Abstract
Background
Epidemiological studies investigating the use of proton pump inhibitors (PPI) on the risk of liver cancer and/or mortality among persons with chronic liver disease (CLD) have reported conflicting results. We conducted a systematic review and meta-analysis to determine the impact of PPI-use on liver cancer and/or death among patients with CLD.
Methods
The core databases including MEDLINE, EMBASE, and Cochrane library were searched through January 2020. We included studies, evaluating the association between PPIs and liver cancer or mortality among patients with CLD including randomized controlled, nonrandomized controlled, and observational studies. We used inverse-variance random-effects models to estimate the pooled relative risk (RR) and 95% confidence interval (CI) for liver cancer or mortality.
Results
Eleven studies including 173,894 patients were selected. In three studies, individuals with CLD who used PPIs had a 67% greater risk of developing hepatocellular carcinoma (HCC) compared to nonusers (RR, 1.67; 95% CI, 1.12–2.50; I2=92%). Combining data from the eight studies relating PPI to overall mortality, we observed a 57% increased risk of mortality in PPI users with CLD compared to CLD nonusers (RR: 1.57; 95% CI, 1.24–1.99; I2=69%).
Conclusion
PPI-use was associated with an increased risk of HCC and mortality in patients with CLD suggesting that PPI prescriptions in patients with CLD should be considered carefully.
Keywords: proton pump inhibitor, mortality, liver cancer, hepatocellular carcinoma, chronic liver disease, systematic review, meta-analysis
Introduction
Proton pump inhibitors (PPIs) were first introduced in 1989 to treat gastroesophageal reflux disorder (GERD) by blocking acid production by irreversibly inhibiting Hþ/Kþ-adenosine triphosphatase in gastric parietal cells. By 2015, PPIs in the United States ranked among the top 10 national health-related drug expenditures [1–4]. However, in recent years, concern has been raised for potential serious adverse events associated with PPI-use including gastric cancer, pancreatic cancer, major adverse cardiovascular events, and death [5–9]. The most recent research suggests that when PPIs are used appropriately, they are safe medications but should be used for the shortest time period at the smallest effective dose [10,11].
As in the general population, PPIs are also among the most commonly prescribed classes of drugs among patients with cirrhosis [12]. However, PPIs are only recommended in a few specific situations such as during the immediate post variceal banding period and only for short-term use [13]. In fact, PPI is not routinely recommended for patients with decompensated cirrhosis and not even for primary or secondary prophylaxis against gastrointerestinal bleeding among those with significant esophageal varices [14].
Recently, several observational studies examining the association between the use of PPIs and the risk of hepatocellular carcinoma (HCC), a well-known complication of cirrhosis whether due to viral hepatitis or alcoholic or nonalcoholic liver disease [15–18], but they reported conflicting results [18–21]. Therefore, we performed a systematic review and meta-analysis of the relevant published literature to evaluate the association between PPI-use, liver cancer development, and mortality among patients with CLD.
Methods
Literature search
We searched relevant full-text articles using the MEDLINE, EMBASE, and Cochrane library databases through January 31, 2020. The search strategy included “liver disease,” “liver neoplasm,” and “liver cancer” as patient-related terms, and “proton pump inhibitor” as the main drug-related term (Supplementary Table 1). Both MeSH terms and text words were applied to each database as applicable. PPI drug names included in the search strategy were omeprazole, esomeprazole, pantoprazole, rabeprazole, dexlansoprazole, tenatoprazole, and benatoprazole as well as their brand and chemical names.
Study selection
We followed the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) guidelines [22]. We included studies that met the following inclusion criteria: they (1) presented original data from randomized controlled studies, nonrandomized controlled studies, or observational studies that evaluated the association between PPIs and liver cancer or mortality among patients with CLD; (2) included clearly defined outcomes of liver cancer incidence and/or mortality; (3) provided quantitative risk estimates (hazard ratio [HR], relative risk [RR], or odds ratio [OR]) and associated 95% confidence intervals (CI); and (4) were written in English. We excluded non-comparative studies, non-peer reviewed studies, conference abstracts, and review studies. Two investigators independently conducted the study selection, data extraction, and quality assessment (HJS and XJ). When discordance occurred and a consensus could not be reached through discussion by the two primary reviewers, discussion and adjudication with the third investigator (HP) was carried out.
Quality assessment
Since all eligible studies of this systematic review were observational studies, we used the risk of bias assessment tool for non-randomized studies (ROBANS) to assess the quality for all articles included in this study [23]. ROBANS consists of six items (selection of participants, confounding variables, measurement of intervention, blinding for outcome assessment, incomplete outcome data, and funding resources) evaluated on the three levels of bias (low, unclear, or high risk of bias).
Data extraction
Data were extracted using a data frame with predefined variables: country of study, study design, data source, inclusion and exclusion criteria of patients, the number of patients in each group, and cohort characteristics (e.g., mean age, sex, and etiology of liver disease), PPI name with dosage, criteria to define liver cancer incidence and mortality outcomes, study follow-up duration, and other relevant confounders if regression analysis was performed. The study protocol was registered to PROSPERO (CRD42018116354) prior to the study execution.
Data analyses
Our primary outcome was the adjusted estimates of the risk of liver cancer incidence or mortality rates associated with PPI-use among patients with CLD. For studies that reported multiple risk estimates, we used the best-adjusted estimates to obtain the pooled estimate. The summary estimate of the adjusted risk ratio of outcome was generated by weighting the study-specific risk ratios by the inverse of their variance. We considered HRs as RRs [24,25], and we converted ORs to RR using the Zhang and Yu method [26]. We included eight studies that reported HRs and two studies that reported ORs in our analysis to estimate the pooled RR [27,28]. If the included study reported the number of deaths for each group, we pooled the unadjusted RR using inverse-variance random effect models.
Heterogeneity was assessed using the I2 tests and the Q statistic [29]. Significance of the Q-statistic test (P<0.05) indicates a substantial level of heterogeneity. The I2 statistic describes the percentage of the variability in estimates resulting from heterogeneity rather than sampling error, with I2 values of 50% or higher indicating the presence of a significantly high level of heterogeneity [29]. Due to the high level of heterogeneity observed in the preliminary analysis of this study, we used a random-effects model to analyze the pooled estimates.
In addition, we performed subgroup analyses according to the type of CLD (cirrhosis or hepatitis), follow-up period (≤1 year versus >1 year), and study location (Asia versus non-Asia) when there was data available for at least two studies. We used the funnel plot to assess possible publication bias. All statistical analyses were performed using the Review Manager Software version 5.3 (RevMan v5.3, The Cochrane Collaboration, Oxford, UK).
Results
Literature search
Our search strategy initially yielded 9,002 articles for review and screening (Fig. 1). After excluding duplicates, 8,575 articles remained for title or abstract screening. Excluded studies included: no CLD patients, no PPI group, papers not written in English, case reports or series, editorials, reviews, and abstracts. After these articles were excluded, 683 full text articles were reviewed and 672 studies were then excluded. Eleven articles (173,894 patients) met our study inclusion/exclusion criteria and were included in the meta-analysis: three studies provided data for liver cancer incidence analysis [20,21,30] and eight studies for mortality rate analysis [12,18,19,31–35]. No studies provided both liver cancer and mortality outcomes.
Fig. 1.
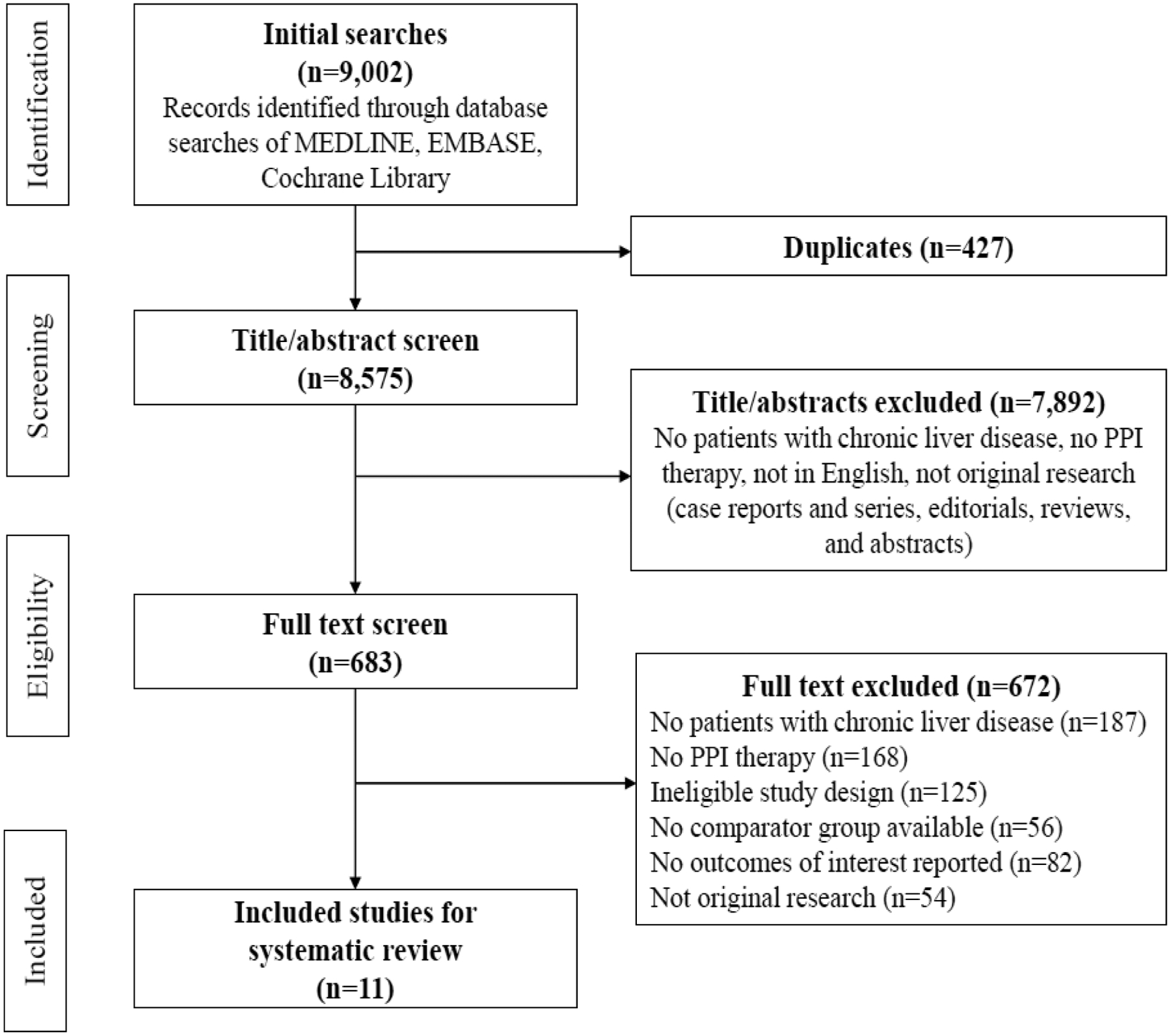
PRISMA flow diagram of study selections
General characteristics of the included studies
Table 1 describes the characteristics of the included studies and their patient cohorts. There were three studies from Taiwan and one from each of the following countries: United States, United Kingdom, Germany, Austria, Italy, Hungary, Singapore, and South Korea. Ten used a cohort study design and one used a nested case-control study design. The etiologies/types of CLD of the study cohorts included ALD, NAFLD, viral hepatitis, cirrhosis of any etiology, autoimmune disease, and other miscellaneous liver diseases. The exposure to PPIs was based on prescribed medications (e.g. omeprazole, esomeprazole, lansoprazole, dexlansoprazole, pantoprazole, and rabeprazole). Table 2 describes the ascertainment methods of exposures and outcomes employed by the included studies.
Table 1.
Study characteristics of included studies
| Author, year | Country | Study design/Data source | Patients | Number of part icipants | Mean age (SD) | Male | Diabetes | Etiology of liver disease/MELD score, median (range) or mean ± SD |
|---|---|---|---|---|---|---|---|---|
| Proton pump inhibitor use and liver cancer | ||||||||
| Kao et al. 2019 [30] | Taiwan | Cohort study/Longitudinal H ealth Insuranc e Database (LH ID), 2003–2013 |
|
|
|
|
|
|
| Li et al. 2018 [21] | US | Cohort study/Electronically retrieved cohort of HCV-infected veterans (ERCHIVES), 2001–2015 | Patients with HCV infection
|
11,526 (5,752 PPI users, 5,774 nonusers) | Median 53 (IQR 49–57) | 96.1% | 6.1% | N/A |
| Shao et al. 2018 [20] | Taiwan | Nested case- control study/National Health Insurance Research Dataset (NHIRD) linked the Death Registry, 2000–2013 | Patients with cirrhosis
|
139,791 (5,545 PPI users, 134,2 46 nonusers) | N/A | N/A | N/A | N/A |
| Proton pump inhibitor use and mortality | ||||||||
| Cole et al. 2016 [31] | UK | Cohort study/Scottish Liver T ransplant Unit (SLTU), 2013 | Patients with liver disease
|
206 (114 PPI us ers, 92 nonusers) | Median 56 (range 50–63) | 65.5% | N/A |
|
| De Roza et al. 2020 [3 4] | Singapore | Cohort study/Changi General Hospital, 2013–2017 | Patients with cirrhosis
|
295 (238 PPI users, 57 nonusers) | PPI 63.3 (12. 4), nonusers 60.0 (13.3) | 68.1% | Type 2 diabetes 53.2% |
|
| Dultz et al. 2015 [12] | Germany | Cohort study/German Unive rsity hospital, 2009–2011 | Patients with cirrhosis
|
272 (213 PPI users, 59 nonusers) | Median 57 (range 25–84) | 66.9% | N/A |
|
| Hung et al. 2018 [18] | Taiwan | Cohort study/National Healt h Insurance Re search Databa se (NHIRD), 2010–2013 | Patients with cirrhosis
|
5,020 (1,004 PPI users, 4,016 nonusers) | PPI users 62. 5 (13.3), nonusers 62.6 (13.6) | 67.5% | N/A |
|
| Janka et al. 2019 [35] | Hungary | Cohort study/Referral Hepatology Center (Division of Gas troenterology, Department of Internal Medicine, Clinical center, University of Debrecen), 2006–2010 | Patients with cirrhosis
|
350 (196 PPI us ers, 154 nonusers) | Median 56 (IQR 50–64) | 53.7% | N/A |
|
| Kwon et al. 2014 [32] | South Korea | Cohort study/Seoul National University Hospital, Seoul National University Boramae Medical Center, 2003–2010 | Patients with cirrhosis
|
533 (82 PPI users, 451 nonusers) | PPI users 61. 9 (9.9), nonusers 62.9 (9. 4) | 76.9% | 18.9% |
|
| Mandorfer et al. 2014 [33] | Austria | Cohort study/Medical University of Vienna, 2006–2011 | Patients with cirrhosis
|
607 (520 PPI users, 87 nonusers) | 57.5 (11.8) | 70.0% | N/A |
|
| Nardelli et al. 2019 [1 9] | Italy | Cohort study/Center for the Study of Portal Hypertension i n Rome, 20142016 | Patients with cirrhosis
|
310 (125 PPI us ers, 185 nonusers) | 62.2 (11.8) | 71.3% | N/A |
|
aDD: average number of defined doses, ALD: alcoholic liver disease, CT: computed tomography, DCC: decompensate cirrhosis, FIB-4: fibrosis-4, H2RA: histamine-2 receptor antagonist, HBV: hepatitis B virus, HCC: hepatocellular carcinoma, HCV: hepatitis C virus, HE: hepatic encephalopathy, HEV: hepatitis E virus, HIV: human immunodeficiency virus, IQR: interquartile range, MELD: model of end-stage liver disease, MRI: magnetic resonance imaging, N/A: not available, NAFLD: non-alcoholic fatty liver disease, PPI: proton pump inhibitor, RNA: ribonucleic acid, SBP: spontaneous bacterial peritonitis, NASH: non-alcoholic steatohepatitis
Table 2.
The definition of exposure and outcome in included studies
| Study | Exposure | PPI/no PPI use defined as | Mortality/Liver ca ncer defined as | Follow-up | The number of events, n/N (%) | Covariates included in ana lysis (Case-control matched var iables) |
|---|---|---|---|---|---|---|
| Proton pump inhibitor use and liver cancer | ||||||
| Kao et al. 2019 [30] | Omeprazole, pant oprazole, lansoprazole, rabeprazol e, esomeprazole, dexlansoprazole | PPI use: ≥28 cDDD (cDDD: 027, 28–119, 120–364, ≥352), c umulative dose = (the numb er of pills dispensed by the p rescribed dose dividing by th e recorded days’ supply, no exposure to PPI for <28 c DDD |
|
|
|
|
| Li et al. 2018 [21] | Omeprazole, eso meprazole, lanso prazole, dexlanso prazole, pantopra zole, rabeprazole | PPI prescription in any Veter ans pharmacy during the stu dy observation; ≥30 cDDD (c DDD: 30–180, 181–540, 541–900, >900), no PPI: <30 cDDD |
|
|
N/A |
|
| Shao et al. 2018 [20] | omeprazole, pant oprazole, lansopr azole, rabeprazol e, esomeprazole | PPI prescriptions in pharmac eutical claims between the i ndex date through 1 year pri or to cancer diagnosis, no PP 1: no prescription in pharmac eutical claims between the i ndex date and 1 year prior to the cancer diagnosis date of their matched cases, cumula tive DDD: sum of the dispens ed DDDsof any PPI |
|
|
PPI users 1,222/5,545 (22.0%), nonusers 11,526/134,246 (8.6%) |
|
| Proton pump inhibitor use and mortality | ||||||
| Coleetal.2016 [31] | Omeprazole, lans oprazole, esomep razole, pantopraz ole | Discharge on a PPI from 2013 on the Track medical recor ds database, indication for P PI prescription: no indication (73.7%), Barrett’s oesophagu s (6.1%), gastric or duodenal ulcer (7.0%), oesophagitis, g astritis, duodenitis (6.1%), G ERD (7.0%) |
|
|
N/A |
|
| De Roza et al. 2020 [34] | PPI | Landmark period of 3 month s before and 6 months after i ndex hepatic decompensatio n admission, PPI users with a cDDD≥28 within the landma rk period |
|
|
PPI users 102/238 (42.9%), nonusers 13/57 (22.8%) |
|
| Dultzetal. 2015 [12] | PPI | PPI treatment based on the assessment at the day of ad mittance to the hospital, PPI treatment was given for stro ng indications (gastrointestinal bleeding, PUD, GERD, end oscopic variceal ligation) or s ymptomatically for epigastri c pain, nausea or vomiting |
|
|
N/A |
|
| Hunget al. 2018 [18] | Omeprazole, rabe prazole, lansopraz ole, pantoprazole, esomeprazole | The average number of defin ed doses (aDD) of PPI was ca Iculated as ‘total number of defined doses of PPI’ divided by ‘total number of hospitali zation days’, defined dosage s: omeprazole 20mg, rabepr azole 20mg, lansoprazole 30 mg, pantoprazole 40mg, eso meprazole 40mg |
|
|
PPI users 704/1,004 (70.1%), nonusers 2,506/4,016 (62.4%) |
|
| Janka et al. 2019 [35] | PPI | Taking drug for at least 80% of the follow-up period |
|
|
PPI users 108/196 (55.1%), nonusers 39/154 (25.3%) |
|
| Kwon et al. 2014 [32] | PPI | The use of any PPIs form ore than 2 days |
|
|
PPI users 39/82 (47.6 %), nonusers 136/451 (30.2%) |
|
| Mandorfer e tal.2014[3 3] | PPI | Information on PPI intake fro m patient’s medical record |
|
|
N/A |
|
| Nardelli etal. 2019 [19] | Omerazole, lanso prazole, pantopra zole, esomeprazol e | Physician admission notes fo r inpatients and medication 1 ists in outpatients notes, PPI treatment at least 4 weeks p rior to the admission, strong indication (gastrointestinal b leeding, PUD, GERD, endosc opic variceal ligation) or sym ptomatically for epigastric pa in, nausea or vomiting, stand ard dosages: omeprazole 20 mg, lansoprazole 30mg, pant oprazole 40mg, esomeprazol e 40mg |
|
|
|
|
ALT: alanine aminotransferase, AST: aspartate aminotransferase, cDDD: cumulative daily drug dose, CHD: chronic heart disease, CHF: congestive heart failure, COPD: chronic obstructive pulmonary disease, FIB-4: Fibrosis-4, GERD: gastro-oesophageal reflux disease, HCC: hepatocellular carcinoma, HCV: hepatitis C virus, HE: hepatic encephalopathy, HF: heart failure, HIV: human immunodeficiency virus, ICD-9-CM: International Classification of Diseases, Ninth Revision, Clinical Modification, MELD: model for end-stage liver disease, MHE: minimal hepatic encephalopathy, MI: myocardial infarction, N/A: not available, NSAIDs: nonsteroidal anti-flammatory drugs, PPI: proton pump inhibitor, PUD: peptic ulcer disease, PVD: peripheral vascular disease, RNA: Ribonucleic acid, SBP: spontaneous bacterial peritonitis, SD: standard deviation, SVR: sustained virologic response, UKELD: UK model for end-stage liver disease
Quality assessment
We assessed the risk of bias for ten cohorts from nine studies since Kao et al. included two separate cohorts (hepatitis B virus [HBV] cohort and hepatitis C virus [HCV] cohort) [30]. All included studies had low risk of bias in the selection of study participants, blinding for outcome assessment, and funding resources (Fig. 2). Since the outcomes of liver cancer and mortality were not associated with subjective judgement, we considered low risk for blinding for outcome assessment. Most (>80%) of the studies also had low risk of bias in the category of confounding variables and incomplete outcome data, while two were considered high risk of bias because the study participants’ demographic data were not included as confounders [12,37]. For bias regarding measurement of intervention, half of the included studies were considered low risk, while the risk was not ascertainable in the remainders because the studies could not identify over-the-counter medications that patients may have purchased.
Fig. 2.
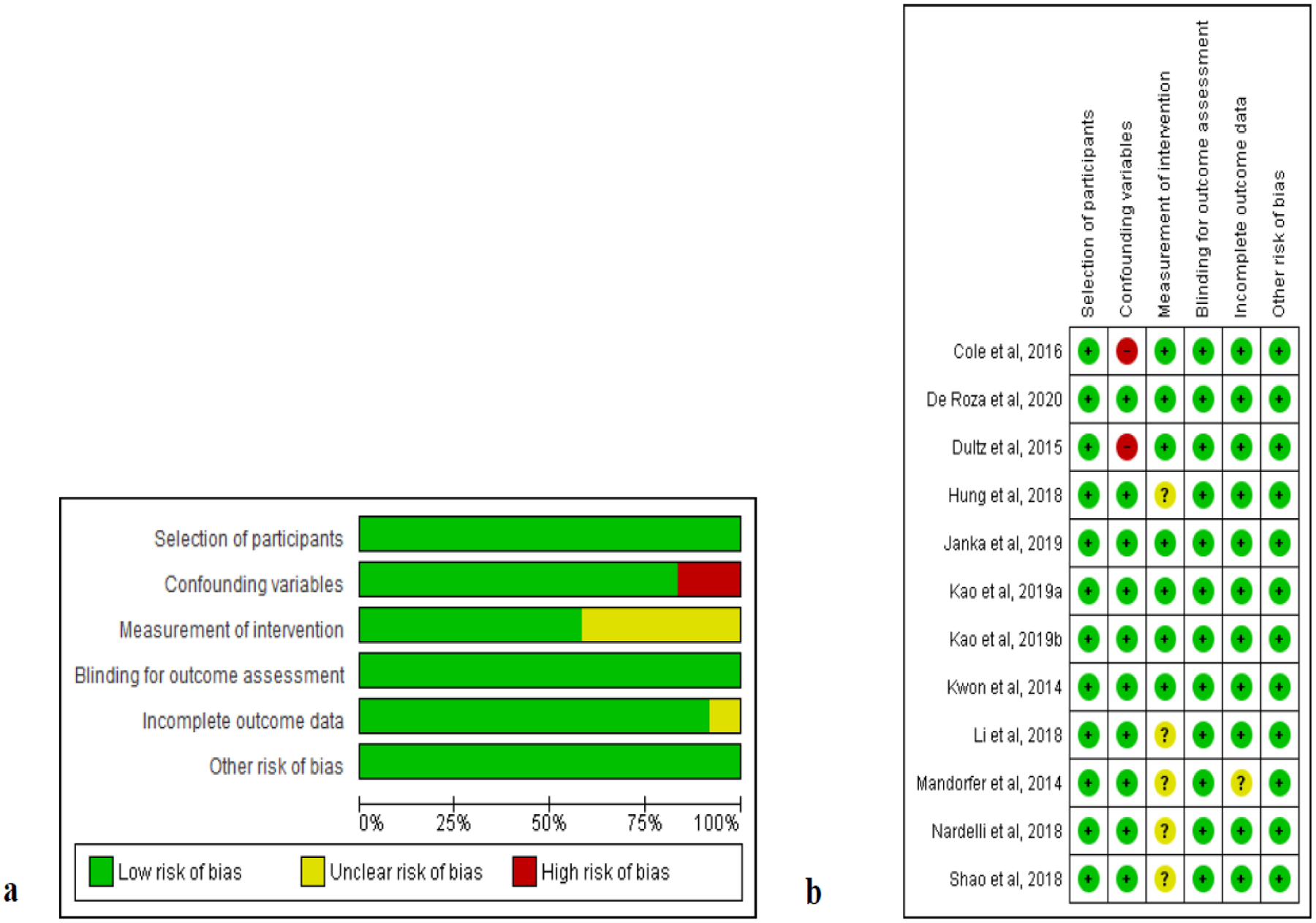
Quality assessment of included studies using Risk of Bias Assessment tool for Nonrandomized Studies (ROBANS) a ROBANS graph and b ROBANS summary +: low risk of bias; ?: unclear risk of bias; −: high risk of bias
Association between PPI-use and liver cancer
Three studies including two from Asia comprising 166,301 patients and evaluating the association between PPIs and HCC were included [20,21,30]. As Kao et al. reported patients with HBV and HCV separately [30]; we had a total of four cohorts in our analysis: one HBV cohort, two HCV cohorts, and one cirrhosis cohort. Overall, the patients’ mean age ranged from 48 to 59 years, with about 48% to 96% male and 6%−19% diabetic patients. Notably, the vast majority of the patients had CLD related to HBV (n=11,154), HCV (n=15,356), or cirrhosis (n=139,791) and less than 3% of HBV and HCV patients had ALD (n= 319) or NAFLD (n=256). All three studies adjusted for relevant demographic, comorbidity and/or concomitant medication covariates in their regression analysis relating HCC outcomes to PPI exposure and showed similar etiology/types of CLD between PPI user and nonuser groups. The Kao et al. and Shao et al. studies defined PPI users by cumulative daily drug dose (cDDD), calculated as the number of pills dispensed by the prescribed dose divided by the recorded days’ supply), of 28 or 30 mg or greater [20,30] while the Li et al. study classified PPI users as those who took at least one PPI prescription at any time during the study period [21] (Table 2). Over a median follow-up time ranging from one to eight years, there were 1,452 cases of incident HCC in 13,037 PPI users (11.1%) and 11,744 cases of incident HCC in 141,738 nonusers (8.3%) from three studies (RR, 1.42; 95% CI 0.68–2.95) (Fig. 3a).
Fig. 3.
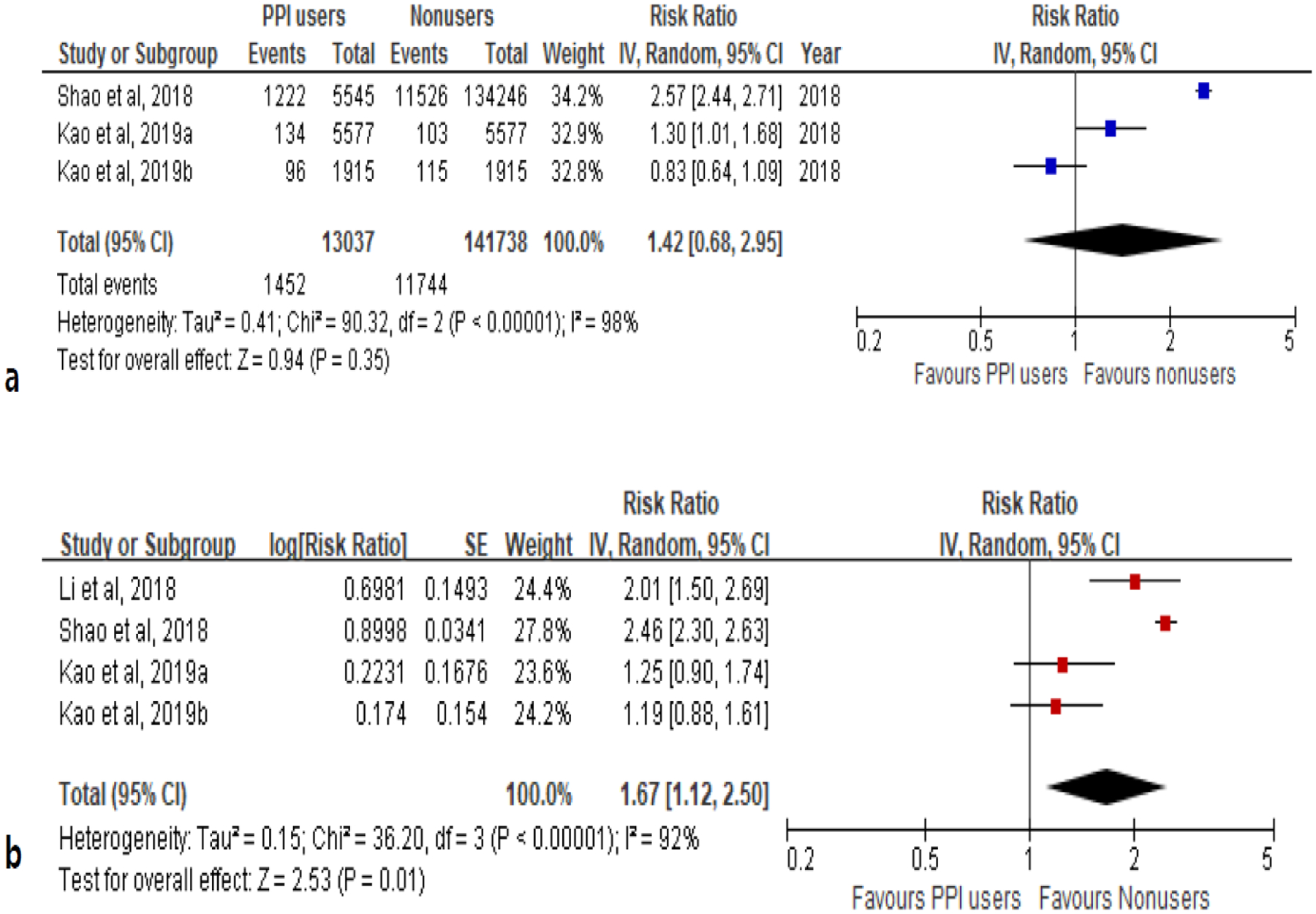
The association between proton pump inhibitor use and the risk of liver cancer in patients with chronic liver disease a unadjusted relative risk and b adjusted relative risk
The pooled risk estimates indicated that PPI users with CLD had a 67% greater risk of developing HCC compared to nonusers (aRR, 1.67; 95% CI, 1.12–2.50) (Fig. 3b). There was evidence of significant heterogeneity (I2=92%, P<0.001), but not publication bias (Fig. 4a). In the subgroup analysis, significantly higher HCC risk was observed in PPI users with hepatitis (PPI users: n=13,244, nonusers: n=13,266) compared to nonuser counterparts (aRR, 1.45; 95% CI, 1.03–2.03). This association was observed among patients with cirrhosis although this finding was not statistically significant (aRR, 1.14; 95% CI, 0.32–4.01) (PPI users: n=5,878, nonusers: n=133,328) (Table 3). We observed that the longer the follow-up of HCC after PPI-use, the higher the pooled RR. Notably, the association between PPI-use and higher HCC risk was not statistically significant in the Asia study.
Fig. 4.
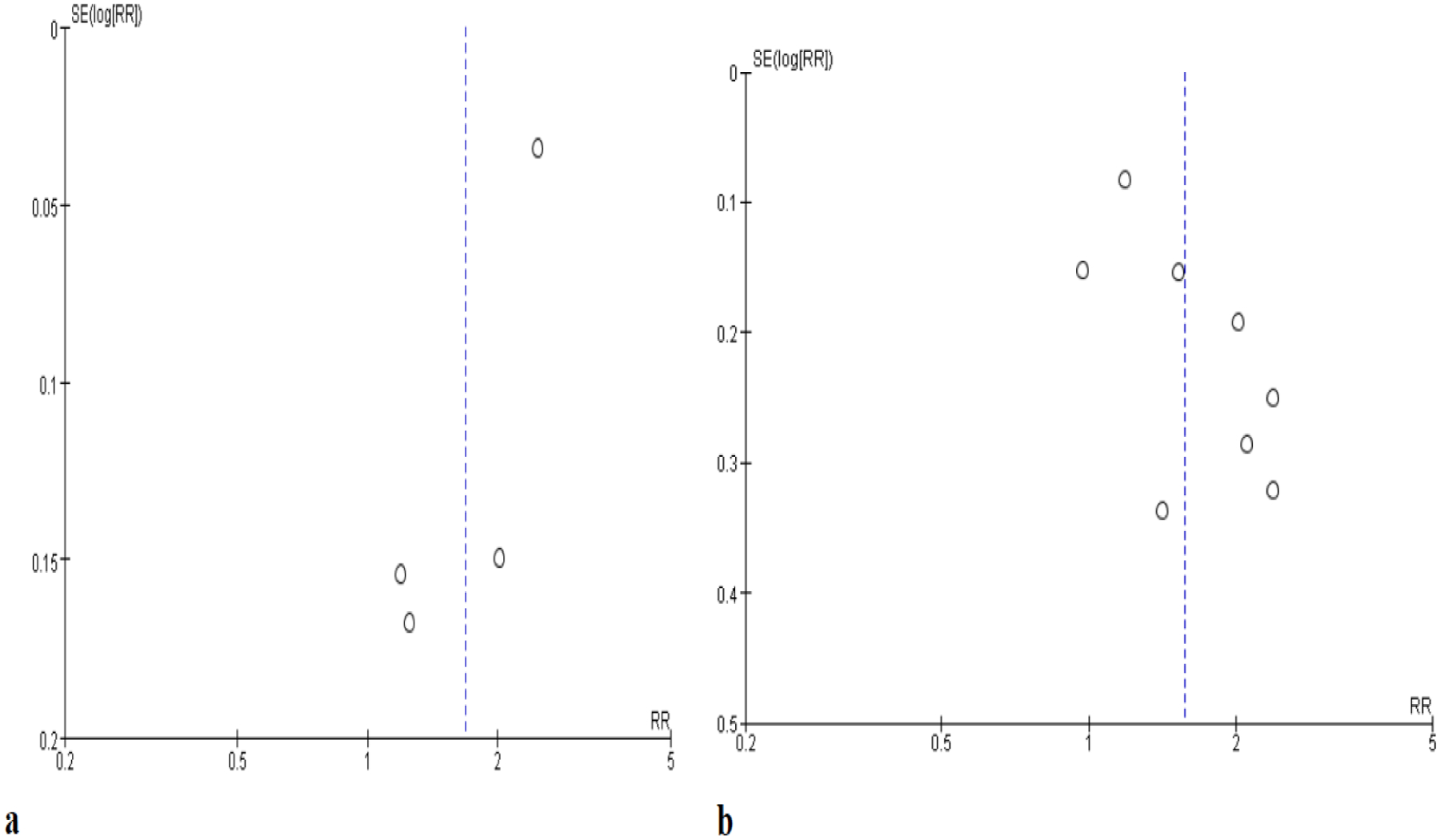
Funnel plot of included studies a liver cancer b mortality
Table 3.
Subgroup analysis of the impact of proton pump inhibitor use on mortality and liver cancer in patients with chronic liver disease
| Subgroup | Studies, n | PPI users, n | Nonusers, n | Random effects, Risk Ratio [95% CI] | Effect, P-value | I2 | Heterogeneity, P-value |
|---|---|---|---|---|---|---|---|
| Proton pump inhibitor use and liver cancer | |||||||
| Liver disease | |||||||
| Non-cirrhotic patients with HBV or HCV | 3 | 13,244 | 13,266 | 1.45 [1.03, 2.03] | 0.03 | 72% | 0.03 |
| Cirrhotic patients | 3 | 5,878 | 134,328 | 1.14 [0.32, 4.01] | 0.84 | 83% | <0.01 |
| Follow-up | |||||||
| 1 year or less | 2 | 7,492 | 7,492 | 1.22 [0.97, 1.52] | 0.08 | 0% | 0.83 |
| More than 1 year | 2 | 11,298 | 140,020 | 2.34 [1.98, 2.77] | <0.01 | 42% | 0.19 |
| Region | |||||||
| Asia | 3 | 13,037 | 141,738 | 1.57 [0.89, 2.74] | 0.12 | 94% | <0.01 |
| Study design | |||||||
| Cohort study | 3 | 13,244 | 13,266 | 1.45 [1.03, 2.03] | 0.03 | 72% | 0.03 |
| Proton pump inhibitor use and mortality | |||||||
| Liver disease | |||||||
| Patients with cirrhosis | 7 | 2,378 | 5,009 | 1.59 [1.23, 2.06] | <0.01 | 73% | <0.01 |
| Follow-up | |||||||
| 1 year or less | 3 | 1,606 | 4,554 | 1.20 [0.97, 1.48] | 0.09 | 54% | 0.11 |
| More than 1 year | 5 | 786 | 547 | 2.05 [1.63, 2.58] | <0.01 | 0% | 0.77 |
| Region | |||||||
| Asia | 3 | 1,324 | 4,524 | 1.43 [1.08, 1.89] | 0.01 | 60% | 0.08 |
| Non-Asia | 5 | 1,168 | 577 | 1.69 [1.12, 2.54] | 0.01 | 75% | 0.003 |
HBV: hepatitis B virus, HCV: hepatitis C virus
Association between PPI-use and mortality
Eight studies investigated the relationship between PPI-use and mortality among patients with CLD (n=7,593 patients: 2,492 PPI users and 5,101 nonusers) [12,18,19,31–35]. One included patients with all types of liver disease [31] whereas seven included patients with cirrhosis [12,18,19,32–35]. Some of the cirrhotic patients from the seven studies also had other liver diseases such as viral hepatitis, ALD or NAFLD, or HCC and the proportion of liver disease among the two groups was similar (Table 1). The majority of the study patients were male (54–77%) with mean age ranging 56 to 63 years. For CLD patients, the model for end-stage liver disease (MELD) score ranged from 11 to 20. Regarding liver disease etiology, ALD accounted for 8–55% among the included studies, NAFLD 3–18%, HBV 13–75%, and HCV 12–27%. Of note, about half of Hung et al. (45%) and Kwon et al. (54%) study patients had HCC [18,32]. The median follow-up time ranged from 30 days to 3.4 years (Table 2). Among eight studies, five reported the number of deaths and adjusted HR [18,19,32,34,35] and three reported mortality data only as adjusted HR [12,31,33]. In the five studies, there were 1,062 deaths among 1,705 PPI users (62.2%) and 2,718 deaths among 4,803 nonusers (56.6%) (RR, 1.82; 95% CI, 1.22–2.72) (Fig. 5a).
Fig. 5.
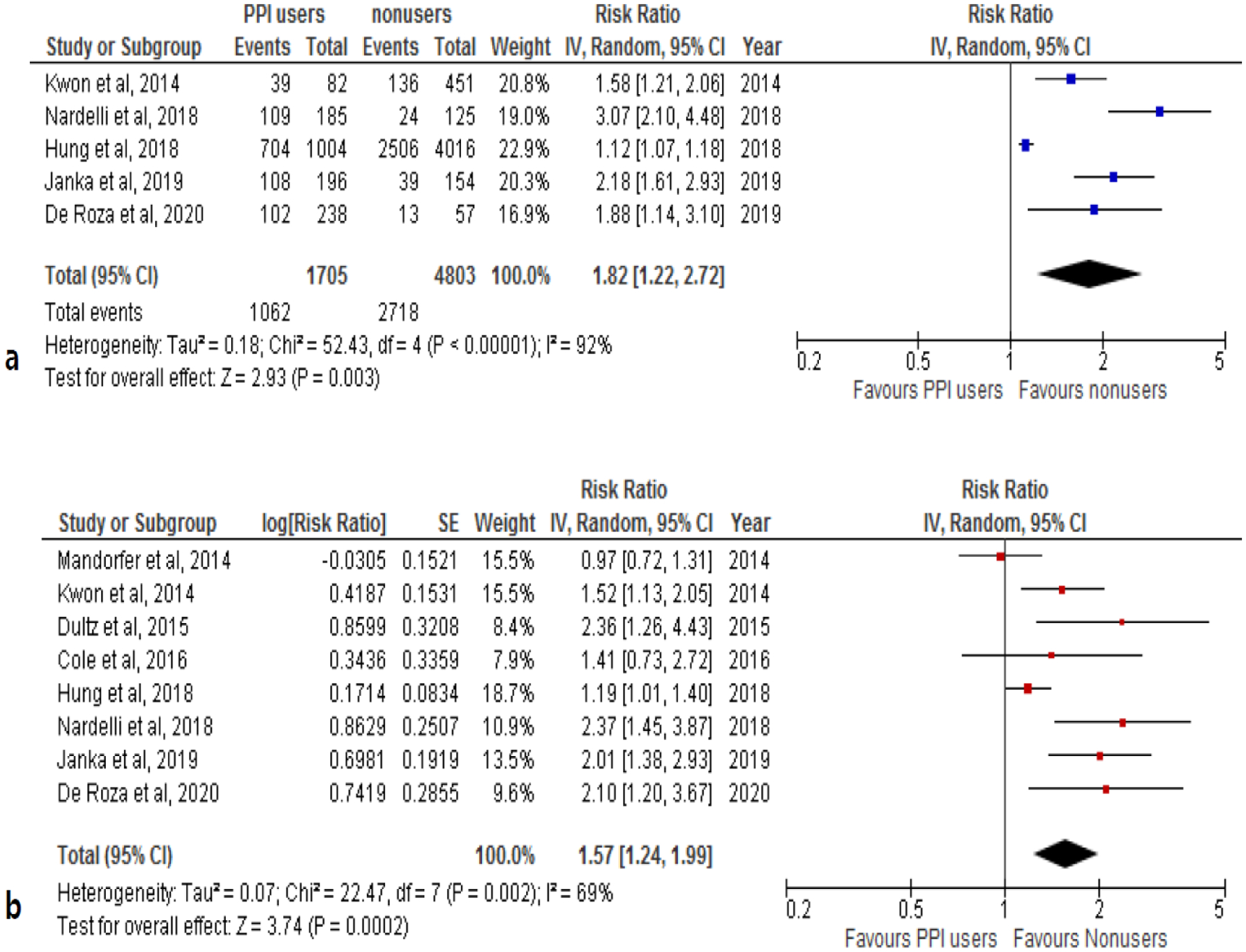
The association between proton pump inhibitor use and the risk of mortality in patients with chronic liver disease a unadjusted relative risk and b adjusted relative risk
Pooled estimates from the eight included studies indicated that PPI users had a 57% increased risk of (RR) mortality compared to PPI nonusers (aRR, 1.57; 95% CI, 1.24–1.99). There was significant heterogeneity (I2=69%, P=0.002) (Fig. 5b) but not publication bias (Fig. 4b). We found a significant association between PPI-use and increased mortality among patients with cirrhosis in seven studies (aRR, 1.59; 95% CI, 1.23–2.06). There was insufficient data to perform sub-analysis for patients with hepatitis. When investigating the effect of follow-up duration, the association between PPI-use and increased mortality was highly significant (aRR, 2.05; 95% CI, 1.63–2.58), while the association was only modest and trending towards significance among those with a one-year follow-up or shorter duration (aRR, 1.20; 95% CI, 0.97–1.48) (Table 3). The significant association between PPI-use and mortality appeared consistent among studies from Asia (aRR, 1.43; 95% CI, 1.08–1.89) and non-Asia (aRR, 1.69; 95% CI, 1.12–2.54).
Discussion
To the best of our knowledge, this is first meta-analysis to evaluate and quantify the association between PPI-use and the risk of liver cancer and mortality among patients with CLD. Overall, we found that patients with CLD who used PPIs had a 67% increased risk of HCC and a 57% increased risk of mortality compared to nonusers, though there were some differences among the various subgroups.
Investigations of the association between PPI-use and hepatic encephalopathy in patients with liver dysfunction [36] and PPI-use and HCC in general population (i.e. people with or without liver disease) [37] reported varied results. One meta-analysis reported that PPIs were associated with a higher hepatic encephalopathy risk among patients with chronic and acute liver dysfunction (OR, 1.76; 95% CI, 1.15–2.69) [36]. Another reported that there was no significant association between PPI-use and the risk of HCC (OR, 1.58; 95% CI, 0.91–2.76) [37]. The differences in our findings may be due to previous studies not considering the impact of CLD as an important risk factor for the incidence of HCC [38]. In fact, HCC almost exclusively occurs in the setting of CLD.
Therefore, to further investigate our findings, we performed subgroup analyses and found that patients with hepatitis were at a higher risk for HCC than nonusers. In addition, patients with >1 year follow-up after initiating PPIs had a two times greater risk for HCC than those with ≤1 year. Unfortunately, we were unable to analyze PPI dosage as only two studies (Li et al. and Shao et al.) reported dose-dependent risk. They found that an increased cumulative daily dose was associated with an increased risk of HCC [20,21]. Though we also found that PPI-use was associated with increased risk of HCC among cirrhotic patients, this finding was not statistically significant, probably due to small sample sizes of PPI users among cirrhotic patients.
Besides the observed increased risk for mortality, we also found that PPI-use was significantly associated with increased risk of death in CLD patients (aRR, 1.57; 95% CI, 1.24–1.99). The association remained significant among patients with cirrhosis and those with >1 year follow-up. Several meta-analyses evaluated the association between PPIs and risk of death in patients with other chronic medical conditions (e.g., spontaneous bacterial peritonitis [SBP]) and their findings are somewhat different [39–42]. Yu et al. conducted a meta-analysis of PPI-use and the risk for mortality among cirrhotic patients with SBP. They reported that the association was not statistically significant. However, they noted that their meta-analysis included only four observational studies including one of low quality and cautioned readers that their results were unstable and further studies were needed [39]. The results of systematic reviews about the association between PPIs and mortality in patients taking clopidogrel were also controversial [40–42].
Although the pathophysiologic mechanism between PPI-use and the risk of liver cancer and death are not well understood, several plausible mechanisms have been suggested. Since PPIs are metabolized in the liver, PPI toxicity may occur in liver impaired patients which could lead to hypergastrinemia causing carcinogenic effects, especially on liver cells [43,44]. In addition, the use of cultured cells from the human liver have exhibited a genetic expression similar to well-known carcinogens in the liver after exposure to PPIs [45,46]. Reducing gastric acid with PPIs also leads to bacterial overgrowth of the stomach by increasing various microbes [48,49]. It has been shown that primary bile acid of the intestine transforms to secondary bile acid contributing to liver disease exacerbation in mice [50–52]. High levels of secondary bile acid in liver and bile duct cells may cause inflammatory, toxic, and deoxyribonucleic acid (DNA) damage that may contribute to HCC and cholangiocarcinoma [53,54]. Furthermore, PPI-use was found to lead to the proliferation of cells with fatal mutations through the induction of oxidative stress and the production of reactive oxygen species that further damage DNA and increases the mutation rate, tumor suppressor genes and oncogenes-increasing the risk of cancers [55–58]. Others have suggested that PPI-use limits the regenerative capacity of livers, reduces proteostasis and lysosomal acidification, and may promote oxidative stress, dysfunction, telomere shortening, aging of human endothelial cells, blockage of the antigen-presenting pathway, inhibiting synthesis and secretion of cytokines, as well as effecting the complement component proteins and coagulation factors. However, the mechanism of the association between changing gene expression and the risk of death is not entirely clear, requiring further study [59–62].
Our study has several strengths. First, this study, to the best of our knowledge, is the first systematic review and meta-analysis to examine the association between PPI-use and liver cancer and mortality in patients with CLD. The results of each observational study were controversial so now we are able to offer the best available evidence through our systematic and meta-analytic approach. Another strength of this study was that the present meta-analysis included a large sample size and high-quality studies. Thus, the precision of the meta-analysis was increased and the results more reliable. Third, we could identify PPI as an independent risk factor for liver cancer or mortality in patients with CLD since we used the best-adjusted estimates to obtain the pooled estimate after controlling for confounders including demographic characteristics, comorbidities, and/or concomitant medications.
Several limitations need to be considered in the interpretation of our findings. First, the number of included studies was small, so we could not examine the magnitude of the association in detail or stratify by dose or different types of CLDs or different categories of PPI. Only two studies reported the dose-related association and found a PPI associated with an increased risk of HCC in a dose-dependent manner. There was one study that investigated a dose-dependent risk for mortality. Second, there was substantial heterogeneity in the population and quality of the original studies. The methods used to ascertain PPI-use and population varied widely across studies, likely contributing to the high degree of heterogeneity in the results. Although a random-effects meta-analysis, which takes into account study variability and confounders, was used to obtain a pooled estimate of studies, unknown factors can affect our results. Third, the included studies of this meta-analysis were cohort or case-control studies. Thus, we could only investigate the association between PPI-use and liver cancer or mortality and the casual relationship could be not confirmed. Fourth, there could be a confounding effect by indication of PPI use among “sicker” patients at higher risk for gastrointestinal bleeding. However, except for the few specific situations such as the immediate postendoscopic variceal banding period, there are generally no proven benefit or recommendation for PPI use in sicker or decompensated liver patients. In addition, PPI use in such situations is usually short-term. Therefore, it is likely that the vast majority of PPI use among cirrhotic patients are for indications that would be similar to the widespread use of PPI in the non-CLD population. Fifth, residual confounding is possible because no information was available for the duration of liver disease.
Conclusion
This systematic review and meta-analysis found that PPI-use was associated with an increased risk for HCC and mortality in CLD patients. We discussed various pathophysiologic mechanisms for these findings to include the direct damage to the liver cells and the impact of the liver disease itself in perhaps hastening liver disease progression. However, these theories require further study before conclusions can be drawn. Therefore, we conclude that PPIs should be used cautiously in patients with CLD.
Supplementary Material
Funding information
Research reported in this publication was supported in part by the National Institute on Drug Abuse of the National Institutes of Health under award number K01DA045618 (to HP).
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflicts of Interest The authors declare that they have no competing interests.
References
- 1.Kantor ED, Rehm CD, Haas JS, Chan AT, Giovannucci EL (2015) Trends in prescription drug use among adults in the United States from 1999–2012. JAMA 314:1818–1831. 10.1001/jama.2015.13766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schumock GT, Li EC, Suda KJ, et al. (2016) National trends in prescription drug expenditures and projections for 2016. Am J Health Syst Pharm 73:1058–1075. 10.2146/ajhp160205 [DOI] [PubMed] [Google Scholar]
- 3.McDonagh MS, Carson S, Thakurta S (2009) Drug class review: proton pump inhibitors: final report update 5. Portland, OR: Oregon Health & Science University. [PubMed] [Google Scholar]
- 4.Katz PO, Gerson LB, Vela MF (2013) Guidelines for the diagnosis and management of gastroesophageal reflux disease. Am J Gastroenterol 108:308–328. 10.1038/ajg.2012.444 [DOI] [PubMed] [Google Scholar]
- 5.Islam MM, Poly TN, Walther BA, et al. (2018) Adverse outcomes of long-term use of proton pump inhibitors: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol 30:1395–1405. 10.1097/MEG.0000000000001198 [DOI] [PubMed] [Google Scholar]
- 6.Batchelor R, Kumar R, Gilmartin-Thomas JFM, Hopper I, Kemp W, Liew D (2018) Systematic review with meta-analysis: risk of adverse cardiovascular events with proton pump inhibitors independent of clopidogrel. Aliment Pharmacol Ther 48:780–796. 10.1111/apt.14955 [DOI] [PubMed] [Google Scholar]
- 7.Laoveeravat P, Thavaraputta S, Vutthikraivit W, et al. (2019) Proton pump inhibitors and histamine-2 receptor antagonists on the risk of pancreatic cancer: a systematic review and meta-analysis. QJM. [Equb ahead of print] 10.1093/qjmed/hcz234 [DOI] [PubMed] [Google Scholar]
- 8.Xie Y, Bowe B, Li T, Xian H, Yan Y, Al-Aly Z (2017) Risk of death among users of proton pump inhibitors: a longitudinal observational cohort study of United States veterans. BMJ Open 6:e015735 10.1136/bmjopen-2016-015735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xie Y, Bowe B, Yan Y, Xian H, Li T, Al-Aly Z (2019) Estimates of all cause mortality and cause specific mortality associated with proton pump inhibitors among US veterans: cohort study. BMJ 365:l1580 10.1136/bmj.l1580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Singh A, Cresci GA, Kirby DF (2018) Proton Pump Inhibitors: Risks and Rewards and Emerging Consequences to the Gut Microbiome. Nutr Clin Pract 33:614–624. 10.1002/ncp.10181 [DOI] [PubMed] [Google Scholar]
- 11.Nehra AK, Alexander JA, Loftus CG, Nehra V (2018) Proton Pump Inhibitors: Review of Emerging Concerns. Mayo Clin Proc 93:240–246. 10.1016/j.mayocp.2017.10.022 [DOI] [PubMed] [Google Scholar]
- 12.Dultz G, Piiper A, Zeuzem S, Kronenberger B, Waidmann O (2015) Proton pump inhibitor treatment is associated with the severity of liver disease and increased mortality in patients with cirrhosis. Aliment Pharmacol Ther 41:459–466. 10.1111/apt.13061 [DOI] [PubMed] [Google Scholar]
- 13.Li DK, Chung RT (2017) Use of proton pump inhibitors in chronic liver diseases. Clin Liver Dis 10:148–151. 10.1002/cld.678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.European Association for the Study of the Liver (2018) EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J Hepatol 69:406–460. [DOI] [PubMed] [Google Scholar]
- 15.Suh JK, Lee J, Lee JH, Shin S, Tchoe HJ, Kwon JW (2018) Risk factors for developing liver cancer in people with and without liver disease. PLoS One 13:e0206374 10.1371/journal.pone.0206374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Said A, Ghufran A (2017) Epidemic of non-alcoholic fatty liver disease and hepatocellular carcinoma. World J Clin Oncol 8:429–436. 10.5306/wjco.v8.i6.429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Piñero F, Pages J, Marciano S, et al. (2018) Fatty liver disease, an emerging etiology of hepatocellular carcinoma in Argentina. World J Hepatol 10:41–50. 10.4254/wjh.v10.i1.41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hung TH, Lee HF, Tseng CW, Tsai CC, Tsai CC (2018) Effect of proton pump inhibitors in hospitalization on mortality of patients with hepatic encephalopathy and cirrhosis but no active gastrointestinal bleeding. Clin Res Hepatol Gastroenterol 42:353–359. 10.1016/j.clinre.2017.11.011 [DOI] [PubMed] [Google Scholar]
- 19.Nardelli S, Gioia S, Ridola L, Farcomeni A, Merli M, Riggio O (2019) Proton pump inhibitors are associated to minimal and overthepatic encephalopathy and increase mortality in cirrhotics. Hepatology 70:640–649. 10.1002/hep.30304 [DOI] [PubMed] [Google Scholar]
- 20.Shao YJ, Chan TS, Tsai K, Wu SY (2018) Letter: association between proton pump inhibitors and the risk of hepatocellular carcinoma. Aliment Pharmacol Ther 48:460–468. 10.1111/apt.15014 [DOI] [PubMed] [Google Scholar]
- 21.Li DK, Yan P, Abou-Samra AB, Chung RT, Butt AA (2018) Proton pump inhibitors are associated with accelerated development of cirrhosis, hepatic decompensation and hepatocellular carcinoma in noncirrhotic patients with chronic hepatitis C infection: results from ERCHIVES. Aliment pharmacol Ther 47:246–258. 10.1111/apt.14391 [DOI] [PubMed] [Google Scholar]
- 22.Liberati A, Altman DG, Tetzlaff J, et al. (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med 151:W65–W94. 10.1016/j.jclinepi. [DOI] [PubMed] [Google Scholar]
- 23.Kim SY, Park JE, Lee YJ, et al. (2013) Testing a tool for assessing the risk of bias for nonrandomized studies showed moderate reliability and promising validity. J Clin Epidemiol 66:408–414. 10.1016/j.jclinepi.2012.09.016 [DOI] [PubMed] [Google Scholar]
- 24.Davies HT, Crombie IK, Tavakoli M (1998) When can odds ratios mislead? BMJ 316:989–991. 10.1136/bmj.316.7136.989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stare J, Maucort-Boulch D (2016) Odds ratio, hazard ratio and relative risk. Metodoloski zvezki 13:59–67. https://www.stat-d.si/mz/mz13.1/p4.pdf [Google Scholar]
- 26.Zhang J, Yu KF (1998) What’s the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. JAMA 280:1690–1691. 10.1001/jama.280.19.1690 [DOI] [PubMed] [Google Scholar]
- 27.Nochaiwong S, Ruengorn C, Awiphan R, et al. (2018) The association between proton pump inhibitor use and the risk of adverse kidneyoutcomes: a systematic review and meta-analysis. Nephrol Dial Transplant 33:331–342. 10.1093/ndt/gfw470 [DOI] [PubMed] [Google Scholar]
- 28.Yang Y, George KC, Shang WF, Zeng R, Ge SW, Xu G (2017) Proton-pump inhibitors use, and risk of acute kidney injury: a meta-analysis of observational studies. Drug Des Devel Ther 11:1291–1299. 10.2147/DDDT.S130568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327:557–560. 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kao WY, Su CW, Tan EC, et al. (2019) Proton Pump Inhibitors and Risk of Hepatocellular Carcinoma in Patients with Chronic Hepatitis B or C. Hepatology 69:1151–1164. 10.1002/hep.30247 [DOI] [PubMed] [Google Scholar]
- 31.Cole HL, Pennycook S, Hayes PC (2016) The impact of proton pump inhibitor therapy on patients with liver disease. Aliment Pharmacol Ther 44:1213–1223. 10.1111/apt.13827 [DOI] [PubMed] [Google Scholar]
- 32.Kwon JH, Koh SJ, Kim W, et al. (2014) Mortality associated with proton pump inhibitors in cirrhoticpatients with spontaneous bacterial peritonitis. J Gastroenterol Hepatol 29:775–781. 10.1111/jgh.12426 [DOI] [PubMed] [Google Scholar]
- 33.Mandorfer M, Bota S, Schwabl P, et al. (2014) Proton pump inhibitor intake neither predisposes to spontaneous bacterial peritonitis or other infections nor increases mortality in patients with cirrhosis and ascites. PLoS One 9:e110503 10.1371/journal.pone.0110503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.De Roza MA, Kai L, Kam JW, et al. (2020) Proton pump inhibitor use increases mortality and hepatic decompensation in liver cirrhosis. World J Gastroenterol 25:4933–4944. 10.3748/wjg.v25.i33.4933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Janka T, Tornai T, Borbély B, et al. (2019) Deleterious effect of proton pump inhibitors on the disease course of cirrhosis. Eur J Gastroenterol Hepatol 32:257–264. 10.1097/MEG.0000000000001499 [DOI] [PubMed] [Google Scholar]
- 36.Bian J, Wang A, Lin J, et al. (2017) Association between proton pump inhibitors and hepatic encephalopathy: A meta-analysis. Medicine (Baltimore) 96:e6723 10.1097/MD.0000000000006723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhao J, Hua L, Li N, An R, Liang C (2018) Letter: proton pump inhibitors use and risk of hepatocellular carcinoma: A meta-analysis of observational studies. Aliment Pharmacol Ther 48:886–888. 10.1111/apt.14962 [DOI] [PubMed] [Google Scholar]
- 38.Janevska D, Chaloska-Ivanova V, Janevski V (2015) Hepatocellular Carcinoma: Risk Factors, Diagnosis and Treatment. Open Access Maced J Med Sci 3:732–736. 10.3889/oamjms.2015.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yu T, Tang Y, Jiang L, Zheng Y, Xiong W, Lin L (2016) Proton pump inhibitor therapy and its association with spontaneous bacterial peritonitis incidence and mortality: A meta-analysis. Dig Liver Dis 48:353–359. 10.1016/j.dld.2015.12.009 [DOI] [PubMed] [Google Scholar]
- 40.Demcsák A, Lantos T, Bálint ER, et al. (2018) PPIs are not responsible for elevating cardiovascular risk in patients on clopidogrel – A systematic review and meta-analysis. Front Physiol 9:1550 10.3389/fphys.2018.01550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kwok CS, Loke YK (2010) Meta-analysis: the effects of proton pump inhibitors on cardiovascular events and mortality in patients receiving clopidogrel. Aliment Pharmacol Ther 31:810–823. 10.1111/j.1365-2036.2010.04247.x [DOI] [PubMed] [Google Scholar]
- 42.Shiraev TP, Bullen A (2018) Proton pump inhibitors and cardiovascular events: a systematic review. Heart Lung Circ 27:443–450. 10.1016/j.hlc.2017.10.020 [DOI] [PubMed] [Google Scholar]
- 43.Fossmark R, Sagatun L, Nordrum IS, Sandvik AK, Waldum HL(2015) Hypergastrinemia is associated with adenocarcinomas in the gastric corpus and shorter patient survival. APMIS 123:509–514. 10.1111/apm.12380 [DOI] [PubMed] [Google Scholar]
- 44.Caplin M, Khan K, Savage K, et al. (1999) Expression and processing of gastrin in hepatocellular carcinoma, fibrolamellar carcinoma and cholangiocarcinoma. J Hepatol 30:519–526. 10.1016/s0168-8278(99)80114-7 [DOI] [PubMed] [Google Scholar]
- 45.Caiment F, Tsamou M, Jennen D, Kleinjans J (2014) Assessing compound carcinogenicity in vitro using connectivity mapping. Carcinogenesis 35:201–207. 10.1093/carcin/bgt278 [DOI] [PubMed] [Google Scholar]
- 46.Lamb J, Crawford ED, Peck D, et al. (2006) The Connectivity Map: using gene-expression signatures to connect small molecules, genes, and disease. Science 313:1929–1935. 10.1126/science.1132939 [DOI] [PubMed] [Google Scholar]
- 47.Tran KT, McMenamin ÚC, Hicks B, et al. (2018) Proton pump inhibitor and histamine-2 receptor antagonist use and risk of liver cancer in two population-based studies. Aliment Pharmacol Ther 48:55–64. 10.1111/apt.14796 [DOI] [PubMed] [Google Scholar]
- 48.Lewis SJ, Franco S, Young G, O’Keefe SJ (1996) Altered bowel function and duodenal bacterial overgrowth in patients treated with omeprazole. Aliment Pharmacol Ther 10:557–561. 10.1046/j.1365-2036.1996.d01-506.x [DOI] [PubMed] [Google Scholar]
- 49.Thorens J, Froehlich F, Schwizer W, et al. (1996) Bacterial overgrowth during treatment with omeprazole compared with cimetidine: a prospective randomised double blind study. Gut 39:54–59. 10.1136/gut.39.1.54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Masclee A, Tangerman A, van Schaik A, van der Hoek EW, van Tongeran JHM (1989) Unconjugated serum bile acid as a marker of small intestinal bacterial overgrowth. Eur J Clin Invest 19:384–389. 10.1111/j.1365-2362.1989.tb00246.x [DOI] [PubMed] [Google Scholar]
- 51.Llorente C, Schnabl B (2015) The gut microbiota and liver disease. Cell Mol Gastroenterol Hepatol 1:275–284. 10.1016/j.jcmgh.2015.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Llorente C, Jepsen P, Inamine T, et al. (2017) Gastric acid suppression promotes alcoholic liver disease by inducing overgrowth of intestinal Enterococcus. Nat Commun 1–14. 10.1038/s41467-017-00796-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jansen PLM (2007) Endogenous bile acids as carcinogens. J Hepatol 47:434–435. 10.1016/j.jhep.2007.06.001 [DOI] [PubMed] [Google Scholar]
- 54.Komichi D, Tazuma S, Nishioka T, Hyogo H, Chayama K (2005) lycochenodeoxycholate plays a carcinogenic role in immortalized mouse cholangiocytes via oxidative DNA damage. Free Radic Biol Med 39:1418–1427. 10.1016/j.freeradbiomed.2005.07.005 [DOI] [PubMed] [Google Scholar]
- 55.Miner PB Jr (2006) Review article: physiologic and clinical effects of proton pump inhibitors on non-acidic and acidic gastro-oesophageal reflux. Aliment Pharmacol Ther 23:25–32. 10.1111/j.1365-2036.2006.02802.x [DOI] [PubMed] [Google Scholar]
- 56.Bernstein H, Bernstein C, Payne CM, Dvorakova K, Garewal H (2005) Bile acids as carcinogens in human gastrointestinal cancers. Mutat Res 589:47–65. 10.1016/j.mrrev.2004.08.001 [DOI] [PubMed] [Google Scholar]
- 57.Martin J, Dufour JF (2008) Tumor suppressor and hepatocellular carcinoma. World J Gastroenterol 14:1720–1733. 10.3748/wjg.14.1720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lee EY, Muller WJ (2010) Oncogenes and tumor suppressor genes. Cold Spring Harb Perspect Biol 2:a003236 10.1101/cshperspect.a003236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kucuk HF, Akyol H, Kaptanoglu L, et al. (2006) Effect of proton pump inhibitors on hepatic regeneration. Eur Surg Res 38:322–328. 10.1159/000094020 [DOI] [PubMed] [Google Scholar]
- 60.Yepuri G, Sukhovershin R, Nazari-Shafti TZ, Petrascheck M, Ghebre YT, Cooke JP (2016) Proton pump inhibitors accelerate endothelial senescence. Circ Res 118:e36–42. 10.1161/CIRCRESAHA.116.308807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wu D, Qiu T, Zhang Q, et al. (2015) Systematic toxicity mechanism analysis of proton pump inhibitors: an in Silico Study. Chem Res Toxicol 28:419–430. 10.1021/tx5003782 [DOI] [PubMed] [Google Scholar]
- 62.Liu W, Baker SS, Trinidad J, et al. (2013) Inhibition of lysosomal enzyme activities by proton pump inhibitors. J Gastroenterol 48:1343–1352. 10.1007/s00535-013-0774-5 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


