconditioning, inactivation of the BNST led to increased short-term post-shock freezing behavior relative to controls, but that this effect reversed during the test the next day. This short-term effect was not due to a general increase in freezing – the increase did not emerge until after the shock, suggesting that having the BNST inactivated caused an exaggerated fear response. A similar result was reported by Meloni, Jackson, Gerety, Cohen, & Carlezon (2006), who found that delivery of muscimol to the BNST led to increased acoustic startle in the presence of a light previously paired with shock (fear potentiated startle). This exaggerated response may have contributed to the weakened freezing response observed during the retention test the next day, possibly because the high fear reflected an overexpected shock value, leading to an enhancement of extinction during the post-shock period, or in the case of Experiment 2, an overexpectation effect with the additional shocks in the strong conditioning protocol (Kremer, 1978). It is worth noting that other studies have not observed short-term increases in freezing or startle (Lee & Davis, 1997; Walker & Davis, 1997), so more work is needed to determine the conditions that lead to these post-shock effects.
Although it is difficult to compare between experiments, it is notable that the post-extinction reconditioning effect that we saw in Experiment 1 was large, but the effect that we saw in Experiment 2 was relatively small. In Experiment 1, and in our previous work with rats (Williams, et al. 2019), we used a strong acquisition protocol that consisted of two days of conditioning. In Experiment 2, because our goal was to evaluate BNST involvement in a single session of conditioning, we only used one day of conditioning. Overall levels of freezing were lower and extinction occurred more quickly in Experiment 2. Thus, it is possible that this extra extinction weakened the reconditioning effect which occurs under some conditions (Bersh & Keltz, 1971;Williams & Lattal, 2019), but we have previously found that with similar parameters to those used here, reconditioning in rats is unaffected by the amount of extinction (Williams, et al. 2019). Instead, it may be that the reconditioning is more likely following multiple acquisition sessions, which may result in the animal expecting that any session with a shock will be followed the next day by another session with a shock, causing an increased freezing response on the post-reconditioning test (Ricker & Bouton, 1996).
There are many unanswered questions about the behavioral and neurobiological mechanisms involved in reconditioning. We found that the BNST is involved in reconditioning, which is consistent with studies that have found a role for the BNST in post-extinction unmasking procedures, such as reinstatement of fear conditioning (Goode et al., 2015; Waddell et al., 2006, 2008) and stress-induced reinstatement of drug-seeking behavior (Erb & Stewart, 1999). Because there are other demonstrations that the BNST is not important for other post-extinction processes, such as contextual renewal (Goode et al. 2015), understanding how the BNST regulates different aspects of extinction and post-extinction behavioral processes will be important for painting a clear picture of the neurobiology of extinction.
Acknowledgements:
This work was supported by grants from the Department of Defense (W81XWH-14-2-0143) and the National Institutes of Health (DA047981).
References
- Alheid GF, & Heimer L (1988). New perspectives in basal forebrain organization of special relevance for neuropsychiatric disorders: the striatopallidal, amygdaloid, and corticopetal components of substantia innominata. Neuroscience, 27(1), 1–39. [DOI] [PubMed] [Google Scholar]
- Alheid GF, DeOlmos CA, & Beltramino CA (1995). Amygdala and Extended Amygdala In Paxinos G (Ed.), The Rat Nervous System (2nd ed., pp. 495–578). San DIego: Wiley/Blackwell; (10.1111). 10.1046/j.1471-4159.1995.65010471.x [DOI] [Google Scholar]
- Bersh PJ, & Keltz JR (1971). Pavlovian reconditioning and the recovery of avoidance behavior in rats after extinction with response prevention. Journal of Comparative and Physiological Psychology, 76(2), 262–266. [DOI] [PubMed] [Google Scholar]
- Bouton ME (2002). Context, ambiguity, and unlearning: sources of relapse after behavioral extinction. Biological Psychiatry, 52(10), 976–986. [DOI] [PubMed] [Google Scholar]
- Buffalari DM, & See RE (2011). Inactivation of the bed nucleus of the stria terminalis in an animal model of relapse: effects on conditioned cue-induced reinstatement and its enhancement by yohimbine. Psychopharmacology, 213(1), 19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M, Walker DL, Miles L, & Grillon C (2010). Phasic vs Sustained Fear in Rats and Humans: Role of the Extended Amygdala in Fear vs Anxiety. Neuropsychopharmacology : Official Publication of the American College of Neuropsychopharmacology, 35(1), 105–135. 10.1038/npp.2009.109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erb S, & Stewart J (1999). A role for the bed nucleus of the stria terminalis, but not the amygdala, in the effects of corticotropin-releasing factor on stress-induced reinstatement of cocaine seeking. The Journal of Neuroscience : the Official Journal of the Society for Neuroscience, 19(20), RC35–RC35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow MS, & Bolles RC (1979). Naloxone and shock-elicited freezing in the rat. Journal of Comparative and Physiological Psychology, 93(4), 736–744. [DOI] [PubMed] [Google Scholar]
- Faul F, Erdfelder E, Lang A-G, & Buchner A (2007). G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behavior Research Methods, 39, 175–191. [DOI] [PubMed] [Google Scholar]
- Goode TD, Kim JJ, & Maren S (2015). Reversible Inactivation of the Bed Nucleus of the Stria Terminalis Prevents Reinstatement But Not Renewal of Extinguished Fear, 2(3), ENEURO.0037–15.2015. 10.1523/ENEURO.0037-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goode TD, & Maren S (2017). Role of the bed nucleus of the stria terminalis in aversive learning and memory. Learning & Memory, 24(9), 480–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goode TD, Ressler RL, Acca GM, Miles OW, & Maren S (2019a). Bed nucleus of the stria terminalis regulates fear to unpredictable threat signals. eLife, 8, e46525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goode TD, Acca GM, & Maren S (2019b). Threat imminence dictates the role of the bed nucleus of the stria terminalis in contextual fear. Neurobiology of learning and memory, 107116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammack SE, Todd TP, Kocho-Schellenberg M, & Bouton ME (2015). Role of the bed nucleus of the stria terminalis in the acquisition of contextual fear at long or short context-shock intervals. Behavioral neuroscience, 129(5), 673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju G, & Swanson LW (1989). Studies on the cellular architecture of the bed nuclei of the stria terminalis in the rat: I. Cytoarchitecture. The Journal of Comparative Neurology, 280(4), 587–602. [DOI] [PubMed] [Google Scholar]
- Ju G, Swanson LW, & Simerly RB (1989). Studies on the cellular architecture of the bed nuclei of the stria terminalis in the rat: II. Chemoarchitecture. The Journal of Comparative Neurology, 280(4), 603–621. 10.1002/cne.902800410 [DOI] [PubMed] [Google Scholar]
- Kim S-Y, Adhikari A, Lee SY, Marshel JH, Kim CK, Mallory CS, et al. (2013). Diverging neural pathways assemble a behavioural state from separable features in anxiety. Nature, 496(7444), 219–223. 10.1038/nature12018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremer EF (1978). The Rescorla-Wagner model: Losses in associative strength in compound conditioned stimuli. Journal of Experimental Psychology: Animal Behavior Processes, 4(1), 22–36. [DOI] [PubMed] [Google Scholar]
- Lattal KM, & Lattal KA (2012). Facets of Pavlovian and operant extinction. Behavioural processes, 90(1), 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, & Davis M (1997). Role of the Hippocampus, the Bed Nucleus of the Stria Terminalis, and the Amygdala in the Excitatory Effect of Corticotropin-Releasing Hormone on the Acoustic Startle Reflex. The Journal of Neuroscience, 17(16), 6434–6446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung HT, Bailey GK, Laurent V, Westbrook RF. (2007) Rapid reacquisition of fear to a completely extinguished context is replaced by transient impairment with additional extinction training. J Exp Psychol Anim Behav Process 33: 299–313. doi: 10.1037/0097-7403.33.3.299 [DOI] [PubMed] [Google Scholar]
- Margeta-Mitrovic M, Mitrovic I, Riley RC, Jan LY, & Basbaum AI (1999). Immunohistochemical localization of GABAB receptors in the rat central nervous system. Journal of Comparative Neurology, 405(3), 299–321. [DOI] [PubMed] [Google Scholar]
- Meloni EG, Jackson A, Gerety LP, Cohen BM, & Carlezon WA (2006). Role of the bed nucleus of the stria terminalis (BST) in the expression of conditioned fear. Annals of the New York Academy of Sciences, 1071(1), 538–541. [DOI] [PubMed] [Google Scholar]
- Pina MM, Young EA, Ryabinin AE, & Cunningham CL (2015). The bed nucleus of the stria terminalis regulates ethanol-seeking behavior in mice. Neuropharmacology, 99, 627–638. 10.1016/j.neuropharm.2015.08.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry CJ, & McNally GP (2012). Naloxone prevents the rapid reacquisition but not acquisition of alcohol seeking. Behavioral Neuroscience, 126(4), 599–604. [DOI] [PubMed] [Google Scholar]
- Rogers JL, Ghee S, & See RE (2008). The neural circuitry underlying reinstatement of heroin-seeking behavior in an animal model of relapse. Neuroscience, 151(2), 579–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz D, & Canbeyli R (1999). Freezing behavior in BNST-lesioned Wistar rats. Annals of the New York Academy of Sciences, 877, 728–731. [DOI] [PubMed] [Google Scholar]
- Sullivan GM, Apergis J, Bush DEA, Johnson LR, Hou M, & LeDoux JE (2004). Lesions in the bed nucleus of the stria terminalis disrupt corticosterone and freezing responses elicited by a contextual but not by a specific cue-conditioned fear stimulus. Neuroscience, 128(1), 7–14. 10.1016/j.neuroscience.2004.06.015 [DOI] [PubMed] [Google Scholar]
- Waddell J, Bouton ME, & Falls WA (2008). Central CRF receptor antagonist a-helical CRF9-41 blocks reinstatement of extinguished fear: the role of the bed nucleus of the stria terminalis. Behavioral Neuroscience, 122(5), 1061–1069. 10.1037/a0013136 [DOI] [PubMed] [Google Scholar]
- Waddell J, Morris RW, & Bouton ME (2006). Effects of bed nucleus of the stria terminalis lesions on conditioned anxiety: aversive conditioning with long-duration conditional stimuli and reinstatement of extinguished fear. Behavioral Neuroscience, 120(2), 324–336. 10.1037/0735-7044.120.2.324 [DOI] [PubMed] [Google Scholar]
- Walker DL, & Davis M (1997). Double dissociation between the involvement of the bed nucleus of the stria terminalis and the central nucleus of the amygdala in startle increases produced by conditioned versus unconditioned fear. The Journal of Neuroscience : the Official Journal of the Society for Neuroscience, 17(23), 9375–9383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DL, Miles LA, & Davis M (2009). Selective Participation of the Bed Nucleus of the Stria Terminalis and CRF in Sustained Anxiety-Like versus Phasic Fear-Like Responses. Progress in Neuro-Psychopharmacology & Biological Psychiatry, 33(8), 1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams AR, Kim ES, & Lattal KM (2019). Behavioral and immunohistochemical characterization of rapid reconditioning following extinction of contextual fear. Learning & Memory, 26, 381–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams AR, & Lattal KM (2019). Rapid reacquisition of contextual fear following extinction in mice: Effects of amount of extinction, acute ethanol withdrawal, and ethanol intoxication. Psychopharmacology, 236, 491–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisden W, Laurie DJ, Monyer H, & Seeburg PH (1992). The distribution of 13 GABAA receptor subunit mRNAs in the rat brain. I. Telencephalon, diencephalon, mesencephalon. The Journal of Neuroscience, 12(3), 1040–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman JM, & Maren S (2011). The bed nucleus of the stria terminalis is required for the expression of contextual but not auditory freezing in rats with basolateral amygdala lesions. Neurobiology of Learning and Memory, 95(2), 199–205. [DOI] [PMC free article] [PubMed] [Google Scholar]


