Abstract
Rationale:
A variety of neural systems are involved in drug addiction, and some of these systems are shared across different addictive drugs. We have found several different types of drug treatments that successfully reduce nicotine self-administration.
Objectives:
The current set of studies is the first in a series to determine if drug treatments that have been found to significantly reduce nicotine self-administration would reduce opiate self-administration.
Methods:
Amitifadine, a triple reuptake inhibitor of dopamine, norepinephrine and serotonin, was assessed in female Sprague-Dawley rats to determine whether it significantly reduces remifentanil self-administration with either acute or chronic treatment.
Results:
Acutely, amitifadine doses of 5, 10 and 20 mg/kg each significantly reduced remifentanil self-administration. In a chronic study, repeated treatment with 10 mg/kg of amitifadine continued to reduce remifentanil self-administration, even after the cessation of treatment. However, amitifadine was not found to attenuate the rise in remifentanil self-administration with continued access. This study and our earlier one showed that the 10 mg/kg amitifadine dose did not significantly affect food motivated responding. Amitifadine did not attenuate remifentanil-induced antinociception as measured on the hot plate test but extended and maintained anti-nociceptive effects.
Conclusions:
These studies show the promise of amitifadine as a treatment for countering opiate self-administration for adjunctive use with opioids for analgesia. Further studies are needed to determine the possible efficacy of amitifadine for combating opiate addiction or preventing it in humans during adjunctive use with opioids for chronic pain.
Keywords: Amitifadine, Remifentanil, Self-administration, Rats, Opioids, Antinociception
Introduction
Opiate addiction and overdose deaths have risen substantially during the past decade (Gomes et al. 2018). Clearly, additional effective treatments for opiate addictions are needed. The neural systems involved with drug addiction are complex. Certainly, dopaminergic innervation of the nucleus accumbens from the ventral tegmental area is central (Langlois and Nugent 2017). However, other neural systems play important roles as well. Other monoamines such as norepinephrine, serotonin and histamine play roles in addictive behavior. We and others have found that a variety of monoaminergic and cholinergic drug treatments significantly reduce nicotine self-administration in rats. These drugs include dopamine (DA) D1 (Hall et al. 2015), serotonin (5HT) 5HT2c (DiPalma et al. 2019; Guy et al. 2014; Higgins et al. 2012; Levin et al. 2011a), histamine H1 receptor (Levin et al. 2011b) acting compounds as well as a monoaminergic reuptake inhibitor (Levin et al. 2015; Levin et al. 2019b). Since multiple drug addictions rely on overlapping neural circuits, there is potential for treatments that attenuate self-administration of one drug of abuse may be effective in reducing self-administration of other drugs of abuse. Wee et al., (2005) observed in a series of potent amphetamine analogs that the analog with the highest 5-HT to DA releasing potency ratio was the least active reinforcer whereas the compound with the lowest 5-HT to DA releasing potency ratio (d-amphetamine) resulted in the highest self-administration. It has been confirmed that 5-HT plays a predominant role in the inhibition of neuronal DA activity (Di Giovanni et al. 2008) and that the ratio of 5-HT to DA reuptake is key for driving drug self-administration (Rosenberg et al. 2013; Rothman and Baumann 2006). This relationship is not an absolute, however, since fluoxetine failed to improve clinical outcomes in cocaine abuse during clinical trials (Grabowski et al. 1995; Schmitz et al. 2001; Winstanley et al. 2011).
Some therapeutic treatments can effectively reduce self-administrtion of more than one type of drugs of abuse. For example, we have found that sazetidine-A, an α4β2 nicotinic partial agonist and desensitizing agent, significantly reduces not only nicotine self-administration in rats, but also reduces alcohol, cocaine and methamphetamine self-administration (Levin et al. 2019a; Rezvani et al. 2010). Another drug lorcaserin, a serotonin 5HT2c agonist, reduces nicotine, cocaine and alcohol self-administration in animal models (Gerak et al. 2016; Rezvani and Levin 2014). However, there needs to be caution when translating experimental animal results to efficacy in humans. In some cases the animal studies are predictive of human efficacy. For example, lorcaserin reduces nicotine self-administration in rats (Higgins et al. 2012; Levin et al. 2011a) and was also found to significantly enhance tobacco smoking cessation (Shanahan et al. 2017). In contrast, even though lorcaserin reduced cocaine self-administration in experimental monkey models (Gerak et al. 2016) but was not found to reduce cocaine reward in humans (Pirtlea et al. 2019).
In the current study, we tested the efficacy of the triple monoamine reuptake inhibitor amitifadine which reduces nicotine self-administration, for reducing the self-administration of remifentanil, a short acting opioid (Michelsen and Hug 1996). In interpreting the usefulness of amitifadine to help overcome or lower risk of opiate addiction, it is important to evaluate its selectivity for opiate self-administration over general motivated behavior. We previously tested the effects of amitifadine on food-motivated responding and found that 20 mg/kg of amitifadine significantly reduced food-motivated responding but that 10 mg/kg did not (Levin et al. 2015). In the current study, we again tested amitifadine effects on food-motivated responding. Finally, if amitifadine has potential to decrease abuse liability of opiates in use as analgesics, the combined analgesic effects should not be less than the opioid alone. Thus, we also tested the effects of amitifadine on remifentanil-induced antinociception usuing the standard hot plate test,
Amitifadine is a serotonin-preferring triple with the following reuptake inhibition profile in human embryonic kidney cells: serotonin transporter (SERT): 12 nM; norepinepherine transporter) NET: 23 nM; (dopamine transporter) DAT: 96 nM, with 8 times greater selectivity at SERT compared with DAT (Skolnick et al. 2003). For perspective, duloxetine is a dual reuptake inhibitor with mood and analgesic claims. It has a similar reuptake inhibitor profile to amitifadine with respect to serotonin and norepinephrine: SERT: 4.6 nM; NET: 16 nM; DAT: 369 nM, but at lower relative binding selectivity of SERT to DAT with a ratio of 80 (Bymaster et al. 2001). Nefopam is a putative triple reuptake inhibitor in worldwide use with perioperative and chronic analgesia indications. Nefopam has the following profile inhibiting monoamine transporters: SERT: 29 nM; NET: 33 nM; DAT: 531 nM, a SERT to DAT ratio of 18 (Gregori-Puigjané et al. 2012) and may have NMDA effects (Fernández-Sánchez et al. 2002). In comparison, cocaine inhibits monoamine transportor activity in synaptosomes to the follow degrees: SERT: 0.300 μM; NET: 0.222 μM; DAT: 0.305 μM , SERT to DAT ratio of 1.15 (Gannon et al. 2018). Thus, the rank order of DAT to SERT selectivity is cocaine > amitifadine > nefopam > duloxetine.
The main difference between these agents is that compared with nefopam and duloxetine, amitifadine has higher DAT vs. SERT selectively . It is believed this may be relevant in the treatment of chronic pain where reduced dopamine neurotransmission may be relevant during opioid-dose reduction and in subpopulations with co-morbid mood symptoms (Jarcho et al. 2012). Patients in pain are believed to perceive pain to a greater degree because of impaired DA signaling, and reduced DA activity is reported to make pain more unpleasant (Tiemann et al. 2014). Thus, a key differentiating factor between amitifadine and other monoaminergic reuptake inhibitors is that amitifadine has effects on 5-HT, NE, and DA neurotransmission with potential mood, anxiety and anti-nociceptive effects. Amitifadine effects on dopamine neurotransmission may reduce the perception and emotional aspects of pain. There is also substantial animal literature suggesting the role of dopamine blockade or depletion in some pain perception (Aksoy et al. 2015; Faramarzi et al. 2016; Tiemann et al. 2014).
In spite of the dopaminergic activity, amitifadine does not cause locomotor activation in rodents (Golembiowska et al. 2012; Skolnick et al. 2003), likely due to the potent serotonin activity and 8x less dopamine potency. Miller et al. (2015) observed that amitifadine has potent SERT/NET pharmacology to treat pain with sufficient dopamine transporter activity to block dilute acid-induced depression of nucleus accumbens DA measured using intracranial self-stimulation (ICSS). Amitifadine did produce less ICSS facilitation than the more DAT-selective inhibitors like cocaine or methylenedioxypyrovalerone (MDPV). The effect profile of amitifadine was more like that of triple releasers like 3,4-Methyl enedioxy methamphetamine (MDMA).
Thus, we tested the effects of amitifadine effects on nociception and on remifentanil-induced antinociception. These studies were focused on determining whether amitifadine might be a good candidate for developing as a new treatment for combating opiate addiction in humans and as an analgesic in combination with opioids to reduce thereinforcing effects of opiates without compromising the antinocicptive effects.
Methods
Subjects
Adult female Sprague-Dawley rats (Charles River Labs, Raleigh, NC, USA) were used in the current atudy. Females were tested to facilitate comparison with our previous research concerning amitifadine effects on nicotine self-administration (Levin et al. 2015; Levin et al. 2019b). The rats were singly (for the self-administration study) or group (for the nociception study) housed in approved standard laboratory conditions in a Duke University vivarium facility next to the testing room to minimize stress induced by transporting the rats. The day-night cycle was reversed (with the dark period from 7:00 AM to 7:00 PM so that the rats were in their active phase during the behavioral testing. All behavioral tests were carried out between 9:00 AM and 4:00 PM. Rats for the self-administration experiment had ad lib access to water and were fed a standard 5001 Rodent Chow (Lab Diet, Brentwood, MO, USA) once daily throughout the study to keep them at approximately 85% ad lib weight with food amounts adjusted from 8–16 g per day as they grew to provide a lean healthy growth curve. Rats for the nociception study had ad lib access to both food and water at all times except during the 30 min hotplate test preparation. All procedures for testing the animals were approved by the Duke University Animal Care and Use Committee and conformed to the Animal Care Guide by NIH.
Preparation of Drugs
Solutions of remifentanil (NIDA Drug Supply, RTI International, Raleigh, NC, USA) and amitifadine (NIDA Drug Supply, RTI International, Raleigh, NC, USA) were prepared in pyrogen-free glassware in sterilized isotonic saline and passed through a 0.2 μm filter (Millipore Corp, Billerica, MA, USA). All solutions were kept refrigerated in the dark between experiments and brought to room temperature before administration. Saline solution was used as control.
Experimental Design and Procedure
Three experiments were conducted: 1) the effects of acute amitifadine on remifentanil self-administration (N = 11); 2) effects of chronic amitifadine on remifentanil self-administration (N = 17 controls; and N = 19 amitifadine-treated); and 3) acute interactions of amitifadine and remifentanil on antinociception (N = 12).
Intravenous Remifentanil Self-administration
Chronically indwelling intravenous jugular catheters were implanted i.v. under ketamine (60 mg/kg) and dexdomitor (15 mg/kg) anesthesia and were flushed daily with a 0.3 ml solution containing 100U/ml heparinized saline. After each self-administration session the remifentanil remaining in each port was drawn out and a sterile lock consisting of heparinized saline 500U/ml with 0.4 mg Gentamicin was infused. Barbiturate injection tests through the catheter were used to verify the patency of catheters. Only data from rats with patent catheters were used for analysis.
For behavioral training, rats were placed in dual lever operant test chambers (Med Associates, Georgia, VT, USA). Each chamber was equipped with a tone generator, house light, cue light above each lever, and a metal tether to cover the drug delivery line. A computer programmed with MED-PC software controls experimental events and data collection. Each catheter was connected to a Micro Liter Syringe Pump, and tethers made of polyethylene tubing with huber needles for access to ports and catheters. During each self-administration session, the rats wore infusion harnesses to connect them to the tethers.
Initially, the rats were trained daily with tutor sessions, lasting 30 min, to press the levers for food pellet reinforcers. Half the animals were rewarded for responding on the right lever and the other half for responding on the left lever. Only the cue light over the correct lever was illuminated while the light over the incorrect lever was off. Pressing on the correct lever was reinforced by immediate delivery of one 45-mg food pellet and activation of the feedback tone for 0.5 sec. There was no timeout period in the tutor sessions.
After the pellet sessions, animals had catheters (Strategic Application Inc., Libertyville, IL, USA) surgically implanted under ketamine anesthesia to provide access for remifentanil self-administration by IV infusion. A plastic SoloPort was attached intraoperatively to a polyurethane catheter and inserted into a subcutaneous interscapular pocket and sutured to underlying fascia. 2–4 days after the surgery, the rats began self-administration sessions with remifentanil (0.3 mg/kg/infusion, i.v.) as the reinforcer.
A lever press on the active side resulted in the activation of the feedback tone for 0.5 sec, and the immediate delivery of one 50-μl infusion of remifentanil in less than 1 sec. Each infusion was immediately followed by a 20-sec timeout in which the house light was illuminated and cue lights were extinguished, and responses were recorded but not reinforced. The benchmark infusion dose of remifentanil was set at 0.3-mg/kg/infusion, i.v. and the fixed ratio (FR) requirement was set at FR1. This is an infusion dose of remifentanil in the range that we have previously found to cause a robust level of self-administration in rats with a relatively level of remifentanil self-administration over the range of 0.09 to 0.9 mg/kg/infusion (Lacagnina et al. 2017). Each remifentanil infusion session lasted one-hour. Initially, all subjects had the same opportunity for self-administering remifentanil for five sessions. Testing was not conducted on the weekends.
The amitifadine doses and saline were given twice in the two phases of the study. The interval between two phases was 3 days. Between each injection test session there was a session with no amitifadine treatment. The second phase was done to replicate our findings and to provide initial information about possible tolerance development. Ametifidine was given sc 20 min before starting the self-adminstartin session.
To test for effects on another form of motivated behavior, acute amitifadine effects on food motivated responding was tested in eleven of the rats from the refentanil self-administration study. As with the tutor sessions the rats responded on an FR1 scheduke for 45-mg food pellets in one-h sessions. As in the acute remifentanil self-administration study amitifadine was injected (SC) acutely in a repeated measures counterbalanced design, but only one injection of eachdose and control was given.
In the chronic study, after the initial five sessions of remifentanil self-administration, subjects were given either the control vehicle or 10 mg/kg amitifadine 20 min before the remifentanil self-administration sessions for the following two weeks and for an additional week after the one-week period of enforced abstinence accomplished by not placing the animals in the test chambers for opportunity for self-administration. Finally, all of the rats were tested for one week with no treatment before the remifentanil self-administration sessions to test for residual effects of the drug treatment.
Hot Plate Test of Antinociception
To determine if drug treatments that counteract opiate self-administration also counteract the analgesic effects of opiates, we tested the acute effect of amitifadine on nociception. The classic hotplate test was used. It is important to recognize that despite its name this test does not produce more than momentary nociception in the rat. The second the rat licked its paws indicating behavioral response to aversive heat nociception, it is removed from the environment. This test is one of the oldest and most widely used experimental methods to assess nociception in rats and mice. The test consists of placing a rodent on an enclosed hot plate and measuring the latency to lick a paw. The advantages of this test is that it is objective, quantifiable, can be administered repeatedly without causing inflammation, and assesses supraspinally-organized responses to a noxious stimulus. There appears to be a good correspondence between drugs that produce antinociception on the hot plate test and drugs used clinically to treat pain in humans. The low intensity hot plate test seems to be especially sensitive to analgesic drugs.
Nociception was assessed using the standard hot plate test (N = 12). The latency to lick a paw when the rat was placed on a 55 °C plate was measured. The temperature of the hot plate was set at 55 °C and monitored continuously. To become adapted to the test environment, rats were exposed to the hot plate instrument for at least 15 min/day for two days without turning on the heat and without any treatment. Then, rats were injected sc with 10 mg/kg of saline or ametifidine and 20 min later were given sc 0.4 mg/kg remifentanil and tested on the hot plate. The rat was removed from the hot plate immediately upon licking a paw or if no response occurred within 30 s.
Statistical Analysis
The data were evaluated with analysis of variance (SuperAnova/StatView, SAS, Cary, NC, USA). Analysis was done for within-and between-subjects factors. For the different studies the factors were: for the Acute Self-administration Study: two within subjects factors (amitifadine dose and repeated testing); for the Acute Food-motivated Study: one within subjects factor (amitifadine dose); for the Chronic Self-adinistration Study: one between subjects factor (amitifadine drug treatment) and one within subjects factor (consecutive session of treatment); and for the Antiociception Study: three within subjects factors (amitifadine treatment, remifentanil treatment and time after treatment). Alpha of p < 0.05 (two-tailed) was used as the threshold for statistical significance.
Results
Acute amitifadine effects on remifentanil self-administration
Acute amitifadine (N = 14) caused a decline in remifentanil self-administration with a significant main effect of treatment (F(3,30) = 5.50, p < 0.005). Comparisons with the saline control treatment (38.4 ± 8.2) showed that each of the treatment dose groups, 5 mg/kg (26.7 ± 6.8, p < 0.025), 10 mg/kg (23.8 ± 7.7, p < 0.01) and 20 mg/kg (19.14 ± 5.8, p < 0.001) caused significant declines in the number of infusions per session (Fig. 1). There was a significant main effect of phase (F(1,10) = 9.17, p < 0 .025) with the rats self-administering more remifentanil during the second phase than the first phase (Fig. 1). The interaction of amitifadine treatment and phase of the tests was not significant (p = 0.53). There were no significant effects of prior treatment on remifentanil self-administration during the no treatment sessions interposed between treatment sessions (p=0.65). Amitifadine effects on correct and incorrect lever pressing was also assessed. Acute amitifadine significantly (F(3,30)= 5.39, p < 0.005) reduced correct lever pressing (Control = 84.0 ± 27.5, Amitifidine 5 mg/kg = 35.5 ± 8.6, p < 0.025; Amitifidine 10 mg/kg = 26.1 ± 10.8, p < 0.005; Amitifidine 20 mg/kg = 19.45 ± 6.8, p < 0.005), but had no significant effect on incorrect lever pressing were seen (Control = 4.6 ± 1.2, Amitifidine 5 mg/kg = 4.0 ± 2.4; Amitifidine 10 mg/kg = 1.5 ± 0.7; Amitifidine 20 mg/kg = 1.3 ± 0.5).
Figure 1.
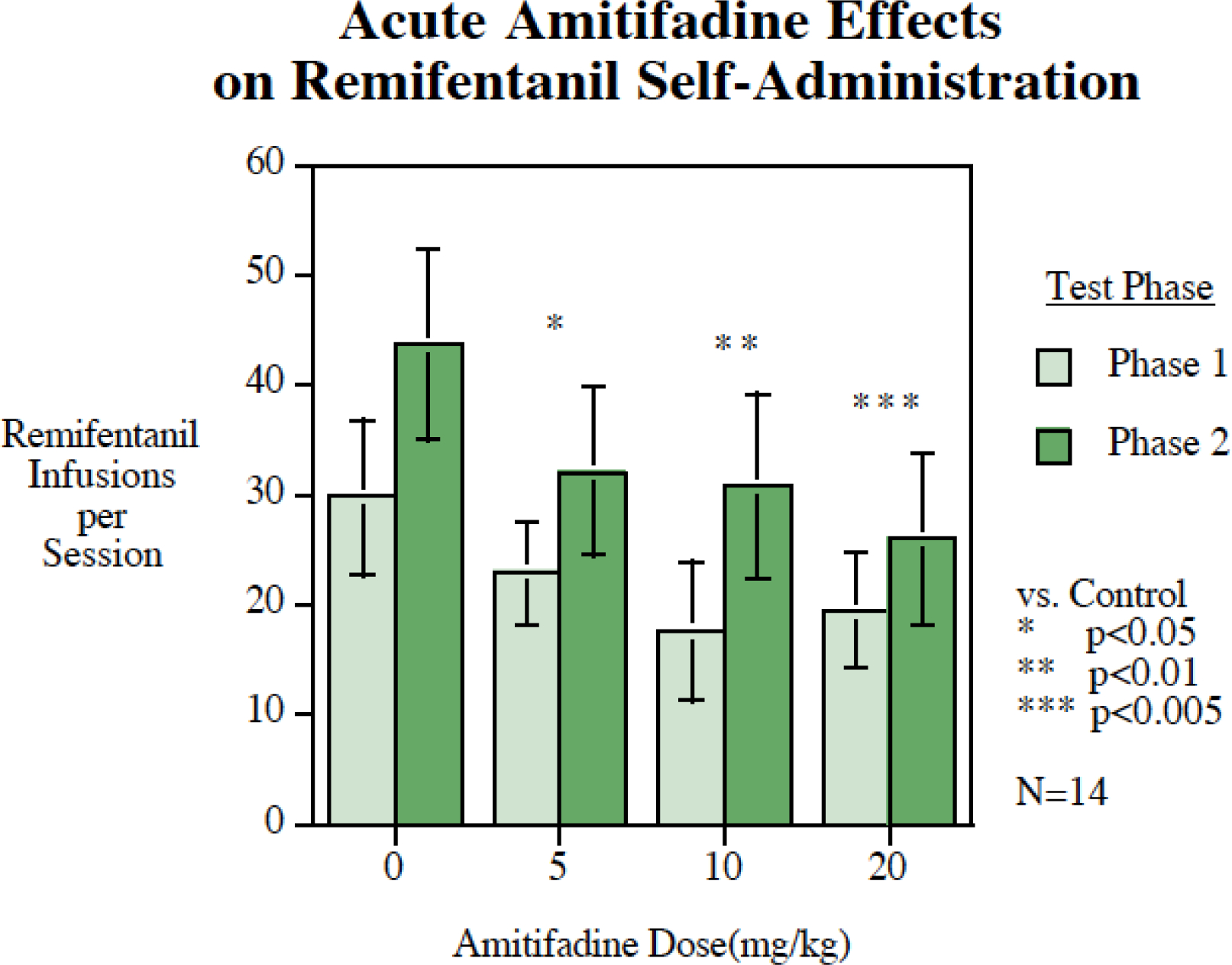
Acute amitifadine effects on remifentanil self-administration (mean ± sem), N = 14. All three doses of amitifadine (5, 10 and 20 mg/kg) significantly reduced remifentanil self-administration. Remifentanil self-administration increased from the first phase of testing to the second but the effectiveness of amitifadine in reducing self-administration was unabated.
Acute amitifadine (N = 11) did not significantly affect food motivated responding (F(3,30) = 2.76, p = 0.06). Nevertheless, with an abudance of caution for determining the dose for the chronic study we compared the potential effect of acute amitifadine doses compared with control on food motivated responding. Only the highest 20 mg/kg dose showed asignificant (p < 0.05) decrease in food motivated responding (Fig. 3, Control 166 ± 14, Amitifadine 5 mg/kg = 175 ± 25, Amitifadine 10 mg/kg = 143 ± 21 and Amitifadine 20 mg/kg = 124 ± 19). Therefore, the chronic study proceeded used the 10 mg/kg amitifadine dose.
Figure 3.
Chronic Amitifadine effects session-by-session (mean ± sem), N = 17 controls, N = 19. Amitifadine caused a significant (p < 0.005) overall decrease in remifentanil self-administration.
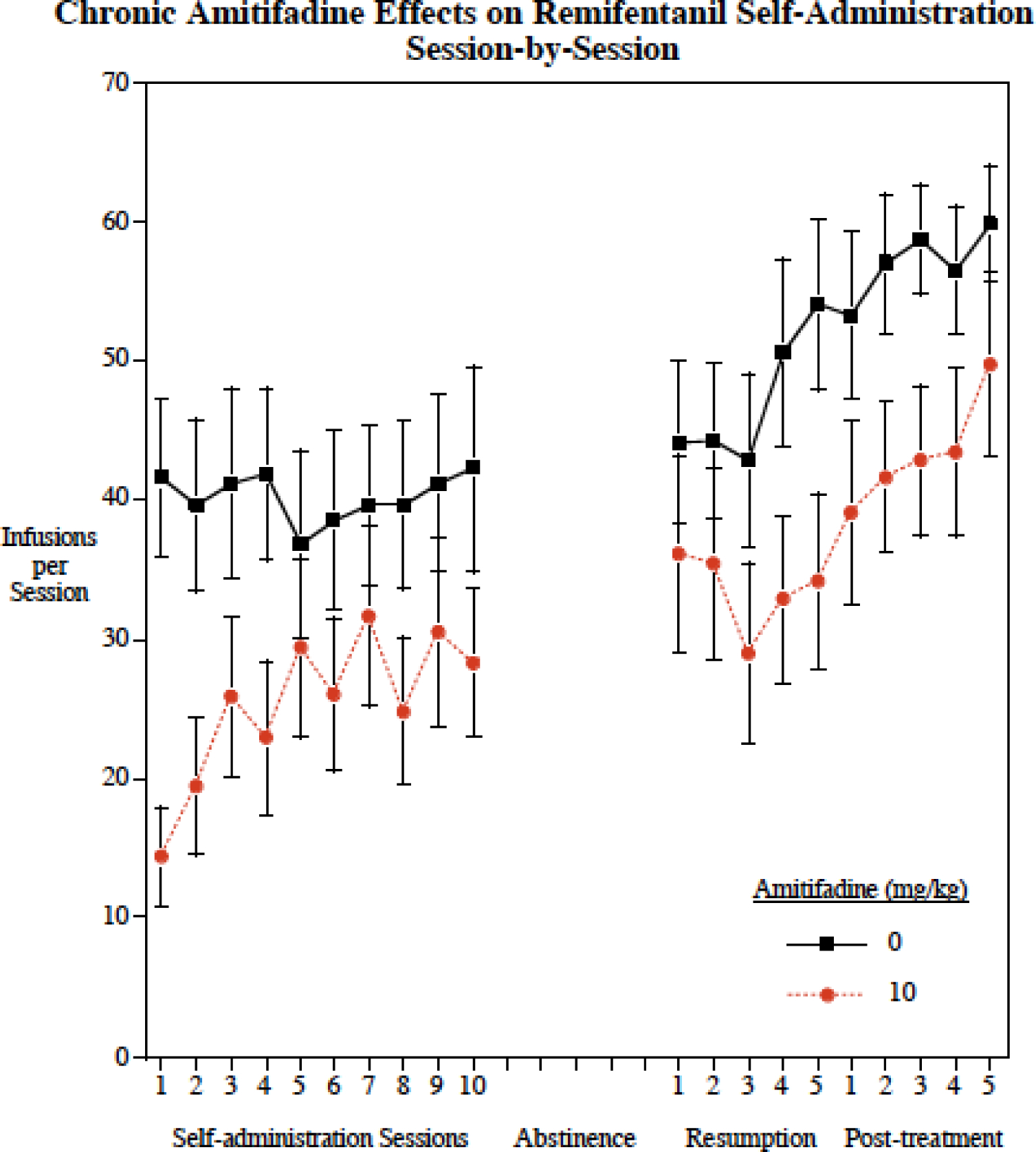
Chronic amitifadine effects on remifentanil self-administration
The 10 mg/kg dose was selected for the chronic study because it was shown in the acute study to significantly reduce remifentanil self-administration and in this and our previous study to be below the threshold for reducing food motivated operant responding and in our previous study ti be below the threshold for reducing locomotor activity (Levin et al. 2015). There was a significant (F(1,33) = 4.41 p<0.05) overall main effect of amitifadine treatment decreasing remifentanil self-administration across the chronic study (Fig. 2). There was also a significant main effect of sessions (F(19,646) = 9.39, p < 0.0005) with an increase in remifentanil self-administration across sessions regardless of the treatment. There was no significant interaction of amitifadine with sessions (p = 0.53)
Figure 2.
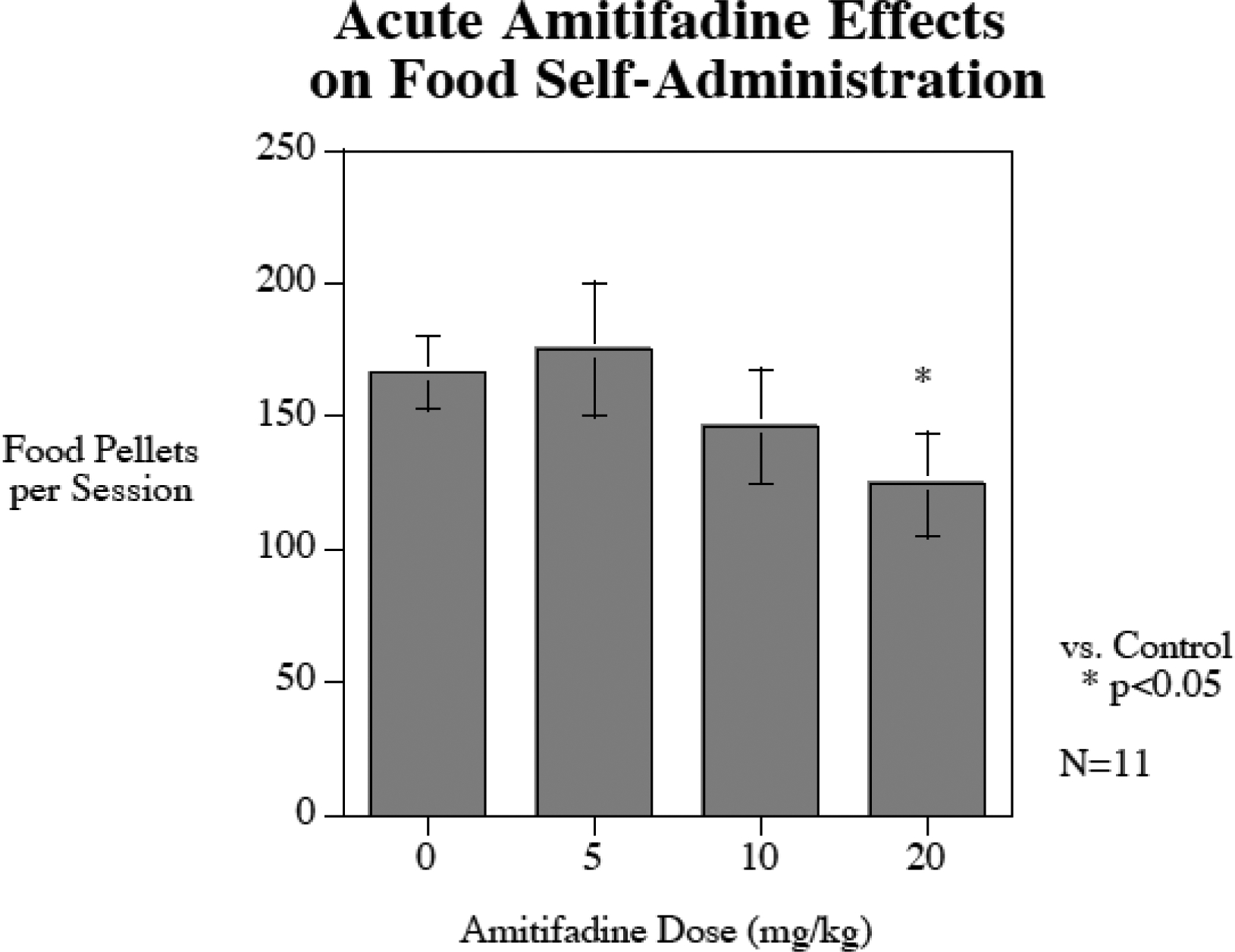
Acute amitifadine effects on food-motovted responding (mean±sem), N = 11. Only the highest 20 mg/kg dose caused a significant (p<0.05) decrease in food self-administration.
Amitifadine effects on correct and incorrect lever pressing was also assessed. Chronic amitifadine significantly (F(1,32)= 5.12, p < 0.05) reduced correct lever pressing (Control = 112.8 ± 24.8; Amitifidine = 62.2 ± 12.9), but had no significant effect on incorrect lever pressing (Control = 8.3 ± 3.1; Amitifadine = 6.0 ± 1.2).
Acute amitifadine interactions with remifentanil-induced antinociception
Figure 4 shows the separate and combined effects of remifentanil and amitifadine on the hotplate test of nociception (N = 12). There was a significant main effect of treatment (F(3,33) = 33.26, p < 0.0005) and a significant interaction of treatment and time (F(9,33) = 29.76, p < 0.0005). Both remifentanil and amitifadine produced antinociceptive effects when given alone. The remifentanil effect was brief and very pronounced with significant antinociceptive effects at 5 min (p < 0.0005) and 10 min (p < 0.0005) after injection, but the effect was of short duration; by 15 min post injection it was no longer significant. In contrast the antinociceptive effect of amitifadine was more modest but more long-lasting, with significant antinociceptive effects 5 min (p < 0.01), 10 min (p < 0.005), 15 min (p < 0.005) and 20 min (p < 0.0005) after injection. The combination of amitifadine with remifentanil did not show any signs of diminishing remifentanil’s antinociceptive effects. Rather, amitifadine outlasted the antinoceptive effects of remifentanil. The combination treatment produced significant antinoceptive effects throughout the test (p < 0.0005).
Figure 4.
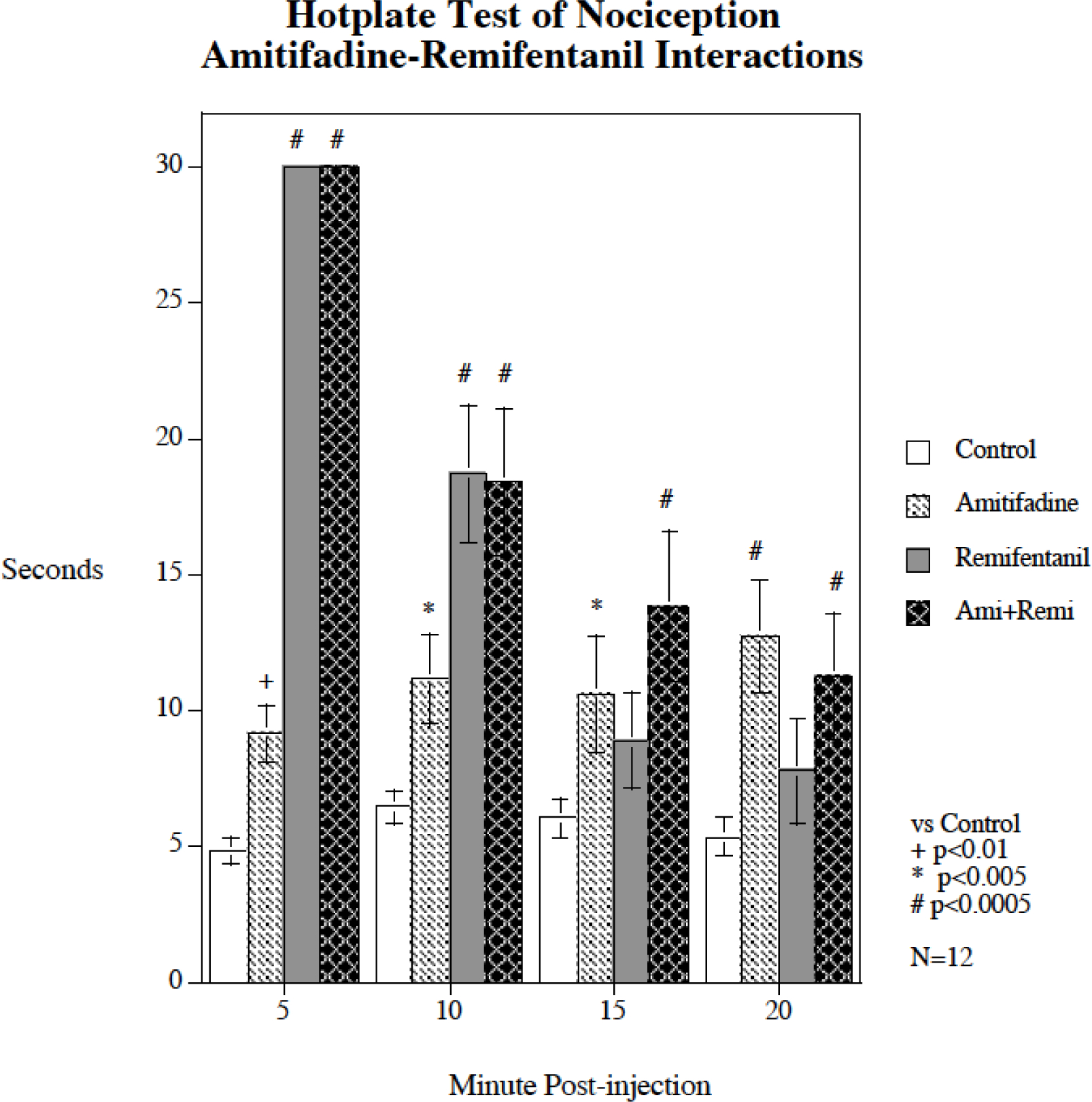
Acute amitifadine interaction with remifentanil hot plate response (mean ± sem), N = 12. Amitifadine did not attenuate the analgesic effect of remifentanil, but rather caused antinociception on its own that outlasted the analgesic effect of remifentanil. There was no evidence that the combination of remifentanil and amitifadine treatment had a significant effect on the antinociceptive effect of the other drug.
Discussion
These studies demonstrated in a rat model show that acute and chronic treatment with the triple re-uptake inhibitor amitifadine can significantly reduce self-administration of the opiate drug remifentanil at an amitifadine dose that did not detract from the analgesic effect of remifentanil. In fact,t amitifadine had a modest antnociceptice effect on its own. ]Amitifadine-induced suppression of remifentanil self-administration was seen across the acute dose range tested (5–20 mg/kg). The doses below 20 mg/kg did not significantly affect food motivated responding and none of the doses significantly affected responding on the non-reinforced lever. In the chronic study, the 10 mg/kg amitifadine dose continued to have an effect supprerssing remifentanil self-admimistration relative to control across two weeks of continued acess, during resumed access after a week of enforced abstinence and even for a week after the end of amitifadine treatment. However, amitifadine (10 mg/kg) did not attenuate the the rise in remifentanil self-administration over the course of the chronic study. The amitifadine-treated rts rose in parallel to but lower than controls through the course of the study. Amitifadine shows promise for reducing the reinforcing value of opiates without compromising their antinociceptive effects, but further study is needed to determine its optimal use..
These effects are consistent with potent amitifadine 5-HT activity inhibiting remifentanil-induced DA release resulting in less self-administration. Separately, 5-HT and NE neurotransmission is known to modulate the descending pain pathways at the dorsal raphe (5-HT) and the locus coeruleus (NE). In addition, some authors have proposed that triple reuptake inhibitors also have anti-nociceptive properties through DA modulation via the periaqueductal gray (Hache et al. 2011). Antinociceptive properties have also been observed in rats with another triple reuptake inhibitor bicifadine, which decreased nociceptive response in a variety of acute and chronic pain models (Basile et al. 2007). In addition, another triple uptake inhibitor, RTI-112 has been found to have antinociceptive effects (Rosenberg et al. 2013).
The 10 mg/kg dose of amitifadine given either acute or chronically was found in the current study to significantly reduce self-administration of remifentanil. Previously, the same dose of 10 mg/kg was shown to significantly reduce nicotine self-administration with both acute and chronic administration at a dose that does not affect food motivated operant responding or locomotor activity (Levin et al. 2015), an effect that was also found in the current study.
The effectiveness of amitifadine was immediate and persisted through the period of continued access to remifentanil, during resumption of access after a week of enforced abstinence and even during a week after cessation of the treatment. The continued lower than control levels of remifentanil self-administration after cessation of the amitifadine self-administration may have resulted from the persistence of the learning of lower response to remifentanil during the chronic treatment or because of carryover of the neural reactions to the chronic amitifadine treatment.In either case, this is a promising effect with regard to the possible therapeutic efficacy of amitifadine for attenuating opiate abuse.
A limitation in the effectiveness of chronic amitifadine was the finding that it was not seen to attenuation of the rise in remifentanil self-admomnmistration over the weeks of access. The amitifadine treated group had consistently lower than control level rates of remifantil over the course of treatment, but both groups rose in remifentanil self-administration throughout the period of access. Further research is needed to determine ways which might effectively suppress this rise in remifentanil self-administration with continued access.
The reduction in remifentanil self-administration induced by amitifadine did not attenuate antinociception because amitifadine showed a modest but significant degree of antinociception of its own. Amitifadine-induced antinociception was, in fact additive to remifentanil providing a more prolonged reduction in nociception on the hot plate test. These antinociception findings are consistent with nefopam in the rat hot plate model (Girard et al. 2001). A major use for nefopam in humans is perioperatively for opioid-sparing, mostly with morphine or remifentanil (Girard et al. 2016) so these data support the potential for future trials of amitifadine in conjunction with opioids.
The current studies provide an important start for a continuing line of investigation to determine possible efficacy of amitifadine or similar treatments to attenuate abuse liability of opiates. The first study examined the dose-effect function and the second study examined the time-effect function of amitifadine reducing remifentanil self-administration. A good next step would be a study providing assessment of dose- and time-effect functions simultaneously.
These studies show that amitifadine treatment holds promise as a treatment for helping to reduce opiate self-administration in direct support to the planned indication for opioid-sparing/taper as an adjunct to opioids in chronic pain. Further research is needed to determine the efficacy of amitifadine in humans and to optimize its use to reduce opiate reinforcement.
Funding Acknowledgement
This research was supported by the P50 grant DA027840 from NIDA.
Footnotes
All procedures for testing the animals were approved by the Duke University Animal Care and Use Committee and conformed to the Animal Care Guide by NIH.
Conflicts of Interest: Anthony McKinney is founder, CEO and shareholder of Ethismos Research, Inc., the IND holder and developer of amitifadine. Jed Rose is on the advisory committee of Ethismos Research, Inc. The other authors have no conflicts of interest.
References
- Aksoy M, Ahiskalioglu A, Ince I, Celik M, Dostbil A, Kuyrukluyildiz U, Altuner D, Kurt N, Suleyman H (2015) The relation between the effect of a subhypnotic dose of thiopental on claw pain threshold in rats and adrenalin, noradrenalin and dopamine levels. Experimental Animals 64: 391–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basile AS, Janowsky A, Golembiowska K, Kowalska M, Tam E, Benveniste M, Popik P, Nikiforuk A, Krawczyk M, Nowak G, Krieter PA, Lippa AS, Skolnick P, Koustova E (2007) Characterization of the antinociceptive actions of bicifadine in models of acute, persistent, and chronic pain. The Journal of Pharmacology and Experimental Therapeutics 321: 1208–1225. [DOI] [PubMed] [Google Scholar]
- Bymaster FP, Dreshfield-Ahmad LJ, Threlkeld PG, Shaw JL, Thompson L, Nelson DL, Hemrick-Luecke SK, Wong DT (2001) Comparative Affinity of Duloxetine and Venlafaxine for Serotonin and Norepinephrine Transporters in vitro and in vivo, Human Serotonin Receptor Subtypes, and Other Neuronal Receptors. Neuropsychopharmacology 25: 871–880. [DOI] [PubMed] [Google Scholar]
- Di Giovanni G, Di Matteo V, Pierucci M, Esposito E (2008) Serotonin–dopamine interaction: electrophysiological evidence Progress in Brain Research. Elsevier, Amsterdam: [DOI] [PubMed] [Google Scholar]
- DiPalma D, Rezvani AH, Willette B, Wells C, Slade S, Hall BJ, Levin ED (2019) Persistent attenuation of nicotine self-administration in rats by co-administration of chronic nicotine infusion with the dopamine D1 receptor antagonist SCH-23390 or the serotonin 5-HT2C agonist lorcaserin. Pharmacology, Biochemistry and Behavior 176: 16–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faramarzi G, Zendehdel M, Haghparast A (2016) D1- and D2-like dopamine receptors within the nucleus accumbens contribute to stress-induced analgesia in formalin-related pain behaviours in rats. European Journal of Pain 20: 1423–1432. [DOI] [PubMed] [Google Scholar]
- Fernández-Sánchez MT, Díaz-Trelles R, Groppetti A, Manfredi B, Brini AT, Biella G, Sotgiu ML, Novelli A (2002) Nefopam, an analogue of orphenadrine, protects against both NMDA receptor-dependent and independent veratridine-induced neurotoxicity. Amino Acids 23: 31–36. [DOI] [PubMed] [Google Scholar]
- Gannon BM, Baumann MH, Walther D, Jimenez-Morigosa C, Sulima A, Rice KC, Collins GT (2018) The abuse-related effects of pyrrolidine-containing cathinones are related to their potency and selectivity to inhibit the dopamine transporter. Neuropsychopharmacology 43: 2399–2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerak LR, Collins GT, France CP (2016) Effects of Lorcaserin on Cocaine and Methamphetamine Self-Administration and Reinstatement of Responding Previously Maintained by Cocaine in Rhesus Monkeys. Journal of Pharmacology and Experimental Therapeutics 359: 383–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard P, Chauvin M, Verleye M (2016) Nefopam analgesia and its role in multimodal analgesia: A review of preclinical and clinical studies. Clinical and Experimental Pharmacology and Physiology 43: 3–12. [DOI] [PubMed] [Google Scholar]
- Girard P, Pansart Y, Coppe MC, Gillardin JM (2001) Nefopam reduces thermal hypersensitivity in acute and postoperative pain models in the rat. Pharmacological Research 44. [DOI] [PubMed] [Google Scholar]
- Golembiowska K, Kowalska M, Bymaster FP (2012) Effects of the triple reuptake inhibitor amitifadine on extracellular levels of monoamines in rat brain regions and on locomotor activity. Synapse 66: 435–444. [DOI] [PubMed] [Google Scholar]
- Gomes T, adrous M, Mamdani MM, Paterson JM, Juurlink DN (2018) The Burden of Opioid-Related Mortality in the United States. JAMA Network Open 1: e180217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabowski J, Rhoades HM, Elk R, Schmitz JM, Davis C, Creson D, Kirby K (1995) Fluoxetine is ineffective for treatment of cocaine dependence or concurrent opiate and cocaine dependence: two placebo-controlled double-blind trials. Journal of Clinical Psychopharmacology 15: 163–174. [DOI] [PubMed] [Google Scholar]
- Gregori-Puigjané E, Setola V, Hert J, Crews BA, Irwin JJ, Lounkine E, Marnett L, Roth BL, Shoichet BK (2012) Identifying mechanism-of-action targets for drugs and probes. Proceedings of the National Academy of Sciences of the United States of America 109: 11178–11183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy EG, Fisher DC, Higgins GA, Fletcher PJ (2014) Examination of the effects of varenicline, bupropion, lorcaserin, or naltrexone on responding for conditioned reinforcement in nicotine-exposed rats. Behavioural Pharmacology 25: 775–783. [DOI] [PubMed] [Google Scholar]
- Hache G, Coudore F, Gardier AM, Guiard BP (2011) Monoaminergic antidepressants in the relief of pain: Potential therapeutic utility of triple reuptake inhibitors (TRIs). Pharmaceuticals 4: 285–342. [Google Scholar]
- Hall BJ, Slade S, Allenby C, Kutlu MG, Levin ED (2015) Neuro-anatomic mapping of dopamine D1 receptor involvement in nicotine self-administration in rats. Neuropharmacology 99: 689–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins GA, Silenieks LB, Rossmann A, Rizos Z, Noble K, Soko AD, Fletcher PJ (2012) The 5-HT2C receptor agonist lorcaserin reduces nicotine self-administration, discrimination, and reinstatement: relationship to feeding behavior and impulse control. Neuropsychopharmacology 37: 1177–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarcho JM, Mayer EA, Jiang ZK, Feier NA, London ED (2012) Pain, affective symptoms, and cognitive deficits in patients with cerebral dopamine dysfunction. Pain 153: 744–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keppel G (1982) Design and Analysis: A Researcher’s Handbook, 2nd edn Prentice-Hall, Inc., Englewood Cliffs, New Jersey [Google Scholar]
- Lacagnina MJ, Kopec AM, Cox SS, Hanamsagar R, Wells C, Slade S, Grace PM, Watkins LR, Levin ED, Bilbo SD (2017) Opioid self-administration is attenuated by early-life experience and gene therapy for anti-inflammatory IL-10 in the nucleus accumbens of male rats. Neuropsychopharmacology 42: 2128–2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langlois LD, Nugent FS (2017) Opiates and Plasticity in the Ventral Tegmental Area. ACS Chemical Neuroscience 8: 1830–1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ED, Johnson J, Slade S, Wells C, Cauley M, Petro A, Rose JE (2011a) Lorcaserin decreases nicotine self-administration in female rats. Journal of Pharmacology and Experimental Therapeutics 338: 890–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ED, Rezvani AH, Slade S, Wells C, Yenugonda VM, Liu Y, Brown ML, Xiao Y, Kellar KJ (2019a) Alpha4beta2 nicotinic receptor desensitizing compounds can decrease self-administration of cocaine and methamphetamine in rats. Eur J Pharmacol 845: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ED, Slade S, Wells C, Pruitt M, Cousins V, Cauley M, Petro A, Hampton D, Rose JE (2011b) Histamine H1 antagonist treatment with pyrilamine reduces nicotine self-administration in rats. Eur J Pharmacol 650: 256–260. [DOI] [PubMed] [Google Scholar]
- Levin ED, Wells C, Johnson JE, Rezvani AH, Bymaster FP, Rose JE (2015) Amitifadine, a triple monoamine reuptake inhibitor, reduces nicotine self-administration in female rats. Eur J Pharmacol 764: 30–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ED, Wells C, Slade S, McKinney A, Rose JE, Rezvani AH (2019b) Potentiating the reduction of nicotine self-administration in rats by co-administering chronic nicotine with amitifadine, a triple monoamine reuptake inhibitor with CYP2B6 inhibitory actions. Nicotine Tob Res in press. [DOI] [PMC free article] [PubMed]
- Michelsen LG, Hug CC (1996. ) The pharmacokinetics of remifentanil. Journal of Clinical Anesthesia 8: 679–682. [DOI] [PubMed] [Google Scholar]
- Miller LL, Leitl MD, Banks ML, Blough BE, Negus SS (2015) Effects of the triple monoamine uptake inhibitor amitifadine on pain-related depression of behavior and mesolimbic dopamine release in rats. Pain 156: 175–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirtlea JL, Hickmana MD, Boinpellya VC, Surinenia K, Thakura HK, Grasing KW (2019) The serotonin-2C agonist Lorcaserin delays intravenous choice and modifies the subjective and cardiovascular effects of cocaine: A randomized, controlled human laboratory study. Pharmacology, Biochemistry and Behavior 180: 52–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezvani AH, Levin ED (2014) Lorcaserin, a selective 5-HT2c receptor agonist, decreases alcohol intake in female alcohol preferring rats. Pharmacology, Biochemistry and Behavior 125: 1–8. [DOI] [PubMed] [Google Scholar]
- Rezvani AH, Slade S, Wells C, Petro A, Li TK, Lumeng L, Xiao Y, Brown ML, Paige MA, McDowell BE, Kellar KJ, Rose JE, Levin ED (2010) Sazetidine-A, a selective α4β2 nicotinic acetylcholine receptor desensitizing agent and partial agonist reduces both alcohol and nicotine self-administration in selectively-bred alcohol preferring (P) rats. Psychopharmacology 211: 161–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg MB, Carroll FI, Negus SS (2013) Effects of monoamine reuptake inhibitors in assays of acute pain-stimulated and pain-depressed behavior in rats. Journal of Pain 14: 246–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman RB, Baumann MH (2006) Balance between dopamine and serotonin release modulates behavioral effects of amphetamine-type drugs. Annals of the New York Academy of Science 1074: 245–260. [DOI] [PubMed] [Google Scholar]
- Schmitz JM, Averill P, Stotts AL, Moeller FG, Rhoades HM, Grabowski J (2001) Fluoxetine treatment of cocaine-dependent patients with major depressive disorder. Drug and Alcohol Dependence 63: 207–214. [DOI] [PubMed] [Google Scholar]
- Shanahan WR, Rose JE, Glicklich A, Stubbe S, Sanchez-Kam M (2017) Lorcaserin for Smoking Cessation and Associated Weight Gain: A Randomized 12-Week Clinical Trial. Nicotine & Tobacco Research 19: 944–951. [DOI] [PubMed] [Google Scholar]
- Skolnick P, Popik P, Janowsky A, Beer B, Lippa AS (2003) Antidepressant-like actions of DOV 21,947: a “triple” reuptake inhibitor. Eur J Pharmacol 461: 99–104. [DOI] [PubMed] [Google Scholar]
- Tiemann L, Heitmann H, Schulz E, Baumkötter J, Ploner M (2014) Dopamine precursor depletion influences pain affect rather than pain sensation. PLoS ONE 9: e96167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wee S, Anderson KG, Baumann MH, Rothman RB, Blough BE, Woolverton WL (2005) Relationship between the Serotonergic Activity and Reinforcing Effects of a Series of Amphetamine Analogs. The Journal of Pharmacology and Experimental Therapeutics 313: 848–854. [DOI] [PubMed] [Google Scholar]
- Winstanley EL, Bigelow GE, Silverman K, Johnson RE, Strain EC (2011) A randomized controlled trial of fluoxetine in the treatment of cocaine dependence among methadone-maintained patients. Journal of Substance Abuse Treatment 40: 255–264. [DOI] [PMC free article] [PubMed] [Google Scholar]


