Abstract
Phagocytosis is a receptor-mediated, actin-dependent process of internalization of large extracellular particles, such as pathogens or apoptotic cells. Engulfment of phagocytic targets requires activity of myosins, actin-dependent molecular motors, which perform a variety of functions at distinct steps during phagocytosis. By applying force to actin filaments, plasma membrane, and intracellular proteins and organelles, myosins can generate contractility, directly regulate actin assembly to ensure proper phagocytic internalization, and translocate phagosomes or other cargo to appropriate cellular locations. Recent studies using engineered microenvironments and phagocytic targets have demonstrated how altering the actomyosin cytoskeleton affects phagocytic behavior. Here, we discuss how studies using genetic and biochemical manipulation of myosins, force measurement techniques, and live cell imaging have advanced our understanding of how specific myosins function at individual steps of phagocytosis.
Keywords: phagocytosis, myosins, actin, macrophage
Phagocytosis is an ancient biological process, utilized initially for nutrient uptake and now a critical activity for immune defense [1]. Professional phagocytes, including neutrophils, dendritic cells, and macrophages, regularly rid the body of pathogens, apoptotic cells and cellular debris. These phagocytic targets are recognized by specific cell surface receptors, which relay distinct downstream signals through protein and lipid kinases and phosphatases, small GTPases, and other signaling proteins [2]. Targets coated by plasma- or cell-derived components (opsonins) are recognized by opsonic receptors, including Fc receptors (FcR) that bind the conserved domain of immunoglobulins and complement receptors (CR), which respond to targets coated in the complement derivative iC3b [1]. Non-opsonic receptors (receptors binding to specific motifs on phagocytic targets) include Dectin-1 receptors that recognize fungal β-glucan and scavenger receptors that interact with both apoptotic and microbial ligands [1, 3]. For internalization, phagocytic receptors can work in concert, with others serving to modulate the phagocytic response [4]. Despite the diversity of phagocytic receptors recognizing various targets, the process of target internalization always requires the actin cytoskeleton [5–9].
Phagocytosis by FcR is classically divided into multiple steps. First, phagocytes search for targets using membrane protrusions, such as filopodia or ruffles, generated by actin polymerization [10]. This active process of probing the cell’s microenvironment can be stimulated, or primed, by soluble molecules such as TLR ligands and growth factors, which increase actin-dependent ruffling [11]. Once contact is made, phagocytic receptors cluster resulting in a more stable adhesion [12]. Downstream signaling from engaged receptors leads to robust actin polymerization that deforms the plasma membrane, causing the cell to encircle the target in a structure known as the phagocytic cup [13]. Closure of the phagocytic cup produces a sealed membrane-bound organelle called the phagosome, which is trafficked along microtubules further into the cell. Fusion of the phagosome with endosomal vesicles and later lysosomes is required for pathogen degradation and antigen presentation [1, 14].
Myosins, actin-dependent molecular motors (see Text Box 1), work to apply direct force on actin filaments, yet their mechanical role in phagocytosis is not fully understood. This is partially due to the multitude of myosin isoforms that are expressed in phagocytes (Figure I), as well as to the relatively fast and complex multi-step nature of phagocytosis. Myosins can regulate actin assembly, actin filament crosslinking and rearrangement, actin-dependent membrane deformation, and protein localization. In this review, we examine our current understanding of the role of myosins in the various steps of phagocytosis, giving special attention to the mechanochemical features of each myosin and how they function in the overall biomechanics of the process. Furthermore, we discuss how the use of force measurements and engineered phagocytic targets and microenvironments has offered new insights into myosin activity during this process.
Text Box 1. Myosins implicated in phagocytosis.
Myosin motors consist of heavy chains, which are responsible for myosin motor activity, and light chains, which are important for myosin force production and regulation [104]. Myosin heavy chains come in a wide variety of isoforms that form the myosin protein superfamily (Figure I). Myosin light chains are typically calmodulins or calmodulin-like calcium-binding proteins, with some myosins having dedicated light chains [105]. While all myosin heavy chains contain an N-terminal motor domain and a neck region that binds light chains, the tail domains of myosins are highly divergent. These diverse tails account for the ability of myosins to localize to specific subcellular regions and interact with cargo. Furthermore, while the overall structure and function of the myosin motor domain is conserved, different myosin isoforms are characterized by distinct mechanochemical properties, such as the rate of ATP hydrolysis, processivity (ability to take multiple steps along an actin filament), duty ratio (fraction of the ATPase cycle spent strongly attached to actin), and velocity of movement along actin [106]. Based on the sequence homology of the motor and structural similarities of tail domains, the myosin superfamily is divided into distinct myosin classes. Evidence for a myosin’s contribution in phagocytosis comes primarily from (I) localization studies, including those examining recruitment signals and motifs that guide myosin enrichment during phagocytosis, (II) chemical inhibition of myosin activity, (III) expression of dominant-negative constructs, and (IV) studies of myosin-null cells.
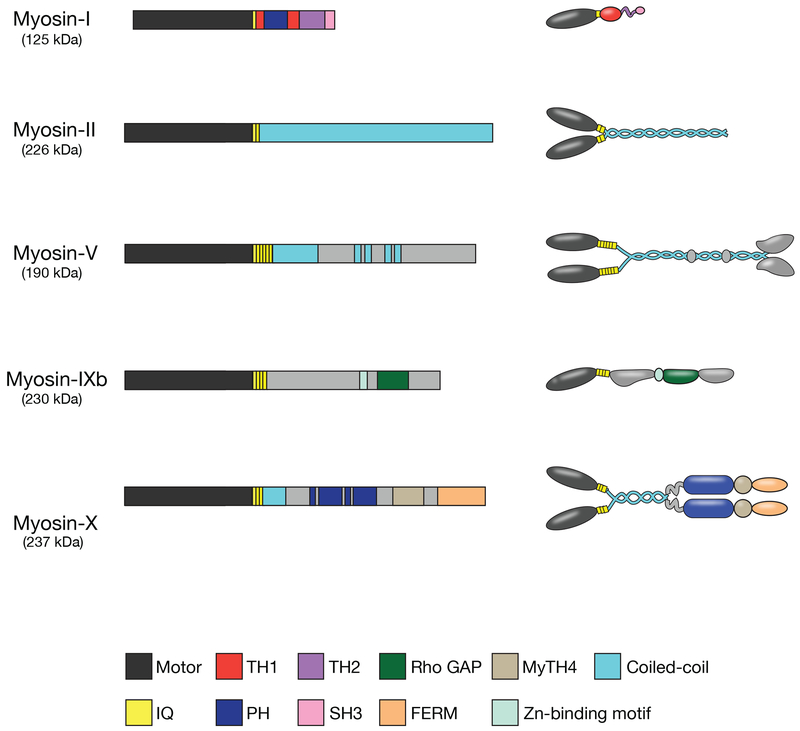
Figure I.
Domain organization and overall structural features of myosin isoforms involved in phagocytosis. Each myosin heavy chain consists of an N-terminal motor domain, a neck domain that binds light chains, and a tail domain that can include coiled-coil motifs to promote dimerization (in myosins-II, -V, and -X), membrane-binding Pleckstrin homology domains (in myosins-I and -X), or protein interaction motifs that promote cargo binding specificity. TH1, tail homology 1; TH2, tail homology 2; PH, Pleckstrin homology; IQ, light chain binding IQ motif; SH3, Src Homology 3; MyTH4, Myosin Tail Homology 4; GAP, GTPase-accelerating protein. Redrawn from [112].
1. Target capture using myosin-X-guided filopodia
Phagocytes capture their targets through actin-based membrane ruffles or filopodia, which can act as cellular tentacles, extending outward to bind a target and retracting back to pull the target to the cell surface [10, 15]. Myosin-X is ubiquitously expressed among all immune cells and well known for its striking localization at filopodial tips [16, 17]. It is a double-headed processive motor with the tail that includes a myosin tail homology 4 domain and band 4.1/ezrin/radixin/moesin domain (MyTH4-FERM) [17, 18]. Myosin-X in macrophages was initially observed to be recruited to the phagocytic cup, and exogenous expression of the myosin-X tail domain as a dominant negative construct reduced phagocytosis of antibody-coated erythrocytes by ~80% [19]. Myosin-X tail specifically reduced uptake of large but not small IgG-coated beads, suggesting that this motor had a specific role in pseudopod extension during phagocytic internalization. However, two new studies now show that myosin-X knockout (KO) macrophages ingest zymosan (yeast particles) and IgG-coated beads or cells at a rate indistinguishable from wild-type cells [20, 21]. Instead, myosin-X-null macrophages seem to form fewer filopodia in response to LPS or E. coli [20]. Thus, despite localization to the phagocytic cup, myosin-X appears to be dispensable for actin dynamics during internalization and likely contributes to the earliest step of phagocytosis, filopodia initiation and maintenance to capture phagocytic targets (Figure II).
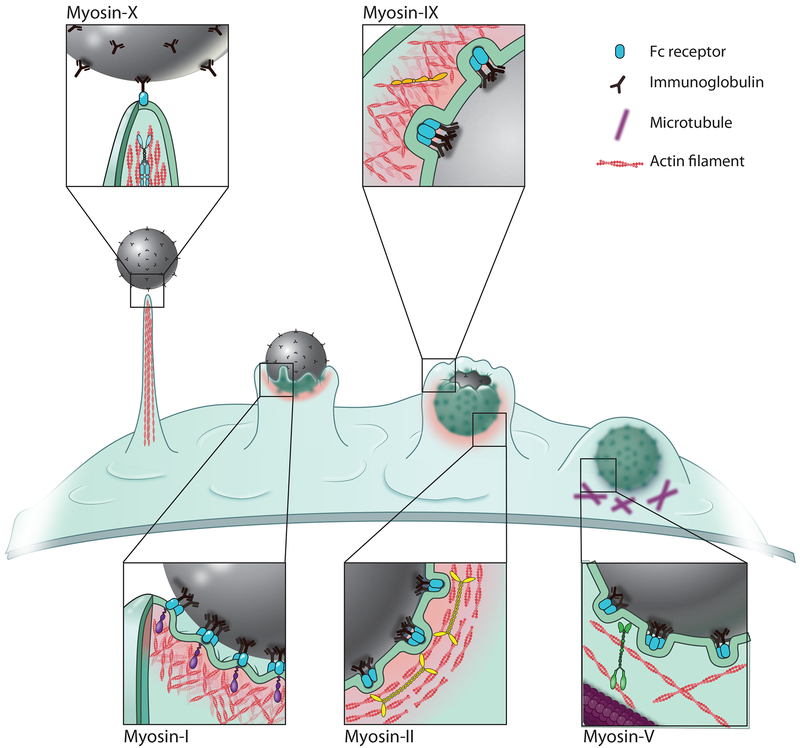
Figure II.
Schematic of the sequential steps of FcR-mediated phagocytosis and the specific myosin isoforms involved. Myosin-X mediated filopodia function in phagocytic target search and capture. Myosin-I (Myo1e/f) and -IX (Myo9b) enable productive actin assembly within the phagocytic cup, with myosin-II generated contractility at the base. Myosin-V (Myo5a) assists in anchoring the phagosome once internalized.
2. Assembling the phagocytic cup: roles for myosin-I and myosin-IX
Once a phagocytic target contacts the surface of the cell, receptor clustering and downstream signaling initiate formation of the phagocytic cup that surrounds the target. The assembly of this actin-based structure likely involves at least two myosin classes: myosin-I and myosin-IX (Figure II).
Myosin-Is are small monomeric motors that represent one of the largest groups in the myosin superfamily. Myosin-Is contain a single heavy chain with a tail domain divided into three conserved regions abbreviated as TH1, TH2, and TH3 for tail homology 1, 2, and 3. The TH1 domain participates in membrane binding, the TH2 domain contains an ATP-insensitive actin binding site, and the TH3 domain is a Src Homology 3 (SH3) domain known to participate in protein-protein interactions [22]. Myosin-Is that contain only the TH1 domain are called “short-tailed”, while those that contain all three tail domains are known as “amoeboid” or “long-tailed” [23].
The participation of myosin-Is in phagocytosis from single-celled organisms to mammalian phagocytes makes this class of myosins an evolutionarily conserved group of phagocytic motor proteins. Myosin-Is in Acanthamoeba castellanii, a free-living soil amoeba, and Entamoeba histolytica, a parasitic protozoan that depends on phagocytosis of human cells, are strongly recruited to phagocytic cups [24, 25]. Of the seven myosin-Is in the slime mold Dictyostelium discoideum, both short-tailed (Myo1E) and long-tailed (Myo1B and Myo1C) isoforms contribute to phagocytosis, with short- and long-tailed myosins likely playing non-overlapping roles in this process [26–30]. Myo1K, a structurally divergent myosin-I, also plays a role in phagocytosis, with Myo1K− cells ingesting ~30% less yeast cells than wild type Dictyostelium [31]. Thus, while myosin-Is are not absolutely essential for phagocytosis, they are required for efficient target uptake and multiple isoforms often need to be depleted to observe an effect on internalization. An unanswered question is why only some members of this myosin class localize to phagocytic cups and, among those, how certain isoforms (e.g. long-tailed vs. short-tailed) perform distinct, non-overlapping functions.
The mammalian genome encodes eight myosin-I isoforms (Myo1a-h; this nomenclature is not related to that of Dictyostelium), with immune cells containing varying combinations of short-tailed (Myo1c and Myo1g) and long-tailed (Myo1e and Myo1f) myosin-Is [32]. In macrophages, Myo1g, Myo1e and Myo1f are recruited to the phagocytic cup during uptake of IgG-coated targets [33–37], yet closer examination reveals slightly different localizations. Myo1g is enriched at the membrane along the sides of the phagocytic cup [37], while Myo1e and Myo1f localize at the leading edge of the phagocytic cup as well as at distinct adhesion sites within the cup [36]. The ability of myosin-Is to bind both actin and membrane and therefore maintain membrane-cortex adhesion has been shown to be important for regulating membrane tension [38], which in turn is important for phagocytosis [39]. Reduced membrane-cytoskeletal adhesion during phagocytosis, as observed in Myo1e and Myo1f double KO macrophages, correlates with the assembly of excessively dense actin meshwork within the phagocytic cup and slower phagocytic internalization [36]. Recently, these phagocytic adhesions have been likened to podosomes [40], specialized actin-based adhesive structures that are capable of degrading extracellular matrix [41]. Moving forward, it will be interesting to determine whether there are mechanistic parallels between the role of myosins in the regulation of podosome dynamics [42, 43] and their contribution to actin dynamics within the phagocytic cup.
The second myosin class believed to participate in phagocytic cup assembly is myosin IX, specifically Myo9b, which has been detected at both phagocytic cups and isolated phagosomes [44, 45]. A genome-wide CRISPR screen using a human macrophage cell line showed that KO of Myo9b inhibited phagocytosis of positively and negatively charged beads of various sizes, as well as IgG-opsonized red blood cells [46]. In dendritic cells derived from the bone marrow of Myo9b KO mice, however, internalization of E. coli was unaffected [47]. Myosin-IX is a unique single-headed processive motor, capable of remaining attached to an actin filament throughout the ATPase cycle by an extended loop within the motor domain [48]. It may be able to act as an actin crosslinker, since purified Myo9a mixed with actin creates an evenly spaced actin lattice structure [49]. In addition, the myosin-IX tail contains a RhoGAP domain, which inactivates specifically the small GTPase Rho [50]. As a result, Myo9b KO macrophages exhibit significantly higher levels of activated RhoA and cannot form membrane protrusions, maintaining a rounded-up appearance [51]. These distinctive motor properties controlling a potential signaling hub suggest a fascinating role for Myo9b in phagocytosis. Given the need for F-actin to remain dynamic during internalization, Rho family GTPases continuously cycle between “on” and “off” states throughout phagocytosis with the help of activating GEFs and deactivating GAPs [52, 53]. Classically, RhoA GTPase is known to be important for CR-mediated phagocytosis, yet dispensable for phagocytosis through FcR [54]. However, Myo9b is clearly recruited to the phagocytic cup around IgG-coated targets [53]. This finding suggests that Myo9b is either not functioning in Rho inactivation at these sites or that the activity of small GTPases during phagocytic cup assembly is more complex than we currently understand.
3. Closing the phagocytic cup and myosin-II generated contractility
A role for myosin-II in phagocytosis has been suspected since its detection at sites of ingestion by early immunofluorescence studies of macrophages [55]. Mammalian cells contain three non-muscle myosin-II isoforms: non-muscle myosin-2A (NM2A), NM2B, and NM2C, encoded by MYH9, MYH10, and MYH14 genes in humans. NM2A is the dominant myosin-II isoform expressed in immune cells [32, 56]. Like skeletal muscle myosin-II, this myosin functions as a heterohexamer consisting of a dimer of heavy chains and four associated light chains: two essential light chains (ELC) and two regulatory light chains (RLC) [57]. These heterohexamers further assemble into filaments or minifilaments, bipolar structures (with myosin motor domains at each end of the filament) that crosslink and contract actin filaments by promoting sliding of actin filaments relative to each other. Regulation of NM2A is accomplished via reversible phosphorylation of the RLC by multiple kinases including myosin light chain kinase (MLCK) and Rho-associated coiled-coil-containing kinase (ROCK) as well as via regulation of the activity of myosin light chain phosphatase by ROCK and other regulators [58]. RLC phosphorylation not only increases the ATPase activity of the motor, but also facilitates assembly of myosin-II filaments [58, 59]. The contribution of myosin-II in phagocytosis has been assessed using chemical inhibition of upstream myosin kinases as well as the motor activity itself and the results differ, leading to uncertainty on whether myosin-II activity is critical for phagocytosis. Yet with the ability of bipolar myosin filaments to contract the F-actin network, NM2A may be contributing to particle internalization via constriction of the phagocytic cup (Figure II). Upon closer examination, differing experimental results may depend on cell type, the type of phagocytosis examined (FcR- or CR-mediated), the timing of experiments (duration of incubation prior to evaluation of phagocytic efficiency), and the target and concentration of the inhibitor used (with different inhibitors affecting myosin ATPase activity either directly or indirectly via inhibition of MLCK or Rho kinases that regulate MLCK activity).
Inhibition of MLCK in neutrophils using ML-7 halts phagocytic internalization [60] while inhibition of MLCK in macrophages using antibodies does not affect phagocytosis [61]. On the other hand, in macrophages treated with ML-7, phagocytic cup closure is delayed, the cups are splayed open and appear not to squeeze IgG-coated red blood cells [62]. NM2A expression level positively correlated with phagocytic ability using a macrophage cell line ingesting sheep red blood cells [63]. Direct inhibition of myosin-II ATPase activity using the drug blebbistatin has been shown to reduce phagocytosis to varying degrees, depending on the length of the experiment and the size of the phagocytic particles [63–65]. Altogether, these observations suggest that, at least in some settings, myosin-II activity contributes to timely phagocytic internalization and may promote close apposition between the phagocytic cup and the surface of the target. Indeed, in a 2D approximation of phagocytosis known as frustrated phagocytosis, macrophages treated with blebbistatin exert significantly reduced contractile forces, as measured by traction force microscopy [66]. It is also possible that myosin-II, like myosin-I and myosin-IX, plays a role in phagocytic cup assembly, although the evidence is again somewhat conflicting. In J774.A1 macrophages, myosin activity is dispensable for phagocytic cup assembly during FcR-mediated phagocytosis but critical for CR-mediated phagocytosis [67], while in RAW macrophages and primary bone marrow-derived macrophages, actin accumulation is drastically reduced by ML-7 and blebbistatin treatment during FcR-mediated phagocytosis [64].
Overall, it appears likely that myosin-II is generating contractile force within the phagocytic cup during internalization to promote efficient closure. While inhibitor studies indicate that myosin-II motor activity is required for efficient target ingestion, the precise timing and location of NM2A contractile activity remain to be elucidated. It is also unknown how actin filaments within the phagocytic cup are arranged for such contractile force. Typically, subcellular actin assemblies subject to myosin-II-mediated contraction are composed of F-actin of opposing polarity with varying degrees of actin and myosin-II filament alignment [68]. Whether phagocytic cups include similar antiparallel arrangements of actin filaments for myosin-II activity is unclear, although using STED microscopy, concentric rings of F-actin, consistent with an ordered and aligned arrangement of actin filaments, have been observed in phagocytic cups of dendritic cells engulfing yeast [69]. Yet for myosin-II to productively act on such filaments, these F-actin bundles would need to be immobilized – anchored at the membrane or crosslinked to each other. One of the candidates for performing this role is alpha-actinin, an actin-binding protein that has been localized to the phagocytic cup [70, 71] and that helps anchor actin filaments to transmembrane proteins at Z-disks or dense bodies of muscle myofibers and at focal adhesions [72]. In the future, it will be important to determine where and in what direction myosin-II-generated force is applied within the cup (for example, does it result in a tangential contraction along the constricting edge of the cup or a uniform radial compression throughout the entire actin-rich cup). Re-examination of myosin-II recruitment, localization, and activation using improved imaging tools and biosensors providing high spatial and temporal precision [73, 74] may help resolve some of the controversies regarding the functions of this myosin class in phagocytosis.
4. Transporting the phagosome with myosin-V
Closure of the phagocytic cup creates a membrane-bound organelle known as the phagosome, which is shuttled further into the cell for processing and degradation [1, 14]. Retrograde transport of the phagosome is known to occur along microtubule tracks [75], yet it also involves myosin-V, a large two-headed myosin motor. In macrophages from dilute-lethal (Myo5a-null) mice, following internalization, phagosomes move toward the cell center twice as fast as in wild type cells, rapidly accumulating in the perinuclear region [76]. As a processive motor with a ~36 nm step size, Myo5a is kinetically adaptable for the transport of large vesicular cargos including organelles [77–80]. Since most actin filaments in mammalian cells are relatively short, the likely roles for Myo5a are (1) participation in short-range/local trafficking of organelles following their long-range delivery along microtubules or (2) competition with faster microtubule-dependent transport by serving as an anchor to maintain organelle distribution in specific cellular regions. The study using dilute-lethal macrophages [76] suggests that Myo5a competes with dynein-mediated centripetal transport on microtubules – a feature that has previously been observed during transport of melanosomes in dilute-lethal melanocytes [81]. The physiological significance of this Myo5a-dependent delay in centripetal movement with frequent stops and reversals of phagosomes is still unknown, although it may be important to promote phagosome interactions with specific organelles at the cell periphery. Furthermore, Myo5a may play a role in vesicle transport during internalization as increased membrane surface area is required for internalization of large targets.
In mammals, the myosin-V tail is linked to cargo via adaptor complexes, and myosin-V motor activity is tightly regulated by adaptor binding, which triggers the unfolding of the autoinhibited protein to bind actin [82]. While the adaptor proteins that link Myo5a to phagosomes remain unknown, a yeast two-hybrid screen has shown that Myo5a interacts with a wide variety of Rab proteins [83], many of which are detected on phagosomes [84]. Thus, some of these Rabs may serve as regulating adaptors of myosin-phagosome complexes.
Concluding remarks
The majority of myosin phagocytosis studies to date have been performed on cells in vitro. However, phagocytes reside in tissues with distinct physical characteristics and encounter phagocytic targets with various geometrical, mechanical, and chemical properties, and the process of phagocytosis is affected by both cell environment and target properties. Since the actin cytoskeleton is influenced by substrate stiffness and geometry, myosin activity during phagocytosis is also likely modulated by cell microenvironment. Engineered substrates and other approaches have been used to test how cell microenvironment, including substrate stiffness, cell confinement, and extracellular pressure, affects phagocytosis [85–87]. These studies show that phagocytosis is stimulated by stiffer substrates, yet inhibited by spatial confinement [85, 86]. Whether other physical features of the microenvironment, such as surface topography or interstitial flow, affect phagocytosis remains to be determined.
Classic targets used to study phagocytosis include both soft sheep red blood cells and stiff polystyrene beads – the latter being used for their easy coating, uniform shape, and availability in a variety of sizes. Yet these polystyrene targets are orders of magnitude more rigid than anything a phagocyte will encounter in vivo. Interestingly, phagocytes have particular preferences when it comes to target size, shape, rigidity, and surface charge, which have been well-studied using targets with more varied properties [88–97]. It is highly likely that the activity and utilization of the various myosin isoforms in phagocytes is influenced by the target’s size, shape, rigidity, and surface properties. Indeed, in one such example of myosin regulation by the mechanical properties of the target, artificial stiffening of red blood cells using glutaraldehyde pretreatment promoted activation of NM2A in macrophages and enhanced FcR-mediated phagocytosis [98], perhaps supporting a system for the selective removal of aging red blood cells in vivo. Conversely, mechanosensitive regulation of CR-mediated phagocytosis appears to be independent of myosin-II [99], thus, different types of phagocytic receptors may engage distinct mechanosensitive pathways (myosin-dependent or independent).
Furthermore, mechanical cues from the target may directly affect the myosin mechanochemical cycle, such as ATP binding, ADP release, and detachment from the actin filament, which can be affected by the force applied to myosin [100]. The effects of force on myosin-actin binding can range from catch-bond-like behavior (detachment rate decreasing under load) to slip-bond behavior (detachment rate increasing under load) to power stroke reversal and even reverse-sliding of myosin toward the minus end of the actin filament under load [100–102]. Such force-sensitive actomyosin interactions could partially account for the sensitivity of phagocytosis to the mechanical properties of the phagocytic target. For example, interactions with stiff targets could result in elevated recruitment of myosins that exhibit catch-bond-like response to load. This recruitment of myosin motors may in turn increase the rigidity of actomyosin networks, creating a positive feedback loop in response to target stiffness. Lastly, myosin activity may also be influenced by the shape of the target, given that myosin-II has been shown to preferentially associate with the regions of low or negative membrane curvature [103].
As techniques that allow a detailed dissection of the mechanosensitivity of phagocytosis and force production continue to be developed (see Text Box 2), future work in this area will likely shed light on the interplay between the properties of phagocytic targets and myosin recruitment, activation, and functions. Examining the F-actin ultrastructure within the phagocytic cup using superresolution microscopy or cryo-EM tomography will also be important since different myosin classes are characterized by a preference for a specific type of actin network (e.g. branched actin for myosin-I, antiparallel bundles for myosin-II). We encourage future research (see Outstanding Questions box) to make use of bioengineered substrates to better examine phagocytosis in a more physiological setting.
Text Box 2. Force measurement techniques in studies of phagocytosis.
Precise measurements of cellular forces during phagocytosis, along with the detection of concomitant changes in myosin activity and localization, are important for deciphering myosin contribution to phagocytic uptake. Specific types of forces associated with phagocytosis include pulling or pushing forces exerted on the target by the phagocyte, squeezing force generated by the contraction of the phagocytic cup, and changes in the cortical tension of the phagocyte during formation and closure of the phagocytic cup. These varied forces can be measured using several techniques. An optical trap holding an antibody-coated bead was used to measure the retraction (pulling) force of capturing filopodia, which ranged from <1 pN to > 15 pN in macrophages and was not affected by the knockdown of Myo5a, knockout of myosin-VI, or inhibition of myosin-II [107]. Recent work combining the 2D frustrated phagocytosis assay with traction force microscopy has enabled the assessment of the contractile forces exerted by the “closing” phagocytic cup, which has been shown to be dependent on myosin-II [66] and not myosin-I [36]. Attaching an antibody-coated bead to an AFM cantilever is another technique recently used to probe the forces generated during phagocytic cup formation [108]. Micropipette aspiration has been used to measure changes in cortical tension during neutrophils phagocytosis of antibody-coated beads [109, 110]. This has led to a model whereby phagocytosis proceeds through the dual action of the cytoskeletal pressure forcing membrane protrusion at the tips of the phagocytic cup and strong membrane-cytoskeleton adhesion at the target surface [109, 110]. Studies using the tether pulling assay by optical tweezers similarly show that, as a cell stretches to engulf a large target, membrane tension increases with enhanced membrane-cytoskeletal adhesion [36, 39]. A new technique [111] involving easily derivatized hydrogel microparticles, in which particle deformation can be used to accurately measure and localize subcellular forces during phagocytic internalization, will undoubtedly help dissect how each myosin is powering ingestion.
Outstanding Questions.
How does myosin activity alter the F-actin organization of the phagocytic cup?
Are myosins transporting any cargo during specific steps of phagocytosis?
Which steps during phagocytic internalization require contractility, what is the geometry/directionality/magnitude of the contractile forces, and which myosin isoforms are involved in force production?
How are specific myosin isoforms temporally and spatially regulated during different forms of phagocytosis?
Are myosins further regulated by external stimuli or by the physical characteristics of the phagocytic target?
How do myosins interact with the internalized phagosome? Are there specific phagosome adaptors used to anchor myosins?
How do the physical characteristics of the phagocyte’s environment in vivo affect myosin activity during phagocytosis?
Highlights.
Phagocytosis in both mammalian and non-mammalian cells requires F-actin and involves multiple myosin isoforms.
Specific steps in phagocytosis rely on the activity of specific myosin isoforms.
Phagocytes encounter targets with varying physical characteristics, which may affect the activity and distribution of the involved myosins.
The physiological setting of a phagocyte affects its acto-myosin cytoskeleton and in turn its phagocytic behavior.
Acknowledgements
This work was supported by the AHA (18PRE34070066) grant to S.R.B., the Italian Association for Cancer Research (AIRC), Investigator Grant (IG) 20716 to N.C.G., and the National Institute of Diabetes and Digestive and Kidney Diseases of the NIH under Award R01DK083345 to M.K. The authors are grateful to Allyson Porter for help in preparing the illustrations and to Dr. Joseph W. Sanger and anonymous reviewers for the helpful suggestions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gordon S (2016) Phagocytosis: An Immunobiologic Process. Immunity 44 (3), 463–475. [DOI] [PubMed] [Google Scholar]
- 2.Groves E et al. (2008) Molecular mechanisms of phagocytic uptake in mammalian cells. Cell Mol Life Sci 65 (13), 1957–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goodridge HS et al. (2012) Mechanisms of Fc receptor and dectin-1 activation for phagocytosis. Traffic 13 (8), 1062–71. [DOI] [PubMed] [Google Scholar]
- 4.Freeman SA and Grinstein S (2014) Phagocytosis: receptors, signal integration, and the cytoskeleton. Immunol Rev 262 (1), 193–215. [DOI] [PubMed] [Google Scholar]
- 5.Newman SL et al. (1991) Differential requirements for cellular cytoskeleton in human macrophage complement receptor- and Fc receptor-mediated phagocytosis. J Immunol 146 (3), 967–74. [PubMed] [Google Scholar]
- 6.Oliveira CA et al. (1996) Effects of latrunculin A on immunological phagocytosis and macrophage spreading-associated changes in the F-actin/G-actin content of the cells. Chem Biol Interact 100 (2), 141–53. [DOI] [PubMed] [Google Scholar]
- 7.Zigmond SH and Hirsch JG (1972) Effects of cytochalasin B on polymorphonuclear leucocyte locomotion, phagocytosis and glycolysis. Exp Cell Res 73 (2), 383–93. [DOI] [PubMed] [Google Scholar]
- 8.Axline SG and Reaven EP (1974) Inhibition of phagocytosis and plasma membrane mobility of the cultivated macrophage by cytochalasin B. Role of subplasmalemmal microfilaments. J Cell Biol 62 (3), 647–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.May RC and Machesky LM (2001) Phagocytosis and the actin cytoskeleton. J Cell Sci 114 (Pt 6), 1061–77. [DOI] [PubMed] [Google Scholar]
- 10.Flannagan RS et al. (2010) Dynamic macrophage “probing” is required for the efficient capture of phagocytic targets. J Cell Biol 191 (6), 1205–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patel PC and Harrison RE (2008) Membrane ruffles capture C3bi-opsonized particles in activated macrophages. Mol Biol Cell 19 (11), 4628–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sobota A et al. (2005) Binding of IgG-opsonized particles to Fc gamma R is an active stage of phagocytosis that involves receptor clustering and phosphorylation. J Immunol 175 (7), 4450–7. [DOI] [PubMed] [Google Scholar]
- 13.Greenberg S and Grinstein S (2002) Phagocytosis and innate immunity. Curr Opin Immunol 14 (1), 136–45. [DOI] [PubMed] [Google Scholar]
- 14.Underhill DM and Goodridge HS (2012) Information processing during phagocytosis. Nat Rev Immunol 12 (7), 492–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vonna L et al. (2007) Micromechanics of filopodia mediated capture of pathogens by macrophages. Eur Biophys J 36 (2), 145–51. [DOI] [PubMed] [Google Scholar]
- 16.Berg JS and Cheney RE (2002) Myosin-X is an unconventional myosin that undergoes intrafilopodial motility. Nat Cell Biol 4 (3), 246–50. [DOI] [PubMed] [Google Scholar]
- 17.Sousa AD and Cheney RE (2005) Myosin-X: a molecular motor at the cell’s fingertips. Trends Cell Biol 15 (10), 533–9. [DOI] [PubMed] [Google Scholar]
- 18.Nagy S et al. (2008) A myosin motor that selects bundled actin for motility. Proc Natl Acad Sci U S A 105 (28), 9616–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cox D et al. (2002) Myosin X is a downstream effector of PI(3)K during phagocytosis. Nat Cell Biol 4 (7), 469–77. [DOI] [PubMed] [Google Scholar]
- 20.Horsthemke M et al. (2017) Multiple roles of filopodial dynamics in particle capture and phagocytosis and phenotypes of Cdc42 and Myo10 deletion. J Biol Chem 292 (17), 7258–7273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bachg AC et al. (2019) Phenotypic analysis of Myo10 knockout (Myo10(tm2/tm2)) mice lacking full-length (motorized) but not brain-specific headless myosin X. Sci Rep 9 (1), 597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim SV and Flavell RA (2008) Myosin I: from yeast to human. Cell Mol Life Sci 65 (14), 2128–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McIntosh BB and Ostap EM (2016) Myosin-I molecular motors at a glance. J Cell Sci 129 (14), 2689–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ostap EM et al. (2003) Dynamic localization of myosin-I to endocytic structures in Acanthamoeba. Cell Motil Cytoskeleton 54 (1), 29–40. [DOI] [PubMed] [Google Scholar]
- 25.Voigt H et al. (1999) Myosin IB from Entamoeba histolytica is involved in phagocytosis of human erythrocytes. J Cell Sci 112 ( Pt 8), 1191–201. [DOI] [PubMed] [Google Scholar]
- 26.Jung G and Hammer JA 3rd (1990) Generation and characterization of Dictyostelium cells deficient in a myosin I heavy chain isoform. J Cell Biol 110 (6), 1955–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jung G et al. (1996) Dictyostelium mutants lacking multiple classic myosin I isoforms reveal combinations of shared and distinct functions. J Cell Biol 133 (2), 305–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Durrwang U et al. (2006) Dictyostelium myosin-IE is a fast molecular motor involved in phagocytosis. J Cell Sci 119 (Pt 3), 550–8. [DOI] [PubMed] [Google Scholar]
- 29.Fukui Y et al. (1989) Myosin I is located at the leading edges of locomoting Dictyostelium amoebae. Nature 341 (6240), 328–31. [DOI] [PubMed] [Google Scholar]
- 30.Chen CL et al. (2012) Myosin I links PIP3 signaling to remodeling of the actin cytoskeleton in chemotaxis. Sci Signal 5 (209), ra10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schwarz EC et al. (2000) Dictyostelium myosin IK is involved in the maintenance of cortical tension and affects motility and phagocytosis. J Cell Sci 113 ( Pt 4), 621–33. [DOI] [PubMed] [Google Scholar]
- 32.Maravillas-Montero JL and Santos-Argumedo L (2012) The myosin family: unconventional roles of actin-dependent molecular motors in immune cells. J Leukoc Biol 91 (1), 35–46. [DOI] [PubMed] [Google Scholar]
- 33.Allen LH and Aderem A (1995) A role for MARCKS, the alpha isozyme of protein kinase C and myosin I in zymosan phagocytosis by macrophages. J Exp Med 182 (3), 829–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Swanson JA et al. (1999) A contractile activity that closes phagosomes in macrophages. J Cell Sci 112 ( Pt 3), 307–16. [DOI] [PubMed] [Google Scholar]
- 35.Maxeiner S et al. (2015) Crucial role for the LSP1-myosin1e bimolecular complex in the regulation of Fcgamma receptor-driven phagocytosis. Mol Biol Cell 26 (9), 1652–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barger SR et al. (2019) Membrane-cytoskeletal crosstalk mediated by myosin-I regulates adhesion turnover during phagocytosis. Nat Commun 10 (1), 1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dart AE et al. (2012) The motor protein myosin 1G functions in FcgammaR-mediated phagocytosis. J Cell Sci 125 (Pt 24), 6020–9. [DOI] [PubMed] [Google Scholar]
- 38.McConnell RE and Tyska MJ (2010) Leveraging the membrane - cytoskeleton interface with myosin-1. Trends Cell Biol 20 (7), 418–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Masters TA et al. (2013) Plasma membrane tension orchestrates membrane trafficking, cytoskeletal remodeling, and biochemical signaling during phagocytosis. Proc Natl Acad Sci U S A 110 (29), 11875–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ostrowski PP et al. (2019) Dynamic Podosome-Like Structures in Nascent Phagosomes Are Coordinated by Phosphoinositides. Dev Cell 50 (4), 397–410 e3. [DOI] [PubMed] [Google Scholar]
- 41.Linder S and Kopp P (2005) Podosomes at a glance. J Cell Sci 118 (Pt 10), 2079–82. [DOI] [PubMed] [Google Scholar]
- 42.Zhang Y et al. (2019) Tail domains of myosin-1e regulate phosphatidylinositol signaling and F-actin polymerization at the ventral layer of podosomes. Mol Biol Cell 30 (5), 622–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van den Dries K et al. (2013) Interplay between myosin IIA-mediated contractility and actin network integrity orchestrates podosome composition and oscillations. Nat Commun 4, 1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Diakonova M et al. (2002) Dynamics of cytoskeletal proteins during Fcgamma receptor-mediated phagocytosis in macrophages. Mol Biol Cell 13 (2), 402–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Goyette G et al. (2012) Proteomic characterization of phagosomal membrane microdomains during phagolysosome biogenesis and evolution. Mol Cell Proteomics 11 (11), 1365–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Haney MS et al. (2018) Identification of phagocytosis regulators using magnetic genome-wide CRISPR screens. Nat Genet 50 (12), 1716–1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xu Y et al. (2014) Dendritic cell motility and T cell activation requires regulation of Rho-cofilin signaling by the Rho-GTPase activating protein myosin IXb. J Immunol 192 (8), 3559–68. [DOI] [PubMed] [Google Scholar]
- 48.Elfrink K et al. (2014) The loop2 insertion of type IX myosin acts as an electrostatic actin tether that permits processive movement. PLoS One 9 (1), e84874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Saczko-Brack D et al. (2016) Self-organization of actin networks by a monomeric myosin. Proc Natl Acad Sci U S A 113 (52), E8387–E8395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Reinhard J et al. (1995) A novel type of myosin implicated in signalling by rho family GTPases. EMBO J 14 (4), 697–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hanley PJ et al. (2010) Motorized RhoGAP myosin IXb (Myo9b) controls cell shape and motility. Proc Natl Acad Sci U S A 107 (27), 12145–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mao Y and Finnemann SC (2015) Regulation of phagocytosis by Rho GTPases. Small GTPases 6 (2), 89–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schlam D et al. (2015) Phosphoinositide 3-kinase enables phagocytosis of large particles by terminating actin assembly through Rac/Cdc42 GTPase-activating proteins. Nat Commun 6, 8623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Caron E and Hall A (1998) Identification of two distinct mechanisms of phagocytosis controlled by different Rho GTPases. Science 282 (5394), 1717–21. [DOI] [PubMed] [Google Scholar]
- 55.Stendahl OI et al. (1980) Distribution of actin-binding protein and myosin in macrophages during spreading and phagocytosis. J Cell Biol 84 (2), 215–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Maupin P et al. (1994) Differential localization of myosin-II isozymes in human cultured cells and blood cells. J Cell Sci 107 ( Pt 11), 3077–90. [DOI] [PubMed] [Google Scholar]
- 57.Heissler SM and Manstein DJ (2013) Nonmuscle myosin-2: mix and match. Cell Mol Life Sci 70 (1), 1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Somlyo AP and Somlyo AV (2003) Ca2+ sensitivity of smooth muscle and nonmuscle myosin II: modulated by G proteins, kinases, and myosin phosphatase. Physiol Rev 83 (4), 1325–58. [DOI] [PubMed] [Google Scholar]
- 59.Scholey JM et al. (1980) Regulation of non-muscle myosin assembly by calmodulin-dependent light chain kinase. Nature 287 (5779), 233–5. [DOI] [PubMed] [Google Scholar]
- 60.Mansfield PJ et al. (2000) Regulation of polymorphonuclear leukocyte phagocytosis by myosin light chain kinase after activation of mitogen-activated protein kinase. Blood 95 (7), 2407–12. [PubMed] [Google Scholar]
- 61.de Lanerolle P et al. (1993) Myosin light chain phosphorylation does not increase during yeast phagocytosis by macrophages. J Biol Chem 268 (23), 16883–6. [PubMed] [Google Scholar]
- 62.Araki N et al. (2003) Phosphoinositide-3-kinase-independent contractile activities associated with Fcgamma-receptor-mediated phagocytosis and macropinocytosis in macrophages. J Cell Sci 116 (Pt 2), 247–57. [DOI] [PubMed] [Google Scholar]
- 63.Tsai RK and Discher DE (2008) Inhibition of “self” engulfment through deactivation of myosin-II at the phagocytic synapse between human cells. J Cell Biol 180 (5), 989–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yamauchi S et al. (2012) Myosin II-dependent exclusion of CD45 from the site of Fcgamma receptor activation during phagocytosis. FEBS Lett 586 (19), 3229–35. [DOI] [PubMed] [Google Scholar]
- 65.Rotty JD et al. (2017) Arp2/3 Complex Is Required for Macrophage Integrin Functions but Is Dispensable for FcR Phagocytosis and In Vivo Motility. Dev Cell 42 (5), 498–513 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kovari DT et al. (2016) Frustrated Phagocytic Spreading of J774A-1 Macrophages Ends in Myosin II-Dependent Contraction. Biophys J 111 (12), 2698–2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Olazabal IM et al. (2002) Rho-kinase and myosin-II control phagocytic cup formation during CR, but not FcgammaR, phagocytosis. Curr Biol 12 (16), 1413–18. [DOI] [PubMed] [Google Scholar]
- 68.Shutova MS and Svitkina TM (2018) Mammalian nonmuscle myosin II comes in three flavors. Biochem Biophys Res Commun 506 (2), 394–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Baranov MV et al. (2016) SWAP70 Organizes the Actin Cytoskeleton and Is Essential for Phagocytosis. Cell Rep 17 (6), 1518–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Allen LA and Aderem A (1996) Molecular definition of distinct cytoskeletal structures involved in complement- and Fc receptor-mediated phagocytosis in macrophages. J Exp Med 184 (2), 627–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Furukawa R and Fechheimer M (1994) Differential localization of alpha-actinin and the 30 kD actin-bundling protein in the cleavage furrow, phagocytic cup, and contractile vacuole of Dictyostelium discoideum. Cell Motil Cytoskeleton 29 (1), 46–56. [DOI] [PubMed] [Google Scholar]
- 72.Sjoblom B et al. (2008) Alpha-actinin structure and regulation. Cell Mol Life Sci 65 (17), 2688–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Markwardt ML et al. (2018) A Genetically Encoded Biosensor Strategy for Quantifying Non-muscle Myosin II Phosphorylation Dynamics in Living Cells and Organisms. Cell Rep 24 (4), 1060–1070 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Smith AS et al. (2018) Myosin IIA interacts with the spectrin-actin membrane skeleton to control red blood cell membrane curvature and deformability. Proc Natl Acad Sci U S A 115 (19), E4377–E4385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Blocker A et al. (1997) Molecular requirements for bi-directional movement of phagosomes along microtubules. J Cell Biol 137 (1), 113–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Al-Haddad A et al. (2001) Myosin Va bound to phagosomes binds to F-actin and delays microtubule-dependent motility. Mol Biol Cell 12 (9), 2742–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mehta AD et al. (1999) Myosin-V is a processive actin-based motor. Nature 400 (6744), 590–3. [DOI] [PubMed] [Google Scholar]
- 78.Wei Q et al. (1997) The predominant defect in dilute melanocytes is in melanosome distribution and not cell shape, supporting a role for myosin V in melanosome transport. J Muscle Res Cell Motil 18 (5), 517–27. [DOI] [PubMed] [Google Scholar]
- 79.Varadi A et al. (2005) Myosin Va transports dense core secretory vesicles in pancreatic MIN6 beta-cells. Mol Biol Cell 16 (6), 2670–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wagner W et al. (2011) Myosin-Va transports the endoplasmic reticulum into the dendritic spines of Purkinje neurons. Nat Cell Biol 13 (1), 40–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wu X et al. (1998) Visualization of melanosome dynamics within wild-type and dilute melanocytes suggests a paradigm for myosin V function In vivo. J Cell Biol 143 (7), 1899–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Li XD et al. (2005) Activation of myosin Va function by melanophilin, a specific docking partner of myosin Va. J Biol Chem 280 (18), 17815–22. [DOI] [PubMed] [Google Scholar]
- 83.Lindsay AJ et al. (2013) Identification and characterization of multiple novel Rab-myosin Va interactions. Mol Biol Cell 24 (21), 3420–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gutierrez MG (2013) Functional role(s) of phagosomal Rab GTPases. Small GTPases 4 (3), 148–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Patel NR et al. (2012) Cell elasticity determines macrophage function. PLoS One 7 (9), e41024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jain N and Vogel V (2018) Spatial confinement downsizes the inflammatory response of macrophages. Nat Mater 17 (12), 1134–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shiratsuchi H and Basson MD (2004) Extracellular pressure stimulates macrophage phagocytosis by inhibiting a pathway involving FAK and ERK. Am J Physiol Cell Physiol 286 (6), C1358–66. [DOI] [PubMed] [Google Scholar]
- 88.Tabata Y and Ikada Y (1988) Effect of the size and surface charge of polymer microspheres on their phagocytosis by macrophage. Biomaterials 9 (4), 356–62. [DOI] [PubMed] [Google Scholar]
- 89.Champion JA et al. (2008) Role of particle size in phagocytosis of polymeric microspheres. Pharm Res 25 (8), 1815–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Beningo KA and Wang YL (2002) Fc-receptor-mediated phagocytosis is regulated by mechanical properties of the target. J Cell Sci 115 (Pt 4), 849–56. [DOI] [PubMed] [Google Scholar]
- 91.Key J et al. (2015) Soft Discoidal Polymeric Nanoconstructs Resist Macrophage Uptake and Enhance Vascular Targeting in Tumors. ACS Nano 9 (12), 11628–41. [DOI] [PubMed] [Google Scholar]
- 92.Anselmo AC et al. (2015) Elasticity of nanoparticles influences their blood circulation, phagocytosis, endocytosis, and targeting. ACS Nano 9 (3), 3169–77. [DOI] [PubMed] [Google Scholar]
- 93.Palomba R et al. (2018) Modulating Phagocytic Cell Sequestration by Tailoring Nanoconstruct Softness. ACS Nano 12 (2), 1433–1444. [DOI] [PubMed] [Google Scholar]
- 94.Champion JA and Mitragotri S (2009) Shape induced inhibition of phagocytosis of polymer particles. Pharm Res 26 (1), 244–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sharma G et al. (2010) Polymer particle shape independently influences binding and internalization by macrophages. J Control Release 147 (3), 408–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Moller J et al. (2012) The race to the pole: how high-aspect ratio shape and heterogeneous environments limit phagocytosis of filamentous Escherichia coli bacteria by macrophages. Nano Lett 12 (6), 2901–5. [DOI] [PubMed] [Google Scholar]
- 97.Paul D et al. (2013) Phagocytosis dynamics depends on target shape. Biophys J 105 (5), 1143–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sosale NG et al. (2015) Cell rigidity and shape override CD47’s “self”-signaling in phagocytosis by hyperactivating myosin-II. Blood 125 (3), 542–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jaumouille V et al. (2019) Coupling of beta2 integrins to actin by a mechanosensitive molecular clutch drives complement receptor-mediated phagocytosis. Nat Cell Biol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Greenberg MJ et al. (2016) A Perspective on the Role of Myosins as Mechanosensors. Biophys J 110 (12), 2568–2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gardini L et al. (2018) Dissecting myosin-5B mechanosensitivity and calcium regulation at the single molecule level. Nat Commun 9 (1), 2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sellers JR and Veigel C (2010) Direct observation of the myosin-Va power stroke and its reversal. Nat Struct Mol Biol 17 (5), 590–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Elliott H et al. (2015) Myosin II controls cellular branching morphogenesis and migration in three dimensions by minimizing cell-surface curvature. Nat Cell Biol 17 (2), 137–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hartman MA and Spudich JA (2012) The myosin superfamily at a glance. J Cell Sci 125 (Pt 7), 1627–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Heissler SM and Sellers JR (2014) Myosin light chains: Teaching old dogs new tricks. Bioarchitecture 4 (6), 169–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Heissler SM and Sellers JR (2016) Kinetic Adaptations of Myosins for Their Diverse Cellular Functions. Traffic 17 (8), 839–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kress H et al. (2007) Filopodia act as phagocytic tentacles and pull with discrete steps and a load-dependent velocity. Proc Natl Acad Sci U S A 104 (28), 11633–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Nelsen E et al. (2019) Combined Atomic Force Microscope and Volumetric Light Sheet System for Mechanobiology. bioRxiv, DOI 10.1101/812396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Herant M et al. (2005) Mechanics of neutrophil phagocytosis: behavior of the cortical tension. J Cell Sci 118 (Pt 9), 1789–97. [DOI] [PubMed] [Google Scholar]
- 110.Herant M et al. (2006) Mechanics of neutrophil phagocytosis: experiments and quantitative models. J Cell Sci 119 (Pt 9), 1903–13. [DOI] [PubMed] [Google Scholar]
- 111.Vorselen D et al. (2018) Superresolved and reference-free microparticle traction force microscopy (MP-TFM) reveals the complexity of the mechanical interaction in phagocytosis. bioRxiv, DOI 10.1101/431221. [DOI] [Google Scholar]
- 112.Krendel M et al. (2005) Myosins: tails (and heads) of functional diversity. Physiology 20, 239–251. [DOI] [PubMed] [Google Scholar]