Abstract
The retrosplenial cortex (RSC) is positioned at the interface between cortical sensory regions and the structures that compose the medial temporal lobe memory system. It has recently been suggested that one functional role of the RSC involves the formation of associations between cues in the environment (S-S learning; Bucci & Robinson, 2014). This suggestion is based, in part, on the finding that lesions or temporary inactivation of the RSC impair sensory preconditioning. However, all prior studies examining the role of the RSC in sensory preconditioning have used cues from multiple modalities (both visual and auditory stimuli). The purpose of the present experiment was to determine if the RSC contributes to unimodal sensory preconditioning. In the present study we found that both electrolytic and neurotoxic lesions of the RSC impaired sensory preconditioning with auditory cues. Together with previous experiments, these findings indicate that the RSC contributes to both multisensory and unimodal sensory integration, which suggests a general role for the RSC in linking sensory cues in the environment.
Keywords: retrosplenial cortex, sensory preconditioning, higher-order conditioning, stimulus-stimulus learning
Introduction
The retrosplenial cortex (RSC) is situated at the interface between sensory cortical regions and the structures that compose the hippocampal/parahippocampal memory system. For instance, RSC receives input from visual, spatial, and auditory cortical regions (van Groen and Wyss, 2003; Wyss and van Groen, 1992; Vogt and Miller, 1983; Todd et al., 2016) and is interconnected with multiple components of the hippocampal formation and parahippocampal regions (e.g., subiculum, and postrhinal, perirhinal, and entorhinal cortices; Burwell and Amaral, 1998; Wyss and van Groen, 1992; Kerr et al, 2007; van Groen and Wyss, 1990a, 1990b; summarized by Sugar et al., 2011). As such, it has been theorized that RSC may function as a hub that contributes to learning and memory by linking together the sensory features that compose a learning environment or event (Bucci & Robinson, 2014; Kobayashi & Amaral, 2007; Wolbers & Buchel, 2005).
This suggestion has been tested using a sensory preconditioning procedure (Brogden, 1939; Blaisdell et al., 2009; Ward-Robinson et al., 2001) in an effort to directly demonstrate that RSC is essential for forming associations between otherwise neutral sensory stimuli (i.e., “S-S associations”; Robinson et al., 2011; 2014). During the first phase (i.e., Preconditioning) of the procedure used by Robinson et al. (2011, 2014), rats received serial presentations of an auditory stimulus (A1) and a visual stimulus (V). On intermixed trials a second auditory stimulus (A2) was presented alone. Importantly, there was no reinforcement during this phase of training. In the subsequent Conditioning phase of the experiment, V was paired with food reward and no other stimuli were presented. Finally, during the critical Test phase, each of the auditory cues was presented alone and non-reinforced. Control rats exhibited greater conditioned responding (food cup behavior) during presentations of A1 than A2, indicative of the typical sensory preconditioning effect. Greater responding to A1 than A2 at test is presumably mediated by a previously established association between A1 and V.
Permanent lesions of RSC carried out prior to training spared conditioning to the light in phase 2, but eliminated the sensory preconditioning effect (Robinson et al., 2011). That is, RSC-lesioned rats responded comparably to A1 and A2 during the test phase. Importantly, temporarily silencing RSC neurons only during preconditioning using a chemogenetic approach also impaired the sensory preconditioning effect (Robinson et al., 2014). These latter data indicate that RSC function is particularly necessary for forming the initial S-S association, since the sensory preconditioning effect was eliminated despite RSC being back online during the conditioning sessions and during the test session.
While these studies indicate that RSC is necessary for forming S-S associations, it remains an open question of whether RSC is needed for all such associations. In particular, studies thus far have demonstrated that RSC is important for forming associations among stimuli of multiple modalities, which is consistent with the fact that RSC is privy to a range of sensory information (i.e., visual, auditory, etc.). However, it is unclear if RSC is also needed for linking stimuli within the same sensory modality. Indeed, linking together sensory information within the same modality could depend on RSC, given recent evidence that secondary auditory cortex is not involved in sensory preconditioning of auditory cues, at least under some conditions (Grosso et al., 2015)
To test this, rats received either neurotoxic or electrolytic RSC lesions (or sham lesions) prior to training in a unimodal sensory preconditioning procedure using auditory stimuli (illustrated in Figure 1). In the Preconditioning phase, one auditory cue (A1) was paired with a second auditory cue (A2). A third cue (A3) was presented alone on intermixed trials. During the Conditioning phase, A2 was paired with food reward. Finally, during the Test phase, A1 and A3 were presented alone and food cup behavior was measured. We predicted that RSC has an essential role in linking together neutral sensory stimuli in the service of learning, and thus damage to RSC would disrupt unimodal sensory preconditioning.
Figure 1.
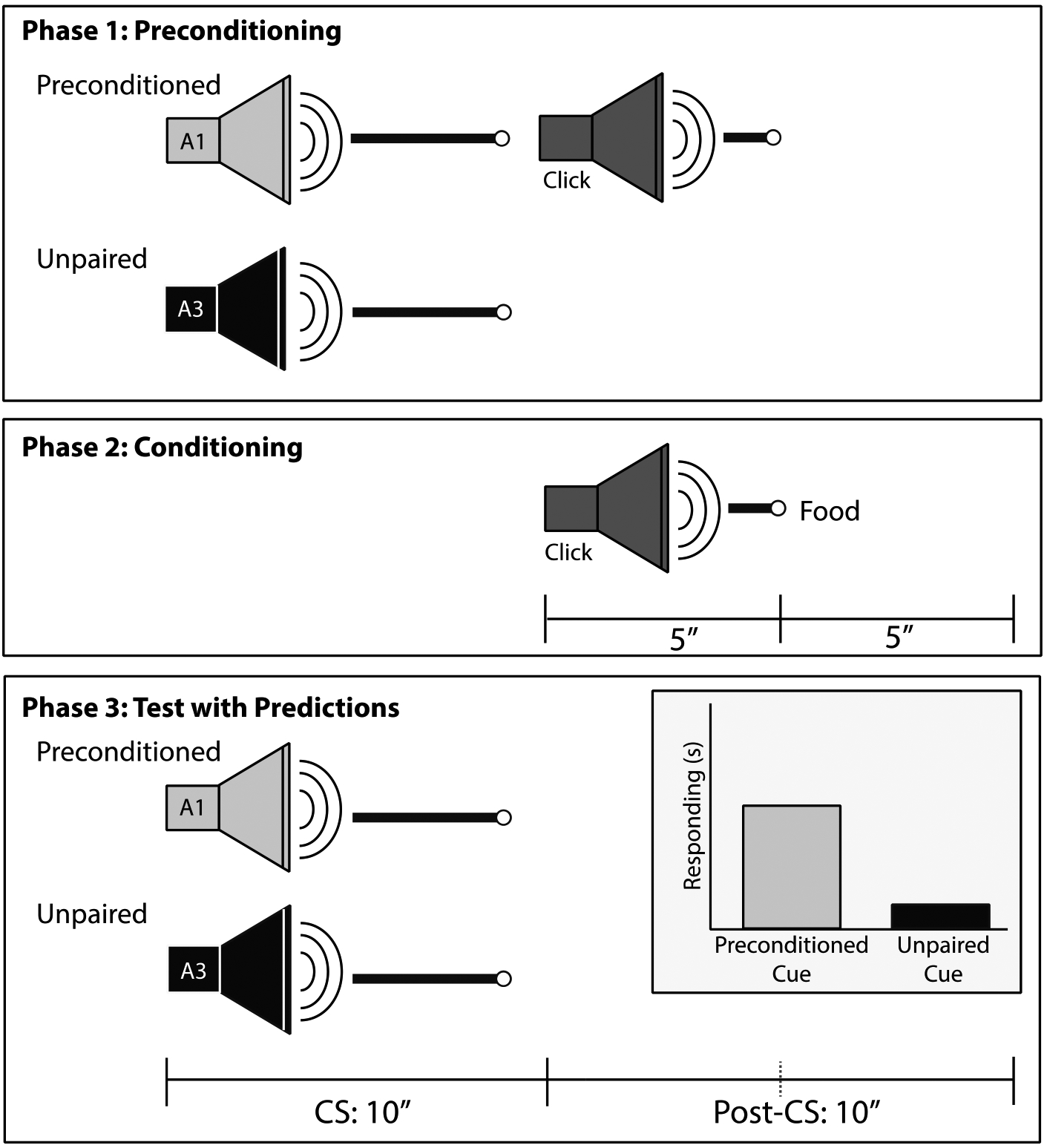
Timeline of Sensory Preconditioning training. During the Preconditioning phase, two neutral auditory cues (A1, A2) were presented serially and a third auditory cue (A3) was presented alone. The preconditioned (A1) and unpaired cue (A3) were either white noise (WN) or a tone (counterbalanced). During the Conditioning phase, a clicker stimulus (A2) was presented after A1 and paired with food reward. During the Test phase, A1 and A3 were presented in intermixed trials. Inset in third panel illustrated expected Test results in intact rats that successfully learned S-S association between preconditioned cue and click; that is, the preconditioned cue should elicit more post-CS responding than the unpaired cue.
Materials and Methods
Subjects
The subjects were 24 naïve Long-Evans male rats obtained from Envigo (Indianapolis, IN) and were ~60 days old upon arrival. All rats were pair-housed and allowed at least 6 days to acclimate to the vivarium. All rats had food (Purina standard chow; Nestle Purina, St. Louis, MO) and water ad libitum during the acclimation period. After the acclimation period all rats underwent surgery as described below. Rats were allowed to return to pre-surgical weight before being food restricted to gradually reduce their weight to 85% of free-feed body weight for behavioral training. Rats were weighed and handled daily and maintained at 85% weight by supplementing food earned during training with standard chow. The rats were maintained on a 14:10 light-dark cycle throughout the experiment and cared for in compliance with association for Assessment and Accreditation of laboratory Animal Care guidelines and the Dartmouth College Institutional Animal Care and Use Committee.
Surgical Procedures
Rats were evenly divided into three groups. One group of rats (n = 8) received bilateral electrolytic RSC lesions, a second group (n = 8) received bilateral neurotoxic (NMDA) RSC lesions, and the final group of rats (n = 8) received sham lesions. Rats were anesthetized with isoflurane gas (1.5 – 3% in oxygen) and placed in a stereotaxic apparatus (Kopf Instruments, Tujunga, CA). The skin was retracted and a craniotomy was performed. For rats receiving either electrolytic or NMDA lesions, skull penetrating burr holes were drilled above the intended lesion site using the coordinates shown in Table 1. The exact co-ordinates differed between electrolytic and neurotoxic because the spread of NMDA drug infusion differs from the spread of an electrically-induced burn. These coordinates were used to ensure maximum RSC damage for each lesion type while also minimizing the potential for non-RSC damage. In the electrolytic lesion group (abbreviated “RSC-Electro”) an epoxy-coated electrode with a 1 mm exposed tip was lowered into each hole and current (2.5 mA) was applied for 15 sec at each site. Rats receiving NMDA neurotoxic lesions (abbreviated “RSC-NMDA”) had a 26-gauge Hamilton syringe lowered into each hole. The syringe was left in place for 1 min prior to the infusion and 4 mins after the infusion to minimize transport of the toxin up the syringe track. NMDA (0.09M) was prepared fresh daily in sterile PBS and infused at a rate of 0.10μL/min. Sham-lesioned rats underwent the craniotomy and received non-penetrating burr holes to avoid damaging the underlying RSC. Rats began sensory preconditioning behavioral training 16 – 28 days post-surgery.
Table 1:
Stereotaxic coordinates
| Anterior-Posterior | Medial-Lateral | Dorsal-Ventral | Volume (μL) | |
|---|---|---|---|---|
| Electrolytic | −2.0 | ±0.3 | −2.0 & −2.7 | N/A |
| −3.5 | ±0.4 | −2.0 & −2.7 | N/A | |
| −5.0 | ±0.4 & ± 1.0 | −2.0 & −2.7 | N/A | |
| −6.5 | ±0.8 & ± 1.5 | −2.0 & −2.8 | N/A | |
| −8.0 | ±1.6 & ± 2.4 | −2.5 & −3.1 | N/A | |
| −9.0 | ±3.4 | −4.0 | N/A | |
| NMDA | −2.2 | ±0.5 | −2.0 | 0.30 |
| −3.9 | ±0.5 | −2.0 | 0.30 | |
| −5.5 | ±0.5 | −2.6 | 0.22 | |
| −5.5 | ±1.0 | −1.8 | 0.30 | |
| −6.7 | ±1.1 | −2.2 | 0.30 | |
| −8.0 | ±1.3 | −1.8 | 0.22 |
Note: Electrolytic lesions were measured from skull level (2.5mA, 15s / site), NMDA lesions were measured from brain surface
Behavioral Apparatus
Eight identical conditioning chambers (Med Associates, Inc., St. Albans, VT, ENV-007; 24 cm W × 30.5 cm L × 29 cm H) were used in the experiment, each housed in an individual sound attenuating chamber (Med Associates, ENV-017M; 66 cm W × 56 cm H × 56 cm D). The conditioning chambers had clear acrylic sidewalls and ceilings and the front and rear walls were made of brushed aluminum. The floors consisted of evenly spaced (1.5 cm) stainless steel rods (5 mm diameter). A food cup was recessed in the center of the front wall of each chamber, and two retractable levers (Med Associates, ENV-112CM) were positioned to the left and right of the food cup (levers remained retracted for the duration of the experiment and were not used). Four panel lights (Med Associates, ENV-221M) were mounted in each chamber: a light was located above each lever and the food cup (~10.8 cm above the grid floor) and an additional light was positioned 16 cm directly above the food cup. A speaker (Med Associates, ENV-224AM) was mounted 20 cm above and to the right of food magazine and was the source of the three auditory stimuli (74 dB, 10Hz clicker; 78 dB, 1500 Hz pure tone; and a 78 dB white noise). Two 45mg dustless grain pellets (BioServ, Flemington, NJ) served as the unconditioned stimulus (US). An infrared photobeam transmitter/receiver was located at the front of the food cup to monitor time (sec) spent in the food cup. A 2.8 W bulb (with a red cover) was mounted to the ceiling of the sound attenuating chamber and remained on during each session to provide diffuse background illumination. Background noise (~68 dB) and airflow was created by an exhaust fan mounted to side of each sound attenuating chamber.
Behavioral Procedures
The behavioral procedures are illustrated in Figure 1. The Preconditioning phase consisted of one session per day for a total of 5 days. Each Preconditioning session was ~60 min in duration, and 12 trials were presented per session: 6 trials consisted of presentations of the “preconditioned” cue, which was either the 10-sec tone or white noise stimulus (counterbalanced), followed immediately by the clicker stimulus (5 sec). The other 6 trials consisted of the “unpaired” cue, which was either the 10-sec tone or white noise (whichever one was not used as the preconditioned cue) presented alone (i.e., not followed by the clicker). Half of the rats in each group had the tone as the preconditioned cue and the other half had the white noise as the preconditioned cue. The two types of trials were intermixed throughout the session. The inter-stimulus interval (ITI) was on average 4.5 mins (range: 2 – 7 mins) and the trial order was random with the exception that no more than two of the same trial types (preconditioned, unpaired) could be presented consecutively.
The Conditioning phase consisted of one session per day for a total of 7 days. Each session was ~60 mins and included 8 trials (average ITI = 7 min; range: 6.2 – 7.7 mins). Each trial consisted of the 5-sec clicker (A2) stimulus terminating with the US delivered into the food cup.
A single Test session occurred 24 hrs after the last conditioning session. The Test session consisted of 12 total trials: 6 presentations of the 10-sec preconditioned cue and 6 presentations of the 10-sec unpaired cue (intermixed trials, ITI = 4.5 min). Half of the rats in each group received the preconditioned cue as the first trial of the Test session, the other half of the rats in each group was presented with the unpaired cue first (the tone and white noise stimuli were counterbalanced as the preconditioned and unpaired cues in all groups). Trial presentation was random following the first designated trial type with the exception that no more than two of the same trial type could occur consecutively.
Behavioral Observations and Analysis
The amount of time spent with the snout in the food cup served as the measure of conditioned food cup responding and was analyzed during, preconditioning, conditioning and during the test session. During preconditioning, we analyzed responding during the presentation of each stimulus (CS-epoch), as well as the 10 seconds prior to (Pre-CS) and 10 seconds following (Post-CS) each stimulus. During conditioning, the data of most interest was time spent in the food cup during presentation of the 5-sec click conditioned stimulus (CS). We also collected data during the 5-sec period before the CS was presented (Pre-CS epoch) and the 5-sec period after the CS ended and food was delivered (Post-CS epoch). The data from each epoch were analyzed using a repeated measures analysis of variance (ANOVA) with Group as the between-subjects factor (RSC sham-lesion, electrolytic lesion, or NMDA lesion) and Session (1–7) as the within-subjects factor.
During the critical Test session, data were collected and analyzed during the Pre-CS epoch, the CS epoch, and the Post-CS epoch (10-sec each). However, the primary measure of interest was the amount of time spent in the food cup during the Post-CS epoch, because this period of time corresponds with the time that the click was presented after the tone (or white noise) during Preconditioning and to the time that food was presented during Conditioning (see Figure 1 for illustration of the different epochs and their alignment in time). That is, if rats formed an association between the tone (or white noise) and the clicker during the Preconditioning phase, food-cup behavior was expected to be particularly high during the Post-CS epoch of the Test session, as observed previously (Blaisdell et al., 2009; Robinson et al., 2011, 2014).
The strength of sensory preconditioning was assessed with two analyses. First, the average response to the preconditioned cue was compared to the average response to the unpaired cue. Second, we used a discrimination ratio to take into account individual differences in food cup responding (Iordanova et al., 2011; Robinson et al., 2011, 2014). Discrimination ratios for each rat were calculated by dividing responding during the Post-CS epoch following the presentation of the preconditioned cue divided by the sum of Post-CS responding observed following the preconditioned and unpaired cues [i.e., Post-CSpreconditioned cue / (Post-CSpreconditioned cue + Post-CSunpaired cue)]. A discrimination ratio of 0.5 would thus indicate no difference in responding to the paired and unpaired cues (i.e., chance) and therefore a lack of a sensory preconditioning effect. A maximal sensory preconditioning effect would be reflected in a discrimination ratio of 1.0, indicating that responding only occurred to the preconditioned cue and never to the unpaired cue. Discrimination ratios for each of the three treatment groups were assessed using one-sample t-tests with a hypothesized value of 0.5 (no sensory preconditioning), consistent with the analysis used in prior studies (Robinson et al., 2011, 2014). An alpha level of 0.05 was used in all analyses.
Lesion Verification and Analysis
After behavioral training was complete, rats were deeply anesthetized with sodium pentobarbital and transcardially perfused with 0.9% saline (~250 ml) followed by 10% buffered formalin (~300 ml). Brains were removed and remained in formalin for 24 hours followed by 72 hrs in 30% sucrose. A freezing microtome was used to collect 60-μm coronal brain sections throughout the RSC (and just anterior and just posterior to RSC), which were kept at 4° in phosphate buffer until being mounted the next day onto gelatin-coated glass slides. The sections (~25/rat) were then thionin-stained and viewed under a compound microscope (Axioskop I, Zeiss, Inc., Thornwood NY). Two analyses were conducted: 1) Sections at six rostro-caudal levels (AP −1.8, −3.0, −4.2, −5.4, −6.6, & −7.8 mm from bregma) were used to obtain areas measurements of RSC damage (defined as missing tissue, gliosis, cortical thinning) and the average percentage of RSC damage was calculated; 2) All ~25 sections collected for each rat were observed under the microscope and noted as having RSC damage or not. This was used to calculate the number of sections across the rostro-caudal extent of RSC that were damaged. Lesion tracings for each rat were made by hand using the Paxinos & Watson (2009) rat brain atlas as a guide and processed using ImageJ.
Results
Histology
As shown in Figure 2, rats in both lesion groups exhibited bilateral RSC damage. On average, 57.5 ± 5.9% of the total area of RSC was damaged on each section analyzed in the electrolytic group (Figure 2, left panel), and 93.8 ± 2.6% of all sections (~26) containing the RSC exhibited damage. Six rats had minor damage to the secondary motor cortex, one rat had minor damage to the cingulum bundle and seven rats had minor damage to the secondary visual cortex. Off target damage was bilateral and did not impact the entire rostro-caudal extent of the structure. For the NMDA lesion group (Figure 2, right panel), an average of 53.8 ± 2.5% of the total area of RSC was damaged on the sections that were analyzed, and 96.1 ± 4.1% of all sections containing the RSC (~25) exhibited damage. Panel A on each side of the figure illustrates a representative photomicrograph of each type of lesion and Panel B on each side of the figure is a schematic drawing of each type of lesion. One rat had superficial unilateral damage to the secondary motor cortex and all eight rats exhibited minor bilateral damage to the posterior secondary visual cortex.
Figure 2.
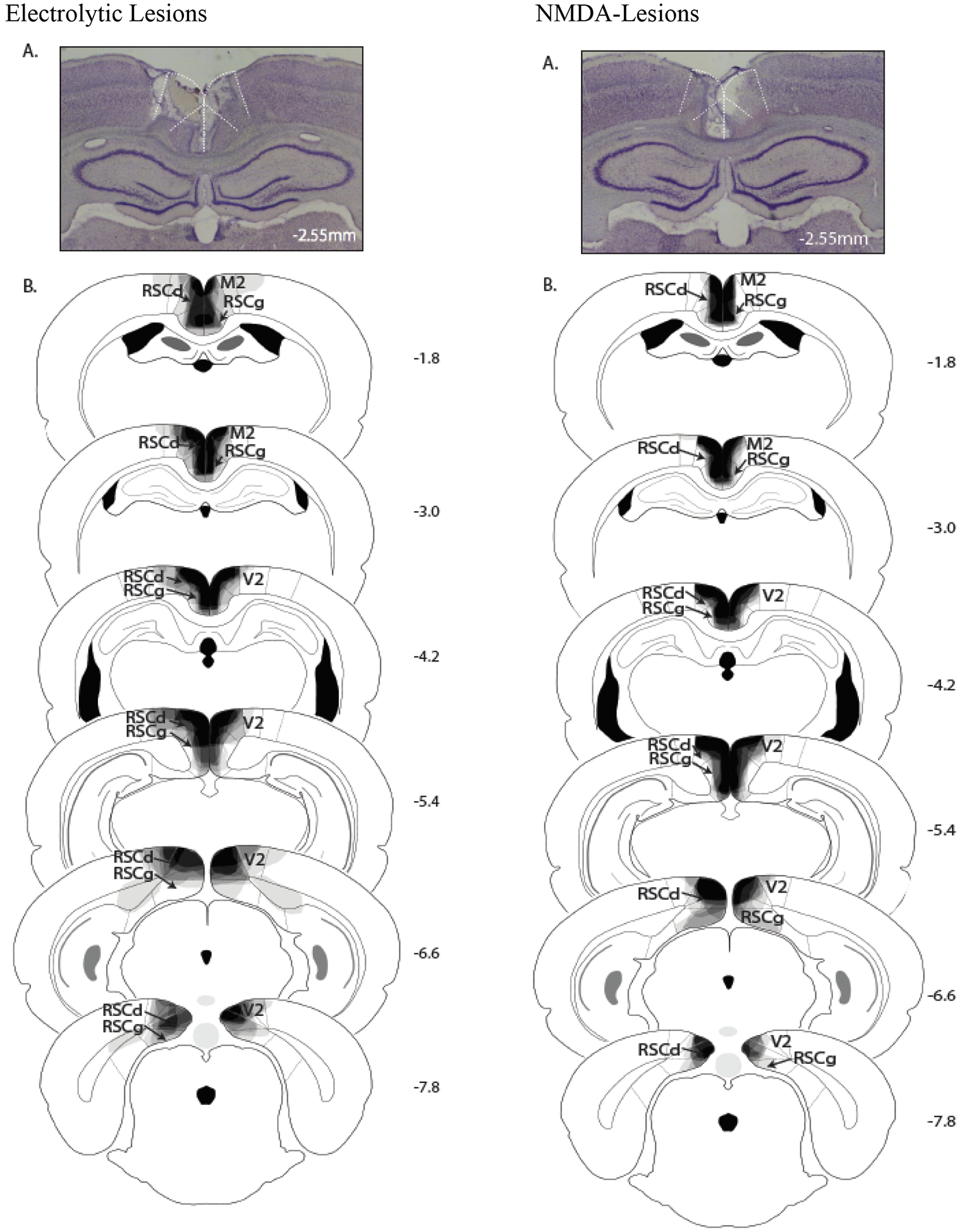
Photomicrographs (A) of representative RSC neurotoxic and electrolytic lesions. Schematic drawings (B) of the extent of damage in RSC-lesioned rats at six levels along the rostro-caudal extent of the RSC (indicated in mm with respect to bregma). At each level, lesion drawings were stacked onto a single image. The degree of shading (gray through black) indicates the number of lesion cases that include that area (black indicates that all cases exhibited damage). M2 = secondary motor cortex; RSCd = restrosplenial dysgranular; RSCg = retrosplenial granular; V2 = secondary visual cortex.
Behavior
Preconditioning.
During preconditioning, the average time in food cup was relatively low, and did not differ between groups. A 3 (Group) × 3 (Cue) × 5 (Session) repeated measures ANOVA of food cup responding during the cue presentations (CS-epochs) revealed a main effect of Cue [F (2, 42) = 8.5, p < 0.001], and a main effect of Session [F (4, 84) = 3.9, p < 0.01]. There was no main effect of group (p = 0.06). Interactions of Cue × Group, Session × Group, and Cue × Session × Group were also not significant (ps > 0.06). Paired samples t-test indicated responding to the click (0.17s; SEM ± 0.04) was significantly lower than the preconditioned (0.35s; SEM ± 0.08) [t (23) = 3.57, p <0.01] and unpaired cues (0.49s; SEM ± 0.09) [t (23) = −3.67, p < 0.01] which did not differ from each other [t (23) = −1.6, p > 0.1]. Thus, although responding to the click stimulus was lower overall, responding did not differ across the preconditioned and unpaired cue.
We then compared responding during the Post-CS for the preconditioned and unpaired cue to ensure there were no pre-existing differences (as noted, this time epoch was expected to be the most sensitive for detecting sensory preconditioning). Note, the 10-s Post-CS period of the preconditioned cue included the 5 second presentation of the click stimulus. A 3 (Group) × 2 (Cue) × 5 (Session) repeated measures ANOVA revealed a main effect of group [F (2, 21) = 7.2, p < 0.01], and a main effect of session [F (4, 84) = 2.6, p < 0.05] which indicated a decrease in responding over session. The main effect of cue was not significant, nor any other interaction. Post-hoc pairwise comparisons using Tukey’s HSD indicated RSC-NMDA group (0.33s; SEM ± 0.06) had significantly higher overall responding than the RSC-Sham (0.14s; SEM ± 0.02) and RSC-Electro groups (0.13s; SEM ± 0.04) (p < 0.02). Importantly however, there were no differences within each group between the preconditioned and unpaired cue: sham [t (7) = −0.75, p = 0.5], RSC-electrolytic [t (7) = −0.18, p = 0.9], and RSC-NMDA cue [t (7) = −0.72, p = 0.5]. A 3 (Group) × 2 (Cue) × 5 (Session) repeated measures ANOVA of food cup responding during the Pre-CS epoch revealed no significant main effects or interactions (ps > 0.2).
Conditioning.
As depicted in Figure 3A, there were no differences in food cup behavior between the three groups during the presentation of the click when it was paired with food. A 3 (Group) × 7 (Session) repeated measures ANOVA revealed a significant main effect of Session [F (6, 126) = 38.1, p < 0.001], indicating that rats increased food cup responding during the CS across the daily sessions. Neither the main effect of group [F (2, 21) = 1.2, p > 0.3] nor the Group × Session interaction [F (12, 126) = 0.7, p > 0.6] were significant. Similarly, a repeated measures ANOVA of food cup responding during the Post-CS epoch (Figure 3B) revealed a significant main effect of Session [F (6, 126) = 81.9, p < 0.001] but no main effect of Group [F (2, 21) = 1.4, p > 0.2], and no Group × Session interaction [F (12, 126) = 1.7, p > 0.1]. Finally, an identical ANOVA on Pre-CS responding did not reveal a significant main effect of session or group (ps > 0.5). The interaction between Group × Session was not significant (p > 0.5). Across all trials and conditioning sessions the average time spent in the food cup was 0.67s (SEM ± 0.28), 0.26s (SEM ± 0.11) and 0.65s (SEM ± 0.26) for Sham, Electrolytic, and Neurotoxic lesioned rats, respectively. In sum, neither electrolytic nor neurotoxic lesions of RSC had an impact on food cup responding during the Conditioning phase. Rats in all groups exhibited increasing levels of conditioned food cup behavior across sessions.
Figure 3.
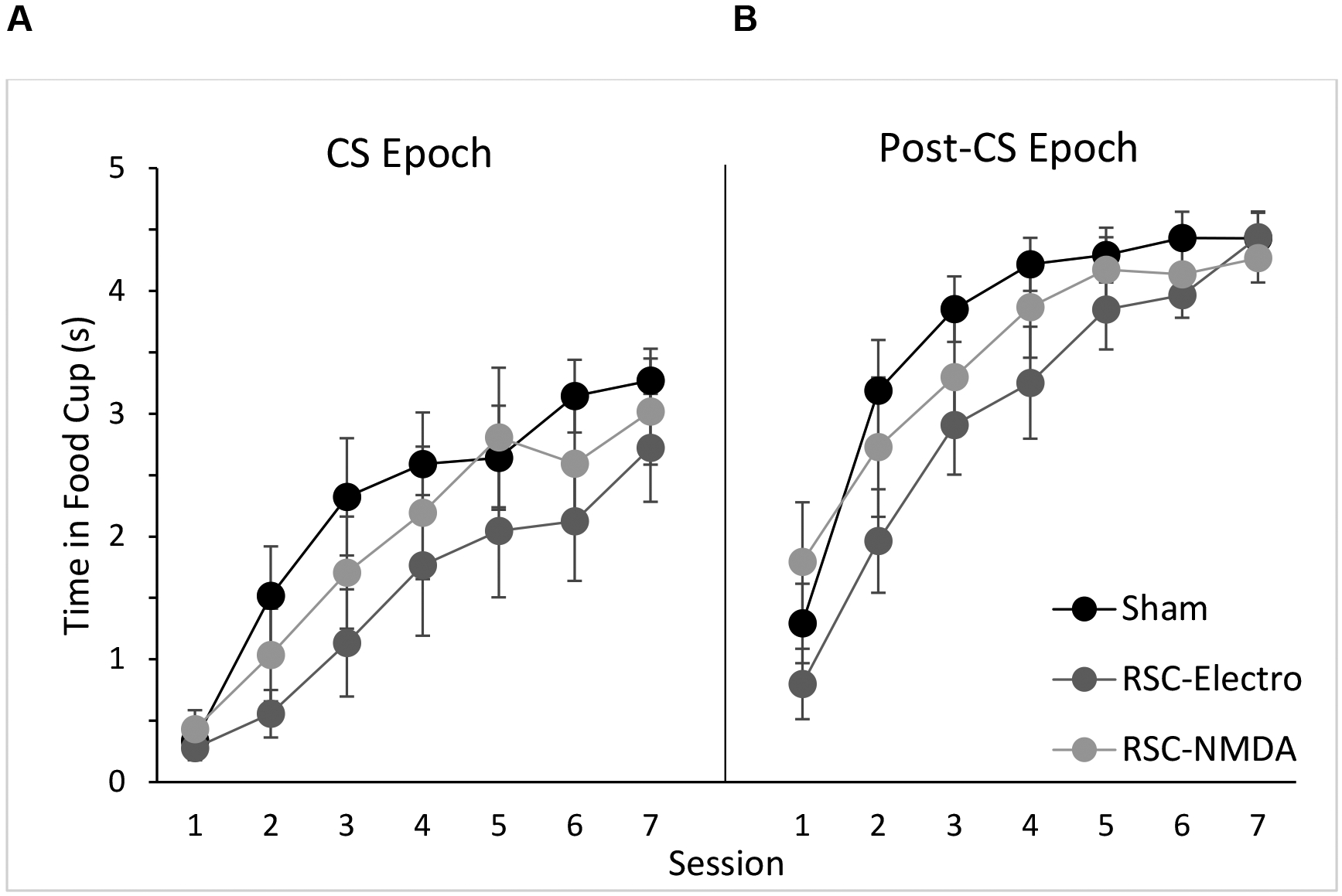
Average food cup behavior during the CS epoch and Post-CS epoch during the Conditioning phase. Data are mean ± S.E.M.
Test.
Responding during the CS-epoch was analyzed with a 3 (Group) × 2 (Cue) × 6 (Trial) repeated measures ANOVA. This analysis revealed a significant main effect of trial [F (5, 105) = 4.4, p < 0.01]. There were no significant main effects of cue, or group (ps > 0.8). There were no significant interactions (ps > 0.3).
As previously noted, we expected responding during the Post-CS epoch to be the most sensitive measure for detecting sensory preconditioning. Responding during this period for the 6 test trials of each cue is presented in Figure 4, along with the mean from each cue. A 3 (Group) × 2 (Cue) × 6 (Trial) repeated measures ANOVA revealed a significant main effect of Cue [F (1, 21) = 4.44, p < 0.05] and a main effect of trial [F (5, 105) = 7.83, p < 0.001]. There were no significant interactions (ps > 0.05). Planned comparisons between each cue within each group revealed that only RSC-Sham rats responded significantly more during the post-CS epoch of the preconditioned cue than the unpaired cue averaged over all trials [t (7) = 4.3; p < 0.005]. The cue comparison was not significant for RSC-Electrolytic rats [t (7) = 0.83, p > 0.4], nor for RSC-NMDA rats [t (7) = −0.11, p > 0.9).
Figure 4.
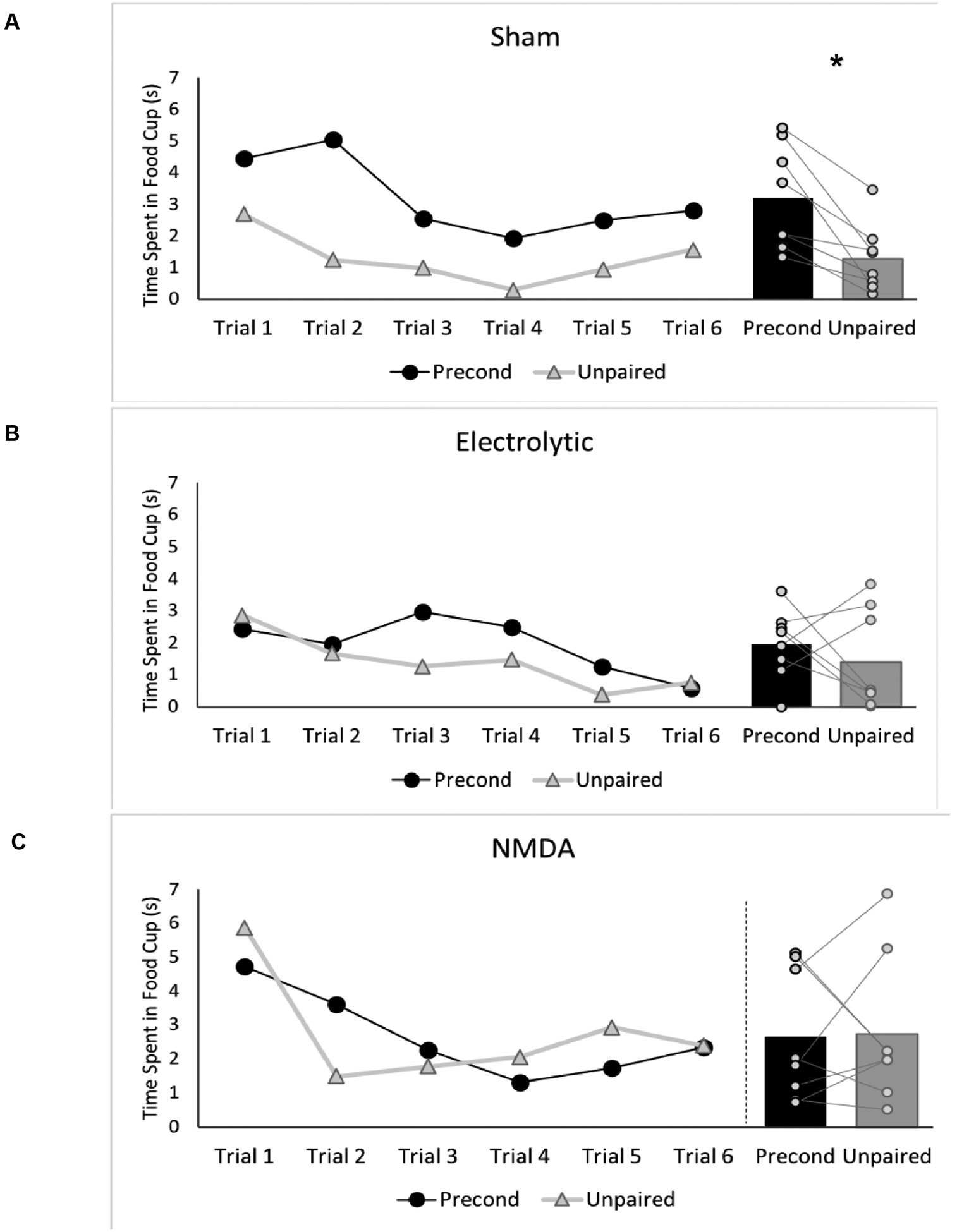
Food cup behavior during the Post-CS epoch of the preconditioned cue and unpaired cue for all six test trials (left panel) and averaged across all test trials for each cue (right panel) for (A) Sham rats, (B) RSC-Electrolytic rats, and (C) RSC-NMDA rats. *p < 0.05.
During the Pre-CS epoch, a 2 (Cue Type) × 3 (Group) repeated measures ANOVA did not reveal any significant main effects of Cue (preconditioned vs. unpaired trials) or of group (ps > 0.3). A Cue Type × Group interaction was not significant (p > 0.5). Furthermore, paired t-tests revealed there were no significant differences in Pre-CS responding between the preconditioned and unpaired cues in any group. For each group and each cue, the average amount of time spent in the food cup during the Pre-CS period was: Sham-lesioned rats (Preconditioned Cue: 0.66s [SEM ± 0.42]; Unpaired Cue: 0.72s [SEM ± 0.26]), Electrolytic-lesioned rats Preconditioned Cue: 0.22s [SEM ± 0.11]; Preconditioned Cue: 0.05s [SEM ± 0.05]), and NMDA-lesioned rats (Preconditioned Cue: 1.0s [SEM ± 0.37; Unpaired Cue: 0.84s [SEM ± 0.30]).
The average discrimination ratio scores for each group are shown in Figure 5. One-sample t-tests using a null hypothesis of a 0.5 discrimination ratio (i.e., no sensory preconditioning) indicated that only the sham-lesioned rats responded significantly more during the preconditioned cue than the non-preconditioned (unpaired) cue, reflected in a discrimination ratio during the food epoch that was significantly greater than 0.5 [t (7) = 5.2, p < 0.01]. Neither the RSC-electrolytic-lesioned nor NMDA-lesioned rats had discrimination ratios significantly higher than 0.5 [t (7) = 0.6, p > 0.5; t (7) = 0.05, p > 0.9, respectively].
Figure 5.
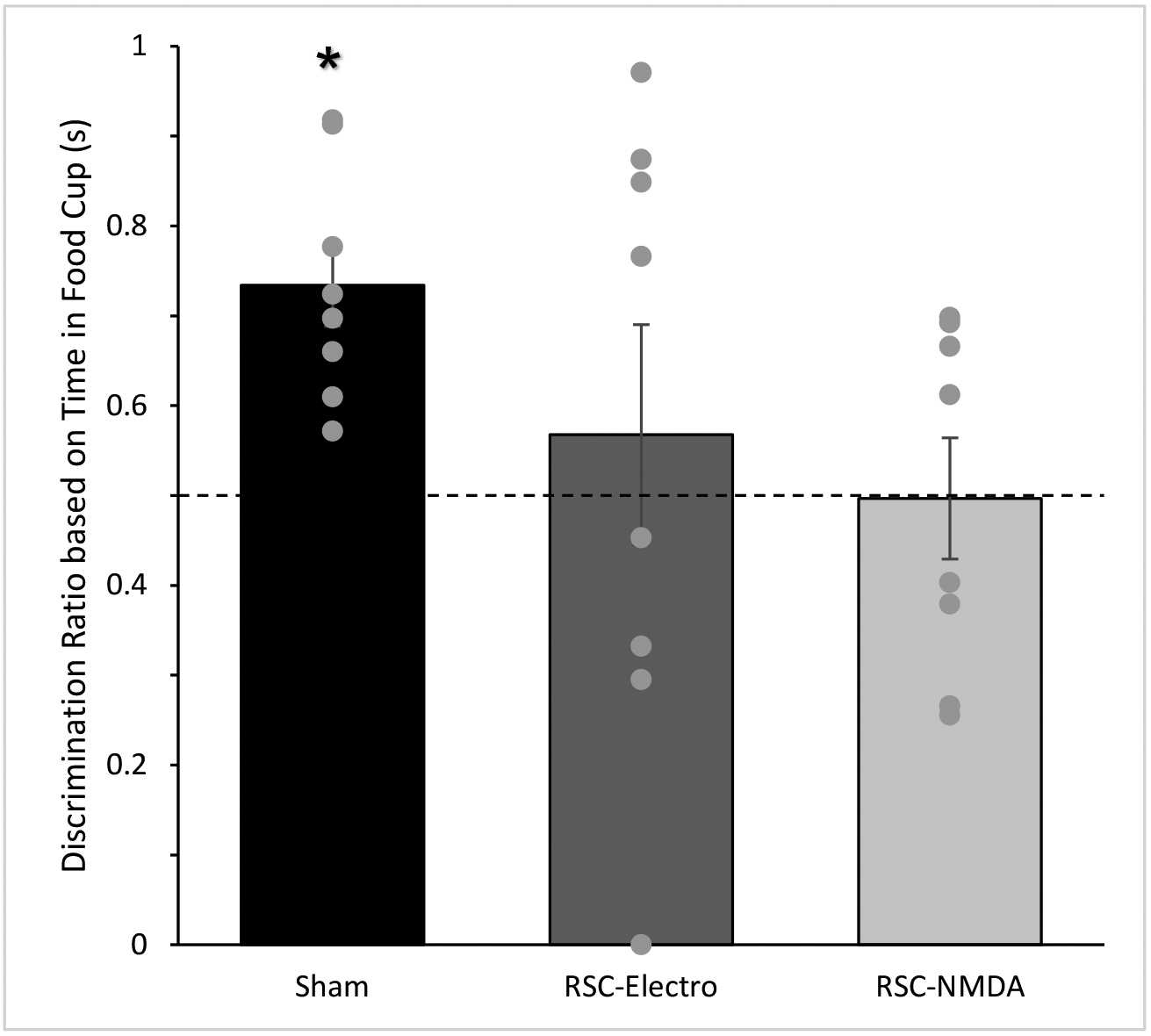
Discrimination ratios during the Post-CS epoch during the Test session. Control rats but not RSC-lesioned rats exhibited discrimination ratios significantly greater than 0.5. Dotted line indicates equal amounts of conditioned responding to both auditory cues (i.e., no sensory preconditioning). Data are means ± SEM, *p < 0.05.
Discussion
Here we used a sensory preconditioning procedure to investigate the role of the RSC in forming associations between neutral sensory stimuli (S-S) when the stimuli are of the same sensory modality (e.g., auditory). Rats received bilateral RSC lesions (neurotoxic or electrolytic) or sham-lesions (control group) prior to behavioral training. During the Preconditioning phase, one auditory cue (A1) was paired with a second auditory cue (click), while a third auditory cue (A3) was presented alone. During this phase of training, intact rats are thought to form an association between the two neutral auditory cues, A1 and the clicker (Rizley and Rescorla, 1972). In the subsequent Conditioning phase, the click was paired with food and no other cues were presented. In the final Test phase, A1 and A3 were presented alone on intermixed trials. Successful sensory preconditioning was observed in the control group, as evidenced by greater conditioned responding (food cup behavior) following presentations of A1 compared to A3 during the test phase, despite the fact that A1 was never directly paired with food reward. In contrast, RSC-lesioned rats exhibited similar levels of responding to the preconditioned cue and the unpaired cue. These effects were obtained even though RSC-lesioned rats exhibited normal conditioning when the click was paired with food in the Conditioning phase. Together, the results suggest that S-S learning during the Preconditioning phase was impaired in RSC lesioned rats and that an intact RSC is needed for within-modality S-S learning.
One possible alternative explanation for the present findings is that RSC-lesioned rats could not form an A2-food association during the Conditioning phase, which would also disrupt the observation of the sensory preconditioning effect. This seems unlikely, however, since conditioning to the click occurred similarly in lesions and control groups, as was also the case in prior sensory preconditioning studies involving RSC (Robinson et al., 2011). Similarly, other studies have demonstrated that single cue delay conditioning is intact following lesions or pharmacological inhibition of RSC (Keene & Bucci 2008a, 2008c; Jiang et al., 2018; Kwapis et al., 2015; cf. Todd et al., 2016). Together, these findings indicate that RSC damage does not impair the ability to form simple associations between single unimodal cues and biological relevant outcomes (US). A second alternative possibility is that RSC lesioned rats were unable to retrieve the click-US association during the Test session. This too seems unlikely since Robinson et al (2014) observed deficits in sensory preconditioning when RSC was silenced during preconditioning, but intact during the test phase of the experiment.
The current study therefore expands upon and replicates the results of prior studies that have tested the effects of RSC manipulations on multimodal sensory preconditioning (i.e., auditory-visual associations; Robinson et al., 2011; 2014). These previous studies have shown that lesioning the RSC prior to training impaired the sensory preconditioning effect using an auditory preconditioned cue that was paired with a visual cue (Robinson et al., 2011). Subsequently, Robinson and colleagues (2014) used a chemogenetic approach (Designer Receptors Exclusively Activated by Designer Drugs, DREADDs) to transiently inactivate the RSC only during the Preconditioning phase, leaving RSC functional during the conditioning and testing phases. Inactivating the RSC during preconditioning eliminated the sensory preconditioning effect in a procedure that also used multimodal stimuli (Robinson et al., 2014). The finding that RSC manipulations impact forming an association between cues of different sensory modalities is consistent with the neuroanatomical nature of RSC, in that RSC receives sensory input from multiple sensory modalities (Sugar et al., 2011; Todd et al., 2016). As such, it has been suggested that RSC may function as a hub for integrating sensory information (Bucci & Robinson, 2014; Kobayashi & Amaral, 2007; Wolbers & Buchel, 2005). It might have been expected that using unimodal cues would obviate the need for RSC in forming sensory associations, in that associations between auditory cues, for example, may only depend on higher order auditory regions such as secondary auditory cortex. However, Grosso et al. (2015) have demonstrated that inactivation of secondary auditory cortex does not impair sensory preconditioning with auditory cues. The authors proposed that the secondary auditory cortex is important for associating sensory cues and their affective (i.e. valance) properties rather than perceptual or S-S learning (Grosso et al., 2015). Although the procedures used by Grosso et al. (2015) differed in many ways from the present study (e.g., age of sensory preconditioning memory by the time of test, type of reinforcement), their findings raise the interesting possibility that the functional contribution of the RSC to sensory associations is distinct from secondary sensory cortices.
Prior studies have examined the role of other brain regions that are interconnected with the RSC, such as hippocampus, in sensory preconditioning procedures. Ward-Robinson et al. (2001) reported no effect of hippocampal lesions on sensory preconditioning during taste-aversion learning (Ward-Robinson et al., 2001), On the other hand, Talk et al. (2002) reported that hippocampal lesions impaired sensory preconditioning with two auditory cues. Interestingly, however, using electrophysiological methods, Talk et al. (2002) did not find a hippocampal neural correlate during the preconditioning phase. The role of the hippocampus in sensory preconditioning is thus somewhat equivocal, and may depend on a host of factors, including sensory modality or when the hippocampal manipulations/measurements were made during the procedures. Nevertheless, together with the findings of Robinson et al. (2011, 2014), the current study suggests that the RSC may have a fundamental role in establishing the initial S-S associations during preconditioning, in the absence of reinforcement. This is consistent with earlier anatomical studies which suggested that, based on its connections, RSC may function to form fundamental associations between cues, and the hippocampus may use those associations as a basis for more complex representations (Kobayashi and Amaral, 2007). For example, S-S relations encoded within the RSC and / or hippocampus might be necessary to later link the preconditioned cue with reward during the conditioning phase. Other areas such as the orbitofrontal cortex (OFC), which also encodes S-S representations, might access this information to guide behavior (Sadacca et al., 2018; Wikenheiser & Schoenbaum, 2016).
In summary, we have found that pretraining RSC lesions impaired the sensory preconditioning effect even when unimodal sensory cues were used in the procedure. Together with prior studies that used multimodal stimuli (Robinson et al, 2011, 2014), these findings suggest that RSC is essential for linking together cues prior to any reinforcement. It is possible that the RSC’s role in S-S associations (in the absence of reinforcement) may be selective; recent studies have demonstrated that the RSC does not necessarily contribute to learning and memory in other conditioning paradigms involving multiple cues (see Nelson et al., 2018; Todd et al., 2019). Nevertheless, the results of this and other sensory preconditioning experiments have suggested a role for the RSC in the formation of S-S associations in the absence of reinforcement, consistent with the framework provided by Bucci and Robinson (2014).
Acknowledgements
This work was supported by National Science Foundation grant IOS1353137 (D.J.B.) and National Institute of Drug Abuse grant T32DA037202 (D.I.F.). T.P.T was supported by the National Institute of Mental Health of the National Institutes of Health under Award Number K01MH116158. The context is solely the responsibility of the authors and does not necessarily represent the official view of the National Institutes of Health.
References
- Alexander AS, and Nitz DA (2015). Retrosplenial cortex maps the conjunction of internal and external spaces. Nat. Neurosci 18, 1143–1151 [DOI] [PubMed] [Google Scholar]
- Auger SD, Mullally SL, and Maguire EA (2012). Retrosplenial Cortex Codes for Permanent Landmarks. PLoS One 7, e43620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaisdell AP (2009). The role of associative processes in spatial, temporal, and causal cognition In Rational Animals, Irrational Animals, Watanabe S, Blaisdell AP, Huber L, and Young A, eds. (Keio University Press; ), pp. 153–172. [Google Scholar]
- Brogden WJ (1939). Sensory preconditioning of human subjects. J. Exp. Psychol 25, 323–332. [DOI] [PubMed] [Google Scholar]
- Bucci DJ, and Robinson S (2014). Toward a conceptualization of retrohippocampal contributions to learning and memory. Neurobiol. Learn. Mem 116, 197–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burwell RD, and Amaral DG (1998). Cortical afferents of the perirhinal, postrhinal, and entorhinal cortices of the rat. J. Comp. Neurol 398, 179–205. [DOI] [PubMed] [Google Scholar]
- Cho J, and Sharp PE (2001). Head direction, place, and movement correlates for cells in the rat retrosplenial cortex. Behav. Neurosci 115, 3–25. [DOI] [PubMed] [Google Scholar]
- Clark BJ, Bassett JP, Wang SS, and Taube JS (2010). Impaired head direction cell representation in the anterodorsal thalamus after lesions of the retrosplenial cortex. J. Neurosci 30, 5289–5302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran KA, Donnan MD, Tronson NC, Guzmán YF, Gao C, Jovasevic V, Guedea AL, and Radulovic J (2011). NMDA receptors in retrosplenial cortex are necessary for retrieval of recent and remote context fear memory. J. Neurosci 31, 11655–11659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriel M, Sparenborg SP, and Stolar N (1987). Hippocampal control of cingulate cortical and anterior thalamic information processing during learning in rabbits. Exp. Brain Res 67, 131–152. [DOI] [PubMed] [Google Scholar]
- Grosso A, Cambiaghi M, Renna A, Milano L, Roberto Merlo G, Sacco T, and Sacchetti B (2015). The higher order auditory cortex is involved in the assignment of affective value to sensory stimuli. Nat. Commun 6, 8886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harker KT, and Whishaw IQ (2004). Impaired place navigation in place and matching-to-place swimming pool tasks follows both retrosplenial cortex lesions and cingulum bundle lesions in rats. Hippocampus, 14, 224–231. [DOI] [PubMed] [Google Scholar]
- Iordanova MD, Good M, and Honey RC (2011). Retrieval-mediated learning involving episodes requires synaptic plasticity in the hippocampus. J. Neurosci 31, 7156–7162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang MY, De Angeli NE, Bucci DJ, and Todd TP (2018). Retrosplenial cortex has a time-dependent role in memory for visual stimuli. Behav. Neurosci 132, 396–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keene CS, and Bucci DJ (2008). Involvement of the retrosplenial cortex in processing multiple conditioned stimuli. Behav. Neurosci 122, 651–658. [DOI] [PubMed] [Google Scholar]
- Keene CS, and Bucci DJ (2009). Damage to the retrosplenial cortex produces specific impairments in spatial working memory. Neurobiol. Learn. Mem 91, 408–414. [DOI] [PubMed] [Google Scholar]
- Keene CS, and Bucci DJ (2008). Contributions of the retrosplenial and posterior parietal cortices to cue-specific and contextual fear conditioning. Behav. Neurosci 122, 89–97. [DOI] [PubMed] [Google Scholar]
- Kerr KM, Agster KL, Furtak SC, and Burwell RD (2007). Functional neuroanatomy of the parahippocampal region: The lateral and medial entorhinal areas. Hippocampus, 17, 697–708. [DOI] [PubMed] [Google Scholar]
- Kobayashi Y, and Amaral DG (2007). Macaque monkey retrosplenial cortex: III. Cortical efferents. J. Comp. Neurol 502, 810–833. [DOI] [PubMed] [Google Scholar]
- Kwapis JL, Jarome TJ, Lee JL, and Helmstetter FJ (2015). The retrosplenial cortex is involved in the formation of memory for context and trace fear conditioning. Neurobiol. Learn. Mem 123, 110–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwapis JL, Jarome TJ, Lee JL, Gilmartin MR, and Helmstetter FJ (2014). Extinguishing trace fear engages the retrosplenial cortex rather than the amygdala. Neurobiol. Learn. Mem 113, 41–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson AJD, Hindley EL, Vann SD, and Aggleton JP (2018). When is the rat retrosplenial cortex required for stimulus integration? Behav. Neurosci 132, 366–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson AJD, Hindley EL, Haddon JE, Vann SD, and Aggleton JP (2014). A novel role for the rat retrosplenial cortex in cognitive control. Learn. Mem 21, 90–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parnell R, Grasby K, and Talk A (2012). The prefrontal cortex is required for incidental encoding but not recollection of source information in rodents. Behav. Brain Res 232, 77–83. [DOI] [PubMed] [Google Scholar]
- Rizley RC, and Rescorla RA (1972). Associations in second-order conditioning and sensory preconditioning. J. Comp. Physiol. Psychol 81, 1–11. [DOI] [PubMed] [Google Scholar]
- Robinson S, Keene CS, Iaccarino HF, Duan D, Bucci DJ, and Author C (2011). Involvement of Retrosplenial Cortex in Forming Associations Between Multiple Sensory Stimuli. Behav Neurosci. 125, 578–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson S, Todd TP, Pasternak AR, Luikart BW, Skelton PD, Urban DJ, and Bucci DJ (2014). Chemogenetic silencing of neurons in retrosplenial cortex disrupts sensory preconditioning. J Neurosci. 34, 10982–10988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadacca BF, Wied HM, Lopatina N, Saini GK, Nemirovsky D, and Schoenbaum G (2018). Orbitofrontal neurons signal sensory associations underlying model-based inference in a sensory preconditioning task. eLife 7, 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DM, Wakeman D, Patel J, and Gabriel M (2004). Fornix lesions impair context-related cingulothalamic neuronal patterns and concurrent discrimination learning in rabbits (Oryctolagus cuniculus). Behav. Neurosci 118, 1225–1239. [DOI] [PubMed] [Google Scholar]
- Sugar J, Witter MP, van Strien NM, and Cappaert NLM (2011). The Retrosplenial Cortex: Intrinsic Connectivity and Connections with the (Para)Hippocampal Region in the Rat. An Interactive Connectome. Front. Neuroinform. 5, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talk AC, Gandhi CC, and Matzel LD (2002). Hippocampal function during behaviorally silent associative learning: Dissociation of memory storage and expression. Hippocampus, 12, 648–656. [DOI] [PubMed] [Google Scholar]
- Todd TP, Mehlman ML, Keene CS, DeAngeli NE, and Bucci DJ (2016). Retrosplenial cortex is required for the retrieval of remote memory for auditory cues. Learn. Mem 23, 278–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd TP, Fournier DI, and Bucci DJ (2019). Retrosplenial cortex and its role in cue-specific learning and memory. Neurosci. Biobehav. Rev 107, 713–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Groen T, and Wyss JM (1990). Connections of the Retrosplenial Granular A Cortex in the Rat. J. Comp. Neurol 300, 593–606. [DOI] [PubMed] [Google Scholar]
- van Groen T, and Wyss JM (1990). The connections of presubiculum and parasubiculum in the rat. Brain Res. 518, 227–243. [DOI] [PubMed] [Google Scholar]
- van Groen T, and Wyss JM (1992). Connections of the Retrosplenial Dysgranular Cortex in the Rat. J. Comp. Neurol 300, 593–606. [DOI] [PubMed] [Google Scholar]
- van Groen T, and Wyss JM (1992). Projections from the laterodorsal nucleus of the thalamus to the limbic and visual cortices in the rat. J. Comp. Neurol 324, 427–448. [DOI] [PubMed] [Google Scholar]
- van Groen T, and Wyss JM (2003). Connections of the retrosplenial granular b cortex in the rat. J. Comp. Neurol 463, 249–263. [DOI] [PubMed] [Google Scholar]
- van Groen T, Kadish I, and Wyss JM (2004). Retrosplenial cortex lesions of area Rgb (but not of area Rga) impair spatial learning and memory in the rat. Behav. Brain Res 154, 483–491. [DOI] [PubMed] [Google Scholar]
- Vann SD, and Aggleton JP (2004). Testing the importance of the retrosplenial guidance system: effects of different sized retrosplenial cortex lesions on heading direction and spatial working memory. Behav. Brain Res 155, 97–108. [DOI] [PubMed] [Google Scholar]
- Vogt BA, and Miller MW (1983). Cortical connections between rat cingulate cortex and visual, motor, and postsubicular cortices. J. Comp. Neurol 216, 192–210. [DOI] [PubMed] [Google Scholar]
- Ward-Robinson J, Coutureau E, Good M, Honey RC, Killcross AS, and Oswald CJP (2001). Excitotoxic lesions of the hippocampus leave sensory preconditioning intact: Implications for models of hippocampal function. Behav. Neurosci 115, 1357–1362. [DOI] [PubMed] [Google Scholar]
- Wikenheiser AM, and Schoenbaum G (2016). Over the river, through the woods: cognitive maps in the hippocampus and orbitofrontal cortex. Nat. Rev. Neurosci 17, 513–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wimmer E, and Shohamy D (2012). Preference by Association: How Memory Mechanisms in the Hippocampus Bias Decisions. Science 338, 270–273. [DOI] [PubMed] [Google Scholar]
- Wolbers T, and Buchel C (2005). Dissociable Retrosplenial and Hippocampal Contributions to Successful Formation of Survey Representations. J. Neurosci 25, 3333–3340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyss JM, and van Groen T (1992). Connections between the retrosplenial cortex and the hippocampal-formation in the rat - a review. Hippocampus 2, 1–12. [DOI] [PubMed] [Google Scholar]


